Abstract
The cholera-like enterotoxins (CLETS), cholera toxin (CT) and Escherichia coli heat-labile toxin (LT), are powerful mucosal adjuvants. Here we show that these toxins also induce a long-lived blockade (of at least 6 months) on the induction of oral tolerance when they are coadministered with the antigen ovalbumin. Strikingly, only enzymatically active CLETS induced this blockade on the induction of oral tolerance. In this regard, the enzymatically inactive mutants of CT and LT, CTK63 and LTK63, and their recombinant B pentamers, rCTB and rLTB, failed to block the induction of oral tolerance, demonstrating a stringent requirement for an enzymatically active A domain in this phenomenon. Together with the results of other recent studies, these results indicate that the enzymatic activity of CLETS, most likely cyclic AMP elevation, is responsible for their adjuvant effects. The results of this study also indicate that measuring the ability of putative mucosal adjuvants to block the induction of oral tolerance may be a superior method for measuring mucosal adjuvanticity.
Oral tolerance is a state of systemic hyporesponsiveness that occurs after the ingestion of protein antigens and is thought to be important for the maintenance of homeostasis between the immune system and food antigens (33). Systemic challenge with an antigen in Freund's adjuvant following prior oral administration of that antigen results in diminished antibody and cytolytic responses (36). Feeding of a single high dose or multiple low doses of a protein antigen induces oral tolerance to that antigen (33). The mechanism of oral tolerance is poorly understood; however, at least two independent mechanisms appear to be involved. First, feeding of a high dose of antigen induces clonal T-cell anergy or deletion, as evidenced by reduced interleukin-2 production and a lack of T-cell proliferation (8, 27, 37). Second, feeding of multiple low doses of antigen induces suppressor T cells that secrete transforming growth factor β (9, 22). Interestingly, mucosal adjuvants such as the cholera-like enterotoxins (CLETS), cholera toxin (CT), and Escherichia coli heat-labile enterotoxin (LT) block the induction of oral tolerance when coadministered with an antigen. In the present study, we determined whether the blockade on the induction of oral tolerance to antigens induced by CLETS is transient or long-lived and what role the enzymatic activity of the toxins plays in this phenomenon.
CT and LT are AB5 enterotoxins consisting of a 27-kDa catalytic A domain anchored in a pentamer of identical 11.7-kDa B subunits (28). The B pentamer of CT (CTB) binds exclusively to GM1 gangliosides on the surfaces of cells, while the B pentamer of LT (LTB) binds to other gangliosides in addition to GM1 (16, 20). These toxins exploit the host protein retention and degradation pathways to gain access to the cytoplasm (reviewed in reference 19). In the cytosol, their A1 subunits catalyze the transfer of an ADP-ribose from NAD to stimulatory alpha subunits of G proteins. After ADP ribosylation, the stimulatory alpha subunits of the G proteins bind to adenylate cyclase and constitutively activate it, leading to a sustained increase in intracellular cyclic AMP concentration (7). This sustained increase in intracellular cyclic AMP levels is responsible for the toxicity to intestinal epithelial cells and for the massive fluid secretion and diarrhea associated with infections by pathogens producing these toxins.
CLETS are also powerful mucosal immunogens and adjuvants that block the induction of oral tolerance and induce strong primary and secondary antibody responses and long-lasting immunologic memory to themselves and to coadministered antigens (23, 34). These toxins are routinely used as adjuvants in mice, where antibody responses to CT and bystander antigens can last for at least 2 years (23, 34). Unfortunately, severe toxic effects make CLETS unsuitable mucosal adjuvants for human use (reviewed in reference 15). To avoid toxicity, enzymatically inactive derivatives of CLETS have been developed either by removing the A domain (32) or by rendering it enzymatically inactive by site-directed mutagenesis (10, 12, 13, 14, 17, 26, 40). Adjuvant studies using recombinant CTB (rCTB) or recombinant LTB (rLTB) have generated conflicting results. Several studies reported an adjuvant effect using rCTB or rLTB (11, 21, 39), while others reported no adjuvant effect (6, 24, 34). Likewise, adjuvant studies using enzymatically inactive mutants of CLETS have generated conflicting results. For instance, CTK63 and LTK63 are two well-studied examples of enzymatically inactive mutants of CLETS (12, 13, 40). These mutants have a lysine residue substitution in the ADP-binding cleft of the A domain. This substitution renders the A domain enzymatically inactive without altering the native structure (10, 14, 17, 26). Several studies have reported that enzymatically inactive mutants retain adjuvanticity (10, 12, 13, 14, 17, 26, 40), while another has reported that they do not (25). Although most of these studies reported adjuvanticity for these mutants, the effects were generally weak compared to wild-type toxins. Of note, the effects of these mutants on the induction of oral tolerance to coadministered antigens have never been directly studied.
