Abstract
Gene expression is regulated by a number of interrelated posttranslational modifications of histones, including citrullination. For example, peptidylarginine deminase 4 (PAD4) converts peptidyl arginine to citrulline in histone H3 and can repress gene expression. However, regulation of gene expression through citrullination of non-histone proteins is less well defined. Herein, we identify a tumor suppressor protein, inhibitor of growth 4 (ING4), as a novel non-histone substrate of PAD4. ING4 is known to bind p53 via its nuclear localization signal (NLS) region and to enhance transcriptional activity of p53. We show that PAD4 preferentially citrullinates ING4 in the same NLS region and thereby disrupts the interaction between ING4 and p53. A citrulline-mimicking Arg-NLS-Gln ING4 mutant, which has all Arg residues in the NLS mutated to Gln, loses its affinity for p53, can no longer promote p53 acetylation, and results in repression of downstream p21 expression. In addition, we found that citrullination leads to increased susceptibility of ING4 to degradation, likely impacting p53-independent pathways as well. These findings elucidate an interaction between posttranslational citrullination, acetylation, and methylation and highlight an unusual mechanism whereby citrullination of a non-histone protein impacts gene regulation.
Keywords: Enzymes, Gene Expression, Histone Modification, p53, Post-translational Modification, Protein Degradation, Protein Domains, Citrullination
Introduction
Protein arginine deiminases (PADs2 or PADIs) catalyze the posttranslational modification of peptidyl arginine and, more slowly, peptidyl monomethylarginine to form peptidyl citrulline. This citrullination reaction is a hydrolytic deimination that eliminates the positive charge of the arginine side chain, can lead to changes in inter- and intraprotein interactions, and can even cause localized protein unfolding (1, 2). The human PAD4 isoform is calcium-regulated, nuclear-targeted, and is of particular interest because of its role in gene regulation as a catalyst of histone modification and chromatin remodeling (3). PAD4 expression is up-regulated in a variety of human malignant cancers, and PAD4-specific inhibitors can kill a selection of cancerous cells lines including HL-60, MCF7, and HT-29 (4). Accordingly, PAD4 has been suggested as a possible target for anticancer therapeutics (5).
Some PAD4-interacting non-histone proteins have also been identified, including the tumor suppressor p53 (6). PAD4 binds p53 and inhibits p53-regulated cell cycle arrest and apoptosis. Through this interaction, PAD4 gets recruited to gene promoters that are targeted by p53 and, in the case of p21, can down-regulate expression. Reciprocally, p53 can regulate the expression of PAD4 through a p53-response element located in a PAD4 gene intron (7). These findings suggest one mechanism whereby PAD4 can promote cancerous growth through its interactions with p53, although other unidentified pathways may also contribute.
PAD4 also acts through citrullination of non-histone proteins, such as p300 (8). The p300 protein, histone methyltransferase coactivator-associated arginine methyltransferase-1 (CARM1) and glucocorticoid receptor interacting protein-1 (GRIP1) form a coactivator complex of nuclear receptor that functions synergistically to regulate estrogen receptor-mediated transcription. The binding between p300 and GRIP1 is critical for their synergistic cooperation as coactivators. Methylation of p300 on Arg-2142 by CARM1 inhibits or loosens the GRIP1 binding. However, PAD4 can citrullinate the same residue to restore or enhance the p300-GRIP1 interaction. Accordingly, PAD4-mediated citrullination can regulate gene transcription by moderating the assembly or conformation of the coactivator complex.
In an effort to discover other pathways that are possibly impacted by citrullination and to identify novel protein substrates of PAD4 in an unbiased manner, we screened a commercially available 38,016-member, redundant, high density protein array derived from a human brain cDNA library (ImaGenes, Berlin, Germany). Initial screens identified a number of putative PAD4 substrates, including the inhibitor of growth protein 4 (ING4). This particular candidate substrate immediately caught our attention because, like PAD4, it can also regulate histone modification and p53 activity. The interactions between PAD4, ING4, and p53 are studied in more depth herein.
EXPERIMENTAL PROCEDURES
Plasmid Construction
The wild-type human ING4 (variant 1) gene was obtained by a PCR from a human brain whole Quick-Clone cDNA library (Clontech, Mountain View, CA), and the sequence was verified by DNA sequencing. Maltose-binding protein-tagged ING4 (MBP-ING4) and its truncations (MBP-ING4-N1 (1–56 amino acids), MBP-ING4-N2 (56–121 amino acids), MBP-ING4-NLS (121–188 amino acids), and MBP-ING4-PHD (188–249 amino acids)) were cloned into the pMal-c2x vector for expression in Escherichia coli strain BL21(DE3). A series of MBP-ING4 mutants with Arg altered to Lys (Arg-NLS-Lys, R133K/R142K/R144K, R142K/R144K, R133K/R166K, or R166K) were generated using PCR based mutagenesis. FLAG-ING4 and its mutants (Arg-NLS-Gln, R133Q/R166Q, R142Q/R144Q, or R166Q) were cloned into a pcDNA3.1(+) vector for expression in human HEK 293T cells or RKO cells. To express glutathione transferase-tagged ING4 (GST-ING4), the ING4 gene was cloned into vector pGEX-6p1.The construction of GST-PAD4, its deletions (GST-IgL1 (1–133 amino acids) and GST-IgL1L2 (1–300 amino acids)), and its inactive mutant GST-C645A were generated using PCR and site-directed mutagenesis. To express MBP-p53 and its deletions (MBP-p53-AD-DBD (1–300 amino acids) and MBP-p53-RD (300–393 amino acids)), the corresponding coding DNA sequences were cloned into a pMal-c2x expression vector.
Recombinant Protein Purification
Plasmids encoding MBP-ING4, its deletions, and its mutants were transformed in to E. coli BL21 (DE3). In each case, expression of recombinant protein was induced by the addition of 0.4 mm isopropyl-β-d-1-thiogalactopyranoside at 37 °C for 1 h. Bacteria from 500 ml of cultures grown in LB media were pelleted by centrifugation at 6371 × g at 4 °C for 10 min and resuspended in 20 ml of column buffer (20 mm Tris, pH 7.5, 200 mm NaCl, 1 mm EDTA) supplemented with a protease inhibitor mixture (Roche Applied Science). Subsequently, cells were sonicated for 12 cycles (10 s on and 50 s off) on ice, and the lysates were centrifuged at 25,000 × g at 4 °C for 30 min. MBP-fusion proteins were purified according to the manufacturer's instructions with Amylose Resin (New England Biolabs, Ipswich, MA).
MBP-p53 and its deletions were purified using the same protocol as above, except that the expression of MBP-p53 and its deletions were induced by 0.2 mm isopropyl-β-d-1-thiogalactopyranoside at 25 °C for 4 h. GST-PAD4, its deletions, and its catalytic inactive C645A mutant were expressed and purified as described previously (9).
Cell Culture and Transfection
HEK 293T and RKO cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Each cell line was cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Invitrogen) at 37 °C under 5% CO2. Transfections were performed using LipofectamineTM 2000 (Invitrogen).
Antibodies
Anti-modified citrulline (Millipore, Billerica, MA), anti-FLAG (M2, Sigma), anti-MBP (New England Biolabs), anti-PAD4 (Abcam, Cambridge, MA), anti-ING4 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-p53 (GeneTex, Irvine, CA), anti-p53 Clone DO-1 (Sigma), anti-acetyl-p53 (Lys-382) (Millipore), anti-β-tubulin (Sigma), anti-p21WAF1(EMD4 Biosciences, Calbiochem), and HRP-conjugated anti-GST (Millipore, Upstate Biotechnology) antibodies were obtained from commercial sources.
Citrullination Assays
In vitro citrullination assays were performed by incubating recombinant GST-PAD4 with MBP-ING4 or its mutants at 25 °C in 100 mm Tris-HCl, pH 7.4, containing 5 mm DTT and 2 mm CaCl2. Reactions were stopped by the addition of SDS-PAGE sample loading buffer. Samples were then subjected to electrophoresis and Western blot. Citrulline was detected by an anti-citrulline (modified) detection kit (Millipore).
For citrullination assays in HEK 293T cells, cells were co-transfected with PAD4 and FLAG-ING4 plasmids. After 24 h, cells were treated with 5 μm calcium ionophore A23187 in Locke's solution for 30 min at 37 °C. Cells were then harvested, and FLAG-ING4 was immunoprecipitated using anti-FLAG M2-agarose affinity gel (Sigma). Citrullination of immunoprecipitated ING4 was visualized as described above.
GST Pulldown Assay, Immunoprecipitation, and Western Blot Analysis
GST pulldown experiments were performed using purified proteins. Briefly, the purified GST fusion proteins were immobilized on glutathione-Sepharose 4 Fast Flow resin (GE Healthcare) and then incubated with other proteins at 4 °C for 2 h with constant shaking. After incubation, the resin was washed thoroughly four times by TBS (100 mm Tris-HCl, 150 mm NaCl, pH 7.4). Any retained proteins were then eluted by addition of 20 mm glutathione in TBS.
For immunoprecipitation and coimmunoprecipitation, cells were lysed by cell lysis buffer (Cell Signaling, Danvers, MA) containing a protease inhibitor mixture (Roche Applied Science). Target proteins were immunoprecipitated using anti-FLAG M2 affinity gel (Sigma) according to the manufacturer's instructions or using specific antibodies. Cell extracts or immunoprecipitates were subjected to Western blot using appropriate antibodies. Results of Western blots were detected and quantified using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Protein Stability Assay
Cycloheximide-inhibited protein stability assays were performed as described elsewhere (10). Briefly, HEK 293T cells were transiently transfected with the FLAG-ING4 constructs together with empty vectors or plasmid encoding the catalytically inactive C645A PAD4 mutant. At 36 h post-transfection, half-life experiments were performed using 100 μg/ml cycloheximide to inhibit protein synthesis. At different time points, cells were harvested and lysed in 1 × SDS-PAGE sample buffer. Cell lysates were boiled for 10 min and subjected to SDS-PAGE and Western blot analysis.
Detection of p53 Acetylation
Detection of p53 acetylation was performed as described previously (11). Briefly, RKO cells were transfected with pcDNA3.1, pcDNA3.1-ING4 (WT), or pcDNA3.1-Arg-NLS-Gln (ING4 mutant) and harvested after 24 h. Before harvesting, cells were treated with 5 μm trichostatin A (Sigma) for 3 h to help the detection of p53 acetylation. Cell lysate (1 mg) was then immunoprecipitated using anti-p53 clone DO-1 (Sigma) with Protein P/G PLUS-agarose (Santa Cruz Biotechnology). Normal mouse IgG was used as a negative control. After washing four times in PBS, samples were analyzed by Western blot. Expression of FLAG-ING4 and its mutant were detected by anti-FLAG antibody. Tubulin was used as a loading control. Anti-p53 (GeneTex) and anti-acetyl-p53 (Ac-p53 (Lys-382)) (Millipore) were used to detect the immunoprecipitated total p53 and the acetylation of p53 at Lys-382, respectively. Cells were treated with etoposide (20 μg/ml) for 8 h as a positive control for p53 acetylation.
RESULTS
PAD4 Citrullinates ING4 in Vitro and in Cells
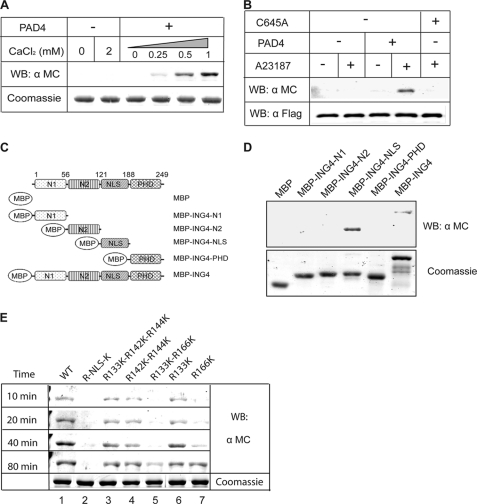
Using commercially available protein arrays (ImaGenes), we identified ING4 as a putative substrate of PAD4 (detailed results will be published elsewhere). To confirm this primary screening result in vitro, purified proteins were prepared. ING4 was cloned, and the encoded protein was expressed with an N-terminal maltose-binding protein tag (MBP-ING4). PAD4 was expressed with an N-terminal glutathione transferase tag (GST-PAD4). These purified proteins were incubated together, and any resulting citrullination was detected by chemical derivatization followed by Western blot. Purified PAD4 is shown to citrullinate ING4 in vitro in a calcium-dependent manner (Fig. 1A), verifying the initial protein array screening results.
FIGURE 1.
PAD4 citrullinates ING4. A, PAD4 citrullinates ING4 in vitro. MBP-ING4 is treated with GST-PAD4 (30 min) with or without calcium at 25 °C. Samples were Western-blotted (WB) using an anti-modified citrulline (α MC) antibody. A duplicate Coomassie-stained gel was run to indicate equal loading. B, PAD4 citrullinates ING4 in HEK 293T cells. PAD4 (or PAD4-C645A) and FLAG-ING4 plasmids were cotransfected into HEK 293T cells. Half of the cells were treated with calcium ionophore A23187 followed by immunoprecipitation using M2-agarose beads. The total amount of immunoprecipitated ING4 was probed by an anti-FLAG antibody (α FLAG) to indicate equal amounts per lane, and the corresponding citrulline content was probed by an anti-modified citrulline. They were detected on the same membrane using the two-color imaging system from Li-COR. C, shown is a schematic of ING4 deletion mutants. D, mapping the citrullinated domain of ING4 is shown. MBP-tagged deletion mutants were treated with GST-PAD4 followed by Western blot, as in A. E, PAD4 preferentially citrullinates ING4 at Arg-166. MBP-tagged ING4 and its mutants were treated with GST-PAD4. At the indicated time points, the reactions were stopped and Western-blotted as in A.
The ability of PAD4 to citrullinate ING4 in cells was also tested. Expression vectors encoding FLAG epitope-tagged ING4 (FLAG-ING4) and encoding PAD4 (or empty vector) were transiently cotransfected into HEK 293T cells. At 24 h after transfection, cells were treated with a calcium ionophore, A23187, to increase intracellular calcium concentrations because PAD4 is activated by elevated Ca2+ concentrations (12). FLAG-ING4 was then immunoprecipitated and probed for citrulline content. In the presence of PAD4 and the calcium ionophore, ING4 is clearly citrullinated (Fig. 1B). Omission of PAD4 or the calcium ionophore blocks citrullination of ING4. To confirm that the ING4 citrullination depends on PAD4 activity, a catalytically inactive mutant (C645A) with the active site Cys mutated to Ala was generated (13) and also tested. Substitution of C645A PAD4 in this assay blocks ING4 citrullination, implicating PAD4 as the citrullinating agent.
To determine which domain of ING4 is preferentially citrullinated, a series of MBP-tagged deletion mutants was prepared to separately investigate the two N-terminal domains, the NLS, and the C-terminal PHD finger (Fig. 1C). Each purified MBP-tagged domain was incubated with PAD4 and calcium in vitro and assayed for citrulline content. Citrullination of full-length ING4 and the NLS domain was clearly observed (Fig. 1D). With longer incubation time or with increased PAD4 concentration, a weak citrullination signal could also be detected in the two N-terminal domains of ING4 but not in the PHD finger (supplemental Fig. S1). To further verify that the NLS region is the preferred site of citrullination in the context of full-length ING4, all four Arg residues in the NLS region (Arg-133, Arg-142, Arg-144, Arg-166) were mutated to Lys. This quadruple mutant (Arg-NLS-Lys) blocked citrullination (Fig. 1E).
To more precisely map the citrullination sites, a series of single (R133K and R166K), double (R142K/R144K and R133K/R166K), and triple (R133K/R142K/R144K) Arg to Lys mutations were also prepared. These Lys substitutions conserve the positive charge of the side chain but are unable to be citrullinated. After short incubation times (10 min), citrulline could be detected in all of the mutants except for those that included R166K, indicating that this position is preferentially citrullinated (Fig. 1E). After longer incubation times (40 min), some citrulline could be observed in the R166K single mutant but not the R166K-R133K double mutant, suggesting that R133 may be a secondary site that is citrullinated less readily. Together, these results indicate that Arg-166 in the NLS region is the preferred site for ING4 citrullination by PAD4.
PAD4 Binds Directly to ING4, Independent of Added Calcium
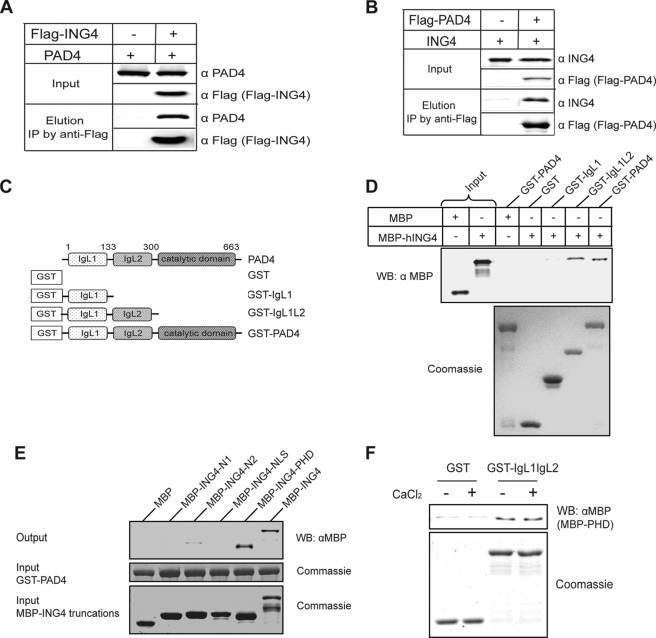
To test for a possible interaction between PAD4 and ING4, PAD4 and FLAG-ING4 were coexpressed in HEK 293T cells. PAD4 was shown to coimmunoprecipitate with FLAG-ING4 (Fig. 2A). Reciprocally, ING4 was shown to coimmunoprecipitate with FLAG-PAD4 in cells co-expressing ING4 and FLAG-PAD4 (Fig. 2B). These results demonstrate that a stable binding interaction is formed between ING4 and PAD4.
FIGURE 2.
PAD4 binds ING4, independent of added calcium. A, PAD4 coimmunoprecipitates (IP) with FLAG-ING4 from HEK 293T cells co-expressing PAD4 and FLAG-ING4. B, ING4 coimmunoprecipitates with FLAG-PAD4 from HEK 293T cells co-expressing FLAG-PAD4 and ING4. C, shown is a schematic of PAD4 deletion mutants. D, the N-terminal IgL of PAD4 was sufficient for the interaction with ING4. Immobilized on the glutathione resin, the full-length and truncations of PAD4 were used to pull down MBP-ING4. The bound MBP-ING4 was detected by Western blot (WB) using an anti-MBP antibody (α MBP). E, the PHD domain of ING4 is the major region involved in PAD4 binding. PAD4 were immobilized on the glutathione resin and incubated with full-length ING4 or its deletion mutants (Fig. 1C). The assay was performed using the same methods as D. F, the PAD4 IgL domains directly interact with the PHD domain of ING4 in a calcium-independent manner. Immobilized on the glutathione resin, GST or GST-IgL was incubated with MBP-PHD in the absence or presence of 2 mm CaCl2. The assay was performed as in D and E.
However, because both ING4 and PAD4 are also known to bind p53 (6, 11), the results above do not indicate whether the ING4-PAD4 interaction is direct or if it occurs indirectly through a mediator protein also found in cell lysates. The possibility of a direct binding between PAD4 and ING4 was tested by pulldown experiments using purified proteins. As shown in Fig. 2D, GST-PAD4 immobilized on glutathione-agarose beads was able to pull down MBP-ING4, demonstrating that a direct binding does occur between these two proteins.
