Abstract
Prokineticins are a pair of signal factors involved in many physiological processes by binding to two closely related G-protein-coupled receptors, PKR1 and PKR2. Recently, mutations in prokineticin 2 (PK2) and PKR2 are found to be associated with Kallmann syndrome and/or idiopathic hypogonadotropic hypogonadism, disorders characterized by delayed puberty and infertility. However, little is known how PKRs interact and activate G-proteins to elicit signal transduction. In the present study, we took advantage of one disease-associated mutation (R164Q) located in the second intracellular (IL2) loop of PKR2, to investigate the role of IL2 loop in the cell signaling, G-protein binding and receptor trafficking. R164Q mutant PKR2 showed normal cell surface expression and ligand binding capacity. However, the PKR2 signaling was abolished by R164Q mutation. We demonstrated that R164Q mutation disrupted the interaction of IL2 loop to the Gαq, Gαi, and Gα16-proteins. A positive-charged amino acid at this position is required for proper function, and the signaling efficacy and potency depend on the net amount of positive charges. We also demonstrated that the interactive partner of Arg-164 may localize in the C-terminal five residues of Gαq-protein. A series of mutation analysis indicated that the basic amino acids at the C terminus of IL2 loop may function cooperatively in GPCRs. Furthermore, R164Q mutation also results in minimal ligand-induced endocytosis of PKR2. As many GPCRs share structural homology in the C terminus of IL2 loop, our findings may have general application in understanding structure and function of GPCRs.
Keywords: Cell Surface Receptor, G Protein-coupled Receptors (GPCR), 7-Helix Receptor, Protein-Protein Interactions, Receptor Endocytosis, Signal Transduction, Prokineticin
Introduction
G-protein-coupled receptors (GPCRs)2 are the largest family of membrane-spanning proteins that transduce numerous extracellular signals including light, odorants, hormones, neurotransmitters, and chemokines to the interior of cells and play fundamental roles in regulation of cellular functions. The structure of GPCRs consists of seven transmembrane (TM) helices, with three intracellular loops (IL1–3) and the cytoplasmic C-terminal tail. The extracellular ligands activate GPCRs and enable the receptors to interact with and activate distinct sets of heterotrimeric G proteins (e.g. Gαβγ) (1).
Prokineticins are a pair of signal factors involved in a variety of physiological processes, including gastrointestinal mobility, circadian rhythms, emotion, nociception, angiogenesis, and neurogenesis (2–5). Prokinticins execute their functions by binding to two closely related GPCRs, PKR1 and PKR2 (6, 7). It has been shown that activation of prokineticin receptors (PKRs) leads to accumulation of inositol phosphate and mobilization of intracellular Ca2+ via Gαq/11 proteins (6). In addition, PKRs may inhibit cAMP accumulation, presumably through Gαi/o proteins (8). Furthermore, PKRs have been shown to stimulate mitogen-activated protein kinase via Gαo protein-mediated signaling (6). However, little is known about the structure-function relationships in PKRs, and more extensive research is necessary to identify the structural determinants involved in the G-protein coupling and activation.
Recently, genetic studies have shown that mutations in prokineticin 2 (PK2) and PKR2 are associated with the Kallmann syndrome (KS) and/or idiopathic hypogonadotropic hypogonadism (IHH), disorders characterized by delayed puberty and infertility (9–13). The hypogonadotropic hypogonadism is due to gonadotropin-releasing hormone (GnRH) deficiency. The PK2 and PKR2 knock-out mice phenocopy the human conditions of KS/IHH (10, 14), confirmed the essential role of PK2/PKR2 signaling for reproductive function. One disease-causing mutation, R164Q, is located at the C terminus of IL2 loop in PKR2. Herein, we took advantage of this naturally occurred mutation and demonstrated the positive charges at the C terminus of IL2 loop plays a key role in the interaction with the C terminus of G-proteins. Disruption of this interaction abolished the receptor function and endocytosis.
EXPERIMENTAL PROCEDURES
Constructs
The cDNAs for human PKR1 and PKR2 was kindly provided by Dr. Qun-Yong Zhou (University of California, Irvine). QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA) was used to introduce various mutations into PKR1 and PKR2. The cDNA for human PKR2 was amplified by polymerase chain reaction (PCR) and cloned into pEGFP-N1 in-frame with enhanced green fluorescence protein (EGFP) to generate PKR2 tagged with EGFP at the C terminus. The cDNA for Gαq was kindly provided by Dr. Olivier Civelli (University of California, Irvine). A human influenza hemagglutinin (HA) tag was added to the N terminus of Gαq to facilitate immunodetection. The cDNA for Gα16 is a kind gift from Dr. Dian-Qing Wu (Yale University) and an N-terminal 3×Flag tag (Sigma) was added to facilitate immunodetection.
Calcium Mobilization Assay
An aequorin-based luminescent assay was used to measure mobilization of intracellular Ca2+ (15). Briefly, Chinese Hamster Ovary (CHO) cells stably expressing the photoprotein aequorin were transiently transfected with wild-type PKR1/PKR2 or their respective mutants. Two days after transfection, the cells were charged in Opti-MEM (Invitrogen, Carlsbad, CA) containing 8 μm coelenterazine cp (Invitrogen) at 37 °C for 2 h. Cells were detached by brief trypsinization and maintained in Hank's balanced salt solution plus 10 mm HEPES, pH 7.5, and 0.1% bovine serum albumin at about 5 × 105 cells/ml. Luminescence measurements were made using a Sirius Single Tube Luminometer (Berthold Detection Systems GmbH, Pforzheim, Germany). PK2 (a kind gift from Dr. Qun-Yong Zhou, University of California at Irvine) were serially diluted in Hank's balanced salt solution plus 10 mm HEPES, pH 7.5, and 0.1% bovine serum albumin. 100 μl of cells were injected into the tubes with 20 μl of PK2. The luminescence was monitored for 25 s (peak 1), then 100 μl of 1% Triton X-100 was injected to lyse the cells, and the luminescence was continuously monitored for another 20 s (peak 2). The area under the curve (AUC) of the peak 1 was divided by the total AUC of peaks 1 and 2, and the maximal responses from wild-type receptors were normalized to 100.
Glutathione S-transferase (GST) Pulldown
An overlapping primer strategy was used to clone the WT, R164Q and H159Q PKR2 IL2 loop into the pGEX-3X vector (GE Healthcare, UK) for expression of fusion proteins with an N-terminal GST. The WT IL2 loop includes amino acid sequences of IDRYLAIVHPLKPRMNYQ. After expression in Escherichia coli, GST fusion proteins were purified from cell extracts by affinity chromatography using glutathione-Sepharose beads (GE Healthcare, UK) according to the manufacturer's instructions. To prepare beads for pulldown assays, GST or GST fusion proteins (5 μg of each) were incubated with 30-μl slurry of glutathione-Sepharose beads equilibrated in buffer A (PBS, 1% Nonidet P 40, 1 mm EDTA supplemented with protease inhibitor mixture) for 1 h at 4 °C with constant agitation. After washing with buffer A, the beads were resuspended in 1 ml of buffer A containing lysates from cells transiently expressed with Flag-Gα16, HA-Gαq, and HA-Gαqi5, respectively. Incubation was performed at 4 °C for more than 1 h with constant agitation. The glutathione beads were collected by centrifugation and washed extensively in buffer A prior to resuspension in 20 μl of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. After separation by SDS-PAGE, proteins were detected by immunoblotting with antibodies against HA or Flag (Sigma), respectively.
Stable Transfection of Human Embryonic Kidney (HEK293) Cells with EGFP-tagged WT or R164Q-PKR2
The HEK293 cells were transfected with 1 μg of DNA using Lipofectamine 2000 reagent (Invitrogen). 24 h after transfection, the cells were selected in the presence of the antibiotic Geneticin (G418) at 1 mg/ml. EGFP expression in clones was initially examined by fluorescence microscopy, and confirmed by immunoblotting using an antibody against GFP (Sigma). Stable transfected HEK293 cells were subcultured onto poly-d-lysine-coated glass slides in 12-well dishes and incubated with cycloheximide (Sigma) for 4 h before experiments.
Analysis of PKR2-EGFP Trafficking
For ligand-induced internalization studies, the cells were treated with PK2 for an indicated period. For washout experiments, cells were washed three times with PBS after 1 h of treatment with 100 nm PK2 and then incubated with culture medium for an indicated time. Cycloheximide was included in all the processes. Alexa Fluor 594-conjugated concanavalin A (Invitrogen) was applied to label the membrane. After washing three times with cold PBS, the cells were fixed with 4% paraformaldehyde for 60 min at 4 °C. Images were acquired by using a laser scanning confocal system installed on a Carl Zeiss microscope with a 63× oil immersion objective. EGFP was excited with a 488-nm argon laser and detected with a 530–560-nm band pass filter. The Alexa Fluor 594 was excited at 590 nm and detected with a long pass band filter at 617 nm.
RESULTS
R164Q Mutation in PKR2 Disrupted Signaling but Not Surface Presentation
We used an aequorin-based calcium measurement to determine the signal transduction of prokineticin receptors after PK2 stimulation (15). As shown in Fig. 1A, PK2 dose-dependently increased the cytoplasmic calcium concentration in cells transfected with wild-type (WT) PKR2; however, R164Q mutation in PKR2 abolished the ligand-induced signaling. To visualize the subcellular location of receptors, we attached an enhanced green fluorescence protein (EGFP) at the C terminus of WT PKR2 and its R164Q mutant. The EGFP-tagged WT PKR2 retained full signaling capacity, whereas EGFP-tagged R164Q-PKR2 showed no signal transduction (Fig. 1A).
FIGURE 1.
The effect of R164Q mutation on the signal transduction and cell surface expression of PKR2. A, R164Q mutation disrupted the signal transduction of PKR2 as assayed with an aequorin-based assay. B, both WT and R164Q-PKR2 were presented on the cell surface. The Alexa Fluor 594-conjugated concanavalin A was used to label the membrane. The fluorescence was monitored with a confocal microscope. Scale bar: 10 μm.
As shown in Fig. 1B, both EGFP-tagged WT and R164Q mutant PKR2 were expressed on the cell surface. Because previous work has indicated R164Q-PKR2 showed intact ligand binding capacity (16), it is very likely that R164Q substitution affects G-protein coupling. However, it is unclear whether R164Q interferes with either the binding of Gα to PKR2 or the catalytic activation of Gα by PKR2.
Arg-164 Plays a Key Role in the Interaction with C Terminus of Gα-protein
To explore the possibility that R164Q affects the binding of Gαq to PKR2, we first determined whether Gαq could directly interact with purified second intracellular loop (IL2) of PKR2. Thus, glutathione S-transferase (GST) fusions of WT and R164Q mutant PKR2-IL2 were expressed in E. coli. The GST fusion proteins were purified with glutathione-Sepharose, and then incubated with cell lysate overexpressed with human Gαq. As shown in Fig. 2A, WT PKR2-IL2 was able to pulldown Gαq, suggesting that these two proteins form direct protein-protein interactions. Importantly, GST alone was not able to pulldown Gαq (Fig. 2A), demonstrating that the interaction was mediated by the IL2 of PKR2. In staggering contrast, the interaction between PKR2-IL2 and Gαq was abolished by the R164Q mutation, suggesting this mutation uncouples functional responses by abrogating receptor/Gαq interaction.
FIGURE 2.
R164Q mutation disrupted the interaction of PKR2-IL2 to Gα-protein. A, GST or GST fusion proteins with IL2 of WT, H159Q, and R164Q-PKR2 were used to pulldown Gαq-protein. B, GST or GST fusion proteins with IL2 of PKR2 were used to pulldown WT or truncated Gαq-protein without the C-terminal five amino acids (Gαq-ΔC5). C, GST or GST fusion proteins with IL2 of WT and R164Q-PKR2 were used to pulldown Gαqi5-protein. The proteins were analyzed by Western blotting with anti-HA antibody to detect the HA tag at the N terminus of Gα-proteins.
Some works have suggested the C-terminal amino acids of Gαq are flexible and interact with a variety of portions in GPCRs, including the IL2 loop (17–21). To study whether the C terminus of Gαq is involved in the interaction with the PKR2 IL2 loop, we made a truncated Gαq without the C-terminal five amino acids (Gαq-ΔC5). As shown in Fig. 2B, WT PKR2-IL2 failed to pulldown the Gαq-ΔC5, suggesting the significant role of these amino acid residues in receptor coupling.
As PKR2 was also reported to couple with Gαi pathway (8) and the IL2 loop seems to interact directly with the C-terminal five amino acids of Gαq protein, we constructed a modified Gαq-protein with the C-terminal five residues replaced by those from the Gαi3 (e.g. Gαqi5). GST-pulldown assay was also use to determine the interaction between PKR2 IL2 and Gαqi5. As shown in Fig. 2C, WT but not R164Q mutant PKR2-IL2 was able to pulldown Gαqi5, implying that R164Q mutation also disrupted the interaction between the IL2 loop and Gαi-protein.
Co-expression of Gαq and Gα16 Failed to Rescue the Signaling Deficiency of R164Q-PKR2
To investigate whether the deficient signaling of R164Q-PKR2 was due to the inadequate levels of Gαq, we co-transfected Gαq with the R164Q-PKR2. As shown in Fig. 3A, R164Q mutant receptor still did not elicit signaling transduction when co-expressed with Gαq. Thus, Gαq overexpression did not overcome the signaling defect of R164Q mutant PKR2, consistent with its deficit in G-protein interaction.
FIGURE 3.
Gαq and Gα16 failed to rescue the function deficiency of R164Q mutation in PKR2. A, PK2-stimulated calcium mobilization was monitored in cells transfected with WT or R164Q mutant PKR2 in combination with Gαq or Gα16 proteins. B, GST or GST fusion proteins with IL2 of WT and R164Q-PKR2 were used to pulldown Gα16-protein. The proteins were analyzed by Western blotting with anti-Flag antibody to detect the 3× Flag tag at the N terminus of Gα16-protein.
The promiscuous murine G-protein α-subunit Gα15 and its human counterpart Gα16 are capable of linking a wide range of Gαs-, Gαi-, and Gαq-coupled receptors to the stimulation of PLC-β and subsequent intracellular Ca2+ release (22). As PKRs have been reported to couple to Gαo and Gαi, besides Gαq, we co-expressed Gα16 with R164Q mutant PKR2 to see if this mutation affects these G-protein coupling. As shown in Fig. 3A, Gα16 co-expression did not rescue the signaling deficit of R164Q mutant receptor, either. We also used the GST pulldown assay to see whether R164Q mutation affects the binding of IL2 loop to Gα16. As shown in Fig. 3B, IL2 from the WT PKR2 was able to pulldown Gα16; however, the R164Q mutation significantly attenuated the binding of PKR2-IL2 to Gα16.