Interestingly, other studies have indicated a prominent role for the enzymatic activity of CLETS in their adjuvant effects. For example, a mutant LT toxin (LTR72) with reduced but not abolished enzymatic activity is a much more potent mucosal adjuvant than the enzymatically inactive LTK63 mutant (18, 29). Another group tested the role of the enzymatically active A domain CT by genetically fusing the active A domain to a synthetic analogue of protein A (1-3). This construct was found to possess potent adjuvant effects (1-3). A role for the enzymatic activity of CT in its adjuvanticity was also indicated when we showed that the A1 subunit of CT possesses potent adjuvant effects when coexpressed in a DNA vaccine (4). Also, we investigated the roles of the enzymatic activity and nontoxic AB complexes of CLETS in their ability to induce the maturation of monocyte-derived dendritic cells (DCs) in vitro. This investigation was of interest, as emerging evidence suggests that DCs may be the principal mediators of the adjuvant effects of CLETS in vivo (30, 38). We found that enzymatic activity is strictly required for CLETS to activate monocyte-derived DCs to mature and that the nontoxic AB complex plays no role in maturation (5).
It is well known that CLETS block the induction of oral tolerance when administered at the same time as the antigen. Likewise, it is well known that CLETS do not break previously established oral tolerance. However, it has never been shown if oral tolerance to an antigen can be induced in animals previously immunized with that antigen in the presence of CT or LT. In this regard, it is not known if the blockade on the induction of oral tolerance to coadministered antigens induced by CLETS is transient or long-lived. As there is conflicting evidence with regard to the roles of the enzymatic activity of CLETS and their nontoxic AB complexes in adjuvanticity, we studied their contributions to the blockade on the induction of oral tolerance. This phenomenon is important because true oral adjuvants must block the induction of oral tolerance to coadministered antigens at the time of immunization and prevent induction of oral tolerance to a coadministered antigen in the event of subsequent exposures.
In the present study, we determined whether the blockade on the induction of oral tolerance induced by CLETS is long- lived and what roles are played by the enzymatic activity and the nontoxic AB complexes of CLETS in this phenomenon. Strikingly, we found that wild-type CLETS induce a long-lived blockade (of at least 6 months) on the induction of oral tolerance to a coadministered antigen (ovalbumin [OVA]) and that this blockade is strictly dependent on enzymatic activity.
MATERIALS AND METHODS
Reagents.
Complete Freund's adjuvant (CFA), incomplete Freund's adjuvant (IFA), and phosphate-buffered saline (PBS) were purchased from GIBCO BRL (Grand Island, N.Y.). CT and rCTB were purchased from List Biological Laboratories (Campbell, Calif.). LT and OVA were purchased from Sigma (St. Louis, Mo.). CTK63, LTK63, and rLTB were a kind gift from Rino Rappouli (Chiron Immunological Research Institute, Siena, Italy). CTK63, LTK63, rCTB, and rLTB were passed over Detoxi-Gel columns (Pierce, Rockford, Ill.) to remove contaminating lipopolysaccharides (LPS). LPS levels were quantified by a Limulus assay (Bio-Whittaker, Walkersville, Md.). There were no more than 100 pg of LPS in the oral inoculations described below.
Animals.
BALB/C mice, 6 to 8 weeks old, were obtained from Harlan (Indianapolis, Ind.).
Immunization strategy for preliminary experiment.