To map the binding interaction in more detail, separate domains were tested in isolation. PAD4 consists of two N-terminal immunoglobulin-like (IgL) domains and a C-terminal catalytic domain that is homologous to other guanidine-modifying enzymes in the pentein superfamily (13). The N-terminal IgL domains were previously reported as the binding domains for p53 and histone deacetylase 2 (HDAC2) (6, 14), and the C-terminal domain was reported as the binding domain for histone deacetylase 1 (HDAC1) (15). To find the domain(s) that specifically interacts with ING4, two GST-PAD4 domain deletions (Fig. 2C) were prepared, immobilized on glutathione-agarose beads, and mixed with MBP-ING4. Deletion of the PAD4 C-terminal catalytic domain does not disrupt PAD4-ING4 binding, but the two IgL domains are required (Fig. 2D). Reciprocally, MBP-tagged ING4 deletions were used to find the PAD4 binding domains (Fig. 1C). Full-length MBP-ING4 and the MBP-tagged PHD finger domain were each retained by bead-bound GST-PAD4, but the other domains were not (Fig. 2E). Taken together, these results indicate that there is direct binding of the PHD finger of ING4 to the IgL domains of PAD4.
The precise mechanism by which PAD4 functions are regulated by calcium has not been fully investigated. Structural studies show that PAD4 contains three Ca2+ binding sties near the IgL domains, which show a significant conformational change upon Ca2+ removal (13). Therefore, we examined whether Ca2+ concentration regulates binding between the PHD finger of ING4 and the IgL domains of PAD4. The GST-tagged IgL1L2 domains were immobilized on glutathione resin and incubated with MBP-PHD in the presence or absence of CaCl2. The two IgL domains of PAD4 are able to pull down the PHD finger of ING4 independent of added calcium (Fig. 2F). This result contrasts with that for citrullination, which requires calcium concentrations approaching 1 mm (Fig. 1A). Therefore, the binding interaction is either not calcium-dependent or can be facilitated by trace amounts of calcium possibly carried over from the protein purification.
Mapping the Interacting Domains of ING4, PAD4, and p53
The experiments above demonstrated binding between the IgL domains of PAD4 and the PHD finger of ING4. Previous reports mapped binding of the same IgL domains to the regulatory domain (RD) of p53 (6). Because of the dimeric nature of PAD4, these IgL interactions would not necessarily be mutually exclusive in the formation of a multiprotein complex. So, the interactions between ING4 and p53 were also investigated. The NLS region of ING4 was reported as the binding region for p53 (11), but the reciprocal binding site on p53 has not been identified. Therefore, to map the ING4 binding site on p53, we constructed and purified MBP-tagged p53 deletions. The p53 protein contains an N-terminal activation domain (AD), a central DNA binding domain (DBD), and a C-terminal regulatory domain (RD) (Fig. 3A). Results from pulldown assays using these isolated domains indicate that the RD domain of p53 is essential and sufficient for ING4 binding (Fig. 3B). Based on these results, a schematic model of the interactions among PAD4, ING4, and p53 is shown in Fig. 3C. We note that this is a schematic model for illustration of the domain interactions and may not represent the complete structure in vivo.
FIGURE 3.
Interactions among PAD4, ING4, and p53. A, shown is a schematic diagram of MBP-p53 and its truncations used in the pulldown experiments. B, the RD of p53 is necessary and sufficient for the interaction with ING4. MBP-tagged domain deletions of p53 were expressed, purified, and bound to glutathione resin. GST pulldown assays were performed as in Fig. 2D. WB, Western blot. C, shown is a schematic of domain interactions among PAD4, ING4, and p53.
Citrullination of the ING4 NLS Region Disrupts p53 Binding
Previous studies have shown that p53 binds to the NLS region of ING4, and mutations in this region lead to diminished p21 expression (16). Because the NLS also contains the preferred sites for citrullination, we used four complementary methods to determine whether citrullination of the ING4 NLS region alters p53 binding.
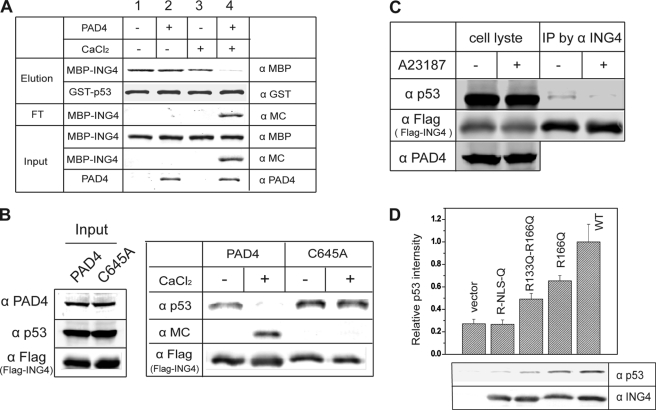
First, purified components were used in a modified pulldown assay. Immobilized GST-p53 was incubated with MBP-ING4, which was previously subjected to a variety of treatments as shown (Fig. 4A). Treatment of ING4 with Ca2+ activated PAD4 and led to the citrullination of ING4 (lane 4). Citrullinated ING4 was not retained by p53 but was instead easily eluted. As controls, the absence of PAD4, calcium, or both did not affect binding (lane 2).
FIGURE 4.
Citrullination of ING4 disrupts p53 binding. A, ING4 citrullination disrupts p53 binding in vitro. Pretreated with combinations of PAD4 and CaCl2 as indicated at top, MBP-ING4 was incubated with resin-bound GST-p53. The input, flow-through (FT), and eluted samples were analyzed by Western blots using anti-PAD4 antibody (α PAD4), anti-modified citrulline (α MC) to detect the citrulline content of ING4, anti-MBP (α MBP) to detect MBP-ING4, and anti-GST antibody (α GST) to detect GST-p53. B, citrullination of ING4 disrupts p53 binding in HEK 293T cell lysates. FLAG-ING4 together with p53 and PAD4 was immunoprecipitated from 293T cells coexpressing FLAG-ING4 and PAD4. Half of the resulting resin was treated with CaCl2 and washed, and the retained proteins were eluted and analyzed by Western blots using anti-modified citrulline, anti-FLAG antibody (α FLAG) to detect FLAG-ING4 and anti-p53 antibody (α p53). C, loss of ING4-p53 interaction in cells with activated PAD4 is shown. HEK 293T cells coexpressing PAD4 and FLAG-ING4 were treated with or without calcium ionophore A23187. FLAG-ING4 was immunoprecipitated (IP), and the coimmunoprecipitated endogenous p53 was detected by anti-p53. Anti-FLAG (α FLAG) was also probed to indicate equal loading of FLAG-ING4. D, citrulline-mimicking mutants of ING4 disrupt binding to p53. FLAG-ING4 and single (R166Q), double (R133Q/R166Q), and tetra (Arg-NLS-Gln) Arg to Gln mutants were generated and expressed in HEK 293T cells. The FLAG epitope immunoprecipitates were analyzed for endogenous p53 binding by Western blot. The ratio of band densities for p53 (α p53) and FLAG-ING4 (α FLAG) are shown and represent three independent experiments.
