Abstract
Enterohemorrhagic Escherichia coli (EHEC) is a group of food-borne pathogens that can cause diarrhea, colitis, and the hemolytic uremic syndrome (HUS). The importance of several of the proposed EHEC virulence factors lacks experimental verification in animal models. The limitations of current animal models led us to reexamine the infant rabbit model for the study of EHEC pathogenicity. Here, we report that intragastric inoculation of a Shiga toxin 2 (Stx2)-producing E. coli O157:H7 clinical isolate into infant rabbits led to severe diarrhea and intestinal inflammation but no signs of HUS. We constructed a set of isogenic derivatives of this isolate with deletions in several putative virulence genes, including stx2, eae, tir, and ehxA, to investigate the contribution of individual virulence factors to EHEC pathogenicity. stx2 increased the severity and duration of EHEC-induced diarrhea. Furthermore, although stx2 had no role in EHEC intestinal colonization nor was it required for EHEC-induced inflammation, stx2 altered how the host responded to EHEC infection by promoting heterophilic infiltration of the colonic epithelium and lamina propria. Intragastric inoculation of purified Stx2 also induced inflammation and diarrhea in this model. Diarrhea and intestinal inflammation were also dependent on EHEC colonization, as EHEC derivatives with deletions in eae and tir did not colonize, form attaching and effacing lesions, or develop clinical signs of disease. Our studies indicate that infant rabbits are a useful model for investigation of the intestinal stage of EHEC pathogenesis and suggest that Shiga toxin may play a critical role in causing diarrhea and inflammation in patients infected with EHEC.
Enterohemorrhagic Escherichia coli (EHEC) is a significant cause of food-borne illness in developed countries around the world (40). EHEC includes several serotypes, but E. coli O157:H7 has been the predominant serotype isolated from outbreaks in North America, the United Kingdom, and Japan (19, 36, 54). EHEC infection is usually acquired by the ingestion of contaminated food or water or occasionally by secondary person-to-person spread (27). Following a typical incubation period of 3 to 5 days, individuals with EHEC infection characteristically develop abdominal cramps and nonbloody diarrhea. In most cases, the diarrhea remains nonbloody and the illness resolves (23). However, in some cases the illness progresses to include bloody diarrhea (hemorrhagic colitis) and the potentially fatal hemolytic uremic syndrome (HUS), a triad of clinical features that includes acute renal failure, thrombocytopenia, and microangiopathic hemolytic anemia (46).
The pathogen and host factors that contribute to the clinical manifestations of EHEC infection are the subject of considerable ongoing investigation. Shiga toxin (Stx), an A-B-type toxin that inhibits protein synthesis in eukaryotic cells, is thought to be required for the severe clinical manifestations of EHEC infection, such as hemorrhagic colitis and HUS (27). There are two main types of Stx produced by EHEC, Stx1 and Stx2. Epidemiologic data suggest that isolates producing Stx2 alone are more likely to cause severe disease than those producing only Stx1 or a combination of Stx1 and Stx2 (3, 41, 44). Evidence of a role for Stx in the development of severe EHEC-related disease comes from several studies using animal models. For example, intravenous administration of Stx1 in baboons reproduced the clinical features of HUS (52). Stx produced by EHEC in the gastrointestinal tract is thought to cross the epithelial cell barrier and enter the systemic circulation, where it can damage endothelial cells (reviewed in reference 56) and thereby cause injury to sensitive distal organs, such as the kidneys and brain. While the correlation between Stx and the development of HUS is relatively well established, the contribution of Stx to EHEC-induced diarrhea and intestinal inflammation has received less attention.
EHEC colonization of the human colon is thought to be another key determinant of virulence. In tissue culture, EHEC, like enteropathogenic E. coli and Citrobacter rodentium (a murine pathogen), forms attaching and effacing (A/E) lesions, and such lesions are believed to mediate colonization (16). A/E lesions are characterized by intimate bacterial adherence to intestinal epithelial cells, localized loss (effacement) of microvilli from epithelial cells, and the accumulation of a pedestal of polymerized actin and other cytoskeletal elements beneath and around the adherent bacterium (reviewed in reference 17). The genes necessary for the formation of A/E lesions are located in the locus of enterocyte effacement (LEE) pathogenicity island. LEE encodes a type III secretion system that may deliver several effector proteins, including Tir, EspF, EspG, EspH, and Map, to the cytoplasm of host epithelial cells (17, 49). Two LEE-encoded genes, eae (intimin) and tir (translocated intimin receptor), have been shown to play a central role in the formation of A/E lesions by EHEC in several cell culture models (12, 25, 51). Intimin has also been shown to play a role in EHEC pathogenesis with animal models (8, 34, 58). Intimin is an outer membrane protein that mediates attachment to enterocytes (12). The receptor for intimin, Tir, is a type III translocated protein that is inserted into the host membrane (9, 10). It is not known whether A/E lesion formation is required for EHEC colonization of the human colon and EHEC pathogenicity; in fact, A/E lesions have not been observed in biopsy specimens taken from patients with EHEC infection (39). Besides stx and the LEE-encoded factors, several other genes are thought to contribute to EHEC pathogenicity. Of these other factors, most is known about enterohemolysin (encoded by ehxA), which has been shown by epidemiologic studies to be frequently associated with severe disease (2, 3, 50).
The host factors that influence the development of EHEC-related disease are poorly understood. Although initial reports suggested that EHEC infection in humans does not provoke a significant intestinal inflammatory response (22), more recent studies indicate that this is not the case. In fact, fecal leukocytes are seen more frequently in patients infected with E. coli O157:H7 than in those infected with some other enteric pathogens, including Salmonella and Shigella spp. (53). Colonic biopsies from patients infected with EHEC reveal neutrophil infiltration in the lamina propria and crypts (21, 29, 30). It is not known whether the host inflammatory response contributes to EHEC-related disease, but studies from an animal model suggest that this may be the case (13). The bacterial factor(s) that directly or indirectly modulate the host response to EHEC infection are still largely unknown.
A variety of animal species, including mice, pigs, baboons, macaques, rabbits, ferrets, and cows, have been used as models to study the virulence of E. coli O157:H7 or the effects of Stx on disease (reviewed in references 7, 35, and 38), but no model reproduces all aspects of EHEC-related disease. Several of these models are limited by high cost (e.g., nonhuman primates) and/or by the requirement for complex animal facilities (e.g., gnotobiotic piglets). Infant rabbits may provide a less expensive, more readily available animal model in which to examine the contribution of specific putative virulence factors to EHEC pathogenicity. To date, while several studies have used infant rabbits as a model to study EHEC infection (13, 14, 33, 42, 45), none have taken advantage of genetically defined EHEC mutants. Using 3-day-old infant rabbits, Pai et al. (42) found that intragastric inoculation of EHEC caused diarrhea, colonic inflammation and death; the severity of EHEC-related disease diminished when older (11-day-old) rabbits were used. They suggested that differences in diarrhea and mortality observed in infant rabbits inoculated with different EHEC isolates were due to variations in the amounts of Stx produced by these isolates during infection. However, this conclusion must be tempered by the fact that nonisogenic EHEC isolates were used in this study.