Groups of five mice were immunized twice orally 1 week apart by using a ball-tipped feeding needle with 100 μg of OVA and 20 μg of CT in a total volume of 200 μl of PBS. Oral tolerance and systemic immunization control mice received PBS alone. One week later, all mice except the systemic immunization control mice were fed 25 mg of OVA in 250 μl of PBS once per day for 5 days by using a ball-tipped feeding needle. One week after the last OVA feeding, all mice were immunized intraperitoneally (i.p.) with 100 μg of OVA in CFA. Two weeks later, all mice were bled from the tail vein, an equal volume of blood from each mouse within a group was pooled, and the sera were isolated. Endpoint titers of anti-OVA immunoglobulin G (IgG) from pooled sera were determined by enzyme-linked immunosorbent assay (ELISA) (below).
Immunization strategy for long-term experiment.
Groups of five mice were immunized twice orally 1 week apart by using a ball-tipped feeding needle with 100 μg of OVA and 20 μg of CT or LT in a total volume of 200 μl of PBS. Oral tolerance and systemic immunization control mice received PBS alone. Six months later, all mice except the systemic immunization control mice were fed 25 mg of OVA in 250 μl of PBS once per day for 5 days by using a ball-tipped feeding needle. One week after the last OVA feeding, all mice were immunized i.p. with 100 μg of OVA in CFA. Two weeks later, all mice were given booster inoculations of 100 μg of OVA in IFA i.p. Four weeks after the booster inoculations, all mice were bled from the tail vein and serum from individual mice was isolated. Endpoint titers of anti-OVA IgG from the serum of individual mice were determined by ELISA (below).
Immunization strategy for mutant toxin experiment.
Groups of five mice were immunized twice orally 1 week apart by using a ball-tipped feeding needle with 100 μg of OVA and 20 μg of CT, LT, CTK63, LTK63, rCTB, or rLTB in a total volume of 200 μl of PBS. Oral tolerance and systemic immunization control mice received PBS alone. One week later, all mice except the systemic immunization control mice were fed 25 mg of OVA in 250 μl of PBS once per day for 5 days with a ball-tipped feeding needle. One week after the last OVA feeding, all mice were immunized i.p. with 100 μg of OVA in CFA. Two weeks later, all mice were given booster inoculations of 100 μg of OVA in IFA i.p. Four weeks after the booster inoculations, all mice were bled from the tail vein and serum from individual mice was isolated. Endpoint titers of anti-OVA IgG from the serum of individual mice were determined by ELISA (below).
ELISA.
Solid-phase ELISA was used to determine OVA-specific antibody titers in the sera. Briefly, 96-well microtiter plates (Nunc, Rochester, N.Y.) were coated with 10 μg of OVA/ml in PBS at 37°C for 1 h. The plates were washed three times with Tris-buffered saline (TBS) and then blocked with BLOTTO (5% wt/vol nonfat dried milk in TBS) at room temperature for 30 min. Serially diluted sera were added to the wells and incubated at room temperature for 1 h and then washed three times with TBS. Peroxidase-conjugated goat anti-mouse IgG (Kirkegaard & Perry, Gaithersburg, Md.) diluted 1/1,000 in BLOTTO was added and incubated at room temperature for 1 h. The plates were washed three times with TBS before TMB peroxidase substrate (Kirkegaard & Perry) was added and they were incubated for 1 to 3 min. The reaction was stopped by adding 1 N H2SO4. Absorbance was read at 450 nm by using a Victor 1420 multilabel counter (EG&G Wallac, Turku, Finland).
Endpoint titer determination.
ELISA endpoint titers were calculated as the inverse of the last serum dilution that produced an increase in the absorbance at 450 nm greater than the mean of the negative control wells plus three standard error values.
RESULTS
Oral immunization with CT or LT and OVA prevents the subsequent induction of oral tolerance to OVA.
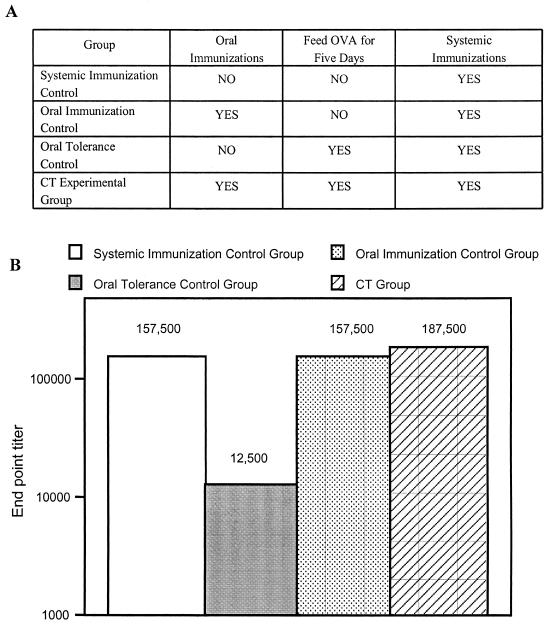
It is well known that the simultaneous feeding of CT and antigen renders the antigen immunogenic and prevents the induction of oral tolerance; however, it has not been determined if the blockade on the induction of oral tolerance induced by CT is transient or long-lived. In addition, true oral adjuvants must block the induction of oral tolerance to coadministered antigens at the time of immunization and prevent induction of oral tolerance to a coadministered antigen in the event of subsequent exposures. For this reason, in a preliminary experiment we determined if oral immunization with CT and OVA could inhibit the induction of oral tolerance to OVA 1 week after immunization. For this experiment, groups of five mice were immunized as described in Materials and Methods and outlined in Fig. 1A. As shown in Fig. 1B, oral tolerance to OVA was not induced in mice previously immunized orally with CT and OVA. In this regard, anti-OVA endpoint titers for mice previously immunized orally with OVA and CT and subsequently fed large doses of OVA followed by a systemic challenge with OVA in CFA (CT group) are similar to those for systemic immunization control mice challenged with OVA in CFA. The anti-OVA endpoint titers for the mice in the systemic immunization control group and the CT group were more than 10-fold higher than those for mice in the oral tolerance control group that were fed large doses of OVA without receiving oral immunizations with OVA and CT. These results also indicate that CT blocks the subsequent induction of oral tolerance to coadministered antigens.
FIG. 1.
Cholera toxin induces a blockade on the induction of oral tolerance. (A) Table displaying immunization strategy. (B) Endpoint titers of OVA-specific IgG in total serum are shown. Five mice per group were immunized as outlined in panel A and detailed in Materials and Methods. Blood was collected from the tail vein at the indicated time point, and an equal volume from each mouse within a group was pooled before isolating the sera. Endpoint titers were determined by serial dilutions by using IgG ELISA with OVA-coated plates.
Interestingly, the anti-OVA endpoint titers for mice in the oral immunization control group (mice immunized orally with CT and OVA and systemically with OVA in CFA) were equal to those for mice in the systemic immunization control group. This result indicates that oral immunization with OVA and CT before systemic immunization with OVA in CFA does not boost anti-OVA endpoint titers over those for mice immunized with OVA in CFA alone.
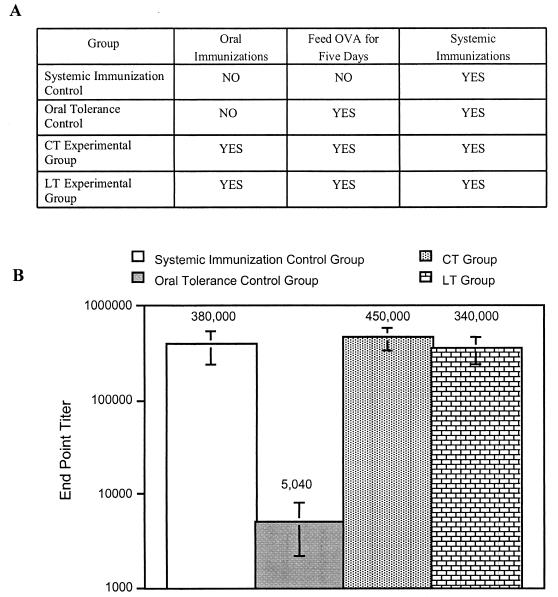
The preliminary experiment above showed that the blockade on the induction of oral tolerance induced by CT lasts at least 1 week. Next, we determined if this blockade is long-lived and if the related enterotoxin LT shares this ability. For this experiment, groups of five mice were immunized as described in Materials and Methods and outlined in Fig. 2A. As shown in Fig. 2B, oral tolerance to OVA was not induced 6 months after oral immunization with OVA and CT or LT. In this regard, anti-OVA endpoint titers for mice previously immunized orally with OVA and CT or LT and 6 months later fed large doses of OVA followed by systemic challenges with OVA were similar to those for systemic immunization control mice. The anti-OVA endpoint titers for the mice in the systemic immunization control group, the CT group, and the LT group were greater-than-60-fold higher than those for mice in the oral tolerance control group that were fed large doses of OVA without receiving oral immunizations with OVA and CT or LT.