Second, proteins from cell lysates were used to test binding. PAD4 and FLAG-ING4 were coexpressed in HEK 293T cells. The complex FLAG-ING4-p53 was immunoprecipitated from cell lysates and subsequently treated with calcium to activate any PAD4 that had co-precipitated. After washing, proteins that were retained on the resin were then eluted and analyzed by Western blots for ING4, citrulline, and p53. The data in Fig. 4B indicate that ING4 is retained on the beads during all treatments. However, Ca2+-activated PAD4 citrullinates ING,4 and citrullinated ING4 no longer binds p53. When the inactive C645A PAD4 mutant is coexpressed with FLAG-ING4, ING4 retains its binding to p53 even after treatment with Ca2+, indicating that the dissociation of p53 from ING4 depends on PAD4 catalytic activity.
Third, the effect of ING4 citrullination on the ING4-p53 interaction within cells was tested. HEK 293T cells coexpressing PAD4 and FLAG-ING4 were treated with or without calcium ionophore. ING4 was immunoprecipitated from cell lysates through the FLAG epitope. Starting with similar levels of p53 and ING4 in lysates, the amount of p53 that coimmunoprecipitated with ING4 decreased in cells that had been treated with a calcium ionophore to activate PAD4 (Fig. 4C).
Finally, mutations of the ING4 NLS region were used to mimic specific citrullination of this region within cells. Because citrulline is not a ribosome-encoded amino acid, Arg to Gln mutations were used to replace the guanidine side chain of Arg with a neutral amide to mimic the urea side chain of citrulline. This mutation is not expected to totally mimic the effects of citrullination, but it is the substitution that best matches citrulline and has been used in previous studies (17). To target the citrullination sites identified above, three mutations were constructed; they are single mutant R166Q, double mutant R166Q/R133Q, and tetramutant Arg-NLS-Gln, which has all four Arg residues in the NLS region mutated to Gln. Wild-type FLAG-ING4 and its mutants were expressed in HEK 293T cells and then immunoprecipitated. Endogenous p53 was found to coimmunoprecipitate less efficiently as more Arg residues of the NLS region were mutated to Gln (Fig. 4D). Taken together, these four experiments confirm the interaction of the ING4 NLS region with p53 and demonstrate that citrullination of this region disrupts its interaction with p53 both in vitro and in cells.
Citrullination of ING4 Inhibits p53 Acetylation and p21 Expression
ING4 is known to enhance p53 transcriptional activity, in part by inducing acetylation of p53 at Lys-382, thereby promoting downstream p21 expression (11). The ING4-mediated up-regulation of p21 expression depends on the direct interaction between the NLS region of ING4 and p53 (16). Above, we demonstrate that PAD4-mediated citrullination of ING4 in the NLS region disrupts its binding to p53. Therefore, we hypothesized that citrullination of the ING4 NLS region down-regulates the transcriptional activity of p53 by repressing the acetylation of p53. To test this, we first compared the acetylation status of p53 in cells expressing wild-type ING4 or the citrulline-mimicking Arg-NLS-Gln ING4 mutant. Because ING4-promoted p53 acetylation has previously been reported in RKO cells (11), we chose the RKO cell line for the following assay. Endogenous p53 in RKO cells was immunoprecipitated and probed with an antibody for acetylation of Lys-382 on p53 (Fig. 5A). Overexpression of wild-type ING4 was shown to increase p53 acetylation. In contrast, overexpression of the citrulline-mimicking tetramutant ING4 showed p53 acetylation only on par with the empty-vector control. Therefore, citrulline-mimicking ING4 mutations block the ability of ING4 to promote acetylation of p53 in RKO cells.
FIGURE 5.
Citrulline-mimicking mutant of ING4 (Arg-NLS-Gln) inhibits p53 acetylation and p21 expression. A, mutant Arg-NLS-Gln blocks ING4-induced p53 acetylation. Endogenous p53 was immunoprecipitated from RKO cells expressing FLAG-ING4, Arg-NLS-Gln, or empty vector. The immunoprecipitates (IP) were probed by Western blot using an anti-acetyl Lys-382 p53 antibody (α Ac-p53 (Lys-382)) to detect p53 acetylation on Lys-382 and anti-p53 (α p53) to detect the total amount of immunoprecipitated p53. Expression of FLAG-ING4 and Arg-NLS-Gln were also tested in cell lysates using anti-FLAG (α FLAG). Anti-tubulin antibody was used for a loading control. B, citrulline-mimicking ING4 mutations block ING4 promotion of p21/waf1 expression. RKO cells expressing FLAG-ING4, Arg-NLS-Gln, or empty vector were lysed and Western-blotted using an anti-p21/WAF1 antibody (α p21), anti-FLAG (α FLAG), and anti-tubulin (α tubulin). The ratio of band densities for p21 and tubulin are shown and represent three independent experiments.
The acetylation status of p53 at Lys-382 correlates with activity and is tightly regulated by a number of different pathways including the histone acetyltransferase CBP (cAMP-response element-binding protein-binding protein)/p300 (18) and distinct HDACs including HDAC1 (19) and hSirt1 (20, 21). Because ING4 was shown to be important for the recruitment of p300 to p53, citrullination of ING4 and its subsequent loss of affinity for p53 would be expected to inhibit recruitment of p300 and the subsequent acetylation.
To further examine the effect of ING4 citrullination on p53 transcriptional activity, the expression of p21 was assayed by Western blot. In cultured RKO cells, overexpression of wild-type ING4 clearly increases p21 expression in comparison with the citrulline-mimicking ING4 mutant Arg-NLS-Gln, which displays a p21 level more similar to that of the empty-vector control (Fig. 5B). However, an alternative explanation for the observed loss of ING4-promoted p53 functions is that citrullination of the ING4 NLS region changes its compartmentalization. We eliminated this possibility by investigating the localization of wild-type and Arg-NLS-Gln ING4 mutant in HEK 293T and RKO cells. In both cell lines expression of ING4 mutant Arg-NLS-Gln remained inside the nucleus (supplemental Fig. 2). Therefore, citrullination of ING4 can block its downstream p53-mediated effects.