Here, we used 3-day-old infant rabbits to investigate the contribution of several putative virulence factors in EHEC-induced disease and inflammation. We constructed a set of isogenic derivatives of a human Stx2-producing E. coli O157:H7 clinical isolate with deletions in several putative virulence genes, including stx2, eae, tir, and ehxA. stx2 increased the severity and duration of EHEC-induced diarrhea and modulated the host response to EHEC, while eae and tir were required for EHEC intestinal colonization.
MATERIALS AND METHODS
Bacterial strain construction.
Five EHEC O157:H7 isolates (905, 205, 2107, 3206, and 2108, named H2M, H2L, H2I, H2C, and H2E in a previous publication [48]) were tested for pathogenicity in the infant rabbit model. Based on our results, we decided to use strain 905, an Stx2-producing clinical isolate from a patient with HUS, as the base strain for generating a set of strains containing deletions of stx2AB, eae, tir, and ehxA. These 905 derivatives were constructed using the PCR based “one-step gene inactivation system” described by Datsenko and Wanner (6). The plasmid pKD4 (6) was used as a template to amplify a kanamycin resistance gene for these studies. PCR primers were designed using DNA sequences derived from the E. coli O157:H7 reference strain EDL933 (43) and are as follows: for stx2AB, JRW1 (5′-ATGAAGTGTATATTATTTAAATGGGTACTGTGCCTGGTGTAGGCTGGAGCTGCTTCG-3′) and JRW2 (5′-TTATGCCTCAGTCATTATTAAACTGCACTTCAGCAACATATGAATATCCTCCTTA-3′); for eae, JRW7 (TTATTCTACACAAACCGCATAGACATTTGGAGTATTGTGTAGGCTGGAGCTGCTTCG-3′) and JRW8 (5′-ATGATTACTCATGGTTGTTATACCCGGACCCGGCACCATATGAATATCCTCCTTA-3′); for tir, JRW16 (5′-GACGAAACGATGGGATCCCGGCGCTGGTGGGTTATTGTGTAGGCTGGAGCTGCTTCG-3′) and JRW17 (5′-ATGCCTATTGGTAATCTTGGTCATAATCCCAATGTGCATATGAATATCCTCCTTA-3′); and for ehxA, JRW9 (5′-ACAGTAAATAAAATAAAGAACATTTTCAATAATGCGGTGTAGGCTGGAGCTGCTTCG-3′)and JRW10 (5′-GACAGTTGTCGTTAAAGTTGTTGAGTGTGTGTTGTTCATATGAATATCCTCCTTA-3′). PCR products were electroporated into 905 previously transformed with the lambda Red-encoding plasmid pKD46 (6). Recombinants containing the kanamycin resistance gene in place of the gene of interest were selected on L-agar plates containing 50 μg of kanamycin ml−1, and the deletion of the gene of interest was confirmed by PCR analyses. The growth rates of each of the 905 derivatives we constructed did not differ from that of 905 during in vitro growth in L broth at 37°C.
Animal protocols.
Litters of 2-day-old New Zealand White rabbits were obtained from a commercial breeding company (Pine Acre Rabbitry, Norton, Mass.). Each litter was housed as a group and nursed by the mother. Three-day-old rabbits were intragastrically inoculated with 905, one of its derivatives, purified Stx2, or phosphate-buffered saline (PBS) using size 5 French catheters with flexible tips (Arrow International, Reading, Pa.). Bacterial doses of 5 ×108 CFU per 90 g of rabbit body weight were used in most experiments. For infant rabbit experiments, bacteria were grown overnight in L broth at 37°C, harvested by centrifugation, and then resuspended in sterile PBS (pH 7.2) and adjusted to a cell density of ∼109 CFU ml−1. For experiments using Stx2, doses of 100 μg of Stx2 kg−1 of rabbit weight were administered on day zero and again on day 1 of the experiment (a typical total dose was 10 to 15 μg of Stx2 per rabbit). Postinoculation, the infant rabbits were weighed daily and observed twice daily for clinical signs of illness. Diarrhea was scored as follows: none, no diarrhea (normal pellets are dark green, hard, and formed); mild, diarrhea consisting of a mix of soft yellow-green unformed and formed pellets, resulting in light staining of the hind legs; severe, diarrhea consisting of unformed or liquid stool, resulting in significant staining of the perineum and hind legs. In most experiments, rabbits were necropsied 7 days postinoculation. Rabbits inoculated with Stx2 were necropsied 2 days postinoculation. All rabbits were necropsied by intracardiac injection with 1 ml of saturated KCl solution following isoflurane anesthesia (Aerrane, Baxter, Deerfield, Ill.). At necropsy, the intestinal tract from the duodenum to the anus was removed and samples were obtained for histologic and microbiologic analyses, as well as for determination of Stx2 concentrations. To limit any litter-specific effects, at least two different litters were used to test each type of inoculum studied.
Histology.
Tissues were fixed in 10% neutral-buffered formalin, routinely processed for histology, and stained with hematoxylin and eosin (H&E). The samples were semiquantitatively assessed for infiltration of heterophils, mononuclear cells, and edema or congestion by a comparative pathologist blinded to the sample identity. Sections were evaluated for heterophil inflammation using the following criteria: 0, none; 1, scattered individual cells or small clusters limited to the superficial lamina propria; 2, multifocal aggregates involving the entire mucosa surface with small numbers in the lumen; 3, coalescing heterophilic mucosal inflammation with abundant cell extrusion into the lumen; and 4, necrotizing inflammation with ulceration, large heterophilic intraluminal rafts, and extension into submucosal and deeper layers. Sections were evaluated for mononuclear cells (predominantly lymphocytes and plasmacytes) using the following criteria 0, normal; 1, slightly increased numbers in the lamina propria; 2, moderately increased numbers with mild separation of the crypts; 3, markedly increased mononuclear cells with decreased crypts and prominent intramucosal follicles; 4, effacing mononuclear cell inflammation with large mucosal and/or submucosal follicles ± extension into deeper layers. Edema, congestion, and hemorrhage were subjectively evaluated as follows: 0, none; 1, mild vascular congestion and/or edema limited to the lamina propria; 2, moderate, involving both mucosa and submucosa; 3, severe congestion and edema ± small hemorrhages of mucosa and submucosa and edema of the serosa; and 4, severe diffuse transmural congestion, edema, and multifocal hemorrhage. Samples for transmission electron microscopy from the ceca and distal colons of rabbits necropsied 2 days postinoculation were fixed in 2.5% glutaraldehyde (pH 7.3) buffered in 0.1 M sodium cacodylate. Ultrathin sections of these samples were then stained with uranyl acetate and lead citrate, post fixed with osmium tetroxide, and examined on a Phillips CM-10 transmission electron microscope.
EHEC intestinal colonization.
The numbers of EHEC CFU in tissue samples and in stool pellets were determined by plating. Tissue and stool samples were homogenized in sterile PBS, serially diluted, and plated on either sorbitol MacConkey (SMAC) plates for enumeration of non-sorbitol-fermenting 905 or Luria-Bertani agar plates containing kanamycin (50 μg ml−1) for enumeration of 905Δstx2, 905Δeae, 905Δtir, and 905ΔehxA. Preliminary experiments revealed that there were equal plating efficiencies of these 905 derivatives on Luria-Bertani agar plates containing kanamycin and on SMAC plates. Kanamycin-containing plates were used instead of SMAC plates to facilitate the detection of 905 derivatives against the background of sorbitol-fermenting flora present in the rabbits. Tissue and stool samples from PBS-treated control rabbits were plated on SMAC plates to assess the extent of cross-contamination between rabbits in the same litter. Stool pellets present in the tissue samples of all animals were removed prior to the determination of bacterial CFU.
Stx2 quantification.
Purified Stx2 was obtained from the Center for Gastroenterology Research on Absorptive and Secretory Processes (Tufts-New England Medical Center, Boston, Mass.). Purified Stx2 was heat inactivated by boiling for 8 h. The absence of Stx2 activity in heat-inactivated-toxin preparations was established by measuring the amount of 3H-leucine incorporation in HCT-8 cells as described previously (55). Total (extracellular and periplasmic) Stx2 concentrations in polymyxin B (2 mg ml−1)-treated homogenized tissue and stool samples were determined using an enzyme-linked immunoassay as described previously (1). Adherent stool was removed from tissue samples prior to measuring Stx2 concentrations.
Statistical analysis.
Weight gain (expressed as a percentage of rabbit weight at the start of the experiment), bacterial counts (after log transformation), and Stx2 concentrations were analyzed using the Student t test. In samples where no bacterial colonies were detected at the lowest dilution, the mean values presented in Fig. 3A to D were calculated using the lower limit of detection as a value. Therefore, the means presented in the figures overestimate the true value. Histology scores are ordinal nonparametric data and were analyzed using the Mann-Whitney U test on Prism software (GraphPad, San Diego, Calif.).
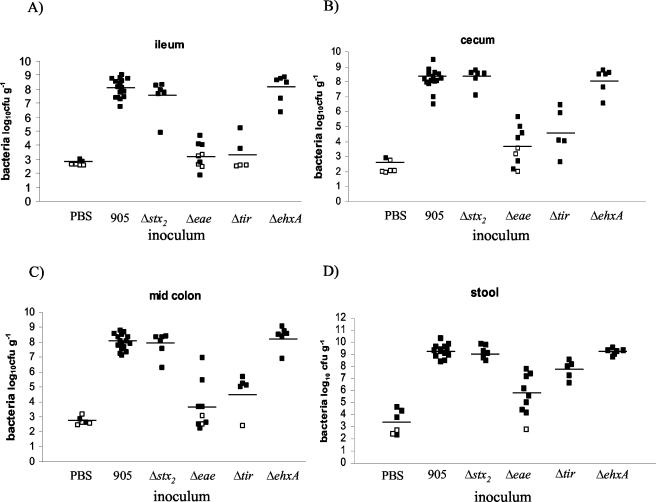
FIG. 3.
Recovery of 905 or one of its derivatives (CFU g−1) from intestinal segments or stool from infected rabbits. Numbers of bacterial CFU were determined in sections taken from the ilea (A), ceca (B), mid-colons (C), and stools (D) of rabbits 7 days postinoculation. Open symbols represent samples below detection limits. Bars represent the mean value for each treatment.
RESULTS
Definition of infection parameters.
Initially, we carried out experiments to explore the influence of EHEC strain, inoculum size, and length of infection on the development of clinical and histopathologic markers of disease following intragastric inoculation of 3-day-old rabbits with human EHEC isolates. All five O157:H7 Stx2-producing EHEC isolates tested caused diarrhea and some degree of colonic inflammation in the rabbits. We selected 905 because this isolate tended to produce more-severe colonic inflammation than the other four isolates tested. 905 inoculum sizes ranging from 5 × 106 to 1 × 1010 CFU all led to similar amounts of diarrhea. Since colitis was less pronounced when inocula of less than ∼5 × 108 CFU were used, we chose this dose for subsequent experiments. Using this inoculum size, we found that colonic inflammation peaked at ∼7 days postinoculation. By day 14 postinoculation, colonic inflammation and the numbers of 905 cells recovered from the colon had both declined (data not shown). Based on these findings, in the experiments described below, we monitored rabbits for 7 days following inoculation of 5 × 108 CFU of 905 (or its derivatives). At that point, the rabbits were necropsied for microbiologic and histologic analyses.
905 induced diarrhea and intestinal inflammation in infant rabbits. (i) Clinical signs.
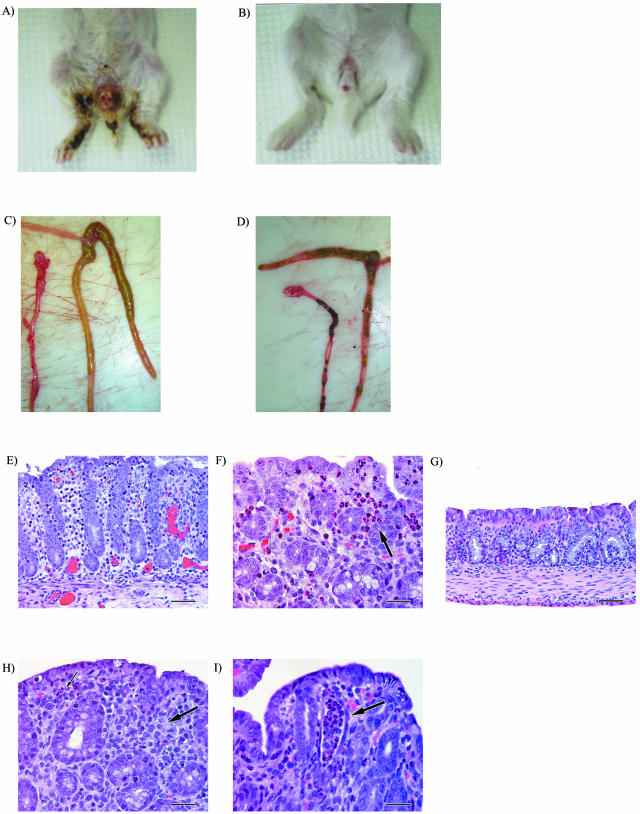
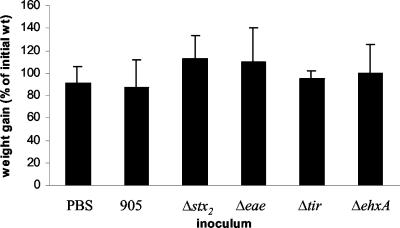
All rabbits intragastrically inoculated with 905 developed severe diarrhea 2 to 3 days postinoculation (Table 1). Diarrhea was made evident by perineal soiling with liquid feces and hyperemic swelling of the anus (Fig. 1A). The 905-inoculated rabbits also developed ruffled coats by day 4 to 5 postinoculation. In contrast to what has been reported previously using this model (e.g., (42), none of the rabbits in our experiments died; this was true even if the duration of the experiment was extended to 14 days postinoculation or when the dose was increased to 1010 CFU. In agreement with previous reports, occult blood was not detected in feces (assessed using the Hemoccult SENSA assay [Beckman Coulter, Inc., Palo Alto, Calif.]). Despite severe diarrhea, all rabbits gained weight and nearly doubled their initial body weight in 7 days (Fig. 2). None of the control rabbits inoculated with PBS developed diarrhea (Table 1). Surprisingly, these PBS-inoculated rabbits gained only slightly more weight than those inoculated with 905, and this difference did not reach statistical significance. At necropsy, the ceca and colons of rabbits inoculated with 905 were distended and filled with unformed stool and fluid (Fig. 1C). In contrast, the ceca and colons of rabbits inoculated with PBS were not distended and contained hard, formed pellets (Fig. 1D).