FIG. 2.
The blockade on the induction of oral tolerance induced by CT and LT lasts for at least 6 months. (A) Table displaying immunization strategy. (B) Groups of mice were immunized as outlined in panel A and detailed in Materials and Methods. Blood was collected from the tail vein and serum was isolated from individual mice. Endpoint titers were determined by serial dilutions by using IgG ELISA with OVA-coated plates. Endpoint titers of OVA-specific IgG in total serum are shown.
Note that giving booster immunizations with OVA in IFA to the mice and waiting an additional 3 weeks before determining IgG concentrations in serum helped reduce the variability between individual mice and enhanced the difference between the endpoint titers for mice in the systemic immunization control group and the oral tolerance group (data not shown). In addition, the variability in endpoint titers between the mice in these groups was less than that for groups of mice that received between 1 and 4 oral immunizations of CT with OVA from other immunization experiments (data not shown). Together, the results of these experiments indicate that the blockade on the induction of oral tolerance to coadministered antigens induced by CT and LT is long-lived and possibly lifelong.
An enzymatically active A domain is necessary for CLETS to block the induction of oral tolerance to OVA.
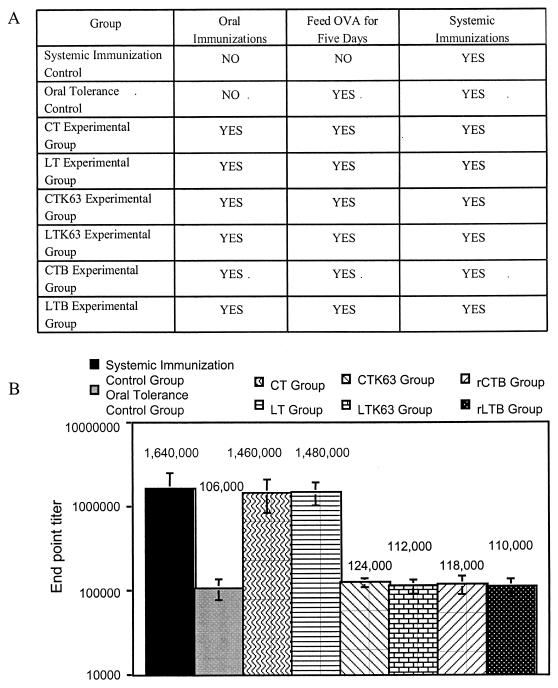
There is considerable interest in identifying nontoxic derivatives of CT and LT that retain adjuvant activity. The enzymatically inactive nontoxic derivatives of CT and LT, CTK63, LTK63, CTB, and LTB, have all been reported to retain adjuvant effects, although the reported effects are generally weaker than those for the native toxins (10, 12, 13, 14, 17, 26, 40). As was stated previously, true oral adjuvants must block the induction of oral tolerance to coadministered antigens at the time of immunization and prevent induction of oral tolerance to a coadministered antigen in the event of subsequent exposures. The results above clearly demonstrate that the wild-type CLETS are true oral adjuvants. We speculated that constructs based on CLETS that lack enzymatic activity may not be true oral adjuvants and may not prevent the induction of oral tolerance to the coadministered antigen upon subsequent exposures. For this reason, we determined if CTK63, LTK63, rCTB, or rLTB shared the ability of the wild-type CLETS to induce a blockade on the induction of oral tolerance.