Earlier reports demonstrate that overexpression of PAD4 can inhibit p53 acetylation and downstream p21 expression and also demonstrate that decreased PAD4 expression or its selective pharmacological inhibition leads to increased p53 acetylation and p21 expression (6). The results described herein help to better define the mechanism of this pathway through the participation of ING4 citrullination.
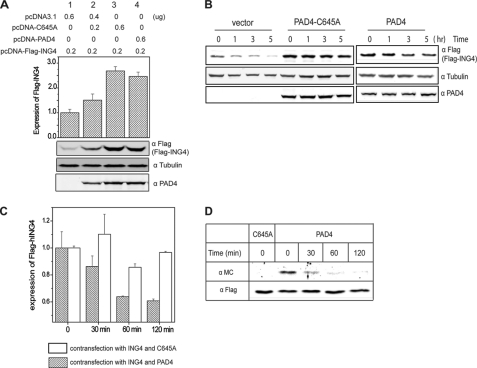
PAD4 Binding Stabilizes ING4, but Citrullination Promotes Its Degradation
The overexpressed ING4 protein has a short half-life in cells and is degraded through the ubiquitin-proteasome pathway (10). Mutations in the NLS region have been shown to promote its degradation (10). Because PAD4 preferentially citrullinates the NLS region of ING4, we investigated whether PAD4 affects levels of ING4 in cells. Wild-type PAD4 was overexpressed in HEK293T cells and shown to increase the cellular amount of ING4 as compared with an empty-vector control (Fig. 6A). Overexpression of the catalytically inactive PAD4 mutant C645A also leads to accumulation of FLAG-ING4 in a dose-dependent manner, possibly reflecting that the increased stabilization of ING4 is due to PAD4 binding, not its activity. To investigate the half-life of ING4 in more detail, cells were treated with cycloheximide to block production of new proteins, and the level of ING4 was monitored over time by Western blot. ING4 in the mock-transfected cells (empty vector) displays a shorter half-life than in cells expressing unactivated wild-type PAD4 or a catalytically inactive PAD4 mutant C645A (Fig. 6B). These results suggest that binding of PAD4 to ING4 stabilizes ING4 and protects it from rapid degradation.
FIGURE 6.
Inactive PAD4 stabilizes ING4 and citrullination of ING4 promotes its degradation in HEK 293T cells. A, cells expressing FLAG-ING4 and wild-type or C645A PAD4 (transfected at two concentrations, shown above) were analyzed by Western blot using anti-FLAG (FLAG-ING4), anti- tubulin (α tubulin), and anti-PAD4 (α PAD4). Relative band densities for FLAG-ING4 and tubulin were determined as in Fig. 5B and are shown at the top. B, inactive PAD4 slows degradation of ING4 in cells. Cells expressing FLAG-ING4 and C645A PAD4, empty vector, or wild-type PAD4 in the absence of a calcium ionophore, were treated with cycloheximide and harvested at later time points for Western blot using anti-FLAG (FLAG-ING4), anti-tubulin, and anti- PAD4. C, active PAD4 promotes ING4 degradation in cells. Cells expressing FLAG-ING4 and wild-type or C645A PAD4 were treated with 5 μm A23187, 2 mm CaCl2, and 100 μg/ml cycloheximide. Cells were harvested at later time points, and the expression of FLAG-ING4 was examined using Western blot as described in B. Data represent three independent experiments and are relative values based on the density at time zero. D, citrullinated ING4 was preferentially degraded. Cells expressing FLAG-ING4 and wild type or C645A PAD4 were treated with 5 μm A23187 in Locke's solution at 37 °C for 30 min and then changed to fresh medium containing 100 μg/ml cycloheximide. Cells were harvested at later time points, and FLAG-ING4 was immunoprecipitated. Citrullination content of ING4 was detected by anti-modified citrulline (α MC). α FLAG was also probed as the loading control.
Because citrullination of proteins is known to increase their susceptibility to proteolysis, we next investigated whether citrullination affects ING4 degradation. HEK 293T cells were cotransfected with FLAG-ING4 and wild-type or C645A PAD4 followed by the treatment with a calcium ionophore to activate PAD4 and cycloheximide to block the synthesis of new proteins. Subsequently, cells were harvested at various time points and analyzed by Western blot to determine the relative levels of FLAG-ING4 (Fig. 6C). Cells cotransfected with inactive C645A PAD4 did not show an appreciable loss of ING4 for at least 2 h after the treatment of calcium ionophore. However, cells expressing wild-type PAD4 clearly showed a time-dependent degradation of ING4 after activation by Ca2+ (Fig. 6C). To determine whether the citrullinated fraction of ING4 is particularly susceptible to degradation, total ING4 was pulled down at the indicated time points after PAD4 activation, normalized for equal loading, and then probed for citrulline content to specifically visualize the stability of the modified fraction (Fig. 6D). Samples from each time point were normalized against the level of total FLAG-ING4. Notably, the citrullinated fraction of ING4 is preferentially degraded and could not even be detected after 2 h. These results indicate that citrullination of ING4 promotes its degradation.
DISCUSSION
PAD4 is known to regulate gene expression by catalyzing the posttranslational citrullination of a number of Arg residues in histone substrates, namely histones H3 (Arg-2, -8, -17, and -26) and H4 (Arg-3) (22, 23). PAD4 can be recruited to histones by its interaction with other proteins. For example, the N-terminal IgG-like domains of PAD4 bind to the regulatory domain of p53 (6). As a result, PAD4 gets recruited to the promoters of p53-targeted genes where it citrullinates histones and represses the transcription of p53-targeted genes, notably p21. PAD4 can also citrullinate non-histone proteins implicated in carcinogenesis, like transcriptional coactivator p300 and chaperone protein nucleophosmin (NPM1) (7, 8). Citrullination of p300 is also expected to have an effect on gene regulation. The p300 protein is a component of the coactivator complex for estrogen receptor-mediated transcription. Its citrullination regulates the assembly or conformation of the coactivator complex and is thereby expected to lead to a down-regulation of transcription (8). To our knowledge there are not any other reports of non-histone protein citrullination leading to gene regulation.
In an effort to identify non-histone substrates of PAD4, high density protein arrays were used to screen for candidate substrates, and ING4 was found to be a putative PAD4 substrate. ING4 belongs to the ING type II tumor suppressor family and also plays a role in various cellular processes including p53-meidated apoptosis (24). The NLS region of ING4 binds to p53, which leads to enhanced acetylation of p53 on Lys-382 and the up-regulation of p53 transcriptional activity (11, 16). Accordingly, the expression of ING4 is tightly controlled in normal cells but is strikingly found to be consistently down-regulated in a variety of cancer types (25–28). Because both PAD4 and ING4 are known to regulate p53 activity, we sought to characterize in more detail the putative physical and functional interactions between PAD4 and ING4.