Mutational Analysis of PKR2 Amino Acid 164
Cumulatively, our data suggest that the arginine at the position 164 of PKR2 is critical for the interaction with G-proteins. To further understand how the precise physiochemical characteristics of the residue 164 might dictate the strength of PKR2 functional coupling, site-directed mutagenesis was performed to convert Arg-164 to glutamic acid (R164E), lysine (R164K), histidine (R164H), alanine (R164A), or threonine (R164T). PK2 stimulated Ca2+ mobilization was measured in cells transiently transfected with individual PKR2 variants.
As shown in the Fig. 4A, replacement of Arg-164 with small (R164A), acidic (R164E) and threonine (R164T) amino acid residues totally disrupted the receptor signaling. However, change of Arg-164 to positive-charged amino acids lysine (R164K) and histidine (R164H) resulted in receptors with partial activity (Fig. 4B). Interestingly, both signaling potency (the half-maximal effective concentration, EC50) and efficiency (the maximal response) correlated well with the net positive charges at position 164 (Fig. 4, C and D). The WT receptor with an arginine, a strong positive-charged residue, at position 164 showed the highest signaling capacity; whereas the replacement of arginine with lysine, a moderate positive-charged residue, moderately attenuated the receptor function. Furthermore, the substitution of arginine with histidine, a weak positive-charged residue significantly impaired the signaling capacity. Nevertheless, R164K and R164H mutant PKR2s were apparently better than other mutant receptors (R164A, R164E, R164T, and R164Q) in the signal transduction, indicating the requirement of positive charges at this position for receptor function.
FIGURE 4.
Mutation analysis of the Arg164 of PKR2. A, mutation of Arg-164 to E, A, and T abolished PKR2 function; B, mutation of Arg-164 to K and H partially restored the activity. C and D, the EC50 (C) and maximal response (D) of PKR2 variants correlate with the net amount of positive charge at position 164. The EC50 and maximal response were deduced from B, and the positive charge was represented by the side chain pKa of amino acids R, K, and H, respectively.
A Cluster of Basic Amino Acids at the C Terminus of PKRs-IL2 Function Cooperatively
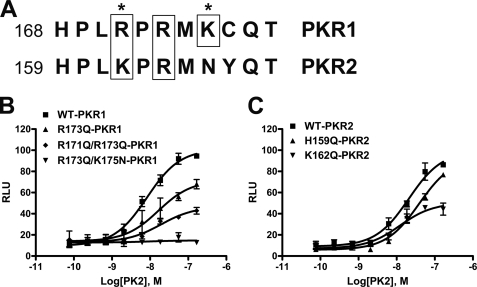
The PKR1 and PKR2 shared about 85% amino acid identity, with the most divergent part residing at the N terminus (6). However, it is reported that the signal transduction efficacy of PKR1 is somewhat higher than that of PKR2 (6, 7). We noticed some interesting differences in the IL2 loop, especially at the C-terminal portion, between PKR1 and PKR2. As shown in Fig. 5A, there are a cluster of three basic amino acids forming a BXBXB (B represents basic amino acids, and X represents any amino acids) motif in the C-terminal IL2 loop of PKR1. However, the third B is replaced with a neutral amino acid, asparagines (N), in PKR2; and the first B is arginine in PKR1 but lysine in PKR2. As our data have indicated that the basic residues in this portion significantly affect the efficacy of function, we speculate the differences in the IL2 loop of PKR1 and PKR2 may contribute to the variation in function. To test this hypothesis, we first replaced the Lys-175 (the third B) of PKR1 with asparagine (e.g. K175N), which resulted in ∼2-fold reduction in the signaling capacity of PKR1 (Table 1). Conversely, substitution of the Asn-166 of PKR2, at the corresponding position of the third B, to lysine (N166K) increased PKR2 function ∼2 times (Table 1).
FIGURE 5.
Mutation analysis of basic amino acids in the IL2 of PKR1 and PKR2. A, sequence comparison of the C-terminal IL2 loop from human PKR1 and PKR2. The basic amino acids were boxed, and the difference between PKR1 and PKR2 were marked with asterisks. B, functional analysis of the single or double basic amino acid mutant PKR1. C, function analysis of the H159Q and R162Q mutant PKR2.
TABLE 1.
The signaling parameters of PKR1, PKR2, and their mutants
| Receptors | EC50 | Maximal responsea |
|---|---|---|
| nm | ||
| PKR1 | ||
| WT | 8.29 ± 1.25 | 100 |
| R173Q | 16.40 ± 1.79 | 71.53 ± 8.59 |
| K175N | 14.66 ± 1.28 | 100 ± 9.59 |
| R171Q/R173Q | 19.26 ± 1.44 | 46.57 ± 3.43 |
| R173Q/K175N | NDb | ND |
| PKR2 | ||
| WT | 15.85 ± 1.45 | 100 |
| H159Q | 39.09 ± 1.27 | 93.23 ± 6.34 |
| R162Q | 12.1 ± 1.45 | 50.25 ± 3.71 |
| R164Q | ND | ND |
| N166K | 8.28 ± 1.27 | 90.69 ± 4.62 |
a The maximal responses of WT PKR1 or PKR2 was set to 100.
b ND, not detectable.
