Abstract
Much data implicate saturated fatty acids in deleterious processes associated with obesity, diabetes, and the metabolic syndrome. Many of these changes may be due to aberrant generation of bioactive lipids when saturated fatty acid availability to tissues is increased. On the other hand, studies are emerging that implicate the monounsaturated fatty acid oleate in protection from saturated fat mediated toxicity; however, the mechanisms are not well understood. Our data demonstrate a novel role for palmitate in increasing mRNA encoding DES1, which is the enzyme responsible for generating ceramide from its precursor dihydroceramide and thus controls synthesis of the bioactive lipid ceramide. Moreover, co-treatment with oleate prevented the increase in ceramide, and this occurred through attenuation of the increase in message and activity of DES1. Knockdown of DES1 also protected from palmitate-induced insulin resistance, and overexpression of this enzyme ameliorated the protective effect of oleate. Together, these findings provide insight into the mechanisms of oleate-mediated protection against metabolic disease and provide novel evidence for fatty acid-mediated regulation of a key enzyme of ceramide biosynthesis.
Keywords: Diabetes, Fatty Acid, Insulin Resistance, Obesity, Sphingolipid, DES1, Ceramide, Dihydroceramide, Dihydroceramide Desaturase, Lipidomics
Introduction
The rise in obesity in recent years has initiated a pandemic of metabolic disease including heart disease and diabetes (1, 2). Perturbations in lipid metabolism and/or endocrine function that occur in obesity likely mediate the development of a disorders, including insulin resistance, nonalcoholic fatty liver disease, pancreatic cell death, hypertension, and the “metabolic syndrome” (3, 4). Although the mechanistic links between obesity and these pathophysiological processes remain incompletely understood, one proposed mechanism is that the elevation in plasma lipids associated with obesity overloads tissues with precursors for synthesis of bioactive lipids, including diacylglycerols (DAG)2 and ceramides (5, 6).
Epidemiological data link diets high in saturated fatty acids with increased incidence of metabolic disease (6, 7). Supporting a mechanistic connection between saturated fatty acids and metabolic syndrome, many studies demonstrate that the saturated fatty acid palmitate promotes insulin resistance in heart, adipose, and skeletal muscle (8–10). On the other hand, data are emerging which support that unsaturated fatty acids such as oleate have protective effects against palmitate toxicity, though the mechanisms are not fully understood (11, 12).
Several studies demonstrate that ceramide plays a key role in insulin resistance in human skeletal muscle cells (13–15). Biosynthesis of sphingolipids including ceramide begins with condensation of serine with acyl-CoA such as palmitoyl-CoA. Exposing muscle cells to palmitate increased ceramide synthesis and inhibited insulin stimulation of Akt/protein kinase B, a serine/threonine kinase that is a central mediator of insulin-stimulated anabolic metabolism (13); moreover, inhibiting ceramide synthesis negated the antagonistic effect of saturated free fatty acids toward Akt/protein kinase B (15). Studies indicate that palmitate increases both ceramide and diacylglycerol and that inhibition of synthesis of either of these species independently can restore insulin sensitivity (15–17); however, the relative importance of these lipids in skeletal muscle insulin resistance remains controversial (18).
Because studies indicate protective effects of oleate on palmitate-dependent pathological outcomes (11, 12), we sought to determine whether oleate would attenuate production of bioactive sphingolipids and, moreover, whether this could be a mechanism for oleate-mediated protection from insulin resistance. Data revealed a novel role for oleate in preventing palmitate-induced ceramide synthesis, and this occurred through preventing palmitate-induced up-regulation of dihydroceramide desaturase 1 (DES1), which is the enzyme responsible for generating ceramide from its precursor dihydroceramide, thus controlling cellular ratios of dihydroceramide to ceramide. This activity of oleate protected cells from the loss of insulin-mediated Akt phosphorylation caused by palmitate, as knockdown of this enzyme also protected from palmitate-induced insulin resistance, and overexpression of this enzyme overcame the protective effects of oleate. These data indicate novel, antagonistic regulatory roles for palmitate and oleate on DES1, support a key role of DES1 in mediating fatty acid-induced insulin resistance, and provide mechanistic insights into the protective effects of oleate in human health.
EXPERIMENTAL PROCEDURES
Materials
Fetal bovine serum, horse serum, 0.5% trypsin EDTA, and Lipofectamine 2000 were from Invitrogen. DMEM with 4 mm l-glutamine was from ATCC; sodium oleate and human insulin solution, bicine, trichloroacetic acid, NADH, and CHAPS were from Sigma-Aldrich; palmitic acid (C16:0) was from (Matreya LLC). Anti-Akt, phospho-Akt (Ser473) and anti-phospho-GSK-3α/β(Ser21/9) antibody were from Cell Signaling Technology; Anti-β-actin antibody was from Santa Cruz Biotechnology. Goat anti-rabbit IgG conjugate with HRP was from Bio-Rad.
Cell Culture
Mouse C2C12 myoblasts were from ATCC and were maintained at 37 °C in DMEM containing 10% FBS. For differentiation to contractile myotubes, the myoblasts were grown to confluency, and the media were replaced with DMEM containing 10% horse serum. Myotubes were used for plasmid DNA or RNA transfection at 4–8 days after differentiation.
FFA Treatment
Free fatty Acids (FFAs) were prepared as described previously (19). In brief, palmitate stock solution (125 mm) was prepared in ethanol, and oleate stock solution was prepared in water. The FFA stock solutions were diluted 1:100 in DMEM containing 2% fatty acid-free BSA and 1% FBS, after brief sonication and 15 min incubation at 55 °C, cooled down to 37 °C, and administrated to myotubes. The myotubes were incubated with serum-free DMEM for 3 h before treatment with FFAs for 16 h.
Lipidomic Measurement by LC/MS
Lipidomic profiling was performed as described previously (20).
Triglyceride Determination
Cell pellets were extracted in 1-butanol:Triton-X 100:methanol (4:1:1, v/v). The samples were incubated at 4 °C overnight, probe-sonicated for 10 s, and incubated at room temperature for 2 h. Samples were centrifuged for 5 min at 4500 × g, and the protein concentration of the supernatant was determined. Triglycerides were measured according to the method described in the triglyceride determination kit (Sigma Aldrich, TR0100).