First, the binding domains of each protein were mapped. PAD4 and ING4 coimmunoprecipitate from HEK293T cell lysates, indicating that they can form a stable complex. Pulldown experiments using purified full-length and truncated proteins demonstrate a binding interaction between the N-terminal IgG-like domains of PAD4 and the PHD finger of ING4 and that binding can occur in a manner independent of added Ca2+. These interactions are added to the known domain interactions between PAD4 and p53 and are summarized in Fig. 3C.
Next, Ca2+-dependent citrullination of ING4 by PAD4 was demonstrated both in vitro and in HEK293T cells. The preferred citrullination sites were mapped to the NLS region of ING4, showing a strong preference for citrullination at Arg-133 and -166. The surrounding sequence context of these residues, 131KGRTQ135 and 164LVRTS168, is not entirely consistent with the proposed consensus site for citrullination by PAD4 because they do not contain small residues in the −2 position (29, 30). However, the NLS region of ING4 is known to be highly disordered (31, 32), which likely facilitates its use as a PAD4 substrate. The finding that PAD4 citrullinates the NLS of ING4 is significant because the same NLS region is also the site of p53 binding (16) and suggests a possible functional interaction between PAD4-mediated ING4 citrullination and p53 activation by ING4.
Therefore, we investigated the downstream effects of ING4 citrullination on its interaction with p53 both in vitro and in cells. Notably, we find that both PAD4-catalyzed ING4 citrullination and the citrulline mimicking Arg-NLS-Gln mutant of ING4 inhibit p53 binding. These modifications also antagonize the ING4 effect on p53 function, namely by inhibiting both ING4-promoted p53 acetylation and downstream p21 expression. These results help elucidate the mechanism whereby this posttranslational modification impacts gene regulation. PAD4 is not only recruited by p53 to downstream p53-targeted promoters for citrullination of histones, but it also functions by citrullination of an upstream regulatory protein, ING4, thereby inhibiting the ING4-mediated activation of p53. In general, this finding is consistent with previous examples of citrullination altering protein-protein interactions (33). In specifics, blocking p53 binding to the NLS region of ING4 also appears to be a conserved regulatory strategy. A cancer-promoting protein from Epstein-Barr virus, EBNA3C, is known to bind to the same NLS region of ING4 and is thought to competitively block p53 binding and subsequent activation (34). Apparently, the disordered NLS region provides a readily accessible motif at which to regulate ING4-p53 interactions both reversibly, in the case of EBNA3C binding, and irreversibly, as is presumed with citrullination. This finding is consistent with other examples of intrinsically disordered regions allowing multiple binding partners and posttranslational modification tuning these interactions (35).
Another noted general effect of protein citrullination is an increase in susceptibility to proteolysis (2). Therefore, we investigated the effects of ING4 citrullination on its stability in cells. Specifically, we find that Ca2+-dependent citrullination leads to a modified ING4 that has a significantly shorter half-life than unmodified ING4. Because binding is not Ca2+-dependent, these proteins may exist in a stable complex during basal cellular conditions. Upon an influx of Ca2+, possibly during apoptosis (36), PAD4 would become activated and citrullinate ING4, leading to its rapid degradation. Regulation of the ING4 effective concentration via this posttranslational modification would be expected to have a more global effect on ING4 function and also block its participation in p53-independent pathways.
It is also notable that the interactions between PAD4, ING4, and p53 represent functional cross-talk of at least three types of posttranslational modification. ING4 is a native subunit of an HBO1 histone acetyltransferase complex and contains a C-terminal conserved PHD finger that recognizes methylated histone H3 (H3K4me3) (37, 38). This interaction between ING4 and H3K4me3 promotes HBO1 acetylation of H3. In contrast, PAD4 is known to decrease the methylation of histone H3 and H4 by converting monomethyl arginine to citrulline (3, 22, 23). Additionally, PAD4 interacts with histone deacetylases (HDAC1 and HDAC2) and facilitates histone deacetylation (14, 15). In broad terms, these observations suggest that ING4 activity correlates with histone methylation and acetylation, but PAD4 activity promotes histone demethylimination and deacetylation. Our current finding that PAD4 citrullinates ING4 further contributes to its ability to inhibit histone acetylation through repression of ING4 activity.
In summary, ING4 is identified as a novel substrate of PAD4 both in vitro and in cultured cells. Protein binding occurs through the IgG-like domains of PAD4 and the PHD finger of ING4. Subsequent citrullination of ING4 occurs preferentially within the NLS region and disrupts its interaction with p53 to inhibit ING4-promoted p53 acetylation and downstream p21 expression. Citrullination also promotes degradation of modified ING4, presumably impacting its role in p53-independent pathways. To our knowledge, in addition to PAD4 citrullination of p300 (8), ING4 provides the second example of a non-histone protein whose citrullination impacts regulation of gene expression. This intriguing example of cross-talk between epigenetic citrullination, acetylation, and methylation posttranslational modifications demonstrates that the cancer-promoting activities of PAD4 that proceed through p53 are not only mediated by down-regulation of p53 activity but also by blockade of its activation.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant GM69754. This work was also supported by grants from the Robert A. Welch Foundation (F-1572) and a seed grant from the Texas Institute for Drug and Diagnostic Development (Welch Foundation Grant H-F-0032).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- PAD4
- peptidylarginine deminase 4
- NLS
- nuclear localization signal
- ING4
- inhibitor of growth 4
- IgL
- immunoglobulin-like
- CARM1
- coactivator-associated arginine methyltransferase-1
- GRIP1
- glucocorticoid receptor interacting protein-1
- MBP
- maltose-binding protein
- HDAC
- histone deacetylase
- RD
- regulatory domain
- AD
- activation domain
- DBD
- DNA binding domain
- RD
- regulatory domain
- PHD
- plant homeo domain.
