Abstract
Vascular calcification is strongly linked with increased morbidity and mortality from cardiovascular disease. Vascular calcification is an active cell-mediated process that involves the differentiation of vascular smooth muscle cells (VSMCs) to an osteoblast-like phenotype. Several inhibitors of this process have been identified, including insulin-like growth factor-I (IGF-I). In this study, we examined the role of the IGF receptor (IGFR) and the importance of IGFR glycosylation in the maintenance of the VSMC phenotype in the face of factors known to promote osteogenic conversion. IGF-I (25 ng/ml) significantly protected VSMCs from β-glycerophosphate-induced osteogenic differentiation (p < 0.005) and mineral deposition (p < 0.01). Mevalonic acid depletion (induced by 100 nm cerivastatin) significantly inhibited these IGF protective effects (p < 0.01). Mevalonic acid depletion impaired IGFR processing, decreased the expression of mature IGFRs at the cell surface, and inhibited the downstream activation of Akt and MAPK. Inhibitors of N-linked glycosylation (tunicamycin, deoxymannojirimycin, and deoxynojirimycin) also markedly attenuated the inhibitory effect of IGF-I on β-glycerophosphate-induced mineralization (p < 0.05) and activation of Akt and MAPK. These results demonstrate that alterations in the glycosylation of the IGFR disrupt the ability of IGF-I to protect against the osteogenic differentiation and mineralization of VSMCs by several interrelated mechanisms: decreased IGFR processing, reduced IGFR cell-surface expression, and reduced downstream signaling via the Akt and MAPK pathways. IGF-I thus occupies a critical position in the maintenance of normal VSMC phenotype and protection from factors known to stimulate vascular calcification.
Keywords: Calcification, Glycosylation, Glycosylation Inhibitors, Insulin-like Growth Factor (IGF), Smooth Muscle, HMG-CoA Reductase Inhibitors
Introduction
The importance of vascular calcification in the development and progression of cardiovascular disease is well established (1, 2), and it is known to be a predictor of future cardiovascular events (3). Vascular calcification is an active cell-mediated process, involving the osteoblastic conversion of vascular smooth muscle cells (VSMCs),2 calcifying vascular cells, pericytes, and adventitial myofibroblasts (4–9). In vivo, vascular calcification has been shown to result from an endochondral ossification-like process, resulting in the formation of bone as well as both marrow and cartilage (10–12).
A previous study from Demer and co-workers (13) has shown that insulin-like growth factor-I (IGF-I) prevents the osteoblastic conversion of calcifying vascular cells. The role of IGF-I in the terminal differentiation of multiple cell types is well known (14–16), but its role in phenotype maintenance is less well understood. We have previously shown that IGF signaling can be attenuated in 3T3-L1 cells using HMG-CoA reductase inhibitors through their effects on IGF receptor (IGFR) glycosylation and Ras prenylation (17). Therefore, by removing this protective pathway, we hypothesized that HMG-CoA reductase inhibitors would also render vascular cells more vulnerable to osteogenic differentiation and subsequent mineralization. This hypothesis is consistent with a recent study showing that atorvastatin promotes osteogenic differentiation and calcium deposition by VSMCs (18) and osteoblast cell lines (19, 20) in vitro. However, there are also reports that HMG-CoA reductase inhibitors inhibit the osteoblastic conversion of human VSMCs (21, 22) and TGFβ-induced calcific nodule formation by valvular interstitial cells (23, 24). The reason for these discrepancies is not clear but may reflect differences in the factors used to stimulate differentiation in these studies.
Therefore, the purpose of this study was (i) to determine the importance of IGF signaling in promoting the proliferation and preventing the osteogenic differentiation of VSMCs, (ii) to determine whether HMG-CoA reductase inhibitors modulate the inhibitory effect of IGF-I on the osteogenic differentiation of VSMCs, and (iii) to elucidate the mechanism by which this modulation occurs. Our data show that cerivastatin, through alterations in IGFR glycosylation, attenuates the protective effect of IGF-I on VSMCs by preventing IGFR processing and cell-surface localization.
EXPERIMENTAL PROCEDURES
Materials
HMG-CoA reductase inhibitors (atorvastatin and cerivastatin) were provided by AstraZeneca and Bayer plc. Deoxymannojirimycin (DMJ) and protease inhibitor mixture I (comprising 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (500 μm), aprotinin (150 nm), E-64 protease inhibitor (1 μm), EDTA (0.5 mm) and leupeptin (1 μm)) were purchased from Calbiochem. β-Glycerophosphate (β-GP), tunicamycin, deoxynojirimycin (DNJ), phosphatase inhibitor mixtures 1 (comprising cantharidin, bromotetramisole, and microcystin LR) and 2 (comprising sodium orthovanadate, sodium molybdate, sodium tartrate, and imidazole) were purchased from Sigma. Rabbit anti-IGFR polyclonal antibodies were purchased from Santa Cruz Biotechnology. Anti-total and anti-phospho-p44/p42 MAPK rabbit polyclonal antibodies and anti-total and anti-phospho-Akt rabbit polyclonal antibodies were purchased from Cell Signaling Technology. Anti-rabbit IgG secondary antibody (whole molecule) was purchased from Sigma. Des-1–3-IGF-I, LONG®R3IGF-I, and recombinant human IGF-I (rhIGF-I) were purchased from GroPep Bioreagents.
Cell Culture
VSMCs were isolated by explant culture from adult bovine aortas and maintained at 37 °C in 5% CO2. Cells were characterized by positive staining for α-smooth muscle cell actin and by negative staining for von Willebrand factor. VSMCs were grown in high glucose (4500 mg/liter) DMEM (Sigma) supplemented with 4 mm glutamine, 1 mm sodium pyruvate, 10% FCS, 1% nonessential amino acids (Invitrogen), and penicillin/streptomycin (growth medium). In all experiments, cells were plated at 1.32 × 104 cells/cm2 and used between passages 4 and 12. Mineralization medium consisted of growth medium supplemented with 5 mm β-GP. Cerivastatin (1–200 nm), atorvastatin (10–1000 nm), tunicamycin (1–100 ng/ml), DMJ (0.5–1 mm), and DNJ (1–2 mm) were preincubated with cells in growth medium for 24 h before the addition of mineralization medium with and without these inhibitors. Cells were refed with fresh medium/treatment every 2 days. All experiments were performed a minimum of three times, and triplicate samples were used in each experiment.
Proliferation of VSMCs
Proliferation of VSMCs was assessed by [3H]thymidine incorporation. After the initial plating, cells were maintained in growth medium for 24 h. Cells were then transferred to serum-free medium with and without cerivastatin or atorvastatin for 24 h. rhIGF-I (10 ng/ml) was added to each well except the untreated control wells, and cells were incubated for a further 20 h. [3H]Thymidine (0.05 μCi/ml; GE Healthcare) was added, and cells were incubated for a further 4 h. Cells were washed with ice-cold PBS before incubation with 10% trichloroacetic acid at 6 °C for 1 h. Cells were washed with ice-cold PBS and lysed in 500 μl of 0.1 m NaOH. Cell lysate (450 μl) was combined with 4 ml of scintillation fluid (OptiPhase HiSafe 2, PerkinElmer Life Sciences) and counted in a β-counter. The remaining 50 μl was used to measure the total protein content using the Pierce BCA protein assay.
Alkaline Phosphatase
Alkaline phosphatase activity was used to assess conversion of VSMCs to an osteoblastic phenotype (25). Confluent VSMCs were incubated with and without β-GP in the presence or absence of cerivastatin or glycosylation inhibitor (tunicamycin, DMJ, or DNJ). On day 4, cells were placed on ice for 15 min and washed with ice-cold PBS. Cells were lysed with 1% Triton X-100 in 10 mm Tris-HCl containing 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (500 μm), aprotinin (150 nm), E-64 protease inhibitor (1 μm), EDTA (0.5 mm), and leupeptin (1 μm), and in a 96-well plate, cell lysate was diluted (1:20) with diethanolamine buffer (1 m diethanolamine and 0.5 mm MgCl2, pH 9.8) before the addition of p-nitrophenyl phosphate (3.75 mg/ml in diethanolamine buffer). The rate of change in absorbance over 5 min was measured at 410 nm. The total protein content of the cell lysate was determined by BCA protein assay. Results are expressed as micromolar substrate converted per mg of total protein/min.
Calcium Assay
On day 8 post-incubation with mineralization medium, the deposited calcium was dissolved in 0.6 n HCl for 1 h on a rotating shaker, and the calcium content of the sample was determined using the O-cresolphthalein method. Samples were incubated with 1.5 m 2-amino-2-methyl-1-propanol, pH 10.7, and color reagent (150 μm O-cresolphthalein and 7 mm 8 hydroxyquinoline, pH 1.5) on a plate shaker for 10 min before absorbance at 560 nm was determined. The remaining cells were lysed in 0.1 m NaOH, and total protein was determined by the BCA protein assay. Results are expressed as milligrams of calcium/mg of total protein.
Western Blotting
IGFR processing and membrane localization were assessed by immunoblotting. Confluent VSMCs were treated with and without β-GP and cerivastatin. Cells were then washed three times with cold PBS before being scraped off into radioimmune precipitation assay buffer (50 mm Tris-HCl, 1% Nonidet P-40, 0.25% deoxycholic acid, 150 mm NaCl, 1 mm EDTA, 1 mm activated sodium vanadate, 1 mm sodium fluoride, and protease inhibitor mixture I). Cell lysates were adjusted for total protein (BCA protein assay), and 20 μg of total protein was diluted to an equal volume in loading buffer (0.125 m Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.25% bromphenol blue). Samples were analyzed by SDS-PAGE before transfer to nitrocellulose membranes and immunoblotting. Samples analyzed for the IGFR were run on 6% polyacrylamide gels; MAPK and Akt analysis was performed using 12.5% gels. In experiments using phospho-specific MAPK and Akt antibodies, cells were serum-starved for 5 h, and the medium was changed every 30 min before incubation with IGF-I. Cells were lysed in radioimmune precipitation assay buffer supplemented with phosphatase inhibitors as well as protease inhibitors (phosphatase inhibitor mixtures 1 and 2). For IGFR immunoblotting, primary and secondary antibodies were used at 1:5000 and 1:10,000 dilutions, respectively. Total and phospho-specific MAPK and Akt immunoblotting was carried out using primary and secondary antibodies at 1:7500 and 1:15,000 dilutions, respectively. Immunoreactivity was detected by enhanced chemiluminescence using Immobilon Western chemiluminescent HRP substrate (Millipore) and Kodak BioMAX film. All gels are representative of three replicates.
