Abstract
We previously proposed a model that DALLY, a Drosophila glypican, acts as a trans co-receptor to regulate BMP signaling in the germ line stem cell niche. To investigate the molecular mechanisms of contact-dependent BMP signaling, we developed novel in vitro assay systems to monitor trans signaling using Drosophila S2 cells. Using immunoblot-based as well as single-cell assay systems, we present evidence that Drosophila glypicans indeed enhance BMP signaling in trans in a contact-dependent manner in vitro. Our analysis showed that heparan sulfate modification is required for the trans co-receptor activity of DALLY. Two BMP-like molecules, Decapentaplegic (DPP) and Glass bottom boat, can mediate trans signaling through a heparan sulfate proteoglycan co-receptor in S2 cells. The in vitro systems reflect the molecular characteristics of heparan sulfate proteoglycan functions observed previously in vivo, such as ligand specificity and biphasic activity dependent on the ligand dosage. In addition, experiments using a DALLY-coated surface suggested that DALLY regulates DPP signaling in trans by its effect on the stability of DPP protein on the surface of the contacting cells. Our findings provide the molecular foundation for novel contact-dependent signaling, which defines the physical space of the stem cell niche in vivo.
Keywords: Bone Morphogenetic Protein (BMP), Carbohydrate Function, Drosophila, Glycosaminoglycan, Heparan Sulfate, Dally, Decapentaplegic, Trans Co-receptor
Introduction
Bone morphogenetic proteins (BMPs)2 play critical roles in cell-cell communication during animal development. Remarkably, these molecules mediate both long and short range signaling dependent on context. For example, Decapentaplegic (DPP), a Drosophila homologue of BMPs, acts as a long range morphogen in the developing wing and as a contact-dependent niche factor in the female germ line stem cell (GSC) niche. The molecular basis underlying this differential activity of BMPs is not fully understood.
Signaling and distribution of BMPs in a tissue are modulated by a class of carbohydrate-modified molecules, heparan sulfate proteoglycans (HSPGs) (1–3). In addition to BMPs, HSPGs serve as co-receptors for a number of other growth factors and morphogens, including FGF, WNT, and Hedgehog (4). HSPGs generally are thought to regulate growth factor signaling on the surface of the signal-receiving cells (5). It has been reported, however, that HSPGs can regulate signaling in trans from neighboring cells in some cases (6, 7).
Recent studies demonstrated that HSPGs are essential regulators of the GSC niche in the Drosophila ovary (8, 9). Although it has been well established that DPP regulates the asymmetric division of a GSC (10), the mechanism by which this secreted molecule differentially regulates two daughter cells has been a mystery. We have found previously that DALLY, a Drosophila HSPG of the glypican type, is expressed specifically in the somatic niche cells (the cap cells) contacting GSCs and is required for GSC maintenance (8). Ectopic dally expression in somatic cells in a wide region of the germarium was sufficient to maintain all the contacting germ line cells as GSC-like undifferentiated cells, thus expanding the GSC niche. One model that accounts for the spatially restricted activation of DPP signaling in the GSC niche of wild-type Germaria is that DALLY acts as a trans co-receptor of DPP and activates its signaling specifically in a directly contacting GSC.
In this study, to validate our model and further explore the trans co-receptor function of HSPGs, we developed novel in vitro assay systems using Drosophila tissue culture S2 cells. These experimental systems demonstrated that DALLY serves as a trans co-receptor for DPP and enhances its signaling in a contact-dependent manner. This finding provides a mechanism, which defines the physical space of the niche by a coordinated action of BMPs and HSPGs. These assay systems also revealed some mechanistic aspects of BMP trans signaling. First, HS chains are critical for the trans co-receptor activity of DALLY. Second, we show that Glass bottom boat (GBB) and Dally-like protein (DLP) can also mediate signaling in trans. We observed ligand HSPG selectivity, which is consistent with previous in vivo observations. Third, DALLY shows biphasic activity; it up-regulates and down-regulates DPP signaling dependent on ligand concentration. Finally, DALLY immobilized on a slide glass disrupts DPP degradation in cells seeded on the DALLY-coated slide, suggesting that DALLY exerts enhanced activity of DPP signaling in trans by stabilizing DPP protein on the surface of the contacting cells. Thus, a combination of novel in vitro assays will provide us an opportunity to further explore the molecular mechanisms of contact-dependent BMP-HSPG signaling.
EXPERIMENTAL PROCEDURES
Fly Stocks
The detailed information for fly strains used is described in Flybase. All flies were maintained at 25 °C. Oregon R and y w were used as a wild-type strain. dallygem and dallyΔP-527 were used as loss-of-function mutant alleles of dally (11). For overexpression experiments, expression of UAS-dally-HA (12) and UAS-dlp-HA (13) was induced by apterous (ap)-Gal4.
Expression Constructs
To construct split GFP (spGFP) expression plasmids, the corresponding regions of SpGFP1–10 and SpGFP11 were amplified by PCR from ace-4p::CD4-2::spGFP1-10 and rig-3p::CD4-2::spGFP11 plasmids (gifts from C. Bargmann; Ref. 14) and subcloned into pENTR/D-TOPO (Invotrogen). The inserts were recombined into the pAW destination vector. To construct pAW-HA-dpp and pAW-dally-myc, the corresponding regions were amplified by PCR from pBRAcpA-HA-dpp (15) and pBRAcpA-dally-myc (16), respectively. To construct pAW-dlp-myc, a Myc epitope tag sequence was inserted between Phe76 and Ser77 residues of DLP by fusion PCR. The PCR fragments for HA-dpp, dally-myc, and dlp-myc were cloned into a pDONR221 vector, and the inserts were recombined into a pAW destination vector. A cDNA clone for Mad-FLAG (17) were obtained from M. O'Connor.