Quantitative Real-time PCR (qPCR)
Total RNA from C2C12 myotubes was isolated using the RNeasy mini kit (Qiagen) according to the manufacturer's instructions. The first-strand cDNA was synthesized from 4 μg of total RNA using the SuperScriptTM First-strand Synthesis System for an RT-PCR kit (Invitrogen). Quantitative real-time PCR was performed on a Cycler system (Bio-Rad) as described previously (21). Mouse DES1 and DES2 primers were ordered from SuperArray Bioscience Corporation. β-actin or GAPDH were used as reference genes.
siRNA Transfection
siRNA oligonucleotides were transfected into differentiated myotubes using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. DES1 siRNA and negative control siRNA were from Qiagen. The DES1 siRNA target sequence was 5′-CACCTTTGACATTATCATTTA-3′, with sense strand 5′-CCUUUGACAUUAUCAUUUATT-3′ and antisense strand 5′-UAAAUGAUAAUGUCAAAGGTG-3′. GenBankTM accession no. was NM_007853. At 72 h post-transfection, the cells were treated with FFAs for 14 h and then stimulated with 100 nm insulin for 10 min for analysis of phospho-Akt by Western blot.
Microsome Preparation
Microsomes were prepared as described previously (22) with minor modification. Briefly, C2C12 myotubes were washed twice with cold phosphate-buffered saline, scraped, and lysed with a Dounce Homogenizer (40 strokes) in buffer containing 20 mm Tris-HCl, pH 7.4, 0.25 m sucrose, 1 mm EDTA, and a protease inhibitor mixture. Cells were sonicated for 10 s and chilled on ice for 20 s, and this was repeated two times. Cell lysates were centrifuged at 1,000 × g for 5 min at 4 °C. The supernatants were then centrifuged at 45,000 rpm for 60 min at 4 °C. The microsome pellets were dissolved in 50 mm K2PO4, pH 7.4 buffer. Protein concentration was determined using the Micro BCATM protein assay reagent kit (Pierce).
In Vitro DES1 Activity Assay
DES1 activity assays were performed as described previously with minor modifications (23). Briefly, DES1 activity is determined by following the formation of tritiated water that accompanied the 4,5-double bond formation if the substrate is labeled appropriately. The labeled N-octanoyl-[4,5–3H]-d-erythro-dihydrosphingosine was used as hot substrate. The cold substrate is N-octanoyl-d-erythro-dihydrosphingosine. To prepare the substrate mixture, 2 nm hot substrate (equal to 0.125 uCi, and ∼100,000 decays per minute), 500 nm cold substrate were dissolved in 1.1 mg of CHAPS (in 10 μl of water). 10-μl substrate mixtures and 100 μg of protein were added to the reaction mixture containing 2 mm NADH, 20 mm bicine, pH 8.5, 50 mm NaCl, and 50 mm sucrose, in a final volume of 200 μl. Reaction mixtures were incubated for 20 min at 37 °C. The reactions were stopped by adding 1.5 ml of chloroform/methanol (2:1, v/v) followed by 0.4 ml of water. Samples were vortexed and centrifuged at 3000 rpm for 5 min at room temperature. 800 μl of the aqueous phase was counted by liquid scintillation. The DES1 enzyme activity is expressed as pmol/ml protein/h.
Western Blot
Cells were lysed for 30 min at 4 °C in a buffer containing 50 mm Tris, pH 7.5, 120 mm NaCl, 1 mm EDTA, 15 mm Na4P2O7, 20 mm NaF, 1% Nonidet, 0.1% phenylmethyl sulfluoride, and protease inhibitors (0.08 μm aprotinin, 0.02 μm leupeptin, 0.04 μm bestatin, and 15 μm pepstaitin). To prepare total cell lysates, cell extracts were centrifuged for 15 min at 12,000 × g at 4 °C. Proteins (10 μg) from cell lysates were separated by SDS-PAGE and then transferred to nitrocellulose membranes, which were incubated overnight in 5% milk in PBS containing 0.1% Tween 20. Membranes were subsequently incubated with primary antibody overnight at 4 °C. Goat anti-rabbit IgG-HRP or donkey anti-goat IgG-HRP was used as secondary antibody. Proteins were detected by enhanced chemiluminescence plus Western blotting detection system (Amersham Biosciences).
DES1 Overexpression
Mouse DES1 overexpression vector and empty vector control (pReceiver-M2) were purchased from GeneCopoeia (EX-Mm 19326-M02 and EX-NEG-M2, respectively). The plasmid DNA was amplified in Escherichia coli and then purified by using QIAfilter Maxi kit according to the manufacturer's instructions (Qiagen). Transfection of plasmid DNA into mouse C2C12 myotubes was accomplished using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols.
Isolation of Mouse Primary Myoblasts
Mouse primary myoblasts were isolated as described previously (24). Briefly, muscles were isolated from the hind limbs of 12-week old C57BL/6J male mice (The Jackson Laboratory, Bar Harbor, ME). To disrupt the tissues, they were placed in collagenase/dispase/CaCl2 solution (1.5 units/ml collagenase D from Fisher Scientific, Pittsburgh, PA; 2.4 units/ml dispase II from Invitrogen; and 2.5 mm CaCl2)and incubated at 37 °C for 1 h. Isolated cells were filtered and resuspended in F-10-based primary myoblast growth medium (F10 nutrient mixture (Invitrogen) supplemented with 20% FBS, 25 μg/ml human basic fibroblast growth factor (Invitrogen), and 1% penicillin/streptomycin (Invitrogen)). Cells were plated on calf collagen (Fisher Scientific) coated plates. Cells were maintained in F-10-based primary myoblast growth medium for 7 days. Myoblasts were then maintained in F-10/DMEM-based primary myoblast growth media (40% F-10-based primary myoblast growth medium and 40% DMEM) for an additional 4 days. Confluent cells were differentiated in DMEM supplemented with 10% horse serum. After 7 days of differentiation, myotubes were formed and utilized for experiments.
RESULTS
Oleate Attenuates Palmitate-induced Ceramide Production
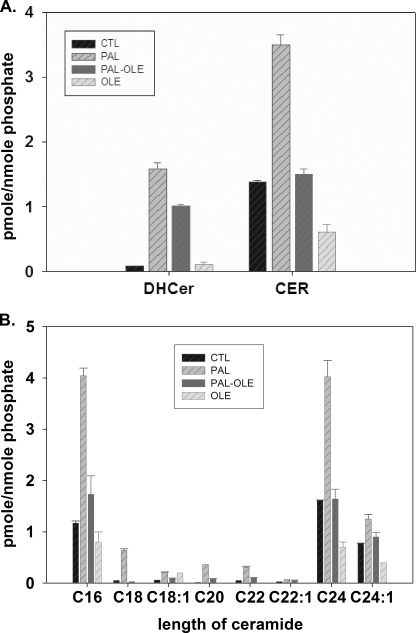
Previous studies by our group showed that palmitate caused broad changes in cell sphingolipids. Specifically, most sphingolipids were elevated by palmitate treatment, which occurred primarily via de novo synthesis (21). One limitation of this study is that cells in vivo are exposed to mixtures of fatty acids, rather than single fatty acids. Moreover, other studies have indicated a role for oleate attenuation of palmitate-induced increases in DAG (11, 25). Thus, we hypothesized that oleate may also attenuate palmitate-induced increases in sphingolipids. To test this, C2C12 myotubes were treated with palmitate in the presence of 0.75 mm oleate and total sphingolipids were determined by LC/MS as described under “Experimental Procedures.” These data indicated that co-treatment with oleate could completely inhibit palmitate-induced ceramide (Fig. 1A). Intriguingly however, dihydroceramide, the immediate metabolic precursor of ceramide, was only partially inhibited (∼33%, Fig. 1A).