IGFR Localization
To determine the effect of HMG-CoA reductase inhibitors on the cell-surface expression of the IGFR, confluent VSMCs (in T75 flasks) were treated with and without cerivastatin for 4 days, and membranes were prepared as described previously (17). Briefly, cells were washed three times with ice-cold PBS, scraped into 50 mm HEPES and protease inhibitor mixture (Calbiochem), and sonicated (3 × 5 s, MSE sonicator, amplitude of 16 μm). Intact cells were pelleted (500 × g, 5 min) and discarded. The total protein of the remaining cell lysate supernatant was determined, and equal amounts of protein were taken for membrane isolation. Membranes were pelleted at 100,000 × g for 45 min 4 °C. Membrane pellets were resuspended in SDS loading buffer and run on a 6% polyacrylamide gel, and the IGFR was visualized by Western immunoblotting.
Statistics
Results are expressed as the mean ± S.D. For statistical analysis, we used SPSS 15.0 for Windows. To determine statistical significance between different treatments, we used the one-way analysis of variance with Dunnett's post hoc test. A p value <0.05 was considered significant.
RESULTS
HMG-CoA Reductase Inhibitors Inhibit the Proliferation and Osteogenic Differentiation of VSMCs
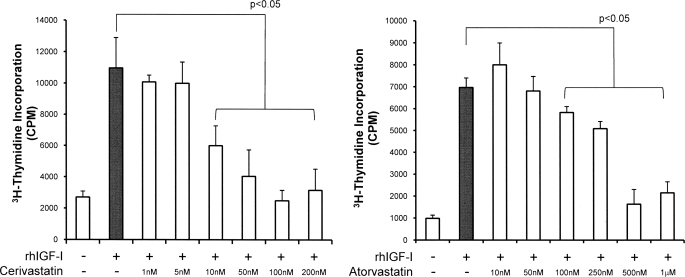
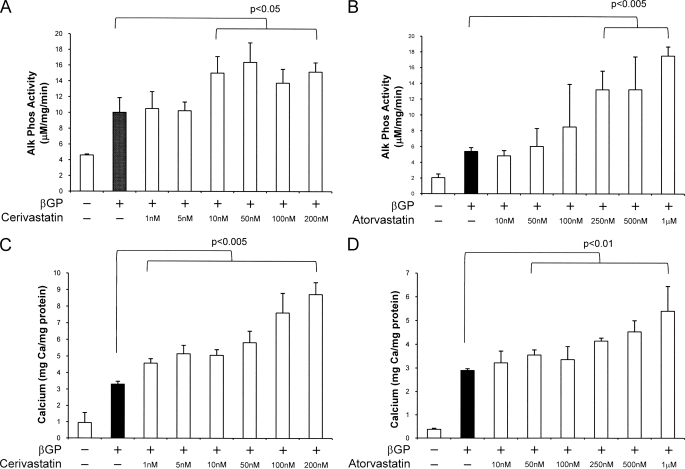
Both cerivastatin and atorvastatin (24-h treatment prior to IGF-I treatment) potently and dose-dependently inhibited IGF-I-stimulated proliferation of VSMCs as measured by [3H]thymidine incorporation (10 ng/ml IGF-I and 10 nm cerivastatin, p < 0.05; 100 nm atorvastatin, p < 0.05) (Fig. 1). Both cerivastatin and atorvastatin also significantly and dose-dependently potentiated the β-GP-stimulated osteogenic differentiation of VSMCs as measured by alkaline phosphatase activity (10 nm cerivastatin, p < 0.05; 250 nm atorvastatin, p < 0.005) (Fig. 2, A and B) and mineral deposition (1 nm cerivastatin, p < 0.005; 50 nm atorvastatin, p < 0.01) (Fig. 2, C and D).
FIGURE 1.
Effect of HMG-CoA reductase inhibitors on IGF-stimulated proliferation of VSMCs. Cells were maintained in serum-free medium with or without cerivastatin or atorvastatin (1 nm to 1 mm) for 24 h and then stimulated with rhIGF-I (10 ng/ml) for 20 h before the addition of [3H]thymidine (final concentration of 0.05 μCi/ml) for a further 4 h. Cells were washed, lysed, and counted in a scintillation counter. Uptake is expressed as counts/min and is the mean ± S.D. of five experiments performed in triplicate.
FIGURE 2.
Effect of HMG-CoA reductase inhibitors on VSMC alkaline phosphatase expression and calcium deposition. Cells were maintained in growth medium with or without cerivastatin (A and C) or atorvastatin (B and D) (1 nm to 1 mm) for 24 h and then stimulated with β-GP (5 mm) for 96 h for alkaline phosphatase activity or for 8 days for calcium deposition. A and B, cells were washed and lysed, and alkaline phosphatase activity was determined. Alkaline phosphatase (Alk Phos) activity is expressed as micromolar p-nitrophenyl phosphate converted per mg of total protein/min and is the mean ± S.D. of five experiments performed in triplicate. C and D, cells were washed, deposited calcium was dissolved in 0.6 n HCl, and the calcium content was determined by the O-cresolphthalein method. Mineralization is expressed as milligrams of calcium/mg of total cellular protein and is the mean ± S.D. of five experiments performed in triplicate.