We then replaced the Arg-173 of PKR1, equivalent to Arg-164 in PKR2, with glutamine (R173Q) and measured its activity with the aequorin-based calcium assay. As shown in Fig. 5B, however, R173Q-PKR1 retained the signaling capacity, albeit the potency and efficacy were reduced. We thus speculate that the first and/or the third basic amino acids in the BXBXB motif may function cooperatively with the second basic amino acid. We therefore further made double mutations of PKR1: R171Q/R173Q and R173Q/K175N. As shown in the Fig. 5B and Table 1, both double mutations dramatically reduced the PKR1 signaling capacity, and the R173Q/K175N mutation (mimicking the R164Q mutation of PKR2) totally disrupted the signaling.
To further explore the contribution of basic amino acids in the IL2 loop to the function of PKRs, we made H159Q and K162Q mutant PKR2s. As shown in Fig. 5C and Table 1, K162Q-PKR2 showed significant signaling reduction, although it is not as severe as R164Q mutant. On the other hand, H159Q-PKR2 only showed subtle reduction in the signaling capacity as compared with WT receptor. Consistent with this functional results, GST pulldown analysis indicated that H159Q mutant PKR2-IL2 was able to pulldown Gαq-protein (Fig. 2A), indicating His-159 is not necessary for the interaction with G-proteins. Interestingly, a recent study also indicated that the equivalent position of His-159 in M3R receptor is not involved in the contact with Gαq-protein.
WT but Not R164Q, PKR2 Is Rapidly Internalized after Ligand Stimulation
Persistent stimulation of GPCRs often leads to internalization of receptors into the intracellular vesicles (23–25). And such ligand-induced endocytosis may contribute to the physiological regulation of receptors, such as the desensitization and resensitization (26). However, so far little information has been available on the membrane distribution of the PKR2 and their intracellular trafficking. Here, we have taken advantage of the intrinsic fluorescent properties of EGFP, which provides a high signal-to-noise ratio and stoichiometric labeling of the receptor of interest, to investigate internalization and trafficking of PKR2 using EGFP-tagged PKR2 stably expressed in HEK293 cells.
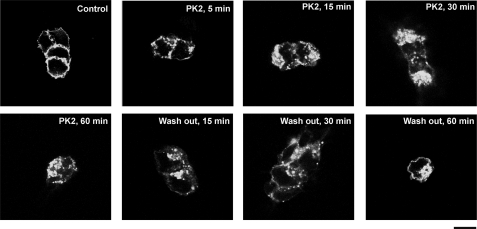
Trafficking of the PKR2-EGFP was studied by using confocal fluorescence microscopy. Incubation of PKR2-EGFP-transfected HEK293 cells with PK2 resulted in a dramatic redistribution of PKR2-EGFP fluorescence from the plasma membrane to an intracellular compartment, leading to a rapid and extensive decrease in surface PKR2-EGFP and with a multitude of punctate structures appearing in the cytoplasm in a dose-dependent manner (Fig. 6). Internalization was detectable within 5 min, and appeared maximal at 30–60 min (Fig. 7).
FIGURE 6.
PK2 dose-dependently stimulated PKR2 endocytosis. PK2 at various concentration (0, 1, 10, 100 nm, and 1 μm) was incubated for 60 min with HEK293 cells stably expressed with PKR2-EGFP, and the location of EGFP was monitored under a confocal microscope. The Alexa Fluor 594-conjugated concanavalin A was used to label the membrane Scale bar: 10 μm.
FIGURE 7.
PKR2-EGFP was internalized after PK2 treatment and restored to the plasma membrane after washout. PK2 (100 nm) was incubated for an indicated period with HEK293 cells stably expressed with PKR2-EGFP, and the location of EGFP was monitored under a confocal microscope. For the washout experiment, PK2 was removed by washing three times with PBS after 60 min of treatment and then incubated in culture medium for indicated time period. Scale bar: 10 μm.
After removal of PK2 (washout), PKR2-EGFP was again seen on the plasma membrane (Fig. 7). The receptor on the membrane is not likely to be newly synthesized proteins, as we have incubated a protein synthesis inhibitor-cycloheximide during this process.
However, R164Q mutant PKR2 failed to undergo endocytosis after PK2 treatment, implying G-protein coupling and signal transduction play a critical role in receptor internalization. As shown in Fig. 8, the R164Q mutant receptors were still on the surface after 30 min treatment with 100 nm PK2, only a small portion of receptors trafficked into the cytoplasm after 60 min of treatment.
FIGURE 8.