DPP-DALLY Cis Signaling Assay
Drosophila S2 cells were obtained from the Drosophila Genomics Resource Center. S2 cells were maintained in Schneider's medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). The cell-based DPP signaling assay was performed as described previously (15, 17), except that cells were transfected using Effectene transfection reagents (Qiagen) in serum-free Schneider's medium using a 12-well plate (1.2 ml of culture per well). S2 cells (1 × 106) were transfected with pAc-Mad-FLAG and pAW-dally-myc and incubated at 25 °C for 72 h. Control cells (without exogenous dally expression) were transfected with pAc5.1 empty vector (Invitrogen). DPP-containing conditioned medium was prepared from S2 cells expressing HA-dpp (transfected with pAW-HA-dpp 5 days before the experiment). Control conditioned medium was prepared in the same manner using cells transfected with pAc5.1. A recombinant DPP peptide (R&D Systems) was used to estimate the DPP amount in the conditioned media. The MAD-expressing cells were incubated with the conditioned medium with orbital shaking at 70 rpm. After incubation at 22 °C for 4 h, cells were lysed in SDS sample buffer. DPP signaling was assayed by immunoblot analysis using rabbit anti-pMAD antibody (EP823Y, 1:2000, Epitomics). Levels of total MAD protein were analyzed using mouse anti-FLAG antibody (M2; 1:1500, Sigma) and used as an internal control.
To confirm the specificity of DPP activity in the conditioned media, DPP-HA was depleted from the media using anti-HA antibody. An aliquot (200 μl) was collected into a siliconized tube and incubated with 0.2 μg of rat anti-HA antibody (Roche Applied Science, antibody 3F10) for 1 h at room temperature. DPP-HA was immunoprecipitated using protein G-conjugated Sepharose (Invitrogen). After immunoprecipitation, this DPP-HA-depleted conditioned medium was used for the signaling assay as described above. Control conditioned medium was prepared in the same manner using anti-Myc antibody.
Immunoblot-based Trans Signaling Assay
DNA transfection was performed as described in “DPP-DALLY Cis Signaling Assay.” To prepare the signal-receiving cells, S2 cells were transfected with pAc5.1 or pAc-Mad-FLAG. To prepare the signal-sending cells, S2 cells were transfected with pAc5.1 or pAW-HA-dpp, in combination with pAc5.1 or pAW-dally-myc. pAW-HA-gbb and pAW-dlp-myc were also used for experiments to test the activities of GBB and DLP. The sending and receiving cells were incubated separately at 25 °C for 72 h. After incubation, the cells were suspended in fresh serum-free Schneider's medium and further incubated at 22 °C for 1 h to minimize basal MAD phosphorylation. After the replacement with fresh serum-free medium, the sending and receiving cells (250 μl each) were co-cultured in a microcentrifuge tube at 22 °C for 4 h in an orbital shaking culture at 70 rpm. The cells were pelleted by a centrifugation at 200 × g, and the cell pellet was analyzed by immunoblotting as described above.
Single-cell BMP-HSPG Trans Signaling Assay
The cells were prepared as described under “Immunoblot-based Trans Signaling Assay,” except that the sending and receiving cells were also transfected with pAW-spGFP1-10 and pAW-spGFP11, respectively. Co-culture was performed by mixing the sending and receiving cells (100 μl each) in an eight-well chamber slide (LabTek) coated with poly-l-lysine. After 4 h of static culture at 22 °C, the cells were fixed in 3.7% formaldehyde and stained with anti-pMAD (EP823Y, 1:1000) and anti-FLAG (M2, 1:1000) antibodies.
Heparin Treatment
To examine the effect of heparin in the cis signaling assay, 5–500 ng/ml of heparin (Sigma) was added to the conditioned media. For the trans signaling assay, heparin was added at the start of co-culturing of the sending and receiving cells. Immunoblotting and immunohistochemical assays were performed as described above.
RNAi Treatment
Templates for double-stranded RNA synthesis were prepared by PCR with gene-specific primers containing the T7 promoter sequence at the 5′ end. The regions corresponding to 1–710 bp of ttv (tout velu) cDNA, 411–922 bp (dally RNAi 1), and 1045 to 1498 bp (dally RNAi 2) of dally cDNA were targeted. Double-stranded RNA was synthesized using a Megascript Kit (Ambion). 500 ng of double-stranded RNA was introduced into the sending cells immediately after DNA transfection and subsequently every 24 h.
Trans Signaling Assay and DPP Stability Assay using DALLY-coated Surface
DALLY was immunoprecipitated with anti-Myc affinity gel (Sigma) from the conditioned medium of S2 cells expressing a Myc-tagged secreted form of DALLY (sec-dally-myc (16)). The beads were washed once with 1 m NaCl and three times with PBS. DALLY was eluted with acid elution buffer (0.1 m glycine-HCl, pH 2.8) followed by neutralization. Eight-well chamber slides were coated with DALLY by a standard technique (18, 19). Mad-transfected cells (the receiving cells) prepared as in “Immunoblot-based Trans Signaling Assay” were seeded onto the DALLY-coated surface. After 5 h of incubation with conditioned medium containing DPP or GBB, pMAD levels were assayed by immunostaining as described above. A BSA-coated slide was used as a control.
Changes in the level of pMAD after a pulse of DPP treatment was monitored as follows. S2 cells transfected with Mad-FLAG were treated with DPP-containing conditioned medium for 30 min. After the cells were washed with fresh Schneider's medium, the cells were incubated on a BSA- or DALLY-coated glass slide. The levels of pMAD throughout the time course (0–6 h after the DPP treatment) were monitored by immunoblotting using an anti-pMAD antibody.