IGFs Prevent the Osteogenic Differentiation of VSMCs
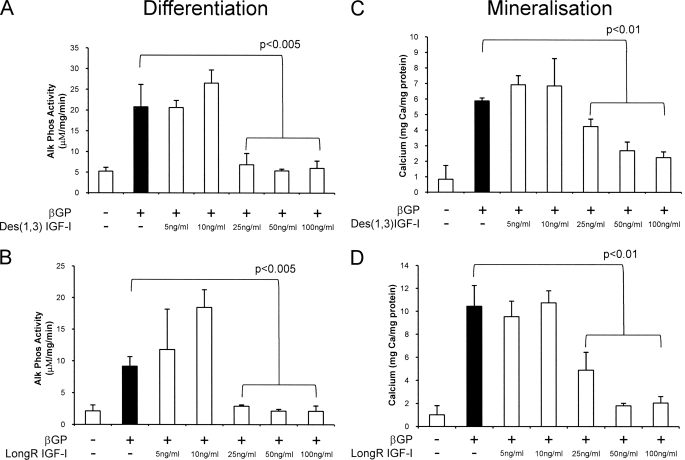
Radcliff et al. (13) reported that IGF-I inhibits the spontaneous as well as bacterial lipopolysaccharide-, TNF-α-, or H2O2-induced osteoblastic conversion of calcifying vascular cells. In this study, we also examined the effect of IGF-I on the osteogenic differentiation and mineralization of VSMCs. In these experiments, we used the IGF analogs LONG®R3IGF-I and des-1–3-IGF-I to prevent potential interference from IGF-binding proteins present in serum. Fig. 3 shows that both IGF analogs significantly inhibited β-GP-induced alkaline phosphatase activity (25 ng/ml, p < 0.005) (Fig. 3, A and B) and mineral deposition (25 ng/ml, p < 0.01) (Fig. 3, C and D).
FIGURE 3.
Effect of IGF-I on VSMC alkaline phosphatase expression and mineral deposition by VSMCs. Cells were maintained in growth medium with or without IGF-I (5–100 ng/ml) for 24 h and then stimulated with β-GP (5 mm) for 4 days (alkaline phosphatase (Alk Phos); A and B) or for 8 days (mineralization; C and D). Alkaline phosphatase activity and calcium deposition were determined. Results are the mean ± S.D. of three experiments performed in triplicate.
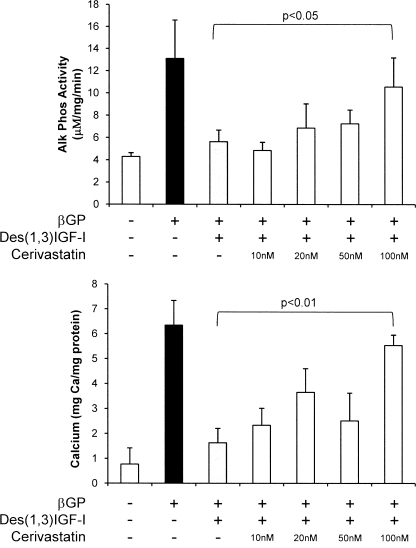
We have previously shown that HMG-CoA reductase inhibitors are potent inhibitors of IGF signaling in 3T3-L1 preadipocytes (17), and so we next investigated whether cerivastatin would modulate inhibition of the osteogenic differentiation and mineralization of VSMCs by IGF. Fig. 4 shows that cerivastatin (100 nm) prevented des-1–3-IGF-I (25 ng/ml) inhibition of both osteogenic differentiation, determined by assessing alkaline phosphatase activity (p < 0.05), and subsequent mineral deposition (p < 0.01) by VSMCs.
FIGURE 4.
Effect of IGF-I and HMG-CoA reductase inhibitors on VSMC alkaline phosphatase expression and mineral deposition by VSMCs. Cells were maintained in growth medium with or without cerivastatin (10–100 nm) and IGF-I (25 ng/ml) for 24 h and then stimulated with β-GP (5 mm) for 4 days (alkaline phosphatase (Alk Phos)) or for 8 days (mineralization). Alkaline phosphatase activity and calcium deposition were determined. Results are the mean ± S.D. of three experiments performed in triplicate.
Importance of IGFR Glycosylation in IGF-mediated Inhibition of the Osteogenic Differentiation of VSMCs
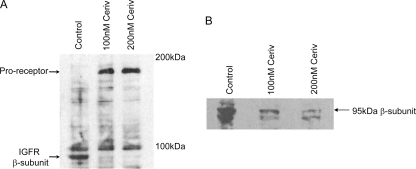
Depletion of cellular mevalonate by HMG-CoA reductase inhibitors leads to depletion of dolichyl phosphate, the initial donor of carbohydrate in protein N-linked glycosylation (26). It has also been reported that aberrant glycosylation of the IGFR results in a buildup of the IGF proreceptor due to the inability of the cell to cleave the proprotein to the mature α- and β-subunits (27). Therefore, to determine whether HMG-CoA reductase inhibitors attenuate IGF protection against osteogenic differentiation by modulating IGFR processing, glycosylation, and localization, VSMCs were treated with and without cerivastatin (100 and 200 nm) for 72 and 96 h, and cell lysates were examined by Western blotting using antibodies against the IGFR. Fig. 5A shows that, in the presence of cerivastatin, there was an increase in the amount of IGF proreceptor detected, suggesting that it is not processed to mature α- and β-subunits as efficiently as in control cells. To examine this further, membranes were isolated after cerivastatin treatment and analyzed by SDS-PAGE and Western immunoblotting for the IGFR β-subunit. In these experiments, we detected a marked decrease in the amount of β-subunit present in the membrane of cerivastatin-treated cells compared with control cells (Fig. 5B). Together, these data suggest that the processing of the proreceptor to mature receptors is decreased in cells treated with HMG-CoA reductase inhibitors, which then leads to decreased numbers of active IGFRs at the cell surface, rendering cells less responsive to IGFs.
FIGURE 5.
Effect of HMG-CoA reductase inhibitors on the processing and cell-surface expression of the IGFR. Cells were maintained in growth medium with or without cerivastatin (Ceriv; 100–200 nm) for 72 and 96 h. A, after 72 h, cells were lysed, and 100 μg of total protein was analyzed for proreceptor content by Western immunoblotting. B, after 96 h, cells were lysed, membranes were prepared by ultracentrifugation, and 20 g of total protein was analyzed for IGFRs by Western immunoblotting.