TABLE 1.
Diarrhea in rabbits inoculated with EHEC strain 905 and its derivatives
| Inoculuma | Total no. of rabbits (no. of litters) | No. with Diarrheab
|
||
|---|---|---|---|---|
| Severe | Mild | None | ||
| 905 | 16 (11) | 16 | 0 | 0 |
| 905Δstx2 | 6 (3) | 0 | 6 | 0 |
| 905Δeae | 9 (2) | 1c | 0 | 8 |
| 905Δtir | 5 (2) | 0 | 0 | 5 |
| 905ΔehxA | 6 (2) | 6 | 0 | 0 |
| PBS | 6 (2) | 0 | 0 | 6 |
All rabbits were inoculated intragastrically with ∼5 × 108 CFU of 905, one of the 905 derivatives or PBS.
Number of rabbits with the respective severity levels of diarrhea. Rabbits were observed twice daily for 7 days following inoculation. Diarrhea was scored as described in the text.
This rabbit had a very watery diarrhea.
FIG. 1.
Gross and histologic findings in infant rabbits infected with EHEC strain 905 or its stx2 derivative, 905Δstx2. Feces-contaminated area of rabbits inoculated with 905 at 7 days postinoculation (A) versus the lack of diarrhea in rabbits inoculated with 905Δstx2 (B) are shown, as well as lack of formed pellets and edematous nature of the colon of rabbits infected with 905 (C) versus normal appearance of colon containing formed ingesta in PBS-treated rabbits (D). (E to I) Representative H&E-stained sections from the mid-colon of infant rabbits infected with 905, 905Δstx2, PBS, or purified Stx2. (E) Suppurative colitis in a rabbit infected with 905 (magnification, ×20). (F) Cluster of heterophils (black arrow) shown at a higher magnification in a 905-infected rabbit (magnification, ×40). (G) No inflammation in the colon of PBS-treated rabbit (magnification, ×20). (H) Lymphocytic colitis with fewer heterophils in rabbit infected with 905Δstx2. (I) Crypt abscess (black arrow) in the distal colon of rabbit inoculated with purified Stx2 (magnification, ×40).
FIG. 2.
Weight gains in infant rabbits inoculated with PBS, 905, or one of its derivatives. Rabbits were weighed prior to inoculation and at 7 days postinoculation, and the difference in weight was expressed as a percentage of the initial rabbit weight. Rabbits infected with 905 gained significantly (P < 0.05) less weight than those infected with 905Δstx2. Error bars represent the standard deviations of the means.
(ii) Bacterial colonization and Stx2 concentrations.
905 colonized all regions of the distal small intestine and large intestine examined. Seven days following inoculation, 905 numbers often exceeded 108 CFU g−1 of tissue in the ileum, cecum, and mid-colon (Fig. 3A to C). The highest numbers of 905 cells were consistently found in stool samples (pellets or liquid feces) (Fig. 3D). 905-inoculated and PBS-treated rabbits were housed in the same cages, but there was little 905 cross-contamination of the control rabbits (compare 905 and PBS in Fig. 3A to C).
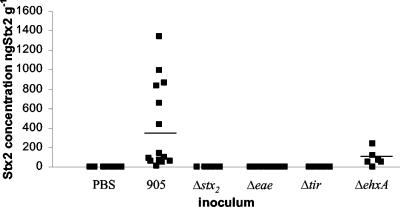
The highest Stx2 concentrations were consistently found in the stool (Fig. 4), and they encompassed a wide range of values (between 40 and 1,340 ng of Stx2 g−1). Over this range, we did not observe any correlation between high levels of Stx2 and more-severe clinical or histologic signs of disease. Stx2 was not detected in any stool or tissue samples taken from rabbits inoculated with PBS.
FIG. 4.
Stx2 concentrations (ng g−1) in stools of rabbits inoculated with PBS, 905, or one of its derivatives. Stool samples were removed from the large intestines of rabbits 7 days postinoculation, and Stx2 concentrations were determined by enzyme-linked immunosorbent assay. Bars represent the mean values.
(iii) Histopathology.
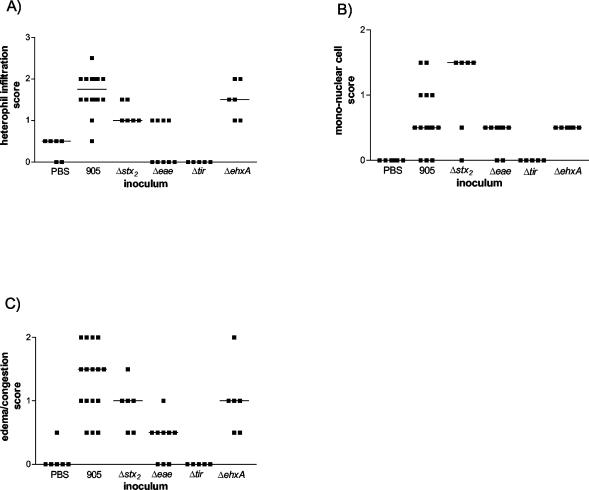
Pathological lesions in 905-inoculated rabbits were confined principally to the colon. Histologically, 905 infection was manifest as suppurative colitis with vascular congestion (Fig. 1E). Colitis was most prominent in the mid and distal parts of the colon in the 905-inoculated rabbits. Compared to the control rabbits, the distal colon of 905-inoculated rabbits contained increased numbers of heterophils (the lapine neutrophil analogue) (P < 0.001) (Fig. 5A) and mononuclear cells (P < 0.001) (Fig. 5B) as well as increased edema and congestion (P < 0.001) (Fig. 5C). Similar findings were observed in the mid-colon (data not shown). Inflammatory cells were most-consistently localized to the mucosal surface epithelium and lamina propria (Fig. 1F), with variable extension into the submucosa. In individual rabbits, the degree of inflammation was more varied in the mid-colon than in the distal colon, where the extent of inflammation was similar in most of the sections examined. Control animals exhibited no or minimal inflammation (Fig. 1G and 5). No histologic abnormalities were observed in the kidneys or brains from any rabbits.
FIG. 5.
Colitis scores from the distal colons of infant rabbits inoculated with PBS, 905, or one of its derivatives. H&E-stained sections were scored for heterophils (A), mononuclear cells (B), and edema or congestion (C). Bars represent the median values.
