Abstract
Jarid2/Jumonji critically regulates developmental processes including cardiovascular development. Jarid2 knock-out mice exhibit cardiac defects including hypertrabeculation with noncompaction of the ventricular wall. However, molecular mechanisms underlying Jarid2-mediated cardiac development remain unknown. To determine the cardiac lineage-specific roles of Jarid2, we generated myocardial, epicardial, cardiac neural crest, or endothelial conditional Jarid2 knock-out mice using Cre-loxP technology. Only mice with an endothelial deletion of Jarid2 recapitulate phenotypic defects observed in whole body mutants including hypertrabeculation and noncompaction of the ventricle. To identify potential targets of Jarid2, combinatorial approaches using microarray and candidate gene analyses were employed on Jarid2 knock-out embryonic hearts. Whole body or endothelial deletion of Jarid2 leads to increased endocardial Notch1 expression in the developing ventricle, resulting in increased Notch1-dependent signaling to the adjacent myocardium. Using quantitative chromatin immunoprecipitation analysis, Jarid2 was found to occupy a specific region on the endogenous Notch1 locus. We propose that failure to properly regulate Notch signaling in Jarid2 mutants likely leads to the defects in the developing ventricular chamber. The identification of Jarid2 as a potential regulator of Notch1 signaling has broad implications for many cellular processes including development, stem cell maintenance, and tumor formation.
Keywords: Cardiac Muscle, Development, Gene Knock-out, Gene Regulation, Genetics, Heart, Transcription Factors, Transcription Repressor, Transcription Target Genes
Introduction
The heart is the first organ to form and function during embryogenesis. Improper formation of the heart leads to congenital heart defects, which are the most common form of human birth defects (1, 2). The formation of the heart is a complex process that requires delicate spatial and biochemical interactions among various cell types. Despite extensive studies to determine the transcriptional regulation of cardiac development, the precise mechanisms of ventricular chamber development remain largely unknown. Mice with a homozygous Jarid2 deletion (Jarid2 KO) exhibit cardiac defects mimicking human congenital cardiac defects including ventricular septal defects (VSD),2 double outlet right ventricle (DORV), and hypertrabeculation associated with noncompaction of the ventricular wall resulting in a thin compact layer (3, 4). These mutant mice survive until birth and offer the unique opportunity to further explore the molecular mechanisms of development and late stage maturation of the ventricular chamber.
Jarid2 is the founding member of the Jumonji family of proteins, all of which contain the JmjC domain that was first defined based on amino acid similarities between Jarid2, Jarid1C (Smcx), and Jarid1A (RBP2) (5–7). Proteins containing the JmjC domain generally function as histone demethylases (8). Intriguingly, Jarid2 contains mutations at key amino acids necessary for enzymatic function and is highly likely enzymatically inactive (9–11). Recent evidence suggests that Jarid2 plays critical roles in the regulation of gene expression, cell growth, and balancing ES cell pluripotency and differentiation (9–14). However, the underlying molecular defects leading to cardiac malformations in Jarid2 mutant hearts during ventricular chamber development remain to be elucidated.
Myocardial trabeculation refers to the process by which the endocardium induces the underlying cardiomyocytes to migrate and proliferate to form the finger-like projections in the heart. Trabeculation plays important roles in the development of the myocardial trabecular layer and the compact layer of the ventricular wall and is critical for normal cardiac contractility in the developing embryo. In addition, adult hypertrabeculation with noncompaction of the ventricular myocardium leading to a thin ventricular wall constitutes a recently defined class of cardiomyopathy in humans (15, 16). There is an orchestrated balance of cells undergoing proliferation, migration, and differentiation to form the normal trabecular and compact layer. However, little is known about the underlying molecular pathways involved in this process. A recent report proposed that trabeculation is a finely controlled event occurring between embryonic day (E) 9.5 and E14.5 in the developing mouse. Mice lacking endothelial Brg1, a chromatin remodeler, fail to develop proper trabeculation (17) because of premature degradation of the cardiac jelly, the extracellular matrix between the myocardium and endocardium.
Mice lacking endothelial Notch1 also exhibit impaired trabeculation (18). Upon Notch1 binding to its ligand, the activated Notch1 intracellular domain (N1ICD) translocates into the nucleus and interacts with the RBPJK/CBF/Su(H) transcriptional repressor (19), converting it into an activator of target genes that include hairy/enhancer of split (HES, HRT1, and Hey1), hairy/enhancer of split-related with YRPW motif (HESR and HRT2) (20), CyclinD1 (21, 22), and EphrinB2 (23). EphrinB2 is a transmembrane ligand of EphB receptor tyrosine kinases. Whole body or endothelium-specific deletion of EphrinB2 leads to embryonic lethality at E9.5 because of a complete failure of myocardial trabeculation (24, 25). EphrinB2 is required for proper expression of Nrg1 (neuregulin 1) (18) that is expressed in the endocardium. Deletion of Nrg1 results in embryonic lethality by E10.5 and impaired myocardial trabeculation (26, 27). Nrg1 binds to the ErbB family of receptor tyrosine kinases expressed in the myