Abstract
Cell cycle regulation is characterized by alternating activities of cyclin-dependent kinases (CDKs) and of the ubiquitin ligase anaphase promoting complex/cyclosome (APC/C). During S-phase APC/C is inhibited by early mitotic inhibitor 1 (Emi1) to allow the accumulation of cyclins A and B and to prevent re-replication. Emi1 is degraded at prophase by a Plk1-dependent pathway. Recent studies in which the degradation pathway of Emi1 was disrupted have shown that APC/C is activated at mitotic entry despite stabilization of Emi1. These results suggested the possibility of additional mechanisms other than degradation of Emi1, which release APC/C from inhibition by Emi1 upon entry into mitosis. In this study we report one such mechanism, by which the ability of Emi1 to inhibit APC/C is negatively regulated by CDKs. We show that in Plk1-inhibited cells Emi1 is stabilized and phosphorylated, that Emi1 is phosphorylated by CDKs in mitotic but not S-phase cell extracts, and that Emi1 phosphorylation by mitotic cell extracts or purified CDKs markedly reduces the ability of Emi1 to bind and to inhibit APC/C. Finally, we show that the addition of extracts from S-phase cells to extracts from mitotic cells protects Emi1 from CDK-mediated inactivation.
Keywords: CDK (Cyclin-dependent Kinase), Cell Cycle, Mitosis, Protein Turnover, Ubiquitin Ligase
Introduction
The eukaryotic cell division is characterized by an ordered unidirectional progression through a series of events culminating in the formation of two genetically identical daughter cells from a single cell. To prevent catastrophes, cell cycle progression must be strictly regulated. In many instances, several mechanisms regulate a single effector or process. Two key mechanisms employed in the regulation of the cell cycle are protein phosphorylation and ubiquitin-mediated protein degradation (1). The family of cyclin-dependent kinases (CDKs)2 orchestrates numerous events by phosphorylating target proteins throughout the cell cycle. The two principal CDKs implicated in the regulation of the cell cycle are CDK2, which associates with regulatory subunits cyclin E or cyclin A to promote the entry into S-phase and the single replication of the chromosomes, and CDK1, which associates with cyclin A or cyclin B to promote entry into mitosis (2).
For cells to enter S-phase and mitosis, cyclins A and B must be allowed to accumulate. Conversely, to exit mitosis, these mitotic cyclins must be rapidly degraded (3). A large multisubunit ubiquitin ligase, the anaphase promoting complex/cyclosome (APC/C) targets cyclins A and B for degradation (4). APC/C is activated upon entry into mitosis due to phosphorylation by mitotic kinases, which promotes the binding of the co-activator Cdc20. Following the metaphase to anaphase transition, APC/C is dephosphorylated and binding of a second co-activator, Cdh1, stimulates APC/C activity until the G1-S transition (4). APC/C ubiquitylates cyclin A at prometaphase, shortly after nuclear envelope breakdown, whereas cyclin B is ubiquitylated by APC/C only following bipolar attachment of chromosomes to the mitotic spindle, at the metaphase to anaphase transition (5, 6).
The accumulation of mitotic cyclins throughout S-phase is dependent upon inhibition of APC/C activity. In Drosophila cells cyclin A is stabilized during S-phase because of the expression of the F-box protein regulator of cyclin A 1 (Rca1), which inhibits APC/CCdh1 in a non-F-box dependent mechanism (7, 8). A vertebrate homologue of Rca1, early mitotic inhibitor 1 (Emi1), was initially identified in Xenopus (9). Emi1 levels oscillate in Xenopus embryonic cell cycles, as well as in human cell lines, rising in S-phase and falling at mitotic entry. Emi1 has been shown to inhibit both APC/CCdc20 and APC/CCdh1 activity in vitro, and the overexpression of Emi1 has been shown to stabilize APC/C substrates in vivo (9, 10). Emi1 expression is vital for proper progression through the cell cycle. Knock-out of Emi1 in mice was shown to be lethal, as no Emi1 null embryos survived beyond 7 days post-gestation (11). Knockdown of Emi1 in human cell lines using siRNA has revealed a role for Emi1 in the prevention of re-replication (12, 13). Depletion of Emi1 from cells caused a destabilization of both cyclin A and geminin during S-phase, and Emi1-depleted cells arrested cell cycle progression due to activation of the DNA damage checkpoint. Emi1-depleted cells exhibited large nuclei and >4n content of DNA, suggesting re-replication. Similar effects on DNA content were seen with depletion of both cyclin A and geminin from HeLa cells by siRNA, and the effect Emi1 depletion on re-replication was abrogated by concomitant depletion of Cdh1 or overexpression of non-degradable cyclin A (12, 13).
Emi1 expression is tightly regulated throughout the cell cycle. Emi1 misregulation has been implicated in several types of tumors, particularly lymphomas, renal, and ovarian clear cell carcinomas, and germ cell tumors. Expression of Emi1 in many tumor types was correlated with advanced grade and a higher malignant potential of the tumor (14). Emi1 levels rise at the G1-S transition as the result of E2F activation and remain high until mitotic entry (15). At mitotic entry, Emi1 is rapidly degraded due to SCFβ-TrCP-mediated ubiquitylation (16, 17). This is promoted by the synergistic activity of two mitotic kinases, cyclin B/CDK1 and polo-like kinase 1 (Plk1). Cyclin B/CDK1 phosphorylates Emi1 on (S/T)P consensus sites, and promotes the Plk1-mediated phosphorylation of a β-TrCP binding motif of Emi1, DSGXXS. The phosphorylation of this motif stimulates the recognition of Emi1 by SCFβ-TrCP thus leading to the degradation of Emi1 (18, 19).
More recent studies have questioned the necessity of Emi1 degradation for successful progression into mitosis. Depletion of Plk1 from cells using siRNA did not cause cells to arrest at prophase, but rather at prometaphase/metaphase due to activation of the spindle assembly checkpoint. Furthermore, cyclin A was degraded in Plk1-depleted cells entering mitosis despite disruption of the Emi1 degradation pathway (20, 21) Inhibition of Plk1 activity in cells using the novel Plk inhibitor, BI2536, resulted in a similar effect. Emi1 was not degraded at mitotic entry and yet cells entered mitosis and cyclin A was degraded at nearly normal kinetics (22). A different approach was used by Di Fiore and Pines (13). By expressing Emi1 mutated in the β-TrCP recognition motif in cells, they were able to show that near physiological expression levels of this non-degradable Emi1 mutant did not prevent half of the cells from completing mitosis. In cells in which non-degradable Emi1 was overexpressed cyclin A degradation was initiated with normal kinetics following nuclear envelope breakdown, and the cells were arrested in a metaphase-like state (13). These studies suggested that Emi1 does not regulate APC/C in early mitosis (23).
Another possibility that could explain how cells progress into mitosis despite the lack of Emi1 degradation is that additional mechanisms, apart from ubiquitin-mediated degradation, regulate Emi1 at mitotic entry. In this study we have explored the role of mitotic phosphorylation in the inactivation of Emi1. We show that Emi1 is negatively regulated by CDKs in mitosis. In mitotic cells in which Plk1 is inhibited, Emi1 is stabilized in a phosphorylated form. In a purified in vitro system and in cell extracts we show that phosphorylation of Emi1 by CDKs markedly diminishes the ability of Emi1 to bind and inhibit APC/C. Finally, we show results suggesting that Emi1 is protected from CDK-mediated phosphorylation during S-phase.
EXPERIMENTAL PROCEDURES
Materials
Ubiquitin, ATP, phosphocreatine, creatine phosphokinase, BSA, ovalbumin, and MgCl2 were purchased from Sigma. HeLa cell growth medium reagents, Dulbecco's modified Eagle's medium, fetal bovine serum, gentamycin, and streptomycin were purchased from Sigma. Electrophoresis reagents were purchased from Bio-Rad. Ubiquitin aldehyde was prepared as described (24). E1 was purified from human erythrocytes as described (25). E2-C/UbcH10 was prepared as described (26). His6-Skp1 and full-length Emi1 baculoviruses were co-infected into BTI-TN-5B1–4 (High Five) insect cells and recombinant Skp1-Emi1 complexes were purified on nickel-agarose as described previously (27). cDNA encoding the 150 C-terminal amino acids of Emi1 (Emi1CT) was subcloned from pCS2 plasmid encoding full-length myc-tagged Emi1 to pGEX-6p-1 plasmid. Point mutations of Emi1CT CDK phosphorylation sites at residues Ser-310, Thr-323, and Ser-383 were performed using the QuikChange method (Stratagene). Wild type GST-Emi1CT and the CDK phosphorylation site mutant GST-Emi1CT 3A were expressed in BL21 (Stratagene) Escherichia coli cells and purified by affinity chromatography on glutathione-Sepharose beads (Amersham Biosciences). His6-Plx (referred to as Plk1) (28) and GST-Δ88-Cyclin B/CDK1 (29) were prepared as described.