FIGURE 1.
Oleate attentuates palmitate-induced ceramide production. Mouse C2C12 myotubes were treated with vehicle control, 1.25 mm palmitate, 1.25 mm palmitate plus 0.75 mm oleate, or 0.75 mm oleate alone. Lipid profiles were determined by LC/MS after 16 h of treatment. A, total dihydroceramide and ceramide. B, N-acyl chain length of ceramides measured. Data are means ± S.E. (n = 2) of three experiments. CTL, control; PAL, palmitate; OLE, oleate; CER, ceramide; DHCer, dihydroceramide.
Dihydroceramide is generated by six distinct (dihydro)ceramide synthase enzymes (26), which catalyze N-acylation of dihydrosphingosine. Each of these enzymes has partially distinct selectivity for acyl-CoAs of various chain lengths, and together they generate dihydroceramides ranging from C14 to C26 or longer N-acyl chains. We observed that the decreases in ceramides occurred similarly in each N-acyl chain-length measured (Fig. 1B), and thus, the mechanism for decreasing ceramide appeared nonspecific with respect to N-acyl chain length. This suggested that oleate mediates its inhibition of ceramide generation by means other than attenuation of specific ceramide synthase isoforms and/or activities. This conclusion, coupled with the observation that dihydroceramides were only partially attenuated by oleate, led to the hypothesis that oleate prevents desaturation of dihydroceramide.
Oleate Prevents DES1 Up-regulation by Palmitate
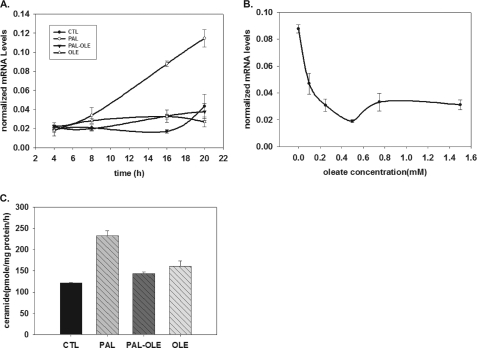
De novo ceramide is derived from the action of dihydroceramide desaturase, DES1, a required enzyme for de novo ceramide synthesis responsible for inserting the 4,5-trans-double bond into the sphingoid backbone of dihydroceramide (27, 28). Though two isoforms of this enzyme occur in mammals, recent data indicate that only DES1 has this enzymatic activity, and thus, is the sole enzyme responsible for conversion of dihydroceramide to ceramide (27). Therefore, we hypothesized that oleate may regulate DES1 expression. To test this, myotubes were treated with palmitate at concentrations from 0.1 to 1.5 mm. RNA was isolated from these cells and used for qPCR. These results demonstrated that palmitate markedly increased the message of DES1 in a dose-dependent manner, which was first observed at 0.2 mm and increased in magnitude up to 1.0 mm (supplemental Fig. 1), concentrations that span healthy physiological range at the lower doses up to pathophysiological elevation at the upper doses (29). Moreover, DES1 message increase was observed as early as 9 h and continued to increase up to 20 h (Fig. 2A). On the other hand, co-treatment with 0.75 mm oleate completely attenuated this increase, showing maximum inhibitory activity as early as 8 h (Fig. 2A). Dose-response experiments were conducted in myotubes treated with palmitate plus oleate at concentrations of 0.1, 0.25, 0.5, 0.75, and 1.5 mm. The results revealed that oleate attenuated DES1 increase at concentrations as low as 0.1 mm, with maximum inhibition at 0.5 mm (Fig. 2B).
FIGURE 2.
Oleate prevents palmitate-induced-DES1 up-regulation in a dose- and time-dependent manner. Mouse C2C12 myotubes were treated with vehicle control, 1.25 mm palmitate, 1.25 mm palmitate plus 0.75 mm oleate, or 0.75 mm oleate alone. DES1 mRNA expression was determined by qPCR. Data are presented as means (n = 6) ± S.E. A, on a 4–20-h time course; B, 1.25 mm palmitate co-treatment with 0–1.5 mm oleate for 16 h. C, at 16 h treatment, DES1 enzymatic activities were determined. Data are presented as mean ± S.E. (n = 3). CTL, control; PAL, palmitate; OLE, oleate.
In sum, these data demonstrated that physiologically relevant concentrations of palmitate and oleate regulate DES1 in C2C12 myotubes according to the concentrations of each fatty acid. To test whether these effects were specific to the C2C12 system or would demonstrate applicability to other skeletal muscle systems, similar experiments were performed in primary isolates of mouse skeletal muscle myoblasts differentiated in vitro to myotubes. Consistent with the C2C12 results, DES1 message also increased in these mouse myotubes treated with 0.5 mm palmitate, and oleate attenuated the induction (supplemental Fig. 2).
To test whether changes in message affected enzyme activity, dihydroceramide desaturase activity was determined in microsomes prepared from C2C12 cells treated with palmitate with and without oleate co-treatment. The data indicated that oleate decreased microsomal DES1 activity by ∼45% (Fig. 2C).
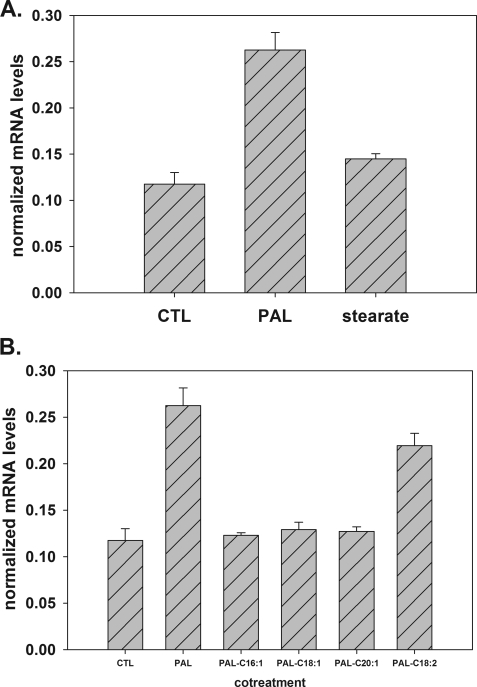
These data indicated a novel role for both palmitate and oleate in regulation of DES1, and thus, we sought to determine the specificity of biologically relevant saturated versus unsaturated FFAs in regulation of DES1. Palmitate and stearate (C18:0) comprise the majority of plasma saturated fatty acids, and thus, myotubes were treated with 1.25 mm palmitate or 1.25 mm stearate. The qPCR measurements of DES1 message indicated that palmitate, but not stearate, increased DES1 (Fig. 3A). This striking difference in activities between these two very similar fatty acids indicates a surprising selectivity for palmitate in regulation of DES1. To evaluate stearate effects on ceramide and dihydroceramide, myotubes were treated with 0.75 mm stearate and cotreatment with 0.75 mm palmitate. Intriguingly, stearate increased both ceramide and dihydroceramide, but, cotreatment could not prevent Cer increase (supplemental Fig. 3, A and B, respectively).
FIGURE 3.
Fatty acid specificity of DES1 message regulation. A, C2C12 myotubes were treated with vehicle control, 1.25 mm palmitate, or 1.25 mm stearate for 16 h. DES1 mRNA expression was determined by qPCR. Data are presented as means (n = 3) ± S.E. B, C2C12 myotubes were treated with vehicle control, 1.25 mm palmitate, or 1.25 mm palmitate plus 0.3 mm C16:1, C18:1, C20:1, and C18:2. At 16 h of treatment, DES1 mRNA expression was determined by qPCR. Data are presented as means (n = 3) ± S.E. CTL, control; PAL, palmitate.
We also sought to determine the specificity of the inhibitory effect of oleate on DES1 increase. Thus, myotubes were treated with palmitate and cotreated with the related and relatively abundant monounsaturated fatty acids palmitoleate (C16:1), oleate (C18:1), or eicosenoic acid (C20:1), and also the essential polyunsaturated linoleic acid (C18:2). The measurements indicated that each monounsaturated species behaved similarly to oleate in attenuation of DES1 message increase by palmitate. On the other hand, inhibition of DES1 message increase by linoleic acid was relatively weak, suggesting that this activity is specific to monounsaturated fatty acids, and, moreover, is not determined by acyl chain length (Fig. 3B).
Oleate Restores Insulin Sensitivity to Palmitate-treated Myotubes
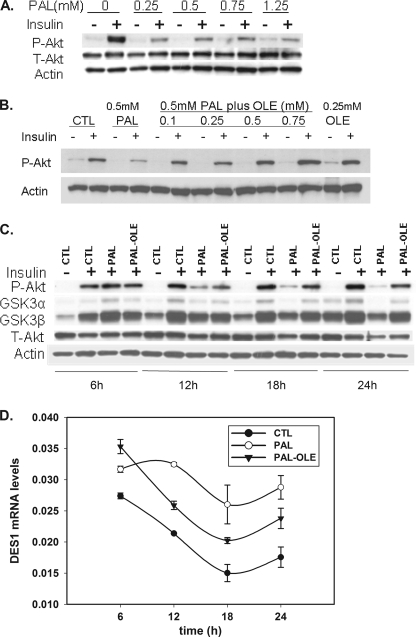
Elevated plasma lipids mediate a number of downstream effects in various tissues to promote diabetes and the metabolic syndrome. A key such activity is the attenuation of insulin sensitivity in skeletal muscle (17, 30, 31). This is thought to arise, at least in part, by the inhibition of Akt phosphorylation in response to insulin by ceramide (6, 32). To test whether oleate could prevent palmitate-induced insulin desensitization, myotubes were treated with palmitate alone or in combination with oleate. Cells were treated with 0.25 mm to 1.25 mm palmitate for 16 h and then stimulated with insulin. Phosphorylation status of Akt was assessed by Western blot. The results indicated Akt phosphorylation was inhibited even at low concentrations, with significant inhibition observed at 0.25 mm palmitate (Fig. 4A). At 0.5 mm palmitate, co-treatment with oleate demonstrated restoration of insulin-dependent Akt phosphorylation at concentrations as low as 0.1 mm (Fig. 4B). To confirm the effects of these treatments on insulin signaling, phosphorylation of the downstream Akt substrates GSK3α/β was assessed by Western blot, and results demonstrated phosphorylation of both isoforms mirrored the Akt phosphorylation state, with no palmitate-mediated inhibition observed at 6 h, followed by robust attenuation of insulin signaling from 12–24 h, and restoration of p-Akt by oleate co-treatment at similar time points (Fig. 4C).
FIGURE 4.
Oleate blocks palmitate-mediated insulin resistance in C2C12 myotubes. A, myotubes were treated with 0.25, 0.5, 0.75, and 1.25 mm palmitate for 16 h and stimulated with 100 nm insulin for 10 min. Akt phosphorylation was measured by Western blot. B, at 0.5 mm palmitate co-treatment with 0.1, 0.25, 0.5, and 0.75 mm oleate for 16 h, cells were stimulated with 100 nm insulin for 10 min. Akt phosphorylation was measured by Western blot. The membranes were stripped and reprobed with an antibody toward β-actin. C, myotubes were treated with 0.75 mm palmitate plus 0.25 mm oleate for 6, 12, 18, and 24 h and then stimulated with 100 nm insulin for 10 min. Akt and GSK 3α/β phosphorylation were measured by Western blot, and the membrane were also reprobed with total Akt and Actin. D, myotubes were treated as the same as C; the DES1 message level was measured by qPCR. Data are representative of two experiments. CTL, control; PAL, palmitate; OLE, oleate; P-Akt, phospho-Akt (Ser473); T-Akt, total Akt; P-GSK3α, phospho-GSK3α (Ser21); P-GSK3β, phospho-GSK 3β (Ser9).
These results were consistent with fatty acid effects on DES1 message, which, at 6 h demonstrated a <15% increase upon palmitate treatment, but increased >50% by 12 h (Fig. 4D), the first point at which an effect on p-Akt was observed (Fig. 4C). This result is also consistent with our previous studies, which indicated relatively small palmitate-induced increases in ceramide prior to 8 h (21). Moreover, the effect of oleate co-treatment on restoration of p-Akt in response to insulin was observed at similar time points as the maximum effect on DES1 expression (Fig. 4, C and D). Additionally, ceramide levels were attenuated under these conditions, with a >30% decrease by 12 h and a >50% decrease at 24 h (supplemental Fig. 4). Together, these results indicate that the effects of palmitate and oleate on DES1 expression, lipid levels, and insulin signaling occurred at comparable lipid concentrations and time points.
Restoration of Insulin Signaling by Oleate Can Be Overcome by Addition of C2-ceramide
Studies indicate that palmitate increases both ceramide and DAG (14, 15); however, the relative importance of these lipids in skeletal muscle insulin resistance remains controversial (33, 34). Thus, we sought to test whether adding back ceramide could overcome the protective effect of oleate. After cotreatment with palmitate and oleate for 14 h, the cells were incubated with exogenous 50 μm C2-ceramide for 2 h before insulin stimulation. Data indicate that C2-ceramide addition overcame the protective effect of oleate on Akt phosphorylation (Fig. 5A).