R164Q mutation inhibited the ligand-induced endocytosis of PKR2. PK2 (100 nm) was incubated for an indicated period with HEK293 cells stably expressed with R164Q mutant PKR2-EGFP, and the location of EGFP was monitored under a confocal microscope. Scale bar: 10 μm.
DISCUSSION
The interaction of GPCRs with heterotrimeric G-proteins is one of the most important processes for receptor signal transduction. However, in the absence of a crystal structure of a GPCR in complex with a G-protein, the specific sites that comprise the receptor-G-protein interface remain relatively undefined. Nevertheless, mutagenesis studies have identified multiple GPCR regions involved in receptor-G protein interactions. These receptor regions include the TM3-IL2 loop junction, the IL2 loop, the cytoplasmic ends of TM5 and TM6, and the C-terminal tail (18, 27). The three key regions of Gα that have been implicated by many studies include the N-terminal helix, the α4-β6 loop and the C-terminal portion (17–21, 28, 29). In an elegant study, Hu et al. identified many receptor/G-protein contact points using M3 muscarinic acetylcholine receptor (M3R)-Gαq as a model system (18). Specifically, they showed that the Leu-173 and Arg-176 from the IL2 loop of M3R directly contact with the C-terminal residues of Gαq protein. In this report, we identified the Arg-164 of PKR2, equivalent to the Arg-176 of M3R, contacts directly with Gα-proteins, probably the C-terminal 5 amino acids. As R164Q mutation affects receptor interaction with various Gα-proteins, the determinants for coupling selectivity may be located in other regions.
The IL2 loop of class A GPCRs can be roughly divided into three portions: the N terminus with a highly conserved (E/D)RY motif; the central portion and the C-terminal motif enriched in basic amino acids. The roles of N-terminal E/DRY motif have been extensively studied in many GPCRs (30). Of these three residues, tyrosine (Y) is least conserved and considered not important for receptor function. The aspartic acid (D) or glutamic acid (E) is conserved, which may regulate receptor activation and G-protein coupling. The arginine (R) is one of the most conserved residues in rhodopsin-like GPCRs, and it is essential for forming intramolecular interactions that constrain receptors in either the inactive or activated conformation, depending on the receptor types (30). For instance, the arginine side chain of rhodopsin receptor forms salt bridges with the preceding aspartic acid side chain and with a glutamic acid side chain in TM 6, and constrain the receptor in an inactive state (31).
The central portion of IL2 loop contains a bulky hydrophobic amino acid (I, L, V, M, or F) at the position 10 in most GPCRs. Moro et al. (32) identified this hydrophobic amino acid is important for the G-protein coupling using alanine mutagenesis scanning. In a seminar report, Wacker et al. analyzed a disease-causing mutation, L148S of GPR54, and demonstrated that L148S does not hinder the association of Gα subunits with GPR54 or its IL2 loop. Instead, L148S impairs the ligand-induced catalytic activation of Gα-protein. Thus, they proposed that Leu-148, or equivalent residue in other type A GPCRs, may stabilize the switch II region of Gα-protein upon ligand stimulation to establish a productive egress route for GDP (33).
The role of C-terminal basic amino acids-enriched motif is relatively less investigated. Xie et al. (34) identified two basic amino acids (K158/R159) at this location of interleukin-8 receptor are necessary for Gα16 but not Gαi-coupling. However, they showed only one of either basic residue is sufficient for Gα16-coupling (34). Iida-Klein et al. demonstrated that a lysine in the C-terminal IL2 of the PTH/PTH-related protein receptor is a key determinant for Gαq-selective coupling. Mutation in this region can selectively abolish the Gαq-coupling of PTH/PTH-related protein receptor but retain the Gαs-coupling capacity (35). However, Saito et al. demonstrated a basic amino acid (Arg-155) in the IL2 of MCHR1 plays a critical role in the G-protein coupling and activation but not selectivity, as both Gαq and Gαi-coupled pathway are disrupted by R155Q mutation (36).
Here, we used GST pulldown assay demonstrating that the Arg-164 in the IL2 of PKR2 is a critical contact point for Gα proteins. Importantly, we identified the C-terminal five amino acids of Gαq were involved in the interaction with IL2 of receptors. Previous reports showed that multiple substitution of the basic amino acids are required to abolish the receptor function (34, 36); however, replacement of Arg-164 alone in PKR2 with amino acids other than positive-charged ones was able to disrupt the receptor function. Nevertheless, studies on the closely related PKR1 indicated that the equivalent Arg-173 does function cooperatively with surrounding basic amino acids.
In contrast to Arg-155 of MCHR1, where the R155K substitution results in a receptor with similar signaling property as R155Q mutation (36); substitution of Arg-164 of PKR2 with positive-charged (K and H), but not other (Q, E, A, and T) residues apparently restored the receptor activity, suggesting the positive charges at this position is required for receptor function. Interestingly, the net amount of positive charges at this position is required for a full signaling efficacy and potency. Our data also implied that variation in this basic amino acids-enriched motif may be one of the underlying causes for the signaling difference between PKR1 and PKR2.
The receptor-mediated endocytosis is an important cellular process in the regulation of receptor signaling, such as down-regulation and desensitization/resensitization. Depending on the particular receptor and cell background, desensitization and internalization are generally followed by resensitization and receptor recycling to the plasma membrane (26). Cheng et al. (37) report that persistent infusion of PK2 intracerebroventricularly seems to desensitize the receptors in vivo. In the present study, by using EGFP-tagged receptor, we demonstrated that PKR2 underwent endocytosis upon ligand stimulation within 5 min. After removal of the ligand, PKR2 trafficked back to the plasma membrane. Importantly, the R164Q mutation that disrupted the signal transduction also inhibited the ligand-induced endocytosis, suggesting that receptor activation is a prerequisite for efficient endocytosis. It is intriguing to utilize the EGFP-tagged receptor described here to investigate how the PKR2 trafficking is regulated.
In conclusion, we have identified a basic amino acid at the C terminus of IL2 loop in PKR2 is vital for the Gα-protein interaction. Disruption of this receptor/Gα-protein interaction abolished the signal transduction as well as ligand-induced receptor trafficking. Importantly, mutations that disrupt this interaction can manifest as human disease. As many GPCRs have such a basic amino acids-enriched motif at the C terminus of IL2 loop, our data may have general application for understanding the structure of GPCRs and its activation mechanism. The receptor/Gα-protein contact point identified in this report may also be served as an anchoring point for the delineation of structure model of the receptor-G-protein interface.
Acknowledgments
We thank Professors Qun-Yong Zhou, Olivier Civelli, and Dian-Qing Wu for reagents.
This project was supported by National Natural Science Foundation of China (Grants 30970958 and 81070481), the Lotus Scholar Professorship Funds from Hunan Province Government, and a research grant provided by the Central South University of China.
- GPCR
- G-protein-coupled receptor
- CHO
- Chinese Hamster ovary
- EGFP
- enhanced green fluorescent protein
- GnRH
- gonadotropin-releasing hormone
- IHH
- idiopathic hypogonadotropic hypogonadism
- IL2
- the second intracellular loop
- PK2
- prokineticin 2
- PKR1 and PKR2
- prokineticin receptor 1 and 2, respectively
- TM
- transmembrane
- WT
- wild type.
REFERENCES
- 1. Jaakola V. P., Ijzerman A. P. (2010) Curr. Opin. Struct. Biol. 20, 401–414 [DOI] [PubMed] [Google Scholar]
- 2. Li M., Bullock C. M., Knauer D. J., Ehlert F. J., Zhou Q. Y. (2001) Mol. Pharmacol. 59, 692–698 [DOI] [PubMed] [Google Scholar]
- 3. Zhou Q. Y., Meidan R. (2008) Results Probl. Cell Differ. 46, 181–199 [DOI] [PubMed] [Google Scholar]
- 4. Li J. D., Hu W. P., Zhou Q. Y. (2009) Neuropsychopharmacology 34, 367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng K. L., Li J. D., Cheng M. Y., Leslie F. M., Lee A. G., Zhou Q. Y. (2005) Science 308, 1923–1927 [DOI] [PubMed] [Google Scholar]
- 6. Lin D. C., Bullock C. M., Ehlert F. J., Chen J. L., Tian H., Zhou Q. Y. (2002) J. Biol. Chem. 277, 19276–19280 [DOI] [PubMed] [Google Scholar]
- 7. Masuda Y., Takatsu Y., Terao Y., Kumano S., Ishibashi Y., Suenaga M., Abe M., Fukusumi S., Watanabe T., Shintani Y., Yamada T., Hinuma S., Inatomi N., Ohtaki T., Onda H., Fujino M. (2002) Biochem. Biophys. Res. Commun. 293, 396–402 [DOI] [PubMed] [Google Scholar]
- 8. Keramidas M., Faudot C., Cibiel A., Feige J. J., Thomas M. (2008) J. Endocrinol. 196, 473–482 [DOI] [PubMed] [Google Scholar]
- 9. Sinisi A. A., Asci R., Bellastella G., Maione L., Esposito D., Elefante A., De Bellis A., Bellastella A., Iolascon A. (2008) Hum. Reprod. 23, 2380–2384 [DOI] [PubMed] [Google Scholar]
- 10. Pitteloud N., Zhang C., Pignatelli D., Li J. D., Raivio T., Cole L. W., Plummer L., Jacobson-Dickman E. E., Mellon P. L., Zhou Q. Y., Crowley W. F., Jr. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dodé C., Teixeira L., Levilliers J., Fouveaut C., Bouchard P., Kottler M. L., Lespinasse J., Lienhardt-Roussie A., Mathieu M., Moerman A., Morgan G., Murat A., Toublanc J. E., Wolczynski S., Delpech M., Petit C., Young J., Hardelin J. P. (2006) PLoS Genet 2, e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abreu A. P., Trarbach E. B., de Castro M., Frade Costa E. M., Versiani B., Matias Baptista M. T., Garmes H. M., Mendonca B. B., Latronico A. C. (2008) J. Clin. Endocrinol. Metab. 93, 4113–4118 [DOI] [PubMed] [Google Scholar]