Preparation of Synchronized Cell Extracts
HeLa S3 cells were grown in suspension in DMEM supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, and 0.1 mg/ml of streptomycin. For synchronization at S-phase, thymidine was added to log phase growing cells (0.4–0.6 × 106 cells/ml) to a final concentration of 2 mm. After 24 h, cells were released from the thymidine block by washing and incubation in fresh medium for 3 h, followed by collection of cells for FACS analysis of DNA content and extract preparation. Cells were synchronized at mitosis by addition of nocodazole to logarithmically growing cells (0.6–0.7 × 106 cells/ml) to a final concentration of 0.2 μg/ml. After 16–18 h, cells were harvested for FACS analysis and extract preparation.
For the preparation of cell extracts, cells from a 2-liter culture were harvested by centrifugation (400 × g, 10 min, 4 °C), washed three times with phosphate-buffered saline (PBS), suspended in 3 volumes of hypotonic buffer (20 mm HEPES-NaOH, pH 7.6, 1.5 mm MgCl2, 0.5 mm KCl, 1 mm dithiothreitol (DTT), 10 μg/ml of leupeptin, and 10 μg/ml of chymostatin), and collected immediately by centrifugation. The pellet was re-suspended in 2 volumes, relative to original cell weight, of the hypotonic buffer described above. After incubation on ice for 30 min, the cells were disrupted by Dounce homogenization (30 strokes). Following centrifugation at 12,000 × g for 30 min, the supernatant (cytoplasmic fraction) was collected, mixed with glycerol (10% final concentration), and stored at −70 °C. For preparation of nuclear extract of S-phase cells, pellets of the hypotonic-mechanical lysis was resuspended in ⅔ volume, relative to the original cell weight of the extraction buffer (20 mm HEPES-NaOH, pH 7.6, 25% glycerol, 1.5 mm MgCl2, 420 mm KCl, 1 mm DTT, 10 μg/ml of leupeptin, and 10 μg/ml of chymostatin), and was subjected to 10 strokes of Dounce homogenization followed by 30 min of gentle agitation at 4 °C. Following centrifugation at 12,000 × g for 10 min at 4 °C, the supernatant (nuclear extract) was collected and stored at −70 °C.
Purification of Mitotic and S-phase Soluble APC/C
Mitotic APC/C (mAPC/C) was purified from extracts of nocodazole-arrested HeLa cells by affinity chromatography on a p13suc1-Sepharose column followed by anion exchange chromatography on a MonoQ HR 5/5 column (GE Healthcare), as described previously (30). Interphase APC/C (iAPC/C) was purified in a single step by anion exchange chromatography. ∼20 mg of S-phase HeLa cell nuclear extract was loaded onto a MonoQ HR 5/5 column (Amersham Biosciences) previously equilibrated with 50 mm Tris-HCl, pH 7.2, 300 mm NaCl, and 1 mm DTT. The column was washed with 25 ml of the above buffer and then subjected to a linear gradient of NaCl (200–700 mm) in 50 mm Tris-HCl, pH 7.2, and 1 mm DTT for 24 min at 1 ml/min. 1-ml fractions were collected into tubes containing 0.2 mg of soybean trypsin inhibitor. Fractions were washed, concentrated, and analyzed for activity and APC/C content as described for mitotic APC/C (30). Peak fractions of Cdh1-dependent APC/C activity, eluted at 450–530 mm NaCl, were combined and stored in small aliquots at −70 °C.
Treatment of Synchronized HeLa Cells by the Plk Inhibitor BI2536
HeLa cells were seeded on 10-cm cell culture plates at 4.5 × 106 cells/plate and cultured at 37 °C for 20 h in DMEM supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, and 0.1 mg/ml of streptomycin. Cells were treated with 2 mm thymidine for 24 h, released from thymidine for 9 h, and treated again with thymidine for 15 h. Following release from the second thymidine block, cells were supplemented with dimethyl sulfoxide or BI2536 (100 nm) and samples were harvested at the times indicated in Fig. 1. Cells were examined by FACS analysis. Cell lysis was performed by incubating cell pellets on ice for 30 min in 3 cell pellet volumes of lysis buffer containing 20 mm HEPES-NaOH, pH 7.4, 1.5 mm MgCl2, 150 mm NaCl, 1 mm dithiothreitol (DTT), 1% Nonidet P-40, 10 μg/ml of leupeptin, 10 μg/ml of chymostatin, 15 mm p-nitrophenyl phosphate, 60 mm β-glycerol phosphate, 0.1 mm vanadate, and 0.5 μm okadaic acid, followed by centrifugation at 10,000 × g for 15 min. 25-μg protein samples were analyzed for cell cycle regulator protein levels by SDS-PAGE followed by immunoblotting with the following antibodies. Monoclonal (mouse) anti-Cdc27 (Apc3) antibody (BD Transduction Laboratories 610455) was used at 1:500 dilution. Monoclonal (mouse) anti-cyclin B (BD Transduction Laboratories C23420) was used at 1:1,000 dilution. Monoclonal (mouse) anti-Emi1 (Invitrogen 37–6600) was used at 1:100 dilution. Monoclonal (mouse) anti-cyclin A (Sigma C-4710) was used at 1:1,000 dilution. Monoclonal (mouse) anti-BubR1 (BD Transduction Laboratories 612503) was used at 1:500 dilution. Monoclonal (mouse) anti-Securin (Medicinal Biological Laboratories K0090-3) was used at 1:2,000 dilution. Monoclonal (mouse) anti-tubulin (Sigma T5168) was used at 1:10,000 dilution.
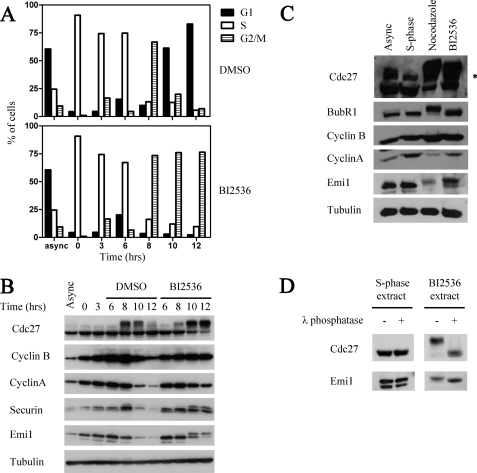
FIGURE 1.
Effects of inhibition of Plk in HeLa cells. A, G2/M arrest in BI2536-treated cells. HeLa cells were released from a double thymidine block. 6 h following release, 100 nm BI2536 or dimethyl sulfoxide (DMSO) as a control were added. Cells were harvested at the indicated times and samples were analyzed by FACS. B, levels of cell cycle proteins in BI2536-treated cells. Cell pellets of the experiment shown in A were lysed in the presence of protease and phosphatase inhibitors as described under “Experimental Procedures” and analyzed by immunoblotting for the specified proteins. C, expression and phosphorylation of cell cycle regulators in nocodazole- or BI2536-treated cells. HeLa cells were released from thymidine block for 11 h into a nocodazole or BI2536 block, as indicated. Asynchronous, S-phase, nocodazole-arrested, or BI2536-arrested cells were harvested, lysed as described above, and analyzed by immunoblotting for the specified proteins. D, Emi1 is phosphorylated in BI2536-treated mitotic cells. 30-μg samples of cell extracts of S-phase cells or BI2536-arrested cells from the experiment shown in C were treated with 10 units/μl of λ phosphatase or buffer alone for 30 min in 30 °C and analyzed by immunoblotting for Cdc27 and Emi1.
Assay of CDK-mediated Inactivation of Emi1 in a Purified System
0.03 pmol of Skp1/Emi1 or GST-Emi1CT was incubated for 30 min at 18 °C in 5 μl of phosphorylation buffer containing 40 mm HEPES-NaOH, pH 7.4, 1 mg/ml of BSA, 10 mm phosphocreatine, 0.1 mg/ml of creatine phosphokinase, 0.5 mm ATP, 5 mm MgCl2, 1 mm DTT, and 1 μm okadaic acid. Prior to incubation, the specified kinases were added to start the phosphorylation reaction. Phosphorylation was terminated by addition of 10 μm staurosporine. This was followed by a second incubation at 30 °C for 60 min following addition of 5 μl of 125I-cyclin B ubiquitin ligase reaction mixture containing 10 mm Tris-HCl, pH 7.6, 1 mg/ml of BSA, 0.5 mm DTT, 50 μm ubiquitin, 1 μm ubiquitin aldehyde, 1 pmol of E1, 5 pmol of E2-C, 1–2 pmol of 125I-cyclin B (1–2 × 105 cpm), and APC/C source and recombinant co-activator (1.8 nm Cdc20 or 5.4 nm Cdh1) as specified. Ubiquitylation of 125I-cyclin B was quenched by boiling in SDS. Samples were subjected to SDS-polyacrylamide electrophoresis on 10% gels, radioautography, and phosphorimager analysis.