FIGURE 5.
Exogenous ceramide overcomes the protective effect of oleate. A, myotubes were treated with vehicle, 1.25 mm palmitate, 1.25 mm palmitate plus 0.75 mm oleate, or 0.75 mm oleate alone. At 14 h of treatment, the samples co-treated with palmitate plus oleate were incubated with 50 μm C2-ceramide for 2 h and then stimulated with 100 nm insulin for 10 min. The proteins were extracted as described under “Experimental Procedures” and used for Western blot with phospho-Akt antibody. Data are representative of two experiments. B, myotubes were treated with vehicle control, 1.25 mm palmitate, 1.25 mm palmitate plus 0.75 mm oleate, or 0.75 mm oleate alone. Total DAG was determined by LC/MS after 16 h of treatment. C, length chain of DAG Data are represented as mean (n = 6) ± S.E. CTL, control; PAL, palmitate; OLE, oleate.
Because palmitate also increases DAG, we sought to determine the effect of oleate on DAG levels in cells. DAG species were measured by LC/MS after palmitate treatment as described under “Experimental Procedures.” Total DAG increased >2-fold in cells treated with 1.25 mm palmitate. Intriguingly, co-treatment with 0.5 mm oleate increased DAG >5-fold relative to control cells (Fig. 5B). These data indicate that oleate does not attenuate DAG synthesis under these conditions and, moreover, that oleate protects from palmitate-induced insulin resistance, strongly supporting that ceramide, not DAG, is the active species in insulin resistance under these conditions.
The LC/MS analysis provided data on the chain length composition of DAG generated by palmitate or palmitate-oleate co-treatment. As shown in Fig. 5C, palmitate increased all DAG species with palmitoyl chains except for C16/C18:1. However, this species was increased >10-fold upon addition of oleate along with palmitate, suggesting that oleate may be limiting for biosynthesis of this DAG species. Additionally, oleate increased most other species with oleoyl chains. These data demonstrated oleate in combination with palmitate increased several distinct DAG species, most notably C16/C18:1, and thus, this species may not promote insulin resistance. These data provide evidence for specific DAG species having specific actions in cells, similar to recent findings for ceramide species (35–37).
Effects of Modulating DES1 Expression on Insulin Signaling
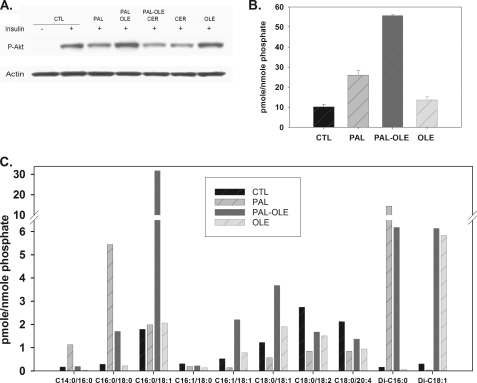
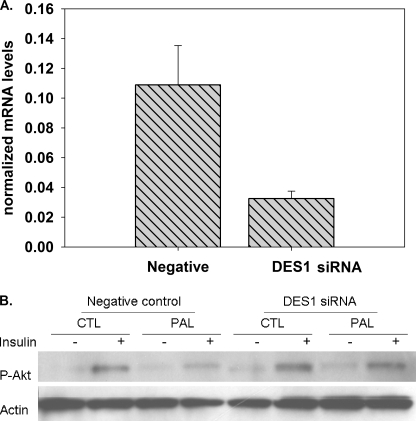
Many protective effects of oleate in palmitate-induced toxicity have been reported previously (11, 12, 25, 38, 39). Coll et al. (11) demonstrated oleate protects palmitate-induced insulin resistance and inflammation. Akito et al. (38) indicated unsaturated fatty acids had protective action against palmitate-evoked events by regulating Cox-2 expression in skeletal muscle. Thus, to test whether oleate-mediated modulation of DES1 expression alone is sufficient to prevent palmitate-induced insulin resistance, myotubes were transfected with DES1 siRNA or unspecific siRNA as control. This treatment decreased DES1 mRNA by 70% compared with the negative control siRNA (Fig. 6A). At 72 h post-transfection, cells were treated with palmitate, and the effects of DES1 knockdown on sphingolipid profiles were determined by LC/MS. Indeed, these measurements indicated DES1 knockdown increased dihydroceramide 140% and decreased ceramide 22% in myotubes treated with 0.5 mm palmitate (supplemental Fig. 5, A and B). To test whether this modulation of sphingolipids was sufficient to restore insulin signaling, insulin stimulation of Akt phospohrylation was determined. DES1 knockdown prevented palmitate-mediated decrease in phospho-Akt inhibition (Fig. 6B). Therefore, we concluded that the activity of oleate in decreasing DES1 mRNA is sufficient to attenuate loss of insulin sensitivity in response to palmitate.
FIGURE 6.
Effects of knockdown DES1 expression on insulin signaling and protection by oleate. A, DES1 siRNA prevents palmitate-mediated phospho-Akt inhibition. Myotubes were cultured in 60-mm dishes, at 4 days after differentiation, cells were transfected with DES1 siRNA or negative control siRNA, and DES1 mRNA levels were measured at 72 h post-transfection. B, at 72 h post-transfection cells were treated with vehicle control or 1.25 mm palmitate for 14 h and then stimulated with 100 nm insulin for 10 min, and phospho-Akt was measured by Western blot. Data are representative of three experiments; CTL, control; PAL, palmitate.
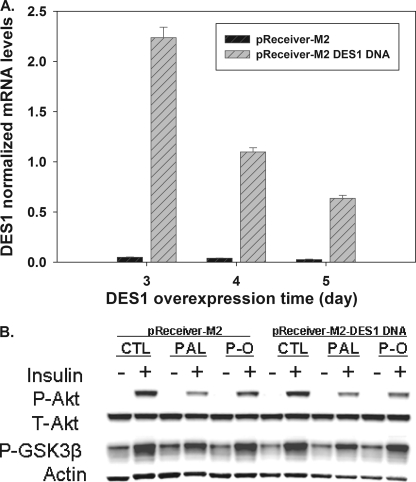
On the other hand, would overriding the oleate-mediated down-regulation prevent this protective effect of oleate? To test this, myotubes were transfected with an overexpression plasmid encoding DES1. As shown in Fig. 7A, DES1 message markedly increased 72 h post-transfection with DES1-encoding vector as compared with empty vector control. At this time point, cells were treated with palmitate or palmitate plus oleate and insulin-stimulated phospho-Akt was measured. As shown in Fig. 7B, forced overexpression of DES1 substantially diminished the protective effect of oleate on insulin signaling. Together, these data demonstrate that regulation of DES1 is sufficient to modulate palmitate-induced insulin resistance, suggesting DES1 can serve as a target for treatment of insulin resistance.