The cell-based DPP stability assay was performed as described previously (15) except that the cells were cultured on a glass slide coated with BSA or DALLY-Myc. S2 cells were incubated with conditioned medium containing DPP for 30 min to allow DPP to bind onto the cell surface. After unbound DPP was washed off, the DPP-HA-bound cells were seeded onto a chamber slide coated with DALLY or BSA. The levels of DPP protein after a 6-h incubation on the coated surface were analyzed by immunoblotting using an anti-HA antibody.
Microscope and Image Quantification
Samples were examined on confocal laser scanning microscopes (Nikon C1 and Zeiss lsm710). Collected images were processed by Adobe Photoshop and analyzed by ImageJ software (National Institutes of Health). For pMAD signal quantification, cells expressing MAD-FLAG were selected and classified into three groups according to the intensity of pMAD staining; <50 (arbitrary units, not detected), 50–175 (weak), and >175 (strong). Cells expressing an extremely high level of MAD-FLAG were eliminated from the quantification analysis as they tend to show nonspecific pMAD background independent of BMP treatment.
RESULTS
DALLY Enhances DPP Signaling in Drosophila S2 Cells
To examine the effect of DALLY expression on DPP signaling in vitro, we performed a cell-based signaling assay using Drosophila S2 cells (20, 21). DPP signaling activity was monitored by phosphorylation of MAD protein using an antibody against phospho-MAD (pMAD). Because endogenous levels of MAD protein in S2 cells are low, the cells were transfected with an epitope-tagged Mad cDNA (Mad-FLAG (20)). The DPP-containing conditioned medium was prepared as described under “Experimental Procedures.” We found that addition of relatively low levels of DPP (equivalent to 2 × 10−9 m of a recombinant DPP peptide, R&D Systems) to the culture medium can stimulate phosphorylation of MAD in a dose-dependent manner (Fig. 1A). The depletion of DPP-HA from the conditioned medium by immunoprecipitation with anti-HA antibody decreased pMAD levels (supplemental Fig. S2A). DPP signaling was restored by the addition of a purified DPP recombinant peptide (R&D Systems). These results confirmed that MAD phosphorylation is dependent on DPP contained in the conditioned media.
FIGURE 1.
DALLY in trans regulates DPP signaling in a contact-dependent manner. A, in vitro DPP signaling assay. S2 cells were transfected with Mad-FLAG cDNA with or without dally-myc cDNA. After 3 days, the cells were treated with undiluted (1×) or 1:10 diluted (0.1×) DPP-containing conditioned medium (supplemental Fig. S1). Control cells were treated with conditioned medium derived from a cell culture transfected with an empty vector. After the cells were incubated for 4 h at 22 °C, pMAD levels were analyzed by immunoblotting using anti-pMAD, anti-FLAG, and anti-Myc antibodies. B, DALLY in trans enhances DPP signaling in vitro. The DPP signaling activity in the receiving cells was assayed by immunoblotting using anti-pMAD antibody. The levels of MAD and DALLY were examined by anti-FLAG and anti-Myc antibodies, respectively. MAD phosphorylation was observed when the receiving cells were co-cultured with the dpp-expressing sending cells, and DPP signaling was enhanced in the presence of DALLY-Myc. C, the spGFP system in S2 cells is shown. spGFP constructs (spGFP1-10 and spGFP11), which encode two complementary portions of GFP, were expressed on the cell surface of each cell. When two cells make contact, spGFP fragments form a complex and reconstitute green fluorescence at the interface of the two cells (green). Nuclei were stained with TOPRO-3 (blue). D, the single-cell BMP-HSPG trans signaling assay is shown. The receiving cells (R) expressing Mad-FLAG and spGFP11 were co-cultured with the sending cells (S) expressing spGFP1-10 and indicated genes. DPP signaling in the receiving cells was assayed using anti-pMAD and anti-FLAG antibodies. Signals for anti-pMAD, anti-FLAG, and spGFP are shown in red, blue, and green, respectively. E, a quantification of pMAD-positive cells is shown. Bar graphs show the percentage of the receiving cells with strong (dark gray) and weak (light gray) pMad signals in the presence (GFP+) or absence (GFP−) of contact with the sending cells. The signal intensity of pMAD was determined as described under “Experimental Procedures.” Only cells with unsaturated anti-FLAG signals were scored. The number of cells scored is indicated below each bar graph (n). Error bars indicate standard error. p values were as follows: *, p < 0.05; **, p < 0.001; N.S., not significant (p > 0.05).
We found that DPP-mediated MAD phosphorylation was enhanced greatly by co-expression of DALLY (Fig. 1A). Similarly, DALLY substantially enhanced MAD phosphorylation in the presence of purified DPP (supplemental Fig. S2B), confirming the specific activity of DALLY in DPP signaling. Altogether, these results showed that DALLY expression in the signal-receiving cells enhances DPP signaling, consistent with the previous in vivo data showing DALLY serves as a co-receptor for DPP (1–3).
It has been suggested that high levels of ligand can bypass the requirement of HSPGs (22). Consistent with this idea, our previous study showed that a mutant form of DPP lacking the DALLY binding site was able to induce MAD phosphorylation in S2 cells when cells are treated with high doses of this mutant ligand (15). In the current assay system, in which a lower dosage (physiological concentrations) of DPP ligand is used, the ligand stimulates signaling in a DALLY-dependent manner. The role of DALLY in DPP signaling under high ligand concentrations will be discussed later.
DALLY Enhances DPP Signaling in Trans
By modifying the protocol described above, we next devised an assay system to evaluate the ability of DALLY to modulate DPP signaling in trans. We separately prepared “signal-receiving cells” that are transfected with a MAD cDNA, and “signal-sending cells” that are transfected with dpp and dally. Cells transfected with an empty vector plasmid (mock transfection for dpp or dally) were used as control sending cells. The sending and receiving cells were co-cultured at a density that allows the cells to contact each other. After 4 h of incubation, DPP signaling was assayed by immunoblotting using anti-pMAD antibody. Although the cell extracts were prepared from a mixture of the sending and receiving cells, the pMAD staining reflects DPP signaling specifically in the receiving cells because endogenous levels of MAD in the sending cells, which were not transfected with Mad, were negligible (supplemental Fig. S3).
Using this assay system, we observed an increase in pMAD levels in the receiving cells by co-culturing with the dpp-expressing sending cells (Fig. 1B). Co-expression of dally in the sending cells substantially elevated pMAD levels, indicating a synergistic enhancement of DPP signaling. The dally-dependent up-regulation of DPP signaling provided evidence for the ability of DALLY to enhance DPP signaling in trans in S2 cells.
We next asked how heparin affects DPP signaling in our assay systems. Addition of heparin to the nondiluted conditioned medium containing DPP-HA (1×) inhibited MAD phosphorylation in the cis signaling assay (supplemental Fig. S4A). DPP signaling in the presence of low levels of DPP-HA (0.1×) was enhanced strongly by DALLY (Fig. 1 and supplemental Fig. S4A) but not by heparin. Instead, the increase of pMAD by DALLY was reversed by heparin treatment (supplemental Fig. S4A). We also observed that heparin inhibited DPP signaling in the trans signaling assay both in the presence and absence of exogenous DALLY. Thus, in both cis and trans assay systems, heparin shows an inhibitory effect on DPP signaling.
DALLY Regulates DPP Signaling in a Contact-dependent Manner
In the immunoblot-based trans signaling assay system described above, the culture may exist as a mixed population where only a portion of receiving cells directly contact DALLY-expressing sending cells. To determine whether DPP and DALLY mediate signaling in a contact-dependent manner, it would be ideal to monitor signaling in a single cell that has (or does not have) this contact. Toward this purpose, we employed the spGFP system (14, 23). In this system, membrane contacts between two cells are detected by complementation of two split GFP proteins, spGFP1-10 and spGFP11. Previously, the membrane-bound forms of spGFP proteins have been used successfully to label membrane contact between two cells (14). To apply the spGFP system to Drosophila S2 cells, we subcloned the spGFP fragments into a S2 cell expression vector and transfected S2 cells with each construct. Fig. 1C shows that reconstitution of GFP specifically marks the interface of two contacting S2 cells.
To analyze the contact dependence of the activity of DALLY, we separately prepared the receiving cells expressing Mad and the sending cells expressing dpp and dally, as described above. These cells were also transfected with each spGFP cDNA. Subsequently, the sending and receiving cells were co-cultured at a density in which some cells directly contact each other and others do not. We monitored in situ DPP signaling using anti-pMAD antibody in a single receiving cell and visualized its contact with a sending cell by spGFPs. Consistent with the results of the immunoblot-based trans signaling assay, DPP expression in the sending cells stimulated signaling in contacting receiving cells (Fig. 1, D and E). The pMAD levels in the receiving cells were further elevated by expression of dally in the sending cells (Fig. 1, D and E). In particular, the proportion of the receiving cells with high levels of pMAD were increased substantially by dally expression in the sending cells (Fig. 1E, dark gray portions of bar graphs). This enhancement was not observed in the absence of contact between the sending and receiving cells as revealed in GFP-negative cells (Fig. 1, D and E). Thus, the single-cell DPP-HSPG trans signaling assay demonstrated that DALLY in trans potentiates DPP signaling in a contact-dependent manner. Together with the previously published in vivo analysis (8), these results establish the molecular basis for the contact-dependent control of the GSC niche.
HS Modification Is Required for Trans Co-receptor Activity of DALLY
Establishment of the novel in vitro assay systems not only demonstrated the trans co-receptor activity of DALLY but also provided us with an opportunity to elucidate the molecular mechanism of BMP-HSPG trans signaling. One important question was whether HS modification is required for the function of DALLY as a trans co-receptor. Recent studies showed that glypicans retain an unexpected level of activity without modification by glycosaminoglycan sugar chain (24, 25).
To investigate the involvement of HS in DPP-DALLY trans signaling, we tested the effect of HS depletion from S2 cells. As we have shown above, the addition of the DPP ligand alone stimulates trans signaling in S2 cells. We assumed that this signaling is supported by endogenous HSPG molecules expressed by S2 cells. RNAi knockdown of ttv, which encodes an HS copolymerase, impaired HS modification of DALLY (supplemental Fig. S5A). In the immunoblot-based trans signaling assay, this treatment blocked the DALLY-dependent phosphorylation of MAD protein (Fig. 2, A and B). Consistent with this result, the single-cell assay also demonstrated that contact-dependent DPP signaling was impaired severely by the ttv RNAi treatment (Fig. 2, C and D). These observations suggested that trans enhancement of DPP signaling by DALLY requires its modification with HS.
FIGURE 2.
HS modification is required for DALLY-mediated pMAD activation in trans. A and B, S2 cells treated with ttv RNAi were analyzed by the immunoblot-based trans signaling assay. The receiving cells expressing Mad-FLAG were co-cultured with the sending cells expressing dpp (A) or both DPP and DALLY (B). Immediately after DNA transfection, dsRNA for ttv was introduced into the sending cells. The image of immunoblotting with anti-pMAD antibody shown in A was taken by a longer exposure than that of B to visualize the effect of DPP without DALLY co-transfection. C and D, effects of ttv RNAi were assayed by single-cell BMP-HSPG trans signaling assay. C, signals for anti-pMAD and anti-FLAG antibodies and spGFP are shown in red, blue, and green, respectively. D, quantification of pMAD-positive cells in the experiment shown in C. Quantification was performed as described in Fig. 1E. RNAi knockdown of ttv blocked DALLY-dependent phosphorylation of MAD protein. R, receiving cells; S, sending cells; *, p < 0.05.
The effect of dally RNAi on DPP trans signaling also was examined. We observed a modest reduction in pMAD levels in both the immunoblot-based (supplemental Fig. S5B) and the single-cell trans signaling (supplemental Fig. S5C) assays. We have tested three different target sequences of dally mRNA, but the effect of dally RNAi in all three cases was not as strong as that of ttv. Because other HSPG core protein genes, including Syndecan (SDC), are expressed abundantly in S2 cells (26), the difference between the results of ttv and dally RNAi suggests that multiple endogenous HSPGs are involved redundantly in DPP signaling. Consistent with this idea, sdc RNAi treatment in the sending cells led to a reduction of pMAD levels (supplemental Fig. S5, B and C).
BMP-HSPG Specificity in Trans Signaling
We next asked whether this novel contact-dependent signaling is mediated by other BMP and/or HSPG molecules. For example, can another Drosophila BMP-like ligand, Glass bottom boat (GBB), also stimulate signaling in trans through HSPG co-receptors as shown for DPP? Is Dally-like protein (DLP), the second Drosophila glypican, able to serve as a trans co-receptor for these BMP ligands? To determine the relationship of the ligands and co-receptors in trans signaling activation, we assayed MAD phosphorylation using different combinations of the two BMP molecules, DPP and GBB, and the two glypicans, DALLY and DLP. Both immunoblot-based and single cell assays revealed that GBB can mediate signaling in trans like DPP (Fig. 3, A and C). Both DALLY and DLP synergistically enhanced GBB signaling (Fig. 3, A and C). On the other hand, DPP signaling was enhanced strongly by DALLY but not DLP (Fig. 3, A and B). This differential activity of the glypicans as a DPP co-receptor is consistent with previous in vivo observations that DALLY but not DLP can potentiate DPP signaling in the developing wing (Fig. 3D) (3). Thus, our in vitro assays reflect the activities and specificity that HSPG co-receptors show in vivo.
FIGURE 3.
Differential activity of DALLY and DLP in BMP trans signaling. Trans signaling was assayed using different combinations of the two ligands (DPP and GBB) and the two glypicans (DALLY and DLP), by the immunoblot-based trans signaling assay (A) and single cell assay (B and C). Quantification for the single cell assay using DPP and GBB are shown in B and C, respectively. p values are represented as in Fig. 1E. D, anti-pMAD staining of wing discs overexpressing dally and dlp in the dorsal compartment by ap-Gal4. D and V indicate the dorsal (dashed line) and ventral compartments, respectively. Expression of dally but not dlp expands the DPP activity gradient (arrows).
Biphasic Activity of DALLY in Trans DPP Signaling
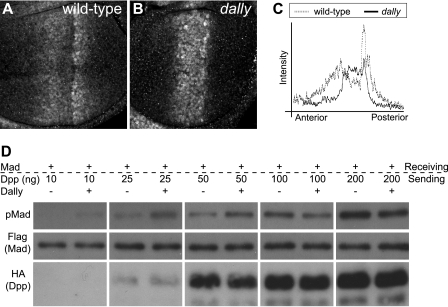
In the developing wing, DPP is produced in a stripe of cells near the anterior-posterior compartment boundary and forms a concentration gradient throughout the wing disc. Thus, the DPP ligand concentration is high near the central region of the wing disc and low in the peripheral domains. We have previously reported that pMAD levels are extremely low in most parts of dally mutant discs (Fig. 4, A–C) (2). Interestingly, however, the mutant discs show abnormally high levels of pMAD in the center of the disc. This observation has suggested that the effects of DALLY depend on the relative levels of ligand and that DALLY may play a negative role where DPP concentration is high.
FIGURE 4.
Biphasic activity of DALLY in DPP signaling. A–C, anti-pMAD staining of wild-type (A) and dally mutant (B) wing discs. Intensity plots of pMAD signals along the anterior-posterior axis in A (wild-type; dotted line) and B (dally; solid line) are shown in C. D, effects of DALLY on DPP signaling under different ligand concentrations. The sending cells were transfected with the indicated amount of dpp cDNA (ng) with or without dally. After co-culture with the receiving cells expressing Mad-FLAG, levels of pMAD and MAD-FLAG were analyzed by immunoblotting using anti-pMAD and anti-FLAG antibodies, respectively. DPP-HA in the conditioned medium was measured using anti-HA antibody.
To examine the biphasic activity of dally, we tested the effect of dally on DPP signaling under different DPP concentrations. pMAD levels increased with the amount of dpp cDNA used for transfection. Co-expression of dally in the sending cells enhanced signaling when transfected with a small amount (<50 ng) of dpp cDNA. However, DALLY showed an inhibitory effect in the presence of high levels of DPP (Fig. 4D). This negative effect was relatively modest but was observed consistently in a highly reproducible manner. Thus, the effect of trans acting DALLY on DPP signaling depends upon the dosage of DPP ligand, exhibiting the biphasic activity consistent with its in vivo behavior.