Phosphorylation of Emi1CT in Cell Extracts
0.25 pmol of GST-Emi1CT was incubated for 30 min at 30 °C in 20 μl of reaction mixture containing the phosphorylation buffer described above and 20 μg of HeLa cell extract. Where specified, 1 μm okadaic acid, 10 μm staurosporine, or 0.1 μg/μl of p27 were added prior to incubation. Phosphorylation was terminated by boiling in SDS, samples were resolved on a 10% SDS-polyacrylamide gel, transferred to nitrocellulose, and blotted with monoclonal anti-Emi1 antibody.
Inactivation of Emi1CT in Cell Extracts
GST-Emi1CT (4 pmol) was incubated at 30 °C for 30 min in a 200-μl reaction mixture containing the above described phosphorylation buffer. Prior to the incubation, 800 μg of HeLa cell extract was added as specified in the figure legends. Following this incubation, 5-μl samples were taken for Western blotting with anti-Emi1 antibody to examine the state of phosphorylation of Emi1. GST-Emi1CT was isolated by binding to 200 μl of glutathione-Sepharose beads previously equilibrated with an equal volume of buffer A (50 mm HEPES, pH 7.4, 150 mm KCl, 2 μg/μl of BSA, and 1 mm DTT). Following rotation at 15 × g at 4 °C for 1 h, the beads were washed three times (400 × g, 5 min, 4 °C) with 1.5 ml of washing buffer containing 20 mm HEPES-NaOH, pH 7.4, 250 mm KCl, and 1 mm DTT and subjected to three rounds of rotation at 15 × g at 4 °C in the presence of 400 μl of elution buffer (50 mm Tris-HCl, pH 8.1, 2 mg/ml of BSA, and 10 mm reduced glutathione), centrifugation at 400 × g for 5 min at 4 °C, and collection of supernatant containing eluted GST-Emi1CT. All three fractions of eluted GST-Emi1CT were concentrated to a ∼50 μl volume in Amicon Ultra centrifugal filter devices (Millipore), washed by addition of 2 ml of buffer containing 40 mm HEPES-NaOH, pH 7.4, 10% glycerol, and 1 mm DTT, and concentrated again to ∼50 μl volume. 5-μl samples of concentrated eluate were subjected to SDS-PAGE parallel to pre-determined GST-Emi1CT standards and Western blotted with anti-Emi1. The concentration of GST-Emi1CT relative to standards was determined by chemiluminescence reading using an ImageQuant RT ECL instrument (GE Healthcare). Eluted GST-Emi1CT was examined at the specified concentrations for ability to inhibit 125I-cyclin B ubiquitylation by APC/C as described above and represented relative to APC/C activity without GST-Emi1CT.
GST Pull-down Assays
Glutathione-Sepharose beads were washed 3 times in buffer A (described above) equilibrated by rotation at 25 × g for 1 h in the presence of buffer A at 4 °C to minimize nonspecific absorption to beads and finally suspended in an equal volume of buffer A. Samples of 30 ng of GST, GST-Emi1CT wt, or GST-Emi1CT 3A were incubated at 30 °C for 30 min in a 40-μl volume with the above described phosphorylation buffer alone, purified kinases, or 200 μg of cell extract as indicated. Phosphorylation was terminated by addition of staurosporine. Where indicated (in experiments performed in purified systems), this was followed by addition of purified mitotic or interphase APC/C and recombinant Cdc20 (1.8 nm) or Cdh1 (1.8 nm) for a second incubation for 30 min at 30 °C in 40 μl of buffer containing 50 mm HEPES-NaOH, pH 7.4, 10% glycerol, 8 μg/μl of BSA, 1 mm DTT, and 150 mm KCl to allow binding. When examining mitotic APC/C binding to Emi1, 1 μm okadaic acid was added, and when interphase APC/C binding to Emi1 was examined, 10 μm staurosporine was added. Where indicated, 5-μl samples were taken at the end of this incubation and supplemented with 125I-cyclin B assay buffer as described above, incubated at 30 °C for 60 min, and analyzed for APC/C ubiquitin ligase activity as described above. The remainder of the binding incubation was added to 20 μl of the 1:1 suspension of glutathione-Sepharose beads in buffer A and rotated at 25 rpm for 2 h at 4 °C. Beads were then washed 3 times in buffer containing 40 mm HEPES-NaOH, pH 7.4, 150 mm NaCl, 0.5 μg/μl of BSA, and 1 mm DTT, followed by elution of bound proteins by addition of 30 μl of electrophoresis sample buffer containing 2.5 μg/μl of BSA. Samples of 15 μl were separated on 8% polyacrylamide gels, transferred to nitrocellulose, and probed with antibodies for the specified proteins.
RESULTS
Effects of Plk Inhibition in Intact Cells
To examine the effect of the inhibition of Plk on cell cycle progression, Emi1 degradation, and APC/C activity in HeLa cells, we used the Plk inhibitor BI2536, which has been shown to be a potent and specific inhibitor of the Plk family of protein kinases in vitro and in vivo (22, 31). HeLa cells were treated with BI2536 or dimethyl sulfoxide 6 h after release from a double thymidine block and cell cycle progression was analyzed by FACS. As opposed to cells treated with dimethyl sulfoxide alone, BI2536-treated cells exhibited a mitotic arrest (Fig. 1A). By 12 h following release from thymidine block, over 90% of cells in the control treatment had successfully exited mitosis, whereas over 75% of BI2536-treated cells were still in G2/M. Samples of cells were lysed in the presence of phosphatase inhibitors and examined for levels of various cell cycle regulatory proteins. When examining BI2536-treated cells as compared with control cells, stabilization of cyclin B and securin and persistence of Cdc27 hyperphosphorylation were apparent 12 h after release from the thymidine block, consistent with a mitotic arrest (Fig. 1B). Emi1 levels in control cells dropped at entry into mitosis, whereas in BI2536-treated cells Emi1 was relatively stable, and persisted even 12 h after release from the thymidine block. These data confirm previously published results (22) and are most likely due to inhibition of the Plk1-dependent SCFβ-TrCP-mediated degradation of Emi1. Notably, Emi1 present in Plk1-inhibited cells displayed an electrophoretic mobility shift that suggested its phosphorylation. Also confirming previous results (22), we observed that cyclin A levels did not remain stable in Plk1-inhibited cells, as a marked (although incomplete) decrease of cyclin A levels was seen at 10 and 12 h following release from the thymidine block. These results suggest that APC/C is activated upon entry into mitosis to promote cyclin A degradation even when Emi1 degradation is blocked, as reported previously (13, 22).
The efficacy of Plk1 inhibition by BI2536 in HeLa cells is demonstrated by lack of mitotic, Plk1-dependent, phosphorylation of BubR1 in BI2536-treated cells 11 h following release from a thymidine block as compared with cells arrested in mitosis by a nocodazole block (Fig. 1C). Comparison of nocodazole-arrested cells to BI2536-arrested cells shows once again that Emi1 persists in BI2536-treated cells in a form exhibiting reduced electrophoretic mobility, whereas cyclin A levels are diminished relative to S-phase cells (3 h following release from a thymidine block). To examine whether the mobility shift of Emi1 in BI2536-arrested cells is due to phosphorylation, extracts of BI2536-treated cells shown in Fig. 1C were subjected to treatment with λ phosphatase or buffer (Fig. 1D). Efficient dephosphorylation is demonstrated by the dephosphorylation of Cdc27 in BI2536-treated extracts treated with λ phosphatase. Similarly to Cdc27, treatment with λ phosphatase resulted in enhanced electrophoretic mobility of Emi1 in BI2536-treated cells, suggesting that the mobility shift of Emi1 seen in Plk1-inhibited cells is due to phosphorylation of Emi1. As a control, no effect on the electrophoretic mobility of either Cdc27 or Emi1 was shown when S-phase cell extracts were treated with λ phosphatase.
We conclude that Plk1 inhibition by BI2536 resulted in a mitotic arrest with relatively high levels of a phosphorylated form of Emi1. Despite the persistence of Emi1, APC/CCdc20 was activated, as suggested by the degradation of cyclin A, implying that the relatively high levels of Emi1 in BI2536-treated mitotic cells were unable to inhibit APC/C. These observations are in agreement with previous results of other investigators (22).