For this experiment, groups of five mice were immunized as described in Materials and Methods and outlined in Fig. 3A. As expected, Fig. 3B shows that both CT and LT blocked the subsequent induction of oral tolerance to OVA. In contrast to the wild-type toxins, Fig. 3 shows that the ADP ribosylation-defective mutants CTK63 and LTK63 and the recombinant B subunits rCTB and rLTB failed to block the induction of oral tolerance to OVA. In this regard, the anti-OVA endpoint titers for mice previously immunized orally with OVA and CTK63, LTK63, rCTB, or rLTB and subsequently fed large doses of OVA followed by a systemic challenge with OVA are similar to those for oral tolerance control mice fed large doses of OVA followed by a systemic challenge with OVA. The results of these experiments indicate that enzymatic activity is required for CLETS to block the induction of oral tolerance.
FIG. 3.
Enzymatic activity is required for CLETS to induce a blockade on the induction of oral tolerance. (A) Table displaying immunization strategy. (B) Groups of five mice were immunized as shown in panel A and detailed in Materials and Methods. Blood was collected from the tail vein, and serum was isolated from individual mice. Endpoint titers were determined by serial dilutions by using IgG ELISA with OVA-coated plates. Endpoint titers of OVA-specific IgG in total serum are shown. The values shown are representative of the results of two experiments.
DISCUSSION
In general, adjuvants boost the immune response to coadministered antigens. This ability may stem from their ability to activate DCs at the immunization site. In this regard, LPS, CT, and LT each activate DCs to mature (5, 35). Recently, we showed that the enzymatically inactive derivatives of CT and LT, CTK63, LTK63, rCTB, and rLTB, failed to activate DCs to mature (5). For this reason, we speculated that these enzymatically inactive derivatives of CLETS may not be true adjuvants. For instance, effective mucosal adjuvants must overcome the induction of mucosal tolerance and induce immunologic responses to coadministered antigens. Wild-type CLETS are powerful mucosal adjuvants that block the induction of oral tolerance and induce both strong primary and secondary antibody responses and long-lasting immunologic memory to themselves and to coadministered antigens. Although applying antigens to mucosal surfaces induces mucosal tolerance, this tolerance is not characterized by a complete lack of immunologic responsiveness to the antigen. By contrast, low level IgA and IgG antibodies can be detected after mucosal administration of antigen. Therefore, it is possible that a putative mucosal adjuvant could increase the IgA and IgG response to coadministered antigens without affecting the induction of mucosal tolerance. Indeed, the results of this study show that enzymatic activity is required for CLETS to block the induction of oral tolerance.
The results of this study further indicate that mutants of CLETS with reduced but not abolished enzymatic activity, such as the LTR72 mutant (18, 31), may be more practical as nontoxic mucosal adjuvants than mutants completely devoid of enzymatic activity. These results also indicate that constructs based on the enzymatic activity of CLETS employing alternate targeting strategies may hold promise as mucosal and systemic adjuvants. In support of this notion, one group has constructed an adjuvant based on the enzymatically active A domain of CT by fusing the A1 domain of CT to a synthetic analogue of protein A (1-3). This construct specifically targets B cells and has been shown to be a potent adjuvant (1-3). Recent evidence indicates that DCs may also be good targets for CTA1-based adjuvants. In this regard, we are currently designing constructs to target CTA1 to DCs. In addition, we recently demonstrated a role for the enzymatic activity of CLETS in their adjuvanticity by showing that the A1 subunit of CT is a potent adjuvant when coexpressed in a DNA vaccine (4).
The immunization regimen described in this study measures the ability of a putative mucosal adjuvant to block the induction of oral tolerance, not the immune response generated from the mucosal immunization. For this reason, this regimen may provide a clearer picture of the adjuvanticity of putative mucosal adjuvants. In addition, this immunization regimen consistently yields clear differences in endpoint titers between mice that have and have not been made orally tolerant, and the variability among endpoint titers within groups is lower than that we observed with other mucosal immunization regimens. For these reasons, measuring the ability of a putative mucosal adjuvant to block the induction of mucosal tolerance to coadministered antigens may provide a superior method for determining its usefulness as a mucosal adjuvant.
Acknowledgments
We thank Rino Rappouli of the Chiron Immunological Research Institute, Siena, Italy, for the generous supply of CTK63, LTK63, and rLTB.
This work was supported by NIH grants AI38192 and AI43046 to G.K.L.