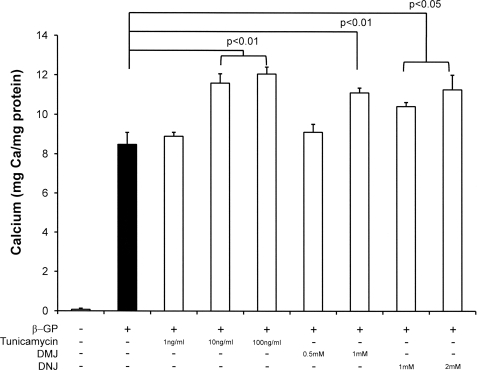
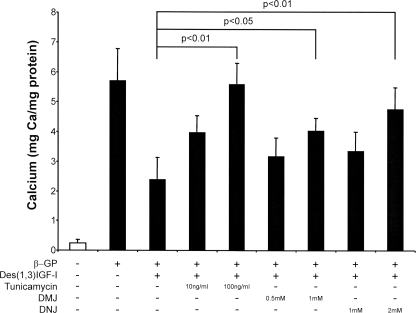
To determine the contribution of impaired glycosylation in vascular calcification, we incubated VSMCs in the presence of specific inhibitors of glycosylation, namely tunicamycin, an inhibitor of oligosaccharyltransferase that prevents attachment of the initial core oligosaccharide, consisting of three glucose, nine mannose, and two N-acetylglucosamine molecules (28); DNJ, which inhibits glucosidases I and II and prevents cleavage of glucose residues from the core oligosaccharide, allowing the carbohydrate chains to be extended (29); and DMJ, a mannosidase inhibitor that prevents the conversion of the long mannose chains to complex oligosaccharides (30). VSMCs were pretreated with these inhibitors for 24 h before co-incubation with β-GP and the inhibitors for 8 days, and calcium deposition was determined. Fig. 6 demonstrates that calcium incorporation was increased with respect to cells treated with β-GP alone with all the inhibitors used (10 ng/ml tunicamycin, p < 0.01; 1 mm DMJ, p < 0.01; 1 mm DNJ, p < 0.05). When cells pretreated with these inhibitors were then treated with IGF-I and β-GP as well, the glycosylation inhibitors were found to prevent the IGF-mediated inhibition of calcification (100 ng/ml tunicamycin, p < 0.01; 1 mm DMJ, p < 0.05; 2 mm DNJ, p < 0.01) (Fig. 7), suggesting that glycosylation of the IGFR is important for mediating the inhibitory effect of IGF-I on mineralization.
FIGURE 6.
Effect of N-linked glycosylation inhibitors on VSMC mineralization. Cells were maintained in growth medium with or without glycosylation inhibitors for 24 h and then stimulated with β-GP (5 mm) for 8 days. Cells were washed, deposited calcium was dissolved in 0.6 n HCl, and the calcium content was determined by the O-cresolphthalein method. Mineralization is expressed as milligrams of calcium/mg of total cellular protein and is the mean ± S.D. of three experiments performed in triplicate.
FIGURE 7.
Effect of IGF-I and N-linked glycosylation inhibitors on VSMC mineralization. Cells were maintained in growth medium with or without des-1–3-IGF-I (25 ng/ml) and glycosylation inhibitor for 24 h and then stimulated with β-GP (5 mm) for 8 days. Cells were washed, deposited calcium was dissolved in 0.6 n HCl, and the calcium content was determined by the O-cresolphthalein method. Mineralization is expressed as milligrams of calcium/mg of total cellular protein and is the mean ± S.D. of three experiments performed in triplicate.
Downstream Signaling
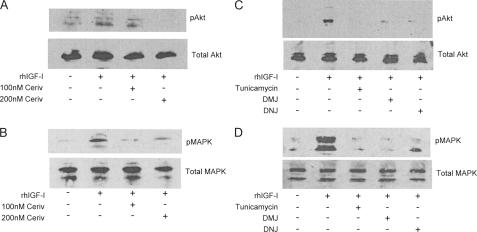
It has been previously reported that the protective IGF signal involves both MAPK and PI3K signaling and that inhibition of either is sufficient to prevent the IGF protection afforded by IGFs (13). Therefore, we investigated the effects of HMG-CoA reductase inhibitors and glycosylation inhibitors on the activation of Akt and MAPK by IGF-I in VSMCs. Phosphorylated Akt and MAPK levels increased with IGF-I treatment compared with untreated controls (Fig. 8, A and B). However, when cells were pretreated with HMG-CoA reductase inhibitors (Fig. 8, A and B) or glycosylation inhibitors (Fig. 8, C and D) and were stimulated with IGF-I, the increase in the phosphorylated levels of both signaling molecules was markedly attenuated.
FIGURE 8.
Effect of cerivastatin and glycosylation inhibitors on IGF-I activation of Akt and MAPK. VSMCs were maintained in growth medium with or without treatment for 4 days. Cells were serum-starved for 5 h before the addition of rhIGF-I (50 ng/ml) for 15 min and lysed in radioimmune precipitation assay buffer containing protease and phosphatase inhibitors. Cell lysates were electrophoresed on SDS-12.5% polyacrylamide gels and analyzed by Western blotting. A and B, VSMCs were treated with and without cerivastatin (100 and 200 nm) before rhIGF-I treatment. Cell lysates were probed with anti-Akt and anti-phospho-Akt antibodies (A) or anti-MAPK and anti-phospho-MAPK antibodies (B). C and D, VSMCs were treated with and without tunicamycin (100 ng/ml), DMJ (1 mm), or DNJ (2 mm) before rhIGF-I treatment. Cell lysates were probed with anti-Akt and phospho-Akt antibodies (C) or anti-MAPK and anti-phospho-MAPK antibodies (D).