Inactivation of Emi1 by Purified Cyclin B/CDK1
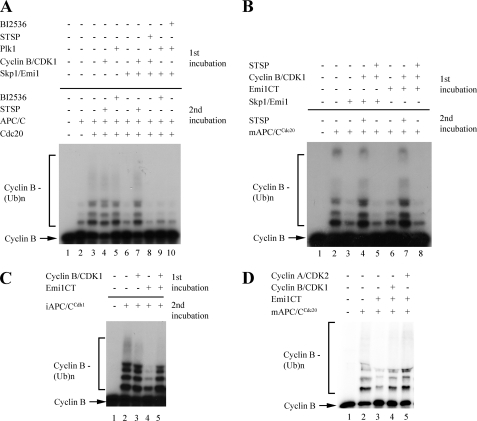
As APC/C is active in cells in which Emi1 is present in a phosphorylated form, it is possible that this phosphorylation may affect the ability of Emi1 to inhibit APC/C. Emi1 undergoes phosphorylation by the mitotic kinases cyclin B/CDK1 and Plk1 at mitotic entry. The role of these two kinases in promoting the degradation of Emi1 has been described (9, 18), yet the effect of mitotic phosphorylation on the ability of Emi1 to inhibit APC/C has not been previously addressed. To explore this question, we reconstituted Emi1-mediated inhibition of APC/C in a purified in vitro system (Fig. 2A). We examined the ubiquitylation of a radioactively labeled N-terminal fragment of cyclin B (125I-cyclin B) by APC/C purified from mitotic HeLa cell extracts (28). Ubiquitylation of cyclin B by purified mitotic APC/C was stimulated by addition of recombinantly purified Cdc20. To study the effect of mitotic kinases on Emi1, a two-stage incubation was carried out. In the first incubation, baculovirus-expressed recombinant full-length Emi1 was phosphorylated by purified recombinant Δ88-cyclin B/CDK1 or baculovirus-expressed recombinantly purified Plk1 at concentrations previously shown to efficiently phosphorylate Emi1 (18). Phosphorylation by cyclin B/CDK1 was terminated by the kinase inhibitor staurosporine, and Plk1 activity was terminated by use of the Plk1 inhibitor BI2536. In the second incubation, APC/C, Cdc20, cyclin B substrate, and the remainder of the components of the cyclin B ubiquitylation assay were added and the formation of cyclin-ubiquitin conjugates was monitored. Emi1 preincubated in buffer efficiently inhibited APC/CCdc20-mediated ubiquitylation of cyclin B. Preincubation of Emi1 with purified non-degradable Δ88-cyclin B/CDK1, however, markedly diminished inhibition of APC/C by Emi1 (Fig. 2A, lane 7 versus 6). Addition of the kinase inhibitor staurosporine to preincubation of Emi1 with Δ88-cyclin B/CDK1 abrogated the effect of Δ88-cyclin B/CDK1 on Emi1, indicating that inactivation of Emi1 by Δ88-cyclin B/CDK1 was dependent on the kinase activity of CDK1. Preincubation of Emi1 with Plk1 did not produce any effect on the ability of Emi1 to inhibit APC/C. As a control, cyclin B/CDK1 and Plk1 themselves had no affect on the activity of APC/C in the second incubation in the presence of staurosporine or BI2536, respectively.
FIGURE 2.
Effect of protein kinases on Emi1-mediated inhibition of APC/C in a purified system. A, inactivation of Emi1 by purified cyclin B/CDK1. In the first incubation, 3 nm full-length recombinant Emi1 in complex with Skp1 (Skp1/Emi1) was treated by a phosphorylation mixture with purified cyclin B/CDK1 or Plk1, and 10 μm staurosporine where indicated. Following this incubation, phosphorylation was inhibited by addition of staurosporine (where not previously added), and a second incubation was carried out in the presence of a ubiquitylation mixture, purified mitotic APC/C, recombinant purified Cdc20, and 125I-cyclin B as substrate. B, inactivation of the GST-tagged C-terminal 150-amino acid fragment of Emi1 by purified cyclin B/CDK1. As in A, 3 nm Skp1/Emi1 or the GST-tagged C-terminal 150 amino acids of Emi1 (GST-Emi1CT) were preincubated with the phosphorylation mixture, and purified cyclin B/CDK1 and staurosporine, where indicated, followed by a second incubation in which the ubiquitylation mixture and 125I-cyclin B were added, along with purified mitotic APC/C, recombinant Cdc20, and staurosporine, as indicated. C, effect of cyclin B/CDK1 on the inhibition of APC/CCdh1 by Emi1. A two-step phosphorylation and ubiquitylation assay as described above. Where indicated, GST-Emi1CT and cyclin B/CDK were added in the first incubation, and purified S-phase APC/C with recombinant Cdh1 was added for the second incubation. Staurosporine was added in all cases following the first incubation. D, inactivation of Emi1 by cyclin B/CDK1 and cyclin A/CDK2. GST-tagged Emi1CT was incubated in the presence of buffer, purified cyclin B/CDK1, or purified cyclin A/CDK2, and then purified on glutathione beads, quantified, and assayed for inhibition of cyclin B ubiquitylation by APC/CCdc20 at 10 nm concentration of each Emi1CT preparation.
Human Emi1 contains eight minimal consensus (S/T)P sites for phosphorylation by CDKs. Three of these sites are located in a C-terminal, 150-amino acid region of Emi1, which contains a zinc binding region, a destruction box, and a newly discovered RL tail, elements shown to be required for inhibition of APC/C by Emi1 (9, 32, 33). We compared the ability of a GST-tagged, bacterially expressed C-terminal 150-amino acid fragment of Emi1 (GST-Emi1CT) to inhibit APC/C. As shown in Fig. 2B, GST-Emi1CT preincubated in buffer inhibited APC/C as efficiently as full-length Emi1, consistent with previous reports (9). Preincubation with cyclin B/CDK1 inactivated both full-length Emi1 and GST-Emi1CT to a similar degree, and this effect was prevented by inhibition of CDK1 kinase activity with staurosporine. These results suggest that phosphorylation of the C-terminal region of Emi1 is sufficient for the inactivation of Emi1.
Emi1 has been shown to inhibit both the early mitotic, hyperphosphorylated, Cdc20-bound form of APC/C, and the interphase, hypophosphorylated, Cdh1-bound APC/C (10). To examine whether CDK-mediated phosphorylation affects the ability of Emi1 to inhibit both forms of APC/C, we preincubated GST-Emi1CT in the presence of buffer or cyclin B/CDK1. The ability of GST-Emi1CT to inhibit cyclin B ubiquitylation by APC/CCdh1 was then examined (Fig. 2C). For this purpose, we used APC/C purified from S-phase HeLa cell nuclear extracts, supplemented with recombinant purified Cdh1. Whereas Emi1 incubated in buffer effectively inhibited APC/CCdh1-mediated ubiquitylation, preincubation of Emi1 with cyclin B/CDK1 prevented Emi1 from inhibiting this form of APC/C, suggesting that phosphorylation of Emi1 by CDKs reduces the ability of Emi1 to inhibit both APC/CCdc20 and APC/CCdh1.
As (S/T)P sites are consensus sites for phosphorylation by the S-phase active CDK2 as well as the mitosis active CDK1, we examined whether purified baculovirus-expressed cyclin A/CDK2 can also inactivate Emi1. GST-Emi1CT was incubated in buffer alone, with cyclin B/CDK1, or with cyclin A/CDK2. Kinase activity levels, as assessed by [32P]phosphate incorporation into histone H1, were equal (data not shown). As full-length cyclin A is an APC/C substrate, and can competitively inhibit APC/C-mediated ubiquitylation of cyclin B, it was necessary to remove cyclin A before addition of the cyclin B ubiquitylation system. For this purpose, following incubation with buffer or kinases, GST-Emi1CT was purified on glutathione-Sepharose beads, quantified, and only then assayed for inhibition of APC/C (Fig. 2D). Emi1 purified following incubation with buffer retained the ability to inhibit APC/C. Surprisingly, the S-phase kinase cyclin A/CDK2 inactivated Emi1 as efficiently as the mitotic cyclin B/CDK1 in this purified in vitro system. As Emi1-mediated inhibition of APC/C is essential for proper execution of S-phase (12, 13), it seems reasonable that additional factors present in S-phase may regulate Emi1-directed CDK activity during S-phase (see below).
Inactivation of Emi1 by Mitotic Cell Extracts
If CDK-mediated phosphorylation were to inactivate Emi1, this would be expected to occur in prophase but not in S-phase, to allow Emi1 to inhibit APC/C throughout the S-phase on the one hand, and to promote cyclin A degradation upon entry into mitosis on the other. To examine the possibility of cell-cycle stage-specific inhibitory phosphorylation of the C-terminal region of Emi1, extracts of cells arrested in mitosis with nocodazole or nuclear extracts of cells in S-phase (3 h following release from thymidine block) were used. Incubation of the C-terminal fragment of Emi1 with mitotic extract did not result in ubiquitylation or destruction of this fragment as it lacks the SCFβ-TrCP binding motif (Fig. 3A). Addition of the phosphatase inhibitor okadaic acid to mitotic extract retarded the electrophoretic mobility of Emi1CT. This retardation is most likely due to phosphorylation of Emi1CT, as the addition of the kinase inhibitor staurosporine abolished this effect. Incubation of Emi1CT with S-phase extract, even in the presence of okadaic acid, did not cause any noticeable change in the electrophoretic mobility of Emi1CT. This suggests that Emi1 is phosphorylated at mitosis, when Emi1 must be inactivated, but not during S-phase, when Emi1 activity is necessary for the prevention of re-replication. The need for addition of okadaic acid may be explained by the presence of nonspecific phosphatase activity in cell extracts, and this activity may be restrained to specific locations in intact cells.