- 13. Sarfati J., Guiochon-Mantel A., Rondard P., Arnulf I., Garcia-Pinero A., Wolczynski S., Brailly-Tabard S., Bidet M., Ramos-Arroyo M., Mathieu M., Lienhardt-Roussie A., Morgan G., Turki Z., Bremont C., Lespinasse J., Du Boullay H., Chabbert-Buffet N., Jacquemont S., Reach G., De Talence N., Tonella P., Conrad B., Despert F., Delobel B., Brue T., Bouvattier C., Cabrol S., Pugeat M., Murat A., Bouchard P., Hardelin J. P., Dode C., Young J. (2010) J. Clin. Endocrinol. Metab. 95, 659–669 [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto S., Yamazaki C., Masumoto K. H., Nagano M., Naito M., Soga T., Hiyama H., Matsumoto M., Takasaki J., Kamohara M., Matsuo A., Ishii H., Kobori M., Katoh M., Matsushime H., Furuichi K., Shigeyoshi Y. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bullock C. M., Li J. D., Zhou Q. Y. (2004) Mol. Pharmacol. 65, 582–588 [DOI] [PubMed] [Google Scholar]
- 16. Monnier C., Dodé C., Fabre L., Teixeira L., Labesse G., Pin J. P., Hardelin J. P., Rondard P. (2009) Hum. Mol. Genet 18, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh Y., Cai K., Khorana H. G. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4883–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu J., Wang Y., Zhang X., Lloyd J. R., Li J. H., Karpiak J., Costanzi S., Wess J. (2010) Nat. Chem. Biol. 6, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai K., Itoh Y., Khorana H. G. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4877–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conklin B. R., Farfel Z., Lustig K. D., Julius D., Bourne H. R. (1993) Nature 363, 274–276 [DOI] [PubMed] [Google Scholar]
- 21. Hamm H. E., Deretic D., Arendt A., Hargrave P. A., Koenig B., Hofmann K. P. (1988) Science 241, 832–835 [DOI] [PubMed] [Google Scholar]
- 22. Offermanns S., Simon M. I. (1995) J. Biol. Chem. 270, 15175–15180 [DOI] [PubMed] [Google Scholar]
- 23. Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. (1987) Cell 48, 913–922 [DOI] [PubMed] [Google Scholar]
- 24. Claing A., Laporte S. A., Caron M. G., Lefkowitz R. J. (2002) Prog. Neurobiol. 66, 61–79 [DOI] [PubMed] [Google Scholar]
- 25. Xia S., Kjaer S., Zheng K., Hu P. S., Bai L., Jia J. Y., Rigler R., Pramanik A., Xu T., Hökfelt T., Xu Z. Q. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15207–15212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferguson S. S. (2001) Pharmacol. Rev. 53, 1–24 [PubMed] [Google Scholar]
- 27. Wess J. (1998) Pharmacol. Ther. 80, 231–264 [DOI] [PubMed] [Google Scholar]
- 28. Scheerer P., Park J. H., Hildebrand P. W., Kim Y. J., Krauss N., Choe H. W., Hofmann K. P., Ernst O. P. (2008) Nature 455, 497–502 [DOI] [PubMed] [Google Scholar]
- 29. Oldham W. M., Hamm H. E. (2008) Nat. Rev. Mol. Cell Biol. 9, 60–71 [DOI] [PubMed] [Google Scholar]
- 30. Rovati G. E., Capra V., Neubig R. R. (2007) Mol. Pharmacol. 71, 959–964 [DOI] [PubMed] [Google Scholar]
- 31. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 32. Moro O., Lameh J., Högger P., Sadée W. (1993) J. Biol. Chem. 268, 22273–22276 [PubMed] [Google Scholar]
- 33. Wacker J. L., Feller D. B., Tang X. B., Defino M. C., Namkung Y., Lyssand J. S., Mhyre A. J., Tan X., Jensen J. B., Hague C. (2008) J. Biol. Chem. 283, 31068–31078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xie W., Jiang H., Wu Y., Wu D. (1997) J. Biol. Chem. 272, 24948–24951 [DOI] [PubMed] [Google Scholar]
- 35. Iida-Klein A., Guo J., Takemura M., Drake M. T., Potts J. T., Jr., Abou-Samra A., Bringhurst F. R., Segre G. V. (1997) J. Biol. Chem. 272, 6882–6889 [DOI] [PubMed] [Google Scholar]
- 36. Saito Y., Tetsuka M., Saito S., Imai K., Yoshikawa A., Doi H., Maruyama K. (2005) Endocrinology 146, 3452–3462 [DOI] [PubMed] [Google Scholar]
- 37. Cheng M. Y., Bullock C. M., Li C., Lee A. G., Bermak J. C., Belluzzi J., Weaver D. R., Leslie F. M., Zhou Q. Y. (2002) Nature 417, 405–410 [DOI] [PubMed] [Google Scholar]