DISCUSSION
Our data implicate the IGF system as key to maintenance of normal VSMC phenotype. Impaired IGF signaling predisposes cells to factors that can induce osteogenic differentiation and contribute to vascular calcification. Specifically, we have shown that correct IGFR processing is crucial to prevent the differentiation of VSMCs to an osteoblast-like phenotype. Thus, IGF-I prevents β-GP-induced osteogenic differentiation of VSMCs, whereas HMG-CoA reductase inhibitors dose-dependently inhibit the protective effect of IGF-I by altering the post-translational glycosylation of the IGFR.
HMG-CoA reductase inhibitors have pleiotropic effects on many different cell types (31–34) and can potently decrease cellular proliferation (17, 33, 35–38). Inhibition of HMG-CoA reductase leads to the cellular depletion of mevalonate, which is a key intermediary in the production of dolichyl phosphate (39, 40). Dolichyl phosphate is vital for the N-linked glycosylation of proteins (41), and we now report that correct IGFR glycosylation is important for the prevention of the osteoblastic conversion of VSMCs by IGF-I. The HMG-CoA reductase inhibitors atorvastatin and cerivastatin potently inhibited IGF-I-mediated proliferation of VSMCs. The different potencies of these drugs in causing this effect is likely to be due to differences in their relative hydrophobicities (17).
Reports of the effect of HMG-CoA reductase inhibitors on vascular calcification are currently conflicting. It has been shown in vitro that cerivastatin and atorvastatin inhibit calcification in a human model of inflammatory vascular calcification (21) and that atorvastatin protects human VSMCs from inorganic phosphate-induced calcification by preventing apoptosis (22). Contrary to this, in a rat model of calcification, using a similar calcification stimulus to our own, atorvastatin dose-dependently increased calcium deposition (18). In addition, lovastatin has been shown to stimulate the expression of BMP-2 in VSMCs (43) and osteoblasts (44). BMP-2 is a causative factor in vascular calcification (45) and has been shown to induce the osteogenic differentiation of VSMCs (46). Furthermore, HMG-CoA reductase inhibitors have been shown to induce apoptosis in pericytes (47), which also appear to contribute to vascular calcification (27), and enhanced apoptosis is associated with enhanced vascular calcification both in vitro and in vivo (48–50). In vivo studies suggest that HMG-CoA reductase inhibitors have a beneficial effect on vascular function due to their predominant action in lowering circulating cholesterol levels. HMG-CoA reductase inhibitors are concentrated largely in the liver in vivo (51), so their direct effects on VSMCs may be relatively small. Indeed, prospective randomized studies have shown no effect of HMG-CoA reductase inhibitors on the progression of vascular calcification and calcific aortic stenosis (52–56). As the in vivo effects of HMG-CoA reductase inhibitors on vascular calcification are likely to include complex interactions with multiple cell types, it is important not to extrapolate the implications of our in vitro data to the clinical environment without further extensive in vivo studies.
IGFR maturation requires N-linked glycosylation for the proreceptor to be processed correctly and for its transport to the cell surface (17, 57, 58). In this study, we have demonstrated that HMG-CoA reductase inhibitors cause a shift from the mature receptor to the proreceptor, leading to decreased expression of the receptor at the cell surface. Several groups have demonstrated that the incorrectly glycosylated proreceptor is degraded (27, 42, 57). We have further confirmed the requirement for correct IGFR glycosylation by demonstrating the exquisite sensitivity of IGF signaling in VSMCs to a range of glycosylation inhibitors that affect the initial addition of carbohydrate, chain extension, and processing of long mannose chains to complex carbohydrate. Furthermore, both MAPK and PI3K arms of the signaling pathway initiated at the IGFR are inhibited by both cerivastatin and these glycosylation inhibitors, suggesting that IGF-I signaling requires the IGFR to be glycosylated.
Treatment with HMG-CoA reductase inhibitors alone resulted in an increase in the degree of mineralization induced by β-GP, in agreement with the study by Trion et al. (18). These authors demonstrated that atorvastatin dose-dependently increased calcium deposition in rat aortic VSMCs when induced to calcify with medium containing CaCl2, ascorbic acid, β-GP, and dexamethasone. We speculate that the true potential of β-GP to induce osteogenic differentiation and subsequent mineralization is masked by the endogenous IGF content of the serum present in the culture medium and that the presence of the HMG-CoA reductase inhibitor prevents this tonic inhibition. In agreement with this hypothesis, we found that preincubation of VSMCs with IGF-I before exposure to β-GP protects the cells from osteoblastic conversion and subsequent mineralization and that the inclusion of an HMG-CoA reductase inhibitor dose-dependently inhibits the IGF protective effect. The true potential of β-GP may also be affected by other serum inhibitors of osteogenic differentiation and mineralization such as fetuin-A and matrix Gla protein, but we cannot speculate whether their effects may be alleviated by HMG-CoA reductase inhibitors. Further understanding of the mechanism by which the IGF system protects VSMCs from osteoblastic conversion will be clinically important in developing agents to prevent vascular calcification.