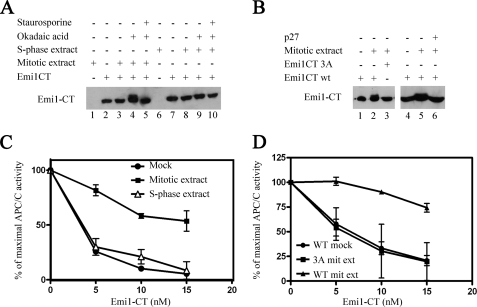
FIGURE 3.
Effect of cell extracts on Emi1 phosphorylation and inhibition of APC/C. A, phosphorylation of recombinant Emi1CT in cell extracts. 10 nm GST-Emi1 C-terminal fragment (Emi1CT) was incubated with the phosphorylation mixture. Where specified, 20 μg of nocodazole-arrested HeLa cell extract (mitotic extract), nuclear extract of HeLa cells 3 h after release from thymidine arrest (S-phase extract), 1 μm okadaic acid, or 10 μm staurosporine were added. Emi1CT phosphorylation was assessed by Western blotting with anti-Emi1 antibody and examination of the Emi1CT electrophoretic mobility shift. B, CDK-dependent phosphorylation of Emi1CT in mitotic cell extracts. 10 nm GST-Emi1CT wild type or mutated in all 3 CDK phosphorylation consensus sites (GST-Emi1CT 3A) was incubated as in A with mitotic extract with or without 0.1 μg/μl of p27, as indicated, and subjected to immunoblotting for Emi1. C, inactivation of Emi1CT by mitotic cell extract. GST-Emi1CT was incubated as described under “Experimental Procedures,” in the presence of phosphorylation buffer alone (●), mitotic extract (■), or S-phase extract (Δ) in duplicates and then purified on glutathione-Sepharose beads as described under “Experimental Procedures.” Following quantitation of these Emi1CT preparations, they were assayed in the indicated concentrations for their ability to inhibit 125I-cyclin B ubiquitylation by APC/CCdc20 in a purified system. The results are expressed as % of maximal APC/C ubiquitin ligase activity. D, effect of mutation of CDK phosphorylation consensus sites of Emi1CT on the inactivation of Emi1CT by mitotic extract. Wild type GST-Emi1CT was incubated as above in the presence of buffer (●) or mitotic extract (▴). GST-Emi1CT 3A was incubated in the presence of mitotic extract (■). Following this incubation, Emi1CT was purified, quantified, and examined for inhibition of APC/C ligase activity as described above.
To examine whether the phosphorylation of GST-Emi1CT is due to CDK activity, we mutated all three serine or threonine residues of the C-terminal Emi1 fragment CDK phosphorylation consensus sites to alanine (see “Experimental Procedures”). This mutant, designated GST-Emi1CT 3A, was not phosphorylated in mitotic cell extracts, as compared with wild type GST-Emi1CT (Fig. 3B, left panel). This indicates that Emi1 is directly phosphorylated by CDKs in mitotic cell extracts. To rule out that this phosphorylation is mediated by staurosporine-sensitive, proline-directed kinases other than CDKs, GST-Emi1CT was incubated in mitotic cell extracts in the presence of recombinant purified CDK inhibitor p27 (Fig. 3B, right panel). Similarly to staurosporine, p27 abrogated the phosphorylation of GST-Emi1CT in mitotic cell extracts, indicating that this phosphorylation is indeed CDK-mediated.
The effect of the phosphorylation of Emi1CT in mitotic extracts on the ability of Emi1CT to inhibit APC/C was examined in the experiment shown in Fig. 3C. GST-Emi1CT was first incubated with extract, then purified using glutathione beads, and finally assayed for the inhibition of APC/CCdc20-mediated ubiquitylation of cyclin B in an in vitro purified system. The purification step following the first incubation was necessary to remove the mitotic extract from Emi1CT, as nocodazole-arrested extracts contain components of the mitotic checkpoint that can inhibit APC/C independently of Emi1. Emi1CT incubated with buffer alone, or with S-phase extract, efficiently inhibited APC/C activity at a 5 nm concentration. Incubation of Emi1CT with mitotic extract, however, resulted in a reduced effect of Emi1CT on the activity of APC/C even in concentrations of up to 15 nm. It is noteworthy that the incubation and purification steps of this experiment caused a loss of up to 5-fold in the efficacy of Emi1CT as an APC/C inhibitor, even when Emi1CT was incubated with buffer alone, and caused some variability in the results obtained. However, in all of several experiments there was a marked reduction of the ability of Emi1CT preincubated with the mitotic cell extract to inhibit APC/C, as compared with mock treated Emi1CT or Emi1CT incubated with S-phase cell extract. These results suggest that an activity present in mitosis, but not S-phase, inactivates Emi1 through a mechanism other than ubiquitin-mediated degradation of Emi1. Similarly to the previous experiments, samples taken from each system following the first incubation showed a mobility shift in Emi1CT incubated with mitotic extract, but not S-phase or mock incubation, in correlation with the inactivation of Emi1CT. This finding suggests that the inactivation of Emi1CT in mitotic extracts is mediated by phosphorylation.
By the use of the GST-Emi1CT 3A mutant, we examined whether the inactivation of Emi1CT in mitotic extracts is mediated by CDKs. Incubation of GST-Emi1CT 3A in mitotic extract did not produce the inactivation seen with wild type GST-Emi1CT. GST-Emi1CT 3A preincubated with mitotic extract was as efficient an inhibitor of APC/C-mediated cyclin B ubiquitylation as wild-type GST-Emi1CT incubated in buffer alone (Fig. 3D).
CDK-mediated Phosphorylation of Emi1 Prevents Its Binding to APC/C
Emi1 has been shown to bind APC/C and co-activators Cdc20 and Cdh1 in Xenopus and HeLa cell extracts (9, 10, 32). A possible mechanism for disruption of Emi1-mediated inhibition of APC/C by phosphorylation may be by interference with the interaction between Emi1, APC/C, and the co-activators. To explore this possibility, GST-Emi1CT was preincubated with buffer or with Δ88-cyclin B/CDK1 and then was further incubated with purified mitotic APC/C and recombinant Cdc20 or purified S-phase APC/C and recombinant Cdh1 (Fig. 4A). Following the second incubation, samples were examined for APC/C cyclin B ubiquitylation activity (lower panels), or subjected to pulldown using glutathione beads and immunoblotting for the Cdc27 subunit of APC/C (upper panels). The results show that binding of APC/CCdc20 or APC/CCdh1 to Emi1 was remarkably reduced following incubation with Δ88-cyclin B/CDK1. In correlation with the reduction of the binding of Emi1 to APC/C and consistent with the results presented above, Emi1CT preincubated with Δ88-cyclin B/CDK1 was unable to efficiently inhibit APC/CCdc20 and APC/CCdh1 in the samples taken prior to addition of glutathione beads.
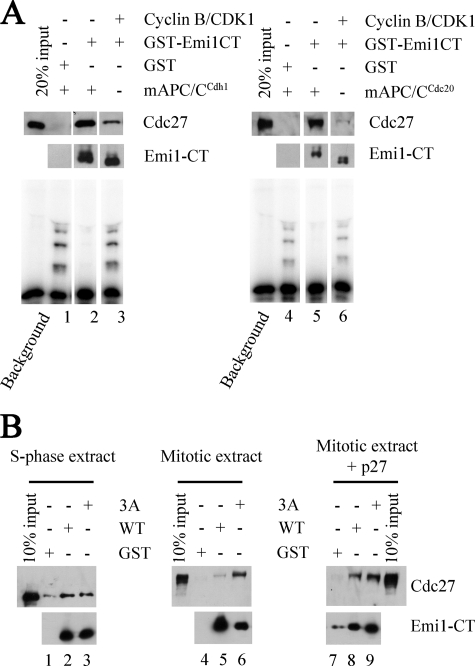
FIGURE 4.
Effect of phosphorylation of Emi1 on its binding to APC/C. A, effect of purified cyclin B/CDK1 on Emi1 binding to APC/C in a purified system. 30 ng of GST-Emi1CT was incubated with phosphorylation buffer alone or with purified cyclin B/CDK1 as indicated. Phosphorylation was terminated by addition of staurosporine. This was followed by addition of purified mitotic or interphase APC/C and recombinant Cdc20 or Cdh1 as indicated and a second incubation was carried out to allow binding. At the end of this incubation 10% of the samples were taken for cyclin B ubiquitylation assay (bottom panels) and the remaining 90% were subjected to GST pulldown. Bead-bound proteins were examined by immunoblotting for Cdc27 and Emi1. B, binding of recombinant Emi1CT to endogenous APC/C in cell extracts. 30 ng of wild type or the 3A mutant GST-Emi1CT were incubated in phosphorylation buffer with 200 μg of S-phase nuclear cell extract, mitotic cell extract, or mitotic cell extracts previously supplemented with 0.1 μg/μl of p27 to inhibit endogenous CDKs. This was followed by GST pulldown and examination of bead-bound proteins by immunoblotting for Emi1 and Cdc27.