FIGURE 7.
Overexpression DES1 diminish the protective effect of oleate in insulin signaling. Mouse DES1 expression plasmids were transfected into mouse C2C12 myotubes as described under “Experimental Procedures.” A, at 3, 4, and 5 days post-transfection, DES1 mRNA was measured by qPCR. B, at 72 h post-transfection, the cells were treated for 16 h with vehicle, 0.5 mm palmitate, 0.5 mm palmitate plus 0.25 mm oleate and then stimulated with 100 nm insulin for 10 min. Phospho-Akt and GSK3β were measured by Western blot. Data are representative of three experiments. CTL, control; PAL, palmitate. P-O, palmitate plus oleate; P-Akt, phospho-Akt(Ser473); T-Akt, total Akt; P-GSK3β, phospho-GSK 3β(Ser9).
DISCUSSION
Though mechanisms of saturated fatty acid-mediated toxicity are not completely understood, a leading hypothesis is that oversupply of these lipids promotes formation of bioactive lipid mediators, a process termed “lipotoxicity” (40, 41). In particular, FFA-mediated generation of DAG and/or ceramide is implicated in lipotoxicity in tissues such as liver (42), heart (43), pancreas (44), and skeletal muscle (45). Indeed, several studies by our group and others support a key role for aberrant biosynthesis of sphingolipids, most notably, ceramide, in diverse lipotoxic processes (46–49).
Much epidemiological data indicates a protective role for unsaturated fatty acids against pathological processes caused by excess saturated fat (11, 12, 38, 50, 51). Thus, we sought to determine the impact of oleate on palmitate-mediated sphingolipid biosynthesis. Surprisingly, we found that oleate attenuated ceramide production at the level of dihydroceramide desaturase and that this occurred by preventing a palmitate-induced up-regulation of DES1. This enzyme plays a crucial role in de novo synthesis of ceramide through addition of the 4,5-trans-double-bond to its immediate metabolic precursor dihydroceramide. Thus, DES1 may significantly impact ceramide signaling processes because the enzyme essentially modulates the dihydroceramide/ceramide ratio in cells (52). As ceramide mediates apoptosis (53), senescence (54), cell growth regulation (55), signaling processes (56), and other diverse functions in cells (57), it may serve as a therapeutic target for a variety of illnesses. Especially promising in this regard is that DES1 null mice demonstrated no susceptibility to insulin resistance caused by nutrient excess or glucocorticoid therapy (58). However, despite these important roles for DES1, its regulation remains poorly understood. Data presented here establish fatty acids in regulation of DES1 at the message level, with palmitate increasing DES1 message and with oleate blocking this increase. These changes in message were mirrored in activity studies, and, moreover, blocking DES1 expression with oleate ameliorated the insulin-resistant phenotype brought on by palmitate. To our knowledge, this is the first study of regulation of DES1 message, and, moreover, indicates a functionality for this regulation pertaining to insulin resistance. These data may provide further mechanistic insights as to how saturated fatty acids drive sphingolipid-mediated lipotoxic processes. Though this study was conducted in a skeletal muscle model system, protective effects of oleate mediated by DES1 may also extend to pancreas, liver, and heart, other tissues suffering from deleterious activities of saturated FFAs.
Sphingolipid metabolic pathways constitute a complex and interlocked “web” of reactions where the presence of many forward and reverse reactions as well as both catabolic and anabolic enzymes lead to a situation where perturbation of one metabolite often has unexpected or counterintuitive “ripple effects” throughout the pathway (59, 60). Consistent with this concept, we observed that oleate co-treatment led to changes in levels of several sphingolipids in addition to dihydroceramides and ceramides, including a slight increase glucosylceramide (supplemental Fig. 6A), a decrease in lactosylceramide (supplemental Fig. 6B), and oleate alone increased sphingomyelin (supplemental Fig. 6C). The mechanisms for these changes remain unknown, as well as their possible effects on cell processes including insulin resistance. On the other hand, in the presence of palmitate, oleate co-treatment significantly attenuated both dihydrosphingosine 1-phosphate and sphingosine 1-phosphate (supplemental Fig. 5D). This decrease is consistent with our previous work demonstrating that oleate attenuated palmitate-induced expression of sphingosine kinase 1 (21). Combined, these data indicate that oleate has multiplex activities on cell sphingolipid profiles, the physiological ramifications of which remain to be determined.
In addition to ceramide synthesis, palmitate also drives generation of DAG, another signaling lipid implicated in insulin resistance. Recent data demonstrated that oleate protected against palmitate-induced insulin resistance in skeletal muscle cells by promoting triacylglycerol accumulation and mitochondrial β-oxidation through peroxisome proliferator activated receptor-α and protein kinase A-dependent mechanisms, thus reducing DAG (11). Although our studies do not rule out a role for DAG in palmitate-mediated insulin resistance, under the conditions used here, we did not observe a decrease in DAG with oleate-palmitate co-treatment. In contrast, oleate stimulated a 5-fold increase in DAG, mostly comprised of the C16:0/C18:1 DAG (Fig. 5C). This argues strongly for ceramide as the major insulin-desensitizing species under these conditions; on the other hand, the specific DAG produced may not have the same signaling properties as other species.
Another surprising finding is the differential regulation of DES1 by saturated FFA. Although palmitate caused a robust up-regulation of DES1 message, stearate had no effect. Because saturated fatty acids are often thought of “en masse,” this finding brings to light the point that each fatty acid is a discrete chemical species and can mediate distinct actions. Intriguingly, cells treated with stearate demonstrated increased dihydroceramide and co-treatment with palmitate caused an additive increase (supplemental Fig. 3B). Additionally, stearate increased ceramides, though no additional increase was observed upon co-treatment with palmitate (supplemental Fig. 3A). These data are intriguing because palmitate is a preferred substrate for the initial enzyme of sphingolipid biosynthesis, serine palmitoyl transferase, yet even though stearate is not a preferred substrate for this enzyme, it also increased ceramide. This finding presents the possibility that saturated fatty acids other than palmitate may also act through increasing sphingolipid synthesis through mechanisms distinct from increased substrate supply to the enzyme.
Each of the monounsaturated FFAs tested demonstrated attenuation of palmitate-mediated DES1 expression; however, the polyunsaturated linoleic acid (C18:2) was not as effective in blocking expression, indicating a preference in this process for monounsaturated fatty acids, which may derive from the 9,10-double bond consistent with the species tested. Because stearoyl CoA-desaturase enzymes introduce this double bond in vivo (61), this enzyme may be an additional target for modulation of insulin resistance.
Although we have demonstrated marked effects of palmitate and oleate on DES1 message levels, the mechanisms remain unclear. Two previous studies have indicated mechanisms by which oleate clears palmitate. One study demonstrated up-regulation of diacylglycerol acyltransferase, thereby shunting palmitate to triacylglycerols (11). Although palmitate increased triacylglycerols, we observed no additional increase in triacylglycerols upon co-treatment with oleate (supplemental Fig. 7), we doubt this mechanism operates under our experimental conditions. The second study demonstrated that oleate up-regulated CPT1, thereby increasing B-oxidation of palmitate and preventing its metabolism to bioactive lipids (25). Whereas, in our hands, oleate did in fact up-regulate CPT1, inhibition of this enzyme with etomoxir was unable to diminish effects of oleate on either ceramides or insulin sensitivity (data not shown). Another intriguing possibility is that oleate could compete with palmitate for esterification to CoA, as ceramide biosynthesis is CoA-dependent; however, because serine palmitoyltransferase is also CoA-dependent, we would expect to observe decreases in ceramide precursors, including dihydrosphingosine and dihydroceramides, which were not observed.
Two additional hypotheses could be that palmitate requires metabolism to sphingolipids to regulate DES1, thus presenting opportunity for a feedback loop that may be biologically advantageous. However, co-treatment with the SPT inhibitor myriocin failed to attenuate DES1 up-regulation, indicating this occurs independently of sphingolipids. Palmitate also induces cell stress, including oxidative stress; however, a recent study indicated that this stress mediated a down-regulation of DES1 activity as opposed to the increase in activity observed in this work (Fig. 2C). Thus, the mechanism(s) by which oleate attenuates palmitate-induced DES1 message increase remains a mystery at present.
In summary, our data demonstrate that fatty acids differentially regulate DES1 and that this regulation plays a functional role in sphingolipid-mediated insulin resistance. Importantly, monounsaturated fatty acids prevent palmitate-mediated up-regulation of this enzyme. These findings provide mechanistic insight into the protective effects of oleate against metabolic disease. Moreover, altering fatty acid profiles to prevent ceramide biosynthesis through DES1 may provide a protective therapeutic strategy for insulin resistance, type 2 diabetes, and the metabolic syndrome.
Supplementary Material
Acknowledgments
We acknowledge Maria Hernandez Corbacho and Dr. Leah Siskind for advice on the DES1 activity assay. LC/MS lipid measurements were performed at the Lipidomics Core Facility at the Medical University of South Carolina.
This work was supported by a Merit Award from the Department of Veterans Affairs (to L. A. C.) and the National Institutes of Health COBRE in Lipidomics and Pathobiology at the Medical University of South Carolina (to L. A. C.). Lipidomics were supported by the Centers of Biomedical Research Excellence in Lipidomics in Pathobiology (NIH P20RR017077).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–7.
- DAG
- diacylglycerol(s)
- DES1
- dihydroceramide desaturase 1
- FFA
- free fatty acid
- qPCR
- quantitative real-time PCR.
REFERENCES
- 1. Lakka H. M., Laaksonen D. E., Lakka T. A., Niskanen L. K., Kumpusalo E., Tuomilehto J., Salonen J. T. (2002) JAMA 288, 2709–2716 [DOI] [PubMed] [Google Scholar]
- 2. Samuel V. T., Petersen K. F., Shulman G. I. (2010) Lancet 375, 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berkowitz D. (1966) JAMA 197, 77–80 [PubMed] [Google Scholar]
- 4. Hansen B. C. (1999) Ann. N.Y. Acad. Sci. 892, 1–24 [DOI] [PubMed] [Google Scholar]
- 5. Moro C., Galgani J. E., Luu L., Pasarica M., Mairal A., Bajpeyi S., Schmitz G., Langin D., Liebisch G., Smith S. R. (2009) J. Clin. Endocrinol. Metab. 94, 3440–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chavez J. A., Summers S. A. (2003) Arch. Biochem. Biophys. 419, 101–109 [DOI] [PubMed] [Google Scholar]
- 7. Lee J. S., Pinnamaneni S. K., Eo S. J., Cho I. H., Pyo J. H., Kim C. K., Sinclair A. J., Febbraio M. A., Watt M. J. (2006) J. Appl. Physiol. 100, 1467–1474 [DOI] [PubMed] [Google Scholar]
- 8. Mazzone T., Chait A., Plutzky J. (2008) Lancet 371, 1800–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabin M. A., Stewart C. E., Crowne E. C., Turner S. J., Hunt L. P., Welsh G. I., Grohmann M. J., Holly J. M., Shield J. P. (2007) J. Cell. Physiol. 211, 244–252 [DOI] [PubMed] [Google Scholar]
- 10. Summers S. A. (2010) Curr. Opin. Lipidol. 21, 128–135 [DOI] [PubMed] [Google Scholar]
- 11. Coll T., Eyre E., Rodríguez-Calvo R., Palomer X., Sánchez R. M., Merlos M., Laguna J. C., Vázquez-Carrera M. (2008) J. Biol. Chem. 283, 11107–11116 [DOI] [PubMed] [Google Scholar]
- 12. Gao D., Griffiths H. R., Bailey C. J. (2009) Br. J. Nutr. 102, 1557–1563 [DOI] [PubMed] [Google Scholar]
- 13. Schmitz-Peiffer C., Craig D. L., Biden T. J. (1999) J. Biol. Chem. 274, 24202–24210 [DOI] [PubMed] [Google Scholar]
- 14. Pickersgill L., Litherland G. J., Greenberg A. S., Walker M., Yeaman S. J. (2007) J. Biol. Chem. 282, 12583–12589 [DOI] [PubMed] [Google Scholar]
- 15. Chavez J. A., Knotts T. A., Wang L. P., Li G., Dobrowsky R. T., Florant G. L., Summers S. A. (2003) J. Biol. Chem. 278, 10297–10303 [DOI] [PubMed] [Google Scholar]
- 16. Itani S. I., Ruderman N. B., Schmieder F., Boden G. (2002) Diabetes 51, 2005–2011 [DOI] [PubMed] [Google Scholar]
- 17. Schmitz-Peiffer C. (2002) Ann. N.Y. Acad. Sci. 967, 146–157 [DOI] [PubMed] [Google Scholar]
- 18. Erion D. M., Shulman G. I. (2010) Nat. Med. 16, 400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chavez J. A., Holland W. L., Bär J., Sandhoff K., Summers S. A. (2005) J. Biol. Chem. 280, 20148–20153 [DOI] [PubMed] [Google Scholar]