Footnotes
- VSMC
- vascular smooth muscle cell
- IGF-I
- insulin-like growth factor-I
- IGFR
- IGF receptor
- DMJ
- deoxymannojirimycin
- β-GP
- β-glycerophosphate
- DNJ
- deoxynojirimycin
- rhIGF-I
- recombinant human IGF-I.
REFERENCES
- 1. Wexler L., Brundage B., Crouse J., Detrano R., Fuster V., Maddahi J., Rumberger J., Stanford W., White R., Taubert K. (1996) Circulation 94, 1175–1192 [DOI] [PubMed] [Google Scholar]
- 2. Frink R. J., Achor R. W., Brown A. L., Jr., Kincaid O. W., Brandenburg R. O. (1970) Am. J. Cardiol. 26, 241–247 [DOI] [PubMed] [Google Scholar]
- 3. Abedin M., Tintut Y., Demer L. L. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1161–1170 [DOI] [PubMed] [Google Scholar]
- 4. Giachelli C. M. (2004) J. Am. Soc. Nephrol. 15, 2959–2964 [DOI] [PubMed] [Google Scholar]
- 5. Proudfoot D., Skepper J. N., Shanahan C. M., Weissberg P. L. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 379–388 [DOI] [PubMed] [Google Scholar]
- 6. Boström K., Watson K. E., Horn S., Wortham C., Herman I. M., Demer L. L. (1993) J. Clin. Invest. 91, 1800–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collett G. D., Canfield A. E. (2005) Circ. Res. 96, 930–938 [DOI] [PubMed] [Google Scholar]
- 8. Cheng S. L., Shao J. S., Charlton-Kachigian N., Loewy A. P., Towler D. A. (2003) J. Biol. Chem. 278, 45969–45977 [DOI] [PubMed] [Google Scholar]
- 9. Demer L. L., Tintut Y. (2008) Circulation 117, 2938–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeziorska M., McCollum C., Wooley D. E. (1998) Virchows Arch. 433, 559–565 [DOI] [PubMed] [Google Scholar]
- 11. Jeziorska M., McCollum C., Woolley D. E. (1998) J. Pathol. 185, 10–17 [DOI] [PubMed] [Google Scholar]
- 12. Qiao J. H., Mertens R. B., Fishbein M. C., Geller S. A. (2003) Hum. Pathol. 34, 402–407 [DOI] [PubMed] [Google Scholar]
- 13. Radcliff K., Tang T. B., Lim J., Zhang Z., Abedin M., Demer L. L., Tintut Y. (2005) Circ. Res. 96, 398–400 [DOI] [PubMed] [Google Scholar]
- 14. Xi G., Kamanga-Sollo E., Pampusch M. S., White M. E., Hathaway M. R., Dayton W. R. (2004) J. Cell. Physiol. 200, 387–394 [DOI] [PubMed] [Google Scholar]
- 15. Smith P. J., Wise L. S., Berkowitz R., Wan C., Rubin C. S. (1988) J. Biol. Chem. 263, 9402–9408 [PubMed] [Google Scholar]
- 16. Valentinis B., Baserga R. (2001) Mol. Pathol. 54, 133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siddals K. W., Marshman E., Westwood M., Gibson J. M. (2004) J. Biol. Chem. 279, 38353–38359 [DOI] [PubMed] [Google Scholar]
- 18. Trion A., Schutte-Bart C., Bax W. H., Jukema J. W., van der Laarse A. (2008) Mol. Cell. Biochem. 308, 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeda T., Kawane T., Horiuchi N. (2003) Endocrinology 144, 681–692 [DOI] [PubMed] [Google Scholar]
- 20. Maeda T., Matsunuma A., Kurahashi I., Yanagawa T., Yoshida H., Horiuchi N. (2004) J. Cell. Biochem. 92, 458–471 [DOI] [PubMed] [Google Scholar]
- 21. Kizu A., Shioi A., Jono S., Koyama H., Okuno Y., Nishizawa Y. (2004) J. Cell. Biochem. 93, 1011–1019 [DOI] [PubMed] [Google Scholar]
- 22. Son B. K., Kozaki K., Iijima K., Eto M., Kojima T., Ota H., Senda Y., Maemura K., Nakano T., Akishita M., Ouchi Y. (2006) Circ. Res. 98, 1024–1031 [DOI] [PubMed] [Google Scholar]
- 23. Benton J. A., Kern H. B., Leinwand L. A., Mariner P. D., Anseth K. S. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1950–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monzack E. L., Gu X., Masters K. S. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaffe I. Z., Tintut Y., Newfell B. G., Demer L. L., Mendelsohn M. E. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 799–805 [DOI] [PubMed] [Google Scholar]
- 26. Schenk B., Fernandez F., Waechter C. J. (2001) Glycobiology 11, 61R0–70R [DOI] [PubMed] [Google Scholar]
- 27. Collier E., Carpentier J. L., Beitz L., Carol H., Taylor S. I., Gorden P. (1993) Biochemistry 32, 7818–7823 [DOI] [PubMed] [Google Scholar]
- 28. McDowell W., Schwarz R. T. (1988) Biochimie 70, 1535–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lodish H. F., Kong N. (1984) J. Cell Biol. 98, 1720–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuhrmann U., Bause E., Legler G., Ploegh H. (1984) Nature 307, 755–758 [DOI] [PubMed] [Google Scholar]