To examine whether this reduction in Emi1-APC/C interaction is regulated during the cell cycle, we examined the interaction of Emi1CT with endogenous APC/C in cell extracts (Fig. 4B). Wild type GST-Emi1CT, or the 3A mutant were incubated with S-phase or mitotic cell extracts in conditions enabling protein kinase activity, and Emi1CT binding to endogenous APC/C in the cell extract was examined by GST pulldown. When incubated with S-phase extract, both wild type and the 3A mutant GST-Emi1CT bound APC/C to a similar degree. In the presence of mitotic extracts, wild type GST-Emi1CT binding to APC/C was markedly reduced relatively to GST-Emi1CT 3A binding to APC/C. Supplementation of the CDK inhibitor p27 to mitotic extracts strongly diminished the differences between wild type and the 3A mutant GST-Emi1CT in the binding of APC/C. These findings suggest that CDK-mediated phosphorylation of GST-Emi1CT in mitotic cell extracts results in a reduction of the interaction between Emi1 and APC/C, thus explaining the inactivation of GST-Emi1CT in mitotic extracts. Taken together, these results suggest that CDK-mediated phosphorylation of Emi1 releases APC/C from inhibition by reducing the affinity of Emi1 to APC/C, and that this mechanism of inactivation occurs in mitosis but not during S-phase.
Protection of Emi1 from CDK-mediated Inactivation in S-phase Cell Extracts
Emi1-mediated inhibition of APC/C is present throughout S-phase (15) and has been shown to be essential for the prevention of re-replication and centrosomal abnormalities that may lead to mitotic catastrophes and aneuploidy (12, 13). Our results show that Emi1 is inactivated by CDKs in mitotic extracts but not in S-phase extracts, in agreement with the role of Emi1 in S-phase and the timing of APC/C activation as the cell enters mitosis. We also showed, however, that Emi1 can be directly inactivated by the S-phase cyclin-CDK complex, cyclin A-CDK2, and that CDK-mediated inactivation of Emi1 affects inhibition of the S-phase form of APC/C, APC/CCdh1. There are several possible explanations for this apparent contradiction. One possibility is that there is less overall CDK activity in S-phase than in mitosis and that Emi1 inactivating phosphorylation occurs only above a certain threshold of CDK activity. Such a principle has been suggested for other CDK substrates to explain the differences in substrate specificity between mitosis and earlier cell cycle stages (34). Indeed, our S-phase extract preparation exhibits a 3-fold reduced CDK-dependent histone H1 kinase activity as compared with mitotic extract (see below).
A second possibility to explain the lack of S-phase inactivation of Emi1 by CDKs is that a certain factor, present and active in S-phase, but not in mitosis, protects Emi1 from CDK-mediated inactivation. Yet another possibility is that some factor, present and active in mitosis, but not S-phase, potentiates CDK activity toward Emi1. The later possibility seems less likely as we have shown that purified CDKs can directly phosphorylate and inactivate Emi1 efficiently.
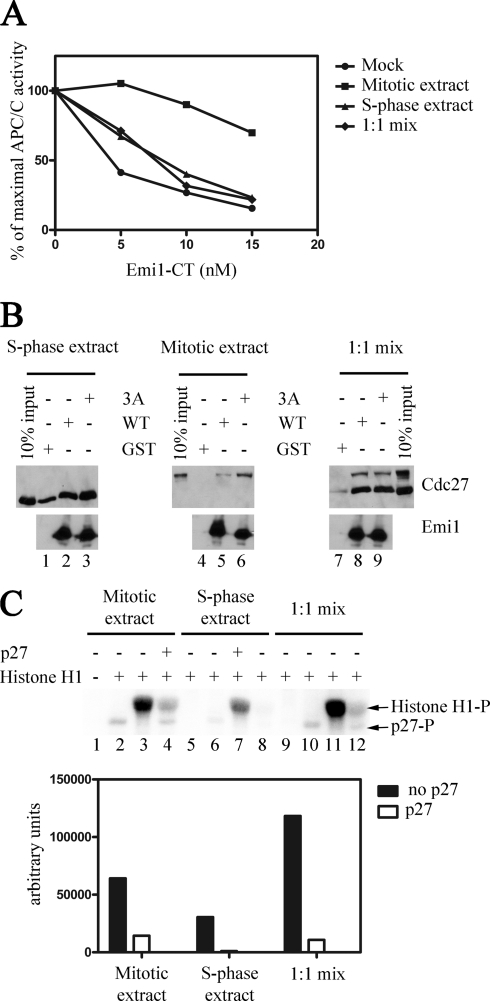
To explore all these possibilities, GST-Emi1CT was incubated with either buffer alone, mitotic extract alone, S-phase extract alone, or a 1:1 mixture of mitotic and S-phase extracts. If a threshold of CDK activity is needed to inactivate Emi1, it would be expected that incubation with the mitotic and S-phase extract mixture would inactivate Emi1 at least as efficiently as mitotic extract alone, if not to a greater extent due to an additive effect of the CDK activity in the S-phase extract. If an Emi1 protective factor is present in S-phase, then mixing mitotic and S-phase extracts would be expected to produce less Emi1 inactivation than mitotic extract alone.
In the experiment shown in Fig. 5A, GST-Emi1CT was incubated in the presence of phosphorylation buffer alone, mitotic extract, S-phase extract, or a 1:1 mixture of mitotic and S-phase extracts, and then purified on glutathione beads. Following quantitation, these Emi1CT preparations were examined for their ability to inhibit APC/CCdc20 in the 125I-cyclin B ubiquitylation assay. In agreement with our previous results, Emi1CT incubated with buffer or S-phase extract efficiently inhibited cyclin B ubiquitylation by APC/C, whereas Emi1CT incubated with mitotic extract exhibited a diminished ability to inhibit APC/C. Incubating Emi1CT with the mitotic and S-phase extract mixture resulted in no apparent inactivation of Emi1CT relative to Emi1CT incubated with buffer alone or S-phase extract, suggesting that a factor existing in S-phase extracts is able to counteract the effect of mitotic extract to inactivate Emi1. This result points to the possibility of an Emi1 protective factor present in S-phase, which prevents CDK-mediated inactivation of Emi1, and thus APC/C activation prior to entry into mitosis.
FIGURE 5.
Effect of mixing S-phase and mitotic extracts on the interaction of Emi1 with APC/C. A, effect of S-phase extract on mitotic extract-mediated inactivation of Emi1. 30 ng of GST-Emi1CT was incubated in the presence of phosphorylation buffer (●), 100 μg of mitotic extract (■), 100 μg of S-phase extract (▴), or 200 μg of a 1:1 ratio mixture of mitotic and S-phase extracts (♦) and then purified on glutathione-Sepharose beads. Following quantitation of these Emi1CT preparations, they were assayed in the indicated concentrations for their ability to inhibit 125I-cyclin B ubiquitylation by APC/CCdc20 in a purified system. The results are expressed as percent of maximal APC/C activity. B, effect of S-phase extract on the ability of Emi1 to bind endogenous APC/C in cell extracts. 30 ng of wild type or the 3A mutant GST-Emi1CT were incubated in phosphorylation buffer with 100 μg of S-phase nuclear cell extract, 100 μg of mitotic cell extract, or 200 μg of a 1:1 mixture of mitotic and S-phase extracts. This was followed by GST pulldown and examination of bead-bound proteins by immunoblotting for Emi1 and Cdc27. C, assay of CDK activity in S-phase, mitotic, and mixed extracts. Incorporation of 32P into histone H1 was assayed by incubation of 5 μg of histone H1 in the presence of 2 mg/ml of S-phase, mitotic or a 1:1 ratio mixture of mitotic and S-phase extracts. CDK activity was inhibited by p27 where indicated. Incorporation of [32P]phosphate was quantified by phosphorimager (arbitrary units). Radioactivity in control lanes lacking histone H1 were subtracted as background.