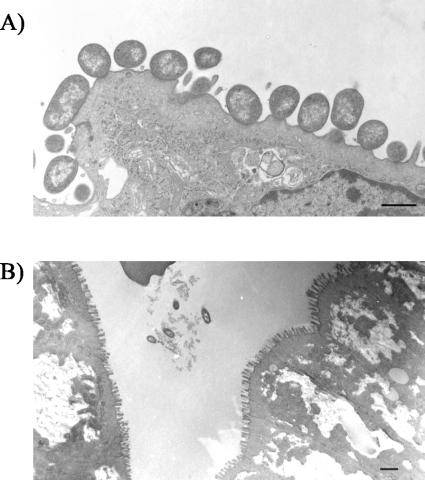
Characteristic A/E lesions were observed in the colons of 905-inoculated rabbits 3 days postinoculation using transmission electron microscopy. There was effacement of epithelial cell microvilli at sites of bacterial attachment, resulting in a diminished brush border (Fig. 6A). In agreement with reports using other E. coli O157:H7 isolates in tissue culture systems and in gnotobiotic piglets, bacteria were found cupped on a small pedestal-like structure.
FIG. 6.
Transmission electron micrographs of tissue sections from the colons of rabbits infected with 905 (A) or 905Δeae (B). Note the loss of microvilli from the epithelial cells in panel A. The colons of rabbits inoculated with 905Δeae contained no adherent bacteria, although bacteria could be seen in the intestinal lumen adjacent to rows of intact microvilli. [magnification, ×4,900 (A) or ×2,200 (B); scale bars, 1 μm].
Stx2 is required for diarrhea and intestinal inflammation.
We utilized two approaches to investigate the contribution of Stx2 to diarrhea and intestinal inflammation in infant rabbits. First, we studied whether an isogenic Δstx2 derivative of 905 caused disease in infant rabbits. Second, we studied whether intragastric inoculation of purified Stx2 caused disease in infant rabbits.
While rabbits inoculated with 905Δstx2 initially developed mild diarrhea at the same time as rabbits inoculated with 905, there was complete resolution of diarrhea in this group of rabbits by day 5 to 6 postinoculation (Table 1). In marked contrast to rabbits inoculated with 905, rabbits inoculated with strain 905Δstx2 had little or no fecal smearing on their rear legs and perinea and exhibited no perianal redness or swelling by day 7 postinoculation (Fig. 1B). Unexpectedly, given the lack of difference in weight gain between 905-infected and PBS-treated rabbits, rabbits inoculated with 905Δstx2 gained significantly (P < 0.05) more weight than those infected with the wild-type strain over the course of the experiment (Fig. 2). At necropsy, the ceca and colons of rabbits inoculated with strain 905Δstx2 contained formed pellets similar to those seen in rabbits inoculated with PBS. These findings suggest that the persistent severe diarrhea observed in 905-inoculated rabbits is Stx2 dependent, whereas the initial diarrhea observed 2 to 3 days post-905 inoculation is Stx2 independent.
Stx2 production does not appear to alter 905 colonization of the infant rabbit intestine. The numbers of 905Δstx2 recovered from all regions of the intestine and the stool were similar to those found when 905 was used as the inoculum (Fig. 3A to D). As expected, Stx2 was not detected in samples taken from rabbits inoculated with 905Δstx2 (Fig. 4).
Interestingly, although the intestinal colonization properties of 905Δstx2 were very similar to those of 905, the deletion of stx2 from 905 clearly influenced the host inflammatory response. Rabbits inoculated with 905Δstx2 had significantly (P < 0.05) lower numbers of heterophils and a trend towards higher numbers of mononuclear cells in the mucosal surface epithelium and lamina propria in the distal colon than rabbits inoculated with 905 (Fig. 1H and Fig. 5). Similar findings were observed in the mid-colon, except that differences between the numbers of mononuclear cells in 905- and 905Δstx2-infected animals reached statistical significance (P < 0.05) in this part of the colon (data not shown). These observations suggest that Stx2 promotes heterophil recruitment into the intestine and alters the nature of the host inflammatory response to EHEC.
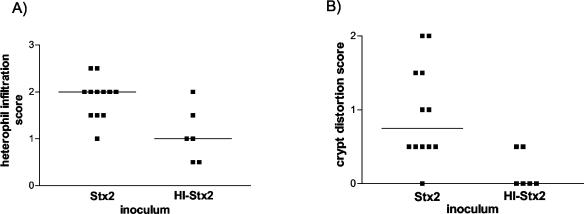
Additional support for the idea that Stx2 can directly cause diarrhea and elicit intestinal inflammation comes from experiments where we intragastrically inoculated infant rabbits with purified Stx2. For these experiments, heat-inactivated Stx2 (HI-Stx2) was administered as a control treatment. Following inoculation of 100 μg of Stx2 kg−1 given as a repeated dose on day zero and on day 1, rabbits developed diarrhea (manifest as fecal staining on the hind legs) by day 2 postinoculation; rabbits inoculated with HI-Stx2 did not exhibit any signs of Stx2-induced disease. Rabbits inoculated with purified Stx2 had significantly (P < 0.05) more heterophils in the distal colonic mucosa than rabbits inoculated with HI-Stx2 (Fig. 7A). Thus, histologic observations from experiments using either purified Stx2 or 905 suggest that Stx2 promotes heterophil infiltration into the colonic mucosa. Unlike rabbits inoculated with 905, rabbits inoculated with purified Stx2 developed crypt abscesses, characterized by crypt ectasia and distortion, with intraluminal plugs of degenerating heterophils and epithelial cells, in the distal colon (Fig. 1I and 7B).
FIG. 7.
Colitis scores from the distal colons of infant rabbits inoculated with purified Stx2 or HI-Stx2. Rabbits were sacrificed 2 days postinoculation. H&E-stained sections were scored as described in Materials and Methods and also assessed for crypt abscesses. Shown are data for heterophils (A) and crypt distortion (B). Bars represent the median values.
eae and tir are required for 905 colonization.
Like many EHEC isolates, 905 contains eae and tir, genes which encode proteins thought to be required for the intimate attachment of EHEC to the intestinal epithelium. Although the importance of eae and tir in EHEC attachment to host cells has been demonstrated in tissue culture epithelial cells, the requirement for these LEE-encoded genes in EHEC colonization in vivo has not been clearly demonstrated. Isogenic derivatives of 905 containing deletions of either eae (905Δeae) or tir (905Δtir) were constructed to investigate the role of eae and tir in 905 colonization of the infant rabbit intestine.
Both eae and tir proved to be critical for 905 colonization of the infant rabbit intestine. The numbers of both 905Δeae and 905Δtir cells recovered from the ilea, ceca, and colons of rabbits inoculated with these 905 derivatives were more than 4 orders of magnitude lower than those found when 905 was used as the inoculum (Fig. 3A to C). In fact, 905Δeae and 905Δtir cells were not detectable in many of these samples. The magnitude of the reduction in the numbers of 905Δeae and 905Δtir cells recovered in stool samples was not as dramatic as that observed in the regions of the intestine that we sampled (Fig. 3D). No adherent bacteria were seen in electron micrographs of any sections taken from the colons of rabbits inoculated with 905Δeae. Instead, the bacteria observed in these micrographs were present in the gut lumen adjacent to intact microvilli (Fig. 6B). These observations suggest that eae mediates 905 adherence to the infant rabbit intestinal epithelium and that adherence is required for efficient colonization.