REFERENCES
- 1. Tarcsa E., Marekov L. N., Mei G., Melino G., Lee S. C., Steinert P. M. (1996) J. Biol. Chem. 271, 30709–30716 [DOI] [PubMed] [Google Scholar]
- 2. Pritzker L. B., Joshi S., Gowan J. J., Harauz G., Moscarello M. A. (2000) Biochemistry 39, 5374–5381 [DOI] [PubMed] [Google Scholar]
- 3. Klose R. J., Zhang Y. (2007) Nat. Rev. Mol. Cell Biol. 8, 307–318 [DOI] [PubMed] [Google Scholar]
- 4. Jones J. E., Causey C. P., Knuckley B., Slack-Noyes J. L., Thompson P. R. (2009) Curr. Opin. Drug Discov. Devel. 12, 616–627 [PMC free article] [PubMed] [Google Scholar]
- 5. Slack J. L., Causey C. P., Thompson P. R. (2011) Cell. Mol. Life Sci. 68, 709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li P., Yao H., Zhang Z., Li M., Luo Y., Thompson P. R., Gilmour D. S., Wang Y. (2008) Mol. Cell. Biol. 28, 4745–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanikawa C., Ueda K., Nakagawa H., Yoshida N., Nakamura Y., Matsuda K. (2009) Cancer Res. 69, 8761–8769 [DOI] [PubMed] [Google Scholar]
- 8. Lee Y. H., Coonrod S. A., Kraus W. L., Jelinek M. A., Stallcup M. R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3611–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stone E. M., Schaller T. H., Bianchi H., Person M. D., Fast W. (2005) Biochemistry 44, 13744–13752 [DOI] [PubMed] [Google Scholar]
- 10. Tsai K. W., Tseng H. C., Lin W. C. (2008) Exp. Cell Res. 314, 3130–3141 [DOI] [PubMed] [Google Scholar]
- 11. Shiseki M., Nagashima M., Pedeux R. M., Kitahama-Shiseki M., Miura K., Okamura S., Onogi H., Higashimoto Y., Appella E., Yokota J., Harris C. C. (2003) Cancer Res. 63, 2373–2378 [PubMed] [Google Scholar]
- 12. Andrade F., Darrah E., Gucek M., Cole R. N., Rosen A., Zhu X. (2010) Arthritis Rheum. 62, 1630–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arita K., Hashimoto H., Shimizu T., Nakashima K., Yamada M., Sato M. (2004) Nat. Struct. Mol. Biol. 11, 777–783 [DOI] [PubMed] [Google Scholar]
- 14. Li P., Wang D., Yao H., Doret P., Hao G., Shen Q., Qiu H., Zhang X., Wang Y., Chen G., Wang Y. (2010) Oncogene 29, 3153–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denis H., Deplus R., Putmans P., Yamada M., Métivier R., Fuks F. (2009) Mol. Cell. Biol. 29, 4982–4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X., Wang K. S., Wang Z. Q., Xu L. S., Wang Q. W., Chen F., Wei D. Z., Han Z. G. (2005) Biochem. Biophys. Res. Commun. 331, 1032–1038 [DOI] [PubMed] [Google Scholar]
- 17. Bates I. R., Libich D. S., Wood D. D., Moscarello M. A., Harauz G. (2002) Protein Expr. Purif. 25, 330–341 [DOI] [PubMed] [Google Scholar]
- 18. Gu W., Roeder R. G. (1997) Cell 90, 595–606 [DOI] [PubMed] [Google Scholar]
- 19. Luo J., Su F., Chen D., Shiloh A., Gu W. (2000) Nature 408, 377–381 [DOI] [PubMed] [Google Scholar]
- 20. Vaziri H., Dessain S. K., Ng Eaton E., Imai S. I., Frye R. A., Pandita T. K., Guarente L., Weinberg R. A. (2001) Cell 107, 149–159 [DOI] [PubMed] [Google Scholar]
- 21. Luo J., Nikolaev A. Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. (2001) Cell 107, 137–148 [DOI] [PubMed] [Google Scholar]
- 22. Wang Y., Wysocka J., Sayegh J., Lee Y. H., Perlin J. R., Leonelli L., Sonbuchner L. S., McDonald C. H., Cook R. G., Dou Y., Roeder R. G., Clarke S., Stallcup M. R., Allis C. D., Coonrod S. A. (2004) Science 306, 279–283 [DOI] [PubMed] [Google Scholar]
- 23. Cuthbert G. L., Daujat S., Snowden A. W., Erdjument-Bromage H., Hagiwara T., Yamada M., Schneider R., Gregory P. D., Tempst P., Bannister A. J., Kouzarides T. (2004) Cell 118, 545–553 [DOI] [PubMed] [Google Scholar]
- 24. Unoki M., Kumamoto K., Takenoshita S., Harris C. C. (2009) Cancer Sci. 100, 1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Q. S., Li M., Zhang L. Y., Jin Y., Tong D. D., Yu Y., Bai J., Huang Q., Liu F. L., Liu A., Lee K. Y., Fu S. B. (2010) Histopathology 57, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li X. H., Kikuchi K., Zheng Y., Noguchi A., Takahashi H., Nishida T., Masuda S., Yang X. H., Takano Y. (2011) Oral. Oncol. 47, 217–223 [DOI] [PubMed] [Google Scholar]
- 27. Li M., Jin Y., Sun W. J., Yu Y., Bai J., Tong D. D., Qi J. P., Du J. R., Geng J. S., Huang Q., Huang X. Y., Huang Y., Han F. F., Meng X. N., Rosales J. L., Lee K. Y., Fu S. B. (2009) J. Pathol. 219, 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang F., Luo L. B., Tao Y. M., Wu F., Yang L. Y. (2009) Cancer Epidemiol. Biomarkers Prev. 18, 409–416 [DOI] [PubMed] [Google Scholar]
- 29. Arita K., Shimizu T., Hashimoto H., Hidaka Y., Yamada M., Sato M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5291–5296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stensland M. E., Pollmann S., Molberg Ø., Sollid L. M., Fleckenstein B. (2009) Biol. Chem. 390, 99–107 [DOI] [PubMed] [Google Scholar]
- 31. Palacios A., Moreno A., Oliveira B. L., Rivera T., Prieto J., García P., Fernández-Fernández M. R., Bernadó P., Palmero I., Blanco F. J. (2010) J. Mol. Biol. 396, 1117–1127 [DOI] [PubMed] [Google Scholar]
- 32. Culurgioni S., Muñoz I. G., Palacios A., Redondo P., Blanco F. J., Montoya G. (2010) Acta. Crystallogr Sect. F Struct. Biol. Cryst. Commun. 66, 567–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Struyf S., Noppen S., Loos T., Mortier A., Gouwy M., Verbeke H., Huskens D., Luangsay S., Parmentier M., Geboes K., Schols D., Van Damme J., Proost P. (2009) J. Immunol. 182, 666–674 [DOI] [PubMed] [Google Scholar]
- 34. Saha A., Bamidele A., Murakami M., Robertson E. S. (2011) J. Virol. 85, 2079–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schreiber G., Keating A. E. (2011) Curr. Opin. Struct. Biol. 21, 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orrenius S., Nicotera P. (1994) J. Neural Transm. Suppl. 43, 1–11 [PubMed] [Google Scholar]
- 37. Palacios A., Muñoz I. G., Pantoja-Uceda D., Marcaida M. J., Torres D., Martín-García J. M., Luque I., Montoya G., Blanco F. J. (2008) J. Biol. Chem. 283, 15956–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palacios A., Garcia P., Padró D., López-Hernández E., Martín I., Blanco F. J. (2006) FEBS. Lett. 580, 6903–6908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.