- 31. Bellosta S., Ferri N., Arnaboldi L., Bernini F., Paoletti R., Corsini A. (2000) Diabetes Care 23, B72–B78 [PubMed] [Google Scholar]
- 32. Bellosta S., Ferri N., Bernini F., Paoletti R., Corsini A. (2000) Ann. Med. 32, 164–176 [DOI] [PubMed] [Google Scholar]
- 33. Bellosta S., Bernini F., Ferri N., Quarato P., Canavesi M., Arnaboldi L., Fumagalli R., Paoletti R., Corsini A. (1998) Atherosclerosis 137, S101–S109 [DOI] [PubMed] [Google Scholar]
- 34. Takemoto M., Liao J. K. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1712–1719 [DOI] [PubMed] [Google Scholar]
- 35. Cuthbert J. A., Lipsky P. E. (1997) Cancer Res. 57, 3498–3505 [PubMed] [Google Scholar]
- 36. Martínez-González J., Badimon L. (1996) Eur. J. Clin. Invest. 26, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 37. Porter K. E., Naik J., Turner N. A., Dickinson T., Thompson M. M., London N. J. (2002) J. Vasc. Surg. 36, 150–157 [DOI] [PubMed] [Google Scholar]
- 38. Wang M., Xie Y., Girnita L., Nilsson G., Dricu A., Wejde J., Larsson O. (1999) Exp. Cell Res. 246, 38–46 [DOI] [PubMed] [Google Scholar]
- 39. Larsson O., Wejde J. (1992) J. Cell Sci. 103, 1065–1072 [DOI] [PubMed] [Google Scholar]
- 40. Langan T. J., Slater M. C. (1991) J. Cell. Physiol. 149, 284–292 [DOI] [PubMed] [Google Scholar]
- 41. Burda P., Aebi M. (1999) Biochim. Biophys. Acta 1426, 239–257 [DOI] [PubMed] [Google Scholar]
- 42. Hwang J. B., Frost S. C. (1999) J. Biol. Chem. 274, 22813–22820 [DOI] [PubMed] [Google Scholar]
- 43. Emmanuele L., Ortmann J., Doerflinger T., Traupe T., Barton M. (2003) Biochem. Biophys. Res. Commun. 302, 67–72 [DOI] [PubMed] [Google Scholar]
- 44. Kanazawa I., Yamaguchi T., Yano S., Hayashi K., Yamauchi M., Sugimoto T. (2009) Horm. Metab. Res. 41, 612–616 [DOI] [PubMed] [Google Scholar]
- 45. Hruska K. A., Mathew S., Saab G. (2005) Circ. Res. 97, 105–114 [DOI] [PubMed] [Google Scholar]
- 46. Li X., Yang H. Y., Giachelli C. M. (2008) Atherosclerosis 199, 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boucher K., Siegel C. S., Sharma P., Hauschka P. V., Solomon K. R. (2006) Microvasc. Res. 71, 91–102 [DOI] [PubMed] [Google Scholar]
- 48. Clarke M. C., Littlewood T. D., Figg N., Maguire J. J., Davenport A. P., Goddard M., Bennett M. R. (2008) Circ. Res. 102, 1529–1538 [DOI] [PubMed] [Google Scholar]
- 49. Proudfoot D., Skepper J. N., Hegyi L., Bennett M. R., Shanahan C. M., Weissberg P. L. (2000) Circ. Res. 87, 1055–1062 [DOI] [PubMed] [Google Scholar]
- 50. Shroff R. C., McNair R., Figg N., Skepper J. N., Schurgers L., Gupta A., Hiorns M., Donald A. E., Deanfield J., Rees L., Shanahan C. M. (2008) Circulation 118, 1748–1757 [DOI] [PubMed] [Google Scholar]
- 51. König J., Seithel A., Gradhand U., Fromm M. F. (2006) Naunyn Schmiedebergs Arch. Pharmacol. 372, 432–443 [DOI] [PubMed] [Google Scholar]
- 52. Cowell S. J., Newby D. E., Prescott R. J., Bloomfield P., Reid J., Northridge D. B., Boon N. A. (2005) N. Engl. J. Med. 352, 2389–2397 [DOI] [PubMed] [Google Scholar]
- 53. Rossebø A. B., Pedersen T. R., Boman K., Brudi P., Chambers J. B., Egstrup K., Gerdts E., Gohlke-Bärwolf C., Holme I., Kesäniemi Y. A., Malbecq W., Nienaber C. A., Ray S., Skjaerpe T., Wachtell K., Willenheimer R. (2008) N. Engl. J. Med. 359, 1343–135618765433 [Google Scholar]
- 54. Schmermund A., Achenbach S., Budde T., Buziashvili Y., Förster A., Friedrich G., Henein M., Kerkhoff G., Knollmann F., Kukharchuk V., Lahiri A., Leischik R., Moshage W., Schartl M., Siffert W., Steinhagen-Thiessen E., Sinitsyn V., Vogt A., Wiedeking B., Erbel R. (2006) Circulation 113, 427–437 [DOI] [PubMed] [Google Scholar]
- 55. Arad Y., Spadaro L. A., Roth M., Newstein D., Guerci A. D. (2005) J. Am. Coll. Cardiol. 46, 166–172 [DOI] [PubMed] [Google Scholar]
- 56. Houslay E. S., Cowell S. J., Prescott R. J., Reid J., Burton J., Northridge D. B., Boon N. A., Newby D. E. (2006) Heart 92, 1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carlberg M., Dricu A., Blegen H., Wang M., Hjertman M., Zickert P., Höög A., Larsson O. (1996) J. Biol. Chem. 271, 17453–17462 [DOI] [PubMed] [Google Scholar]
- 58. Dricu A., Kanter L., Wang M., Nilsson G., Hjertman M., Wejde J., Larsson O. (1999) Glycobiology 9, 571–579 [DOI] [PubMed] [Google Scholar]