Consistent with the marked reduction in the intestinal colonization by 905Δeae and 905Δtir and the absence of Stx2 in stool samples from rabbits inoculated with these strains (Fig. 4), the clinical and histologic signs of infection with these 905 derivatives were greatly attenuated. Eight of nine rabbits inoculated with 905Δeae and five of five rabbits inoculated with 905Δtir had no diarrhea (Table 1). Rabbits inoculated with 905Δeae or 905Δtir also tended to gain more weight than those inoculated with 905, although these differences did not reach statistical significance (Fig. 2). Heterophil infiltration in tissue sections from most of the rabbits inoculated with 905Δeae and all of the rabbits inoculated with 905Δtir did not differ from results with PBS-treated rabbits (Fig. 5). These findings suggest that intestinal colonization mediated by eae and tir is required for 905-induced diarrhea and intestinal inflammation.
ehxA does not contribute to diarrhea.
Epidemiologic data suggest that the enterohemolysin EhxA contributes to EHEC virulence (2, 3, 50). An isogenic derivative of 905 containing a deletion of ehxA was constructed to investigate the importance of EhxA in 905 pathogenicity in infant rabbits. The clinical signs of 905ΔehxA infection of infant rabbits did not differ from those observed with 905. All 905ΔehxA-inoculated rabbits developed severe diarrhea (Table 1), perianal redness, and ruffled coats. Weight gains in the 905- and 905ΔehxA-inoculated rabbits did not differ (Fig. 2). EhxA did not influence intestinal colonization by 905. Equal numbers of 905ΔehxA and 905 cells were recovered from the intestine and the stool (Fig. 3). Amounts of Stx2 found in the stool of 905ΔehxA- and 905-inoculated rabbits were not significantly different (Fig. 4). Compared to 905-inoculated rabbits, there was a tendency toward less heterophil infiltration in the distal colon of 905ΔehxA-inoculated rabbits (Fig. 5A); this difference reached statistical significance in the mid-colon (P < 0.05) (data not shown). Overall, besides a subtle effect on colonic heterophil infiltration, ehxA does not seem to significantly influence 905 pathogenicity in infant rabbits.
DISCUSSION
Promising results using infant rabbits as model hosts for the study of EHEC pathogenicity were published several years ago (13, 14, 42, 45), but studies using genetically defined EHEC mutants in this model have not been presented. Taking advantage of contemporary genetic techniques that facilitate creation of deletion mutants in E. coli, we constructed a set of isogenic derivatives of a human clinical EHEC isolate to reexamine the infant rabbit model of EHEC pathogenicity. Intragastric inoculation of infant rabbits with the human EHEC isolate caused diarrhea and colitis but no signs of HUS. Stx2 increased the severity and duration of EHEC-induced diarrhea and modulated the host inflammatory response to EHEC. Intragastric inoculation of purified Stx2 also caused inflammation and diarrhea in rabbits. Colonization of the infant rabbit intestine by EHEC depended on eae and tir but was not influenced by stx2 or ehxA. Our findings indicate that infant rabbits are a useful model for the study of the intestinal manifestations of EHEC pathogenicity. If results from this model apply to human EHEC infections, then our observations suggest that EHEC-induced diarrhea is primarily caused by Stx2.
An advantage of infant rabbits over mice for study of EHEC pathogenicity is that human EHEC isolates colonize the infant rabbit intestine without the requirement for additional treatments. Mice must be given streptomycin, presumably to eliminate the normal intestinal flora to facilitate EHEC colonization. Furthermore, mice develop neither diarrhea, colitis, nor A/E lesions following EHEC inoculation (60). A limitation of the infant rabbit model is that rabbits do not develop HUS or other evidence of microangiopathy. The reason(s) for this are not known, although the absence of Gb3, the Stx receptor, in rabbit kidneys may explain the absence of HUS in this model (61). Intravenous injection of Stx in adult rabbits can cause microvascular lesions in the brain that resemble thrombotic microangiopathy in humans (61). The lack of this pathological finding in EHEC-infected infant rabbits may be due to insufficient absorption of Stx from the intestine, or alternatively, Gb3 may not yet be expressed in the endothelium of the developing central nervous system in young rabbits.
The mechanism(s) by which Stx causes diarrhea in infant rabbits are not known. The enterotoxigenic effect of Stx on ligated ileal loops of adult rabbits is well documented (28) and is thought to be due to selective damage and loss of villus absorptive epithelial cells. Studies by Keusch and colleagues suggest that these effects are due to Stx binding to Gb3 on these cells (e.g., see references 26, 31, and 37). However, Gb3 does not appear in the rabbit small intestine until day 16 of life (37). A number of studies have described the effects of intravenously administered Stx to adult rabbits (e.g., see references 18 and 47). Typically, these animals develop neurologic signs of disease, but gastrointestinal signs of disease including diarrhea are also common. In this model, Stx appears to act primarily on the vasculature supply of the intestine, especially the cecum (47). It is not known if the intravenous route of Stx administration (as opposed to the intragastric route) influences how the host responds to Stx. Besides physiological alterations in epithelial cell absorptive and barrier functions resulting from direct Stx2-Gb3 interactions (or Stx2 interactions with an alternative receptor), indirect effects of Stx2 on the integrity of the intestinal epithelium may also contribute to diarrhea. For example, the Stx2-dependent influx of heterophils into the colonic mucosa may cause diarrhea. Such a mechanism is suggested by the results of Elliott et al. (13). These workers showed that pretreatment of 10-day-old EHEC-infected rabbits with an antibody to the leukocyte adhesion molecule, CD18, reduced diarrhea, epithelial disruption, and electrolyte transport abnormalities induced by EHEC.
Our findings with both purified Stx2 and 905Δstx2 revealed that Stx2 modulates the host immune response. It has been suggested that gut inflammation may facilitate the severe manifestations of EHEC infection by allowing increased uptake of Stx from the gut lumen into the systemic circulation. Hurley et al. (24) reported that neutrophil transmigration had a significant impact on Stx absorption across a model epithelial barrier. Stx's can elicit production of interleukin 8 and other C-X-C chemokines from cultured intestinal epithelial cell lines (55, 57). In vivo, epithelial cells may respond to Stx2 by expressing proinflammatory cytokines, leading to the recruitment of heterophils into the epithelium and lamina propria as observed in this study. Interestingly, the inflammatory infiltrates elicited by 905 and by 905Δstx2 differed. Inflammatory lesions in 905Δstx2-infected rabbits contained more mononuclear cells than in 905-infected rabbits. Thus, although Stx2 is not required for EHEC-induced inflammation, Stx2 alters how the host responds to EHEC infection both by promoting heterophil infiltration and by decreasing mononuclear cell infiltration. Presumably this modulation of the host response creates some as-yet-unknown benefit to EHEC. If diarrhea results from polymorphonuclear cell infiltration into the colon as discussed above, then Stx2 modulation of the host response may promote EHEC dissemination.