To further explore the possibility of an Emi1 protective factor in S-phase cell extracts, we examined the effect of mixing mitotic and S-phase extracts on Emi1-APC/C binding (Fig. 5B). Wild type and the 3A mutant GST-Emi1CT efficiently pulled down endogenous APC/C in S-phase cell extracts to a roughly similar extent. In mitotic cell extracts, however, wild type GST-Emi1CT was unable to efficiently bind APC/C as compared with the 3A mutant GST-Emi1CT. In a 1:1 mixture of mitotic and S-phase cell extracts no difference was observed between the ability of wild type and mutant GST-Emi1CT to bind both the hypophosphorylated and hyperphosphorylated forms of APC/C. Interestingly, examination of the bead-bound GST-Emi1CT shows that the phosphorylation of Emi1CT observed following incubation in mitotic cell extracts, as seen by an electrophoretic mobility shift, did not occur when S-phase and mitotic cell extracts were mixed. This suggests that the S-phase extract mediates its Emi1 protective effect through either prevention of Emi1 phosphorylation or promotion of its dephosphorylation. That this effect was not due to inhibition of overall CDK activity in mitotic extracts was confirmed by a histone H1 phosphorylation assay (Fig. 5C). CDK-mediated incorporation of [32P]phosphate into histone H1 incubated with S-phase extracts was only one-third of the CDK-mediated [32P]phosphate incorporated into histone H1 incubated in mitotic extracts. Incubation of histone H1 with both mitotic and S-phase extracts resulted in an additive incorporation of [32P]phosphate into histone H1. These results suggest that the lack of Emi1 phosphorylation observed in S-phase extracts is due to an Emi1-directed protective effect and not due to overall inhibition of CDK activity. We conclude from these results that a factor, present in cells during S-phase but not in mitotic cells, acts to prevent or reverse Emi1 phosphorylation, thus enabling Emi1 to bind and inhibit APC/C during S-phase.
DISCUSSION
Recent reports have questioned the importance of Emi1 destruction at prophase for entry into mitosis, and the role of Emi1 as an inhibitor of APC/CCdc20 (13, 22, 23). Disruption of Plk1 function in cells by Plk1-specific siRNA or the small molecule inhibitor BI2536, or overexpression of high levels of non-degradable Emi1 in cells did not hinder the timing and robustness of cyclin A degradation following nuclear envelope breakdown (13, 21, 22). In our study, we explored the possibility of the existence of additional modes of Emi1 regulation at prophase, which would explain how cells are able to enter mitosis despite the lack of Emi1 degradation. Our findings suggest that CDK-mediated phosphorylation can inactivate Emi1 and enable APC/C activation upon entry into mitosis. In support of this notion we report that: 1) Emi1 is phosphorylated directly by CDKs in mitotic but not S-phase cell extracts; 2) Emi1 phosphorylated by CDKs is unable to efficiently inhibit APC/C; 3) Emi1 phosphorylated by CDKs is unable to form a stable complex with APC/C; and 4) Emi1 is inactivated by incubation in mitotic, but not S-phase cell extracts and this effect is CDK-dependent. In further work it will be desirable to test this model in cells, by expression of the non-phosphorylatable Emi1 3A mutant and determining whether APC/C remains associated with it and remains inactive in the presence of the Plk inhibitor.
Plk1-deficient or inhibited cells arrest in prometaphase with high levels of cyclin B (20, 22). CDK activity remains high in these cells, possibly explaining how APC/C inhibition by Emi1 is overcome despite Emi1 stability. Also in agreement with our interpretation, overexpression of non-degradable Emi1 caused a mitotic arrest with relatively high levels of cyclin B compared with control anaphase/telophase cells, indicating incomplete APC/C activation in these cells (13). If Emi1 were truly dispensable for APC/CCdc20 inhibition, one would not expect any cell cycle arrest in cells overexpressing non-degradable Emi1. It is possible that in these cells arrested in mitosis, the relatively high cyclin B levels represent an equilibrium of CDK and APC/C activity. Higher cyclin B levels would promote Emi1 inactivation and APC/C activity to promote cyclin B degradation, and lower cyclin B levels would not prevent Emi1 from inhibiting APC/C, thus allowing cyclin B accumulation. In this manner, cyclin B levels remain at a constant level throughout a continuous mitotic arrest. A somewhat similar mechanism has been described regarding the meiotic homologue of Emi1, Emi2, in eggs arrested by cytostatic factor (CSF) (35). At exit from meiosis I cyclin B is degraded, but its levels drop only partially, and cyclin B/CDK1 activity remains at a relatively high level throughout meiosis II until fertilization, although lower than the level during meiosis I (35). The stability of cyclin B levels is maintained by inhibition of APC/C-mediated degradation. Recent studies have suggested the possibility of a feedback loop acting to regulate cyclin B/CDK1 activity levels through modulation of Emi2-mediated inhibition of APC/C. Cyclin B/CDK1 has been shown to phosphorylate residues on both the C-terminal and N-terminal regions of Emi2. CDK1-mediated phosphorylation of the C-terminal region of Emi2 produces a reduction of Emi2 affinity to APC/C, and therefore interferes with the ability of Emi2 to inhibit APC/C (36, 37). Phosphorylation of the N-terminal portion of Emi2 by cyclin B/CDK1 reduces Emi2 stability in CSF-arrested Xenopus egg extracts by enabling phosphorylation of the β-TrCP recognition motif of Emi2 by Plx1, thus promoting the SCFβ-TrCP-mediated ubiquitylation of Emi2 (38). By these two mechanisms, cyclin B/CDK1 can inactivate Emi2 and promote its own inactivation by APC/C. Emi2 inactivation by cyclin B/CDK1 is negated by the Mos pathway-dependent action of protein phosphatase 2A (PP2A) to dephosphorylate Emi2 at CDK phosphorylation sites (36–40). When Emi2-directed cyclin B/CDK1 activity rises above Emi2-directed PP2A activity, Emi2 is inactivated and degraded to a level sufficient to enable partial activation of APC/C, promoting cyclin B/CDK1 degradation. In this manner cyclin B/CDK1 autoregulates its level of activity, preventing unchecked cyclin B accumulation that might disrupt the rapid transition from CSF arrest to anaphase II at fertilization (36). As expression of non-degradable Emi1 in cells caused them to arrest in mitosis with relatively high levels of cyclin B (13), it is possible that expression of non-degradable Emi1 in cells produces a CSF-like state in which high CDK activity promotes cyclin B degradation, whereas low CDK activity promotes inhibition of APC/C by Emi1 and cyclin B stability, resulting in stable mid-range cyclin B/CDK1 activity levels.
Our results, which show a protective effect of S-phase cell extracts on the ability of Emi1 to bind and inhibit APC/C in the presence of mitotic cell extract, points to a possible Emi1 protective factor in S-phase cells. Such a factor would allow APC/C inhibition throughout S-phase, preventing re-replication and mitotic catastrophes. An Emi1-binding protein, Evi5, has been reported to protect Emi1 from premature SCFβ-TrCP-mediated degradation during S-phase by preventing Plk1-mediated phosphorylation of Emi1 (41). Evi5 is an unlikely candidate for the unknown factor protecting Emi1 from CDK-mediated inactivation as Evi5 is itself a target for Plk1-mediated SCFβ-TrCP-dependent degradation upon mitotic entry. Depletion of Plk1 using siRNA stabilizes Evi5 (41) and yet cyclin A is still degraded at prophase in Plk1-depleted or -inhibited cells (20–22), indicating that the persistence of Evi5 is not sufficient to prevent inactivation of Emi1 in these cells.
Another factor implicated in protection of Emi1 from premature degradation is the peptidyl-prolyl cis/trans isomerase Pin1 (42). Pin1 binds proteins previously phosphorylated by proline-directed kinases such as CDKs and MAP kinases and catalyzes a conformational change in substrates (43). Pin1 binds Emi1 during G2 phase and prevents Emi1 binding to β-TrCP, allowing the stabilization of Emi1 during G2 despite active CDKs and Plk1 (42). Pin1 interaction with Emi1 is dependent on CDK-mediated phosphorylation of an N-terminal serine residue of Xenopus Emi1. Our results show that protection of Emi1 from inactivation in cell extracts does not require the N-terminal portion of Emi1. Furthermore, the finding that Pin1 binds Emi1 only following CDK-mediated phosphorylation is not consistent with the possibility of Pin1 acting to prevent CDK-mediated inactivation of Emi1.
Inactivation of Emi2 by CDKs is held in check by the Mos pathway-dependent activity of PP2A on Emi2. Does such a PP2A activity protect Emi1 during S-phase? Our experiments were conducted in the presence of okadaic acid, an inhibitor of PP2A. Without addition of okadaic acid we were unable to see the phosphorylation of the C-terminal portion of Emi1 in mitotic extracts. It is possible that this represents a possible role for PP2A in the regulation of Emi1 activity and stability, or it may only represent a nonspecific PP2A activity in cell extracts as opposed to intact cells. Further experiments in mitotic extracts and intact cells are needed to explore the possible role of PP2A in the protection of Emi1 from CDK-mediated inactivation. Regardless of the role of PP2A, we have shown that mixing mitotic and S-phase extracts abrogates the CDK-mediated inactivation of Emi1 and enables Emi1 to bind APC/C, even in the presence of okadaic acid. These results point toward an okadaic acid-insensitive Emi1 protective factor active at S-phase, which is unrelated to the possible role of PP2A. No such factor has been suggested to protect Emi2 in CSF-arrested eggs. Finally, PP2A activity toward Emi2 during CSF is dependent upon the Mos-MEK-MAPK-Rsk pathway. In somatic cell cycles, this pathway is inactive, explaining the ability of early embryonic cell division cycles to progress despite residual persistence of Emi2 in early embryonic cell cycles (44). For PP2A to act on Emi1, this activity would have to be independent of the Mos pathway. The identification of the possible factor protecting Emi1 from CDK-mediated inactivation and whether this factor acts by binding to Emi1 and inhibiting the interaction of CDKs with Emi1 or the phosphorylation of Emi1 by CDKs, or whether it acts as a phosphatase, removing CDK-mediated phosphorylation of Emi1, require further research.