- 20. Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Methods 39, 82–91 [DOI] [PubMed] [Google Scholar]
- 21. Hu W., Bielawski J., Samad F., Merrill A. H., Jr., Cowart L. A. (2009) J. Lipid. Res. 50, 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mao C., Xu R., Szulc Z. M., Bielawski J., Becker K. P., Bielawska A., Galadari S. H., Hu W., Obeid L. M. (2003) J. Biol. Chem. 278, 31184–31191 [DOI] [PubMed] [Google Scholar]
- 23. Schulze H., Michel C., van Echten-Deckert G. (2000) Methods Enzymol. 311, 22–30 [DOI] [PubMed] [Google Scholar]
- 24. Springer M., Rando T., Blau H. (1997) in Current Protocols in Human Genetics (Boyle A. ed) pp. 13.4.1–13.4.19, John Wiley & Sons, New York: [DOI] [PubMed] [Google Scholar]
- 25. Henique C., Mansouri A., Fumey G., Lenoir V., Girard J., Bouillaud F., Prip-Buus C., Cohen I. (2010) J. Biol. Chem. 285, 36818–36827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pewzner-Jung Y., Ben-Dor S., Futerman A. H. (2006) J. Biol. Chem. 281, 25001–25005 [DOI] [PubMed] [Google Scholar]
- 27. Michel C., van Echten-Deckert G. (1997) FEBS Lett. 416, 153–155 [DOI] [PubMed] [Google Scholar]
- 28. Geeraert L., Mannaerts G. P., van Veldhoven P. P. (1997) Biochem. J. 327, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roden M. (2004) Physiology 19, 92–96 [DOI] [PubMed] [Google Scholar]
- 30. Sell H., Eckardt K., Taube A., Tews D., Gurgui M., Van Echten-Deckert G., Eckel J. (2008) Am. J. Physiol. Endocrinol. Metab. 294, E1070–1077 [DOI] [PubMed] [Google Scholar]
- 31. Mullen K. L., Pritchard J., Ritchie I., Snook L. A., Chabowski A., Bonen A., Wright D., Dyck D. J. (2009) Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R243–251 [DOI] [PubMed] [Google Scholar]
- 32. Ghosh N., Patel N., Jiang K., Watson J. E., Cheng J., Chalfant C. E., Cooper D. R. (2007) Endocrinology 148, 1359–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boden G., Shulman G. I. (2002) Eur. J. Clin. Invest. 32, 14–23 [DOI] [PubMed] [Google Scholar]
- 34. Kraegen E. W., Cooney G. J. (2008) Curr. Opin. Lipidol. 19, 235–241 [DOI] [PubMed] [Google Scholar]
- 35. Senkal C. E., Ponnusamy S., Bielawski J., Hannun Y. A., Ogretmen B. (2010) FASEB J. 24, 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Senkal C. E., Ponnusamy S., Rossi M. J., Bialewski J., Sinha D., Jiang J. C., Jazwinski S. M., Hannun Y. A., Ogretmen B. (2007) Mol. Cancer. Ther. 6, 712–722 [DOI] [PubMed] [Google Scholar]
- 37. Mesicek J., Lee H., Feldman T., Jiang X., Skobeleva A., Berdyshev E. V., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2010) Cell Signal 22, 1300–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kadotani A., Tsuchiya Y., Hatakeyama H., Katagiri H., Kanzaki M. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E1291–1303 [DOI] [PubMed] [Google Scholar]
- 39. Das S. K., Mondal A. K., Elbein S. C. (2010) J. Lipid Res. 51, 2121–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Unger R. H. (2003) Endocrinology 144, 5159–5165 [DOI] [PubMed] [Google Scholar]
- 41. Summers S. A. (2006) Prog. Lipid. Res. 45, 42–72 [DOI] [PubMed] [Google Scholar]
- 42. Wei Y., Wang D., Topczewski F., Pagliassotti M. J. (2006) Am. J. Physiol. Endocrinol. Metab. 291, E275–281 [DOI] [PubMed] [Google Scholar]
- 43. Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shimabukuro M., Higa M., Zhou Y. T., Wang M. Y., Newgard C. B., Unger R. H. (1998) J. Biol. Chem. 273, 32487–32490 [DOI] [PubMed] [Google Scholar]
- 45. Turpin S. M., Ryall J. G., Southgate R., Darby I., Hevener A. L., Febbraio M. A., Kemp B. E., Lynch G. S., Watt M. J. (2009) J. Physiol. 587, 1593–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kitatani K., Sheldon K., Anelli V., Jenkins R. W., Sun Y., Grabowski G. A., Obeid L. M., Hannun Y. A. (2009) J. Biol. Chem. 284, 12979–12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taha T. A., Hannun Y. A., Obeid L. M. (2006) J. Biochem. Mol. Biol. 39, 113–131 [DOI] [PubMed] [Google Scholar]
- 48. Hannun Y. A. (1996) Science 274, 1855–1859 [DOI] [PubMed] [Google Scholar]
- 49. Ye J. (2007) Endocr. Metab. Immune. Disord. Drug. Targets 7, 65–74 [DOI] [PubMed] [Google Scholar]
- 50. Frangioudakis G., Garrard J., Raddatz K., Nadler J. L., Mitchell T. W., Schmitz-Peiffer C. (2010) Endocrinology 151, 4187–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gaster M., Rustan A. C., Beck-Nielsen H. (2005) Diabetes 54, 648–656 [DOI] [PubMed] [Google Scholar]
- 52. Kraveka J. M., Li L., Szulc Z. M., Bielawski J., Ogretmen B., Hannun Y. A., Obeid L. M., Bielawska A. (2007) J. Biol. Chem. 282, 16718–16728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Obeid L. M., Hannun Y. A. (1995) J. Cell. Biochem. 58, 191–198 [DOI] [PubMed] [Google Scholar]
- 54. Venable M. E., Lee J. Y., Smyth M. J., Bielawska A., Obeid L. M. (1995) J. Biol. Chem. 270, 30701–30708 [DOI] [PubMed] [Google Scholar]
- 55. Spiegel S., Merrill A. H., Jr. (1996) FASEB J. 10, 1388–1397 [DOI] [PubMed] [Google Scholar]
- 56. Arana L., Gangoiti P., Ouro A., Trueba M., Gómez-Muñoz A. (2010) Lipids Health Dis. 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hannun Y. A., Obeid L. M. (2008) Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 58. Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., Summers S. A. (2007) Cell. Metab. 5, 167–179 [DOI] [PubMed] [Google Scholar]
- 59. Alvarez-Vasquez F., Sims K. J., Cowart L. A., Okamoto Y., Voit E. O., Hannun Y. A. (2005) Nature 433, 425–430 [DOI] [PubMed] [Google Scholar]
- 60. Cowart L. A., Shotwell M., Worley M. L., Richards A. J., Montefusco D. J., Hannun Y. A., Lu X. (2010) Mol. Syst. Biol. 6, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miyazaki M., Kim H. J., Man W. C., Ntambi J. M. (2001) J. Biol. Chem. 276, 39455–39461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.