Our observations suggesting that Stx contributes significantly to the development of EHEC-induced diarrhea in infant rabbits are in contrast to findings with 10-day-old rabbits (33) and gnotobiotic piglets (59). Stx did not contribute to diarrhea, mucosal damage, or colonic ion transport in 10-day-old rabbits infected with various strains of EHEC (33); however, the results from this study were obtained using nonisogenic strains of E. coli O157:H7. With gnotobiotic piglets, Tzipori et al. (59) found that all E. coli O157:H7 strains administered to gnotobiotic piglets, regardless of stx, were capable of inducing diarrhea and mucosal damage. The reason(s) that gnotobiotic piglets are apparently insensitive to the intestinal effects of Stx are unknown. In agreement with our observations, Stx has been shown to contribute to the diarrhea caused by Shigella dysenteriae type 1 in a macaque monkey model using isogenic strains (15). The conflicting results obtained from these studies highlight the fact that animal models can yield different information about the role of virulence factors in EHEC pathogenicity, however; our findings together with observations from a number of different studies (reviewed in reference 20) suggest that Stx plays an important role in colonic disease caused by EHEC.
Two LEE pathogenicity island genes, eae and tir, were required for EHEC colonization, A/E lesion formation (only tested for 905Δeae), inflammation, and diarrhea in infant rabbits. In gnotobiotic piglets, EHEC eae was also found to be required for A/E lesion formation, intestinal inflammation, and diarrhea (34), but intestinal colonization did not depend on eae (58). In studies by Tzipori et al., similar numbers of wild-type EHEC and eae EHEC cells were recovered from the intestines of infected gnotobiotic piglets (58). In contrast, in neonatal calves, eae appeared to be required for intestinal colonization (8). These differences in the requirement for eae in intestinal colonization in different animal models (as reflected in the recovery of EHEC cells from the intestines of infected animals) may reflect species-specific differences in the varieties of niches available for intestinal EHEC growth.
Our work suggests that there is some niche where eae or tir are not required for EHEC growth within the intestine of the infant rabbit, since significant numbers of 905Δeae and 905Δtir cells were recovered in stool pellets of infected animals (Fig. 3D). Interestingly, although these pellets contained >107 905Δtir CFU g−1 and >105 905Δeae CFU g−1, no Stx2 was detected there. This dichotomy between cell numbers and Stx2 concentrations may suggest that Stx2 production requires eae- and tir-dependent colonization of the appropriate niche; alternatively, our assays for Stx2 may not be sufficiently sensitive to detect Stx2 in stool samples with reduced numbers of EHEC.
Despite the frequent presence of ehxA in EHEC isolates obtained from patients with severe EHEC-related disease (50), our observations do not support the idea that ehxA is a critical EHEC virulence factor. Other than a subtle reduction in the severity of colitis in sections from the mid-colon in rabbits inoculated with 905ΔehxA, no differences between 905- and 905ΔehxA-infected rabbits were detected. In agreement with our findings, Tzipori et al. found that curing EHEC isolates of the plasmid containing ehxA did not influence EHEC-induced disease in the gnotobiotic piglet model (59).
The infant rabbit model of EHEC infection should be useful in exploring the role of other putative EHEC virulence factors. The list of potential factors continues to grow and includes a catalase peroxidase (encoded by katP) (5), an extracellular serine protease (encoded by espP) (4, 11), and a secreted metalloprotease (encoded by stcE) (32). It will be particularly interesting to investigate the importance of LEE-encoded type III effectors, such as EspF, EspG, EspH, and Map, in this model, since the importance of many of these proteins in EHEC pathogenicity has not been established with an animal model. The recent advances in techniques for the genetic manipulation of E. coli will facilitate these studies. Investigation of different host factors important in EHEC pathogenicity in infant rabbits will also be possible. Such studies may be limited however, by the repertoire of available reagents suitable for use with rabbits.
Acknowledgments
We thank Suqian Lu and Wendy E. Smith for excellent technical support and Sausan T. Campbell for help with the purified Stx2 rabbit experiments. We are grateful to Anne Kane and Sarah McLeod for critical review of the manuscript. We also thank Cathy Linsenmayer for help with the electron microscopy work.
This study was supported by AI-42347 and HMMI (M.K.W.), AI-01715 (C.M.T.), and P30DK-34928 for the Center for Gasteroenterology Research on Absorptive and Secretory Processes.
Editor: A. D. O'Brien
REFERENCES
- 1.Acheson, D. W., M. Jacewicz, A. V. Kane, A. Donohue-Rolfe, and G. T. Keusch. 1993. One step high yield affinity purification of shiga-like toxin II variants and quantitation using enzyme linked immunosorbent assays. Microb. Pathog. 14:57-66. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, T. J., J. B. Kaper, A. E. Jerse, and I. K. Wachsmuth. 1992. Virulence factors in Shiga-like toxin-producing Escherichia coli isolated from humans and cattle. J. Infect. Dis. 165:979-980. [DOI] [PubMed] [Google Scholar]
- 3.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 5.Brunder, W., H. Schmidt, and H. Karch. 1996. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:3305-3315. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A. 2003. Bovine Escherichia coli O157:H7 infection model, p. 329-338. In D. Philpott and F. Ebel (ed.), E. coli: Shiga toxin methods and protocols, vol. 73. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 10.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djafari, S., F. Ebel, C. Deibel, S. Kramer, M. Hudel, and T. Chakraborty. 1997. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol. Microbiol. 25:771-784. [DOI] [PubMed] [Google Scholar]
- 12.Donnenberg, M. S., S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, E., Z. Li, C. Bell, D. Stiel, A. Buret, J. Wallace, I. Brzuszczak, and E. O'Loughlin. 1994. Modulation of host response to Escherichia coli O157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology 106:1554-1561. [DOI] [PubMed] [Google Scholar]
- 14.Farmer, J. J., III, M. E. Potter, L. W. Riley, T. J. Barrett, P. A. Blake, C. A. Bopp, M. L. Cohen, A. Kaufmann, G. K. Morris, R. S. Remis, B. M. Thomason, and J. G. Wells. 1983. Animal models to study Escherichia coli O157:H7 isolated from patients with haemorrhagic colitis. Lancet i:702-703. [DOI] [PubMed] [Google Scholar]
- 15.Fontaine, A., J. Arondel, and P. J. Sansonetti. 1988. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox− mutant of Shigella dysenteriae 1. Infect. Immun. 56:3099-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankel, G., D. C. Candy, P. Everest, and G. Dougan. 1994. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect. Immun. 62:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 18.Fujii, J., Y. Kinoshita, T. Kita, A. Higure, T. Takeda, N. Tanaka, and S. Yoshida. 1996. Magnetic resonance imaging and histological study of brain lesions in rabbits given intravenous verotoxin 2. Infect. Immun. 64:5053-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin, P. M. 1998. Epidemiology of Shiga toxin-producing Escherichia coli infections in humans in the United States, p. 15-22. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 20.Griffin, P. M. 1995. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli, p. 739-761. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, N.Y.