Editor: A. D. O'Brien
REFERENCES
- 1.Agren, L., B. Lowenadler, and N. Lycke. 1998. A novel concept in mucosal adjuvanticity: the CTA1-DD adjuvant is a B cell-targeted fusion protein that incorporates the enzymatically active cholera toxin A1 subunit. Immunol. Cell Biol. 76:280-287. [DOI] [PubMed] [Google Scholar]
- 2.Agren, L., E. Sverremark, L. Ekman, K. Schon, B. Lowenadler, C. Fernandez, and N. Lycke. 2000. The ADP-ribosylating CTA1-DD adjuvant enhances T cell-dependent and independent responses by direct action on B cells involving anti-apoptotic Bcl-2- and germinal center-promoting effects. J. Immunol. 164:6276-6286. [DOI] [PubMed] [Google Scholar]
- 3.Agren, L. C., L. Ekman, B. Lowenadler, and N. Y. Lycke. 1997. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J. Immunol. 158:3936-3946. [PubMed] [Google Scholar]
- 4.Bagley, K. C., M. T. Shata, D. Y. Onyabe, A. L. DeVico, T. R. Fouts, G. K. Lewis, and D. M. Hone. 2003. Immunogenicity of DNA vaccines that direct the coincident expression of the 120 kDa glycoprotein of human immunodeficiency virus and the catalytic domain of cholera toxin. Vaccine 21:3335-3341. [DOI] [PubMed] [Google Scholar]
- 5.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect. Immun. 70:5533-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard, T. G., N. Lycke, S. J. Czinn, and J. G. Nedrud. 1998. Recombinant cholera toxin B subunit is not an effective mucosal adjuvant for oral immunization of mice against Helicobacter felis. Immunology 94:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y., J. Inobe, R. Marks, P. Gonnella, V. K. Kuchroo, and H. L. Weiner. 1995. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376:177-180. (Erratum, 377:257.) [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y., J. Inobe, and H. L. Weiner. 1995. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J. Immunol. 155:910-916. [PubMed] [Google Scholar]
- 10.Covone, M. G., M. Brocchi, E. Palla, W. Dias da Silveira, R. Rappuoli, and C. L. Galeotti. 1998. Levels of expression and immunogenicity of attenuated Salmonella enterica serovar Typhimurium strains expressing Escherichia coli mutant heat-labile enterotoxin. Infect. Immun. 66:224-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Haan, L., W. R. Verweij, I. K. Feil, M. Holtrop, W. G. Hol, E. Agsteribbe, and J. Wilschut. 1998. Role of GM1 binding in the mucosal immunogenicity and adjuvant activity of the Escherichia coli heat-labile enterotoxin and its B subunit. Immunology 94:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douce, G., C. Turcotte, I. Cropley, M. Roberts, M. Pizza, M. Domenghini, R. Rappuoli, and G. Dougan. 1995. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc. Natl. Acad. Sci. USA 92:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douce, G., M. Fontana, M. Pizza, R. Rappuoli, and G. Dougan. 1997. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect. Immun. 65:2821-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujihashi, K., T. Koga, F. W. van Ginkel, Y. Hagiwara, and J. R. McGhee. 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20:2431-2438. [DOI] [PubMed] [Google Scholar]
- 16.Fukuta, S., J. L. Magnani, E. M. Twiddy, R. K. Holmes, and V. Ginsburg. 1988. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 56:1748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghiara, P., M. Rossi, M. Marchetti, A. Di Tommaso, C. Vindigni, F. Ciampolini, A. Covacci, J. L. Telford, M. T. De Magistris, M. Pizza, R. Rappuoli, and G. Del Giudice. 1997. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect. Immun. 65:4996-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliani, M. M., G. Del Giudice, V. Giannelli, G. Dougan, G. Douce, R. Rappuoli, and M. Pizza. 1998. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 187:1123-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazes, B., and R. J. Read. 1997. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36:11051-11054. [DOI] [PubMed] [Google Scholar]