This work was supported by a grant from the Israel Science Foundation (to A. H.).
- CDK
- cyclin-dependent kinase
- APC/C
- anaphase promoting complex/cyclosome
- CSF
- cytostatic factor
- Emi1
- early mitotic inhibitor 1
- Emi1CT
- 150-amino acid residue C-terminal fragment of Emi1
- Plk1
- Polo-like kinase 1.
REFERENCES
- 1. Reed S. I. (2003) Nat. Rev. Mol. Cell Biol. 4, 855–864 [DOI] [PubMed] [Google Scholar]
- 2. Murray A. W. (2004) Cell 116, 221–234 [DOI] [PubMed] [Google Scholar]
- 3. Thornton B. R., Toczyski D. P. (2003) Nat. Cell Biol. 5, 1090–1094 [DOI] [PubMed] [Google Scholar]
- 4. Peters J. M. (2006) Nat. Rev. Mol. Cell Biol. 7, 644–656 [DOI] [PubMed] [Google Scholar]
- 5. den Elzen N., Pines J. (2001) J. Cell Biol. 153, 121–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geley S., Kramer E., Gieffers C., Gannon J., Peters J. M., Hunt T. (2001) J. Cell Biol. 153, 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong X., Zavitz K. H., Thomas B. J., Lin M., Campbell S., Zipursky S. L. (1997) Genes Dev. 11, 94–105 [DOI] [PubMed] [Google Scholar]
- 8. Grosskortenhaus R., Sprenger F. (2002) Dev. Cell 2, 29–40 [DOI] [PubMed] [Google Scholar]
- 9. Reimann J. D., Freed E., Hsu J. Y., Kramer E. R., Peters J. M., Jackson P. K. (2001) Cell 105, 645–655 [DOI] [PubMed] [Google Scholar]
- 10. Reimann J. D., Gardner B. E., Margottin-Goguet F., Jackson P. K. (2001) Genes Dev. 15, 3278–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H., Lee D. J., Oh S. P., Park H. D., Nam H. H., Kim J. M., Lim D. S. (2006) Mol. Cell. Biol. 26, 5373–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Machida Y. J., Dutta A. (2007) Genes Dev. 21, 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Fiore B., Pines J. (2007) J. Cell Biol. 177, 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lehman N. L., Tibshirani R., Hsu J. Y., Natkunam Y., Harris B. T., West R. B., Masek M. A., Montgomery K., van de Rijn M., Jackson P. K. (2007) Am. J. Pathol. 170, 1793–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu J. Y., Reimann J. D., Sørensen C. S., Lukas J., Jackson P. K. (2002) Nat. Cell Biol. 4, 358–366 [DOI] [PubMed] [Google Scholar]
- 16. Guardavaccaro D., Kudo Y., Boulaire J., Barchi M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P. K., Yamasaki L., Pagano M. (2003) Dev. Cell 4, 799–812 [DOI] [PubMed] [Google Scholar]
- 17. Margottin-Goguet F., Hsu J. Y., Loktev A., Hsieh H. M., Reimann J. D., Jackson P. K. (2003) Dev. Cell 4, 813–826 [DOI] [PubMed] [Google Scholar]
- 18. Moshe Y., Boulaire J., Pagano M., Hershko A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 7937–7942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen D. V., Loktev A. V., Ban K. H., Jackson P. K. (2004) Mol. Biol. Cell 15, 5623–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sumara I., Giménez-Abián J. F., Gerlich D., Hirota T., Kraft C., de la Torre C., Ellenberg J., Peters J. M. (2004) Curr. Biol. 14, 1712–1722 [DOI] [PubMed] [Google Scholar]
- 21. van Vugt M. A., van de Weerdt B. C., Vader G., Janssen H., Calafat J., Klompmaker R., Wolthuis R. M., Medema R. H. (2004) J. Biol. Chem. 279, 36841–36854 [DOI] [PubMed] [Google Scholar]
- 22. Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J. J., Hoffmann M., Rettig W. J., Kraut N., Peters J. M. (2007) Curr. Biol. 17, 304–315 [DOI] [PubMed] [Google Scholar]
- 23. Di Fiore B., Pines J. (2008) Chromosoma 117, 333–338 [DOI] [PubMed] [Google Scholar]
- 24. Mayer A. N., Wilkinson K. D. (1989) Biochemistry 28, 166–172 [DOI] [PubMed] [Google Scholar]
- 25. Hershko A., Heller H., Elias S., Ciechanover A. (1983) J. Biol. Chem. 258, 8206–8214 [PubMed] [Google Scholar]
- 26. Aristarkhov A., Eytan E., Moghe A., Admon A., Hershko A., Ruderman J. V. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 4294–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montagnoli A., Fiore F., Eytan E., Carrano A. C., Draetta G. F., Hershko A., Pagano M. (1999) Genes Dev. 13, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golan A., Yudkovsky Y., Hershko A. (2002) J. Biol. Chem. 277, 15552–15557 [DOI] [PubMed] [Google Scholar]
- 29. Sudakin V., Shteinberg M., Ganoth D., Hershko J., Hershko A. (1997) J. Biol. Chem. 272, 18051–18059 [DOI] [PubMed] [Google Scholar]
- 30. Hershko A. (2005) Methods Enzymol. 398, 170–175 [DOI] [PubMed] [Google Scholar]
- 31. Steegmaier M., Hoffmann M., Baum A., Lénárt P., Petronczki M., Krssák M., Gürtler U., Garin-Chesa P., Lieb S., Quant J., Grauert M., Adolf G. R., Kraut N., Peters J. M., Rettig W. J. (2007) Curr. Biol. 17, 316–322 [DOI] [PubMed] [Google Scholar]
- 32. Miller J. J., Summers M. K., Hansen D. V., Nachury M. V., Lehman N. L., Loktev A., Jackson P. K. (2006) Genes Dev. 20, 2410–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohe M., Kawamura Y., Ueno H., Inoue D., Kanemori Y., Senoo C., Isoda M., Nakajo N., Sagata N. (2010) Mol. Biol. Cell 21, 905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hochegger H., Takeda S., Hunt T. (2008) Nat. Rev. Mol. Cell Biol. 9, 910–916 [DOI] [PubMed] [Google Scholar]
- 35. Wu J. Q., Kornbluth S. (2008) J. Cell Sci. 121, 3509–3514 [DOI] [PubMed] [Google Scholar]
- 36. Wu J. Q., Hansen D. V., Guo Y., Wang M. Z., Tang W., Freel C. D., Tung J. J., Jackson P. K., Kornbluth S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 16564–16569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hansen D. V., Pomerening J. R., Summers M. K., Miller J. J., Ferrell J. E., Jr., Jackson P. K. (2007) Cell Cycle 6, 732–738 [DOI] [PubMed] [Google Scholar]
- 38. Wu Q., Guo Y., Yamada A., Perry J. A., Wang M. Z., Araki M., Freel C. D., Tung J. J., Tang W., Margolis S. S., Jackson P. K., Yamano H., Asano M., Kornbluth S. (2007) Curr. Biol. 17, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inoue D., Ohe M., Kanemori Y., Nobui T., Sagata N. (2007) Nature 446, 1100–1104 [DOI] [PubMed] [Google Scholar]
- 40. Nishiyama T., Ohsumi K., Kishimoto T. (2007) Nature 446, 1096–1099 [DOI] [PubMed] [Google Scholar]
- 41. Eldridge A. G., Loktev A. V., Hansen D. V., Verschuren E. W., Reimann J. D., Jackson P. K. (2006) Cell 124, 367–380 [DOI] [PubMed] [Google Scholar]
- 42. Bernis C., Vigneron S., Burgess A., Labbé J. C., Fesquet D., Castro A., Lorca T. (2007) EMBO Rep. 8, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu K. P., Liou Y. C., Zhou X. Z. (2002) Trends Cell Biol. 12, 164–172 [DOI] [PubMed] [Google Scholar]
- 44. Liu J., Grimison B., Lewellyn A. L., Maller J. L. (2006) J. Biol. Chem. 281, 34736–34741 [DOI] [PubMed] [Google Scholar]