- 21.Griffin, P. M., L. C. Olmstead, and R. E. Petras. 1990. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 99:142-149. [DOI] [PubMed] [Google Scholar]
- 22.Griffin, P. M., S. M. Ostroff, R. V. Tauxe, K. D. Greene, J. G. Wells, J. H. Lewis, and P. A. Blake. 1988. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109:705-712. [DOI] [PubMed] [Google Scholar]
- 23.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 24.Hurley, B. P., C. M. Thorpe, and D. W. Acheson. 2001. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect. Immun. 69:6148-6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismaili, A., D. J. Philpott, M. T. Dytoc, and P. M. Sherman. 1995. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect. Immun. 63:3316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacewicz, M., H. Clausen, E. Nudelman, A. Donohue-Rolfe, and G. T. Keusch. 1986. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 163:1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keenan, K. P., D. D. Sharpnack, H. Collins, S. B. Formal, and A. D. O'Brien. 1986. Morphologic evaluation of the effects of Shiga toxin and E coli Shiga-like toxin on the rabbit intestine. Am. J. Pathol. 125:69-80. [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly, J., A. Oryshak, M. Wenetsek, J. Grabiec, and S. Handy. 1990. The colonic pathology of Escherichia coli O157:H7 infection. Am. J. Surg. Pathol. 14:87-92. [DOI] [PubMed] [Google Scholar]
- 30.Kelly, J. K., C. H. Pai, I. H. Jadusingh, M. L. Macinnis, E. A. Shaffer, and N. B. Hershfield. 1987. The histopathology of rectosigmoid biopsies from adults with bloody diarrhea due to verotoxin-producing Escherichia coli. Am. J. Clin. Pathol. 88:78-82. [DOI] [PubMed] [Google Scholar]
- 31.Keusch, G. T., M. Jacewicz, M. Mobassaleh, and A. Donohue-Rolfe. 1991. Shiga toxin: intestinal cell receptors and pathophysiology of enterotoxic effects. Rev. Infect. Dis. 13(Suppl. 4):S304-S310. [DOI] [PubMed] [Google Scholar]
- 32.Lathem, W. W., T. E. Grys, S. E. Witowski, A. G. Torres, J. B. Kaper, P. I. Tarr, and R. A. Welch. 2002. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol. Microbiol. 45:277-288. [DOI] [PubMed] [Google Scholar]
- 33.Li, Z., C. Bell, A. Buret, R. Robins-Browne, D. Stiel, and E. O'Loughlin. 1993. The effect of enterohemorrhagic Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology 104:467-474. [DOI] [PubMed] [Google Scholar]
- 34.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melton-Celsa, A. R., and A. D. O'Brien. 2003. Animal models for STEC-mediated disease. Methods Mol. Med. 73:291-305. [DOI] [PubMed] [Google Scholar]
- 36.Michino, H., K. Araki, S. Minami, T. Nakayama, Y. Ejima, K. Hiroe, H. Taneka, N. Fujita, S. Usami, M. Yonekawa, K. Sadamoto, S. Takaya, and N. Sakai. 1998. Recent outbreaks of infections caused by Escherichia coli O157:H7 in Japan, p. 73-81. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 37.Mobassaleh, M., A. Donohue-Rolfe, M. Jacewicz, R. J. Grand, and G. T. Keusch. 1988. Pathogenesis of shigella diarrhea: evidence for a developmentally regulated glycolipid receptor for shigella toxin involved in the fluid secretory response of rabbit small intestine. J. Infect. Dis. 157:1023-1031. [DOI] [PubMed] [Google Scholar]
- 38.Moxley, R. A., and D. H. Francis. 1998. Overview of animal models, p. 249-260. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 39.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Brien, A. D., and J. B. Kaper. 1998. Shiga toxin-producing Escherichia coli: yesterday, today and tomorrow, p. 1-11. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 41.Ostroff, S. M., P. I. Tarr, M. A. Neill, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 42.Pai, C. H., J. K. Kelly, and G. L. Meyers. 1986. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect. Immun. 51:16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 44.Pickering, L. K., T. G. Obrig, and F. B. Stapleton. 1994. Hemolytic-uremic syndrome and enterohemorrhagic Escherichia coli. Pediatr. Infect. Dis. J. 13:459-475. [DOI] [PubMed] [Google Scholar]
- 45.Potter, M. E., A. F. Kaufmann, B. M. Thomason, P. A. Blake, and J. J. Farmer III. 1985. Diarrhea due to Escherichia coli O157:H7 in the infant rabbit. J. Infect. Dis. 152:1341-1343. [DOI] [PubMed] [Google Scholar]
- 46.Proulx, F., E. G. Seidman, and D. Karpman. 2001. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 50:163-171. [DOI] [PubMed] [Google Scholar]
- 47.Richardson, S. E., T. A. Rotman, V. Jay, C. R. Smith, L. E. Becker, M. Petric, N. F. Olivieri, and M. A. Karmali. 1992. Experimental verocytotoxemia in rabbits. Infect. Immun. 60:4154-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritchie, J. M., P. L. Wagner, D. W. Acheson, and M. K. Waldor. 2003. Comparison of Shiga toxin production by hemolytic-uremic syndrome-associated and bovine-associated Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 69:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roe, A. J., D. E. Hoey, and D. L. Gally. 2003. Regulation, secretion and activity of type III-secreted proteins of enterohaemorrhagic Escherichia coli O157. Biochem. Soc. Trans. 31:98-103. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt, H., and H. Karch. 1996. Enterohemolytic phenotypes and genotypes of Shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 34:2364-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherman, P., R. Soni, M. Petric, and M. Karmali. 1987. Surface properties of the Vero cytotoxin-producing Escherichia coli O157:H7. Infect. Immun. 55:1824-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegler, R. L., T. J. Pysher, V. L. Tesh, and F. B. Taylor, Jr. 2001. Response to single and divided doses of Shiga toxin-1 in a primate model of hemolytic uremic syndrome. J. Am. Soc. Nephrol. 12:1458-1467. [DOI] [PubMed] [Google Scholar]
- 53.Slutsker, L., A. A. Ries, K. D. Greene, J. G. Wells, L. Hutwagner, and P. M. Griffin. 1997. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann. Intern. Med. 126:505-513. [DOI] [PubMed] [Google Scholar]
- 54.Smith, H. R., B. Rowe, G. K. Adak, and R. K. Reilly. 1998. Shiga toxin (verocytotoxin)-producing Escherichia coli in the United Kingdom, p. 49-58. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 55.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 67:5985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorpe, C. M., J. M. Ritchie, and D. W. K. Acheson. 2002. Enterohemorrhagic and other Shiga toxin-producing Escherichia coli, p. 119-154. In M. S. Donnenberg (ed.), Escherichia coli: virulence mechanisms of a versatile pathogen. Academic Press, Boston, Mass.
- 57.Thorpe, C. M., W. E. Smith, B. P. Hurley, and D. W. Acheson. 2001. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect. Immun. 69:6140-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzipori, S., F. Gunzer, M. S. Donnenberg, L. de Montigny, J. B. Kaper, and A. Donohue-Rolfe. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect. Immun. 63:3621-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wadolkowski, E. A., J. A. Burris, and A. D. O'Brien. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58:2438-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zoja, C., D. Corna, C. Farina, G. Sacchi, C. Lingwood, M. P. Doyle, V. V. Padhye, M. Abbate, and G. Remuzzi. 1992. Verotoxin glycolipid receptors determine the localization of microangiopathic process in rabbits given verotoxin-1. J. Lab. Clin. Med. 120:229-238. [PubMed] [Google Scholar]