- 20.Heyningen, S. V. 1974. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183:656-657. [DOI] [PubMed] [Google Scholar]
- 21.Isaka, M., Y. Yasuda, S. Kozuka, Y. Miura, T. Taniguchi, K. Matano, N. Goto, and K. Tochikubo. 1998. Systemic and mucosal immune responses of mice to aluminum-adsorbed or aluminum-non-adsorbed tetanus toxoid administered intranasally with recombinant cholera toxin B subunit. Vaccine 16:1620-1626. [DOI] [PubMed] [Google Scholar]
- 22.Khoury, S. J., W. W. Hancock, and H. L. Weiner. 1992. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J. Exp. Med. 176:1355-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lycke, N., and J. Holmgren. 1987. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand. J. Immunol. 25:407-412. [DOI] [PubMed] [Google Scholar]
- 24.Lycke, N., and J. Holmgren. 1986. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology 59:301-308. [PMC free article] [PubMed] [Google Scholar]
- 25.Lycke, N., T. Tsuji, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277-2281. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti, M., M. Rossi, V. Giannelli, M. M. Giuliani, M. Pizza, S. Censini, A. Covacci, P. Massari, C. Pagliaccia, R. Manetti, J. L. Telford, G. Douce, G. Dougan, R. Rappuoli, and P. Ghiara. 1998. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine 16:33-37. [DOI] [PubMed] [Google Scholar]
- 27.Melamed, D., and A. Friedman. 1993. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur. J. Immunol. 23:935-942. [DOI] [PubMed] [Google Scholar]
- 28.Ohtomo, N., T. Muraoka, A. Tashiro, Y. Zinnaka, and K. Amako. 1976. Size and structure of the cholera toxin molecule and its subunits. J. Infect. Dis. 133(Suppl.):31-40. [DOI] [PubMed] [Google Scholar]
- 29.Pizza, M., M. M. Giuliani, M. R. Fontana, G. Douce, G. Dougan, and R. Rappuoli. 2000. LTK63 and LTR72, two mucosal adjuvants ready for clinical trials. Int. J. Med. Microbiol. 290:455-461. [DOI] [PubMed] [Google Scholar]
- 30.Porgador, A., H. F. Staats, Y. Itoh, and B. L. Kelsall. 1998. Intranasal immunization with cytotoxic T-lymphocyte epitope peptide and mucosal adjuvant cholera toxin: selective augmentation of peptide-presenting dendritic cells in nasal mucosa-associated lymphoid tissue. Infect. Immun. 66:5876-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan, E. J., E. McNeela, M. Pizza, R. Rappuoli, L. O'Neill, and K. H. Mills. 2000. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J. Immunol. 165:5750-5759. [DOI] [PubMed] [Google Scholar]
- 32.Tamura, S., H. Funato, T. Nagamine, C. Aizawa, and T. Kurata. 1989. Effectiveness of cholera toxin B subunit as an adjuvant for nasal influenza vaccination despite pre-existing immunity to CTB. Vaccine 7:503-505. [DOI] [PubMed] [Google Scholar]
- 33.Tomasi, T. B., Jr. 1980. Oral tolerance. Transplantation 29:353-356. [DOI] [PubMed] [Google Scholar]
- 34.Vajdy, M., and N. Y. Lycke. 1992. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 75:488-492. [PMC free article] [PubMed] [Google Scholar]
- 35.Verhasselt, V., C. Buelens, F. Willems, D. De Groote, N. Haeffner-Cavaillon, and M. Goldman. 1997. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 158:2919-2925. [PubMed] [Google Scholar]
- 36.Weiner, H. L., A. Friedman, A. Miller, S. J. Khoury, A. al-Sabbagh, L. Santos, M. Sayegh, R. B. Nussenblatt, D. E. Trentham, and D. A. Hafler. 1994. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu. Rev. Immunol. 12:809-837. [DOI] [PubMed] [Google Scholar]
- 37.Whitacre, C. C., I. E. Gienapp, C. G. Orosz, and D. M. Bitar. 1991. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J. Immunol. 147:2155-2163. [PubMed] [Google Scholar]
- 38.Williamson, E., G. M. Westrich, and J. L. Viney. 1999. Modulating dendritic cells to optimize mucosal immunization protocols. J. Immunol. 163:3668-3675. [PubMed] [Google Scholar]
- 39.Wu, H. Y., and M. W. Russell. 1998. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16:286-292. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]