Effects of Trans Acting DALLY on DPP Protein Stability
Our previous study suggested that in the developing wing, DALLY regulates the distribution and signaling of DPP by disrupting its degradation (15). It is therefore possible that DALLY also affects the stability of DPP in trans, thus enhancing DPP signaling in the contacting cells. We previously monitored DPP degradation in vitro by a “cell-based DPP protein stability assay,” in which we traced internalization and degradation of DPP protein by S2 cells (15). However, this system is not readily applicable to assess effects of trans acting DALLY on DPP protein stability due to difficulty in distinguishing between fractions of DPP protein internalized by the sending or receiving cells. We therefore developed a sending cell-free system to assay the effect of DALLY on DPP stability in trans signaling.
We first asked whether DALLY immobilized on the slide surface can function as a trans co-receptor. The Mad-transfected receiving cells were suspended in a conditioned medium with or without DPP and seeded onto a glass slide coated with BSA or DALLY. After an incubation of 5 h, pMAD levels in the cells were assessed by immunostaining. When cells were incubated on a BSA-coated slide glass, treatment with the DPP-containing conditioned medium only slightly elevated pMAD levels compared with the cells treated with a control conditioned medium (Fig. 5, A and B). MAD phosphorylation was up-regulated substantially in the cells plated on a DALLY-coated but not a BSA-coated glass slide (Fig. 5, A and B). Similarly, GBB-mediated MAD phosphorylation was significantly enhanced by the DALLY-coated surface. This DALLY-dependent enhancement of DPP signaling showed that trans co-receptor activity of DALLY was recapitulated in this sending cell-free in vitro system. It also demonstrated that the effects of DALLY on BMP signaling observed in the above trans assay systems were induced directly by the contact with DALLY rather than secondary effects caused by dally expression in the sending cells.
FIGURE 5.
DPP trans signaling on the DALLY-coated surface. A and B, DALLY immobilized on a glass slide enhances DPP- and GBB-dependent MAD phosphorylation. Mad-FLAG-transfected cells were seeded onto a glass slide coated with BSA or DALLY in the presence of DPP or GBB. After incubation, the cells were stained with anti-FLAG (blue) and anti-pMAD (red) antibodies (A). Shown is a quantification of the pMAD levels shown in A (B). Fluorescence intensity of anti-pMAD staining was calculated using ImageJ software and was normalized by that of anti-FLAG staining. The value of the control cells (Mock, BSA) was defined as 1 arbitrary unit (AU). C, time course of pMAD levels in the cells seeded on the DALLY-coated surface. S2 cells expressing Mad-FLAG were treated with DPP-containing conditioned medium. After washing, the cells were cultured on a BSA- or DALLY-coated slide. At indicated time points, pMAD and MAD-FLAG were detected by immunoblotting. D and E, DPP stability assay using a DALLY-coated surface. S2 cells were incubated with conditioned medium (CM) containing DPP-HA for 30 min. After unbound DPP was washed off, the cells were cultured for 6 h on a chamber slide coated with BSA or DALLY. Levels of DPP-HA protein were analyzed by immunoblotting using anti-HA antibody. DPP-HA levels shown in C were quantified using the Gel Analyzing function of ImageJ software (E).
We next examined the effect of the DALLY-coated surface on the change in levels of DPP signaling during incubation. In the cells seeded on a BSA-coated control slide, pMAD levels dropped relatively quickly within 1.5 h after DPP treatment (Fig. 5C). On the other hand, pMAD gradually reduced over the time course of 0–6 h in the cells on the DALLY-coated surface. This observation suggested that DALLY potentiates DPP signaling in the contacting cells for a longer period after initial stimulation.
Using the DALLY-coated surface, we assessed the effect of trans-acting DALLY on the stability of DPP protein. After DPP-HA was bound onto the surface of S2 cells, the cells were plated onto a DALLY- or BSA-coated slide. Levels of DPP-HA were monitored by immunoblotting using anti-HA antibody (Fig. 5, D and E). Consistent with a previous report (15), nearly 50% of DPP-HA was degraded after a 6-h incubation of cells on a control slide (Fig. 5, C and D). In contrast, the rate of DPP degradation was retarded significantly when the cells were incubated on a DALLY-coated surface. These results support the idea that the trans co-receptor activity of DALLY is exerted by its effect on the stability of DPP protein on the surface of the contacting cells.
DISCUSSION
A Novel Mechanism for Contact-dependent Signaling
Several classes of secreted signaling molecules, including BMP, Wnt, and Hedgehog, act as both long range morphogens as well as short range niche factors (27–29). Interestingly, most of these molecules, if not all, are known to be HS-dependent factors, of which distribution and signal transduction are affected by HSPGs in vivo. For example, DPP regulates the cell fate as a long range morphogen in the developing wing (30) and controls differentiation of the GSCs in a contact-dependent manner in the ovary (10). Our previous study on the function of DALLY in the GSC niche suggested a novel mechanism to achieve contact-dependent signaling by complementation of a signaling receptor complex (8). In this model, receptors and co-receptors are separated between two different cell types. These molecules can “meet” and form a fully active receptor complex only on the contacting membrane at the interface of the two cells. The complementation of the receptor complex can explain the spatial regulation of the GSC niche. The current study provided evidence showing that DALLY indeed enhances BMP signaling in trans in a contact-dependent manner, strongly supporting this model on the role of DALLY in the GSC niche. Thus, differential activities of the ligands in long and short range systems can be explained by distinct functional modes of HSPG co-receptors; they function as canonical co-receptors in morphogen signaling and as trans co-receptors in the stem cell niche. The mechanism for contact-dependent signaling thus far known is communication mediated by membrane-bound ligands, such as the ligand proteins for the NOTCH receptor. The complementation of the receptor complex components provides a new paradigm of cell-cell communication.
Molecular Mechanisms for Trans Signaling
We used newly established assay systems to address several important questions regarding the trans co-receptor activity of HSPGs. As one of our mechanistic questions, we examined whether HS modification is critical for trans signaling. Recent studies have shown that in some in vivo contexts, the activities of glypicans reside in the core protein moiety (24, 25). In contrast, our analyses showed that the trans co-receptor activity of DALLY requires its HS modification. In trans signaling, glypicans would have to access and form a signaling complex with the receptor molecules on the surface of neighboring cells. This unique function needs to overcome the distance between the two cells. In addition, the configuration of HSPGs and the receptors during signaling complex formation would be different between cis and trans signaling. Therefore, it appears reasonable that long and physically flexible HS chains play an essential role in the trans co-receptor activity of glypicans.
Our in vitro systems reproduce several conditions previously reported in vivo. First, the S2 cell systems showed that DPP signaling was significantly up-regulated by DALLY but not DLP. In fact, this specificity has been observed in the developing wing (Fig. 3D) (3). Thus, ligand molecules selectively utilize a specific HSPG as a co-receptor both in vitro and in vivo. A recent study of mammalian glypicans also supports the idea that glypican core proteins show the specificity in their activity to regulate cellular response to Hh (31). Second, the effect of DALLY on DPP signaling was dependent on the DPP ligand dosage; it enhances signaling when DPP concentration is low and represses it in the presence of excess amount of DPP. This biphasic activity also is consistent with in vivo observations (Fig. 4, A and B) (2). Mutation of dally impairs DPP signaling in most parts of the wing disc but increases it near the source of DPP. Similar biphasic activity has been observed in Crossveinless 2 and DLP, which are proposed to act as “exchange factors” (25, 32, 33). Collectively, these observations indicated that our in vitro systems devised in this study recapitulate well the molecular events involved in BMP-HSPG signaling in living animals. These novel assay systems will provide us an opportunity to elucidate the molecular mechanisms by which contact-dependent signaling is regulated in the stem cell niche or in other contexts.
DALLY does not have to be expressed in live cells to exert its trans co-receptor activity: DALLY immobilized on the slide surface can enhance DPP signaling in cells that have direct contact with the surface. This fact shows that DALLY presented on the surface of the sending cells is sufficient to increase BMP signaling in the receiving cells and exclude the involvement of secondary effects caused by dally expression in the sending cells. Using this sending cell-free system, we demonstrated that DALLY slows down the degradation rate of DPP protein in the receiving cells. Given that receptor-mediated endocytosis is a major route for BMP degradation (34, 35), association with HSPGs on the neighboring cells may disrupt this process. Our results, however, do not exclude other possible mechanisms for the ability of DALLY to potentiate BMP signaling in trans. A recent study has shown that HS enhances BMP signaling by facilitating the recruitment of type II receptor subunits to the ligand type I receptor complex (36). In fact, our results showed that the effect of the DALLY-coated substrate was more significant in signaling output (pMAD) compared with that in the ligand levels (Fig. 5). Thus, it is possible that DALLY regulates BMP signaling at multiple steps, such as ligand stability and receptor oligomerization.
The addition of heparin in culture showed inhibitory effects on DPP signaling both in the cis and trans assays. This is consistent with previous reports showing that heparin can inhibit BMP signaling (37–39). As glypican overexpression enhances BMP signaling when the ligand concentration is low, we examined a range of conditions with different DPP and heparin concentrations. In all of the conditions we tested, heparin shows only a negative effect. One possible explanation for this observation is that heparin in the conditioned medium competes with the cell surface HSPGs and sequesters the DPP ligand. It also suggests the possibility that glypican core protein and/or HS fine structures may play an important role in the enhancement of DPP signaling. The contribution of core protein and structural specificity of HS in trans BMP signaling remains to be elucidated.
Supplementary Material
Acknowledgments
We are grateful to C. Bargmann, S. Selleck, M. O'Connor, the Developmental Studies Hybridoma Bank, and the Bloomington Stock Center for reagents. We thank S. Stringer and A. Kleinschmit for helpful discussions and critical reading of the manuscript.
This work was supported in part by National Institutes of Health Grant R01 HD042769. This work was also supported by the American Heart Association (to H. N.) and a Uehara Memorial Foundation Postdoctoral Fellowship (to K. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S5.
- BMP
- bone morphogenetic protein
- HSPG
- heparan sulfate proteoglycan
- DPP
- Decapentaplegic
- GBB
- Glass bottom boat
- DLP
- Dally-like protein
- GSC
- germ line stem cell
- spGFP
- split GFP.
REFERENCES
- 1. Jackson S. M., Nakato H., Sugiura M., Jannuzi A., Oakes R., Kaluza V., Golden C., Selleck S. B. (1997) Development 124, 4113–4120 [DOI] [PubMed] [Google Scholar]
- 2. Fujise M., Takeo S., Kamimura K., Matsuo T., Aigaki T., Izumi S., Nakato H. (2003) Development 130, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 3. Belenkaya T. Y., Han C., Yan D., Opoka R. J., Khodoun M., Liu H., Lin X. (2004) Cell 119, 231–244 [DOI] [PubMed] [Google Scholar]
- 4. Kirkpatrick C. A., Selleck S. B. (2007) J. Cell Sci. 120, 1829–1832 [DOI] [PubMed] [Google Scholar]
- 5. Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. (1991) Cell 64, 841–848 [DOI] [PubMed] [Google Scholar]
- 6. Kramer K. L., Yost H. J. (2002) Dev. Cell. 2, 115–124 [DOI] [PubMed] [Google Scholar]
- 7. Jakobsson L., Kreuger J., Holmborn K., Lundin L., Eriksson I., Kjellén L., Claesson-Welsh L. (2006) Dev. Cell. 10, 625–634 [DOI] [PubMed] [Google Scholar]
- 8. Hayashi Y., Kobayashi S., Nakato H. (2009) J. Cell Biol. 187, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Z., Wang Z. (2009) Development 136, 3627–3635 [DOI] [PubMed] [Google Scholar]
- 10. Xie T., Spradling A. C. (1998) Cell 94, 251–260 [DOI] [PubMed] [Google Scholar]
- 11. Nakato H., Futch T. A., Selleck S. B. (1995) Development 121, 3687–3702 [DOI] [PubMed] [Google Scholar]
- 12. Tsuda M., Kamimura K., Nakato H., Archer M., Staatz W., Fox B., Humphrey M., Olson S., Futch T., Kaluza V., Siegfried E., Stam L., Selleck S. B. (1999) Nature 400, 276–280 [DOI] [PubMed] [Google Scholar]
- 13. Kirkpatrick C. A., Dimitroff B. D., Rawson J. M., Selleck S. B. (2004) Dev. Cell. 7, 513–523 [DOI] [PubMed] [Google Scholar]
- 14. Feinberg E. H., Vanhoven M. K., Bendesky A., Wang G., Fetter R. D., Shen K., Bargmann C. I. (2008) Neuron 57, 353–363 [DOI] [PubMed] [Google Scholar]
- 15. Akiyama T., Kamimura K., Firkus C., Takeo S., Shimmi O., Nakato H. (2008) Dev. Biol. 313, 408–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeo S., Akiyama T., Firkus C., Aigaki T., Nakato H. (2005) Dev. Biol. 284, 204–218 [DOI] [PubMed] [Google Scholar]
- 17. Shimmi O., Umulis D., Othmer H., O'Connor M. B. (2005) Cell 120, 873–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bunch T. A., Brower D. L. (1992) Development 116, 239–247 [DOI] [PubMed] [Google Scholar]
- 19. Maeda N., Noda M. (1996) Development 122, 647–658 [DOI] [PubMed] [Google Scholar]
- 20. Ross J. J., Shimmi O., Vilmos P., Petryk A., Kim H., Gaudenz K., Hermanson S., Ekker S. C., O'Connor M. B., Marsh J. L. (2001) Nature 410, 479–483 [DOI] [PubMed] [Google Scholar]
- 21. Shimmi O., O'Connor M. B. (2003) Development 130, 4673–4682 [DOI] [PubMed] [Google Scholar]
- 22. Häcker U., Lin X., Perrimon N. (1997) Development 124, 3565–3573 [DOI] [PubMed] [Google Scholar]
- 23. Ghosh I., Hamilton A. D., Regan L. (2000) J. Am. Chem. Soc. 122, 5658–5659 [Google Scholar]
- 24. Kirkpatrick C. A., Knox S. M., Staatz W. D., Fox B., Lercher D. M., Selleck S. B. (2006) Dev. Biol. 300, 570–582 [DOI] [PubMed] [Google Scholar]
- 25. Yan D., Wu Y., Feng Y., Lin S. C., Lin X. (2009) Dev. Cell. 17, 470–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasevayuth K., Yanagishita M. (2004) Biochem. Biophys. Res. Commun. 324, 205–211 [DOI] [PubMed] [Google Scholar]
- 27. Kornberg T. B., Guha A. (2007) Curr. Opin. Genet. Dev. 17, 264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nusse R., Fuerer C., Ching W., Harnish K., Logan C., Zeng A., ten Berge D., Kalani Y. (2008) Cold Spring Harb. Symp. Quant. Biol. 73, 59–66 [DOI] [PubMed] [Google Scholar]
- 29. Watabe T., Miyazono K. (2009) Cell Res. 19, 103–115 [DOI] [PubMed] [Google Scholar]
- 30. Nellen D., Burke R., Struhl G., Basler K. (1996) Cell 85, 357–368 [DOI] [PubMed] [Google Scholar]
- 31. Williams E. H., Pappano W. N., Saunders A. M., Kim M. S., Leahy D. J., Beachy P. A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 5869–5874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serpe M., Umulis D., Ralston A., Chen J., Olson D. J., Avanesov A., Othmer H., O'Connor M. B., Blair S. S. (2008) Dev. Cell. 14, 940–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan D., Wu Y., Yang Y., Belenkaya T. Y., Tang X., Lin X. (2010) Development 137, 2033–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jortikka L., Laitinen M., Lindholm T. S., Marttinen A. (1997) Cell Signal. 9, 47–51 [DOI] [PubMed] [Google Scholar]
- 35. Miller A. F., Harvey S. A., Thies R. S., Olson M. S. (2000) J. Biol. Chem. 275, 17937–17945 [DOI] [PubMed] [Google Scholar]
- 36. Kuo W. J., Digman M. A., Lander A. D. (2010) Mol. Biol. Cell. 21, 4028–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takada T., Katagiri T., Ifuku M., Morimura N., Kobayashi M., Hasegawa K., Ogamo A., Kamijo R. (2003) J. Biol. Chem. 278, 43229–43235 [DOI] [PubMed] [Google Scholar]
- 38. Irie A., Habuchi H., Kimata K., Sanai Y. (2003) Biochem. Biophys. Res. Commun. 308, 858–865 [DOI] [PubMed] [Google Scholar]
- 39. Jiao X., Billings P. C., O'Connell M. P., Kaplan F. S., Shore E. M., Glaser D. L. (2007) J. Biol. Chem. 282, 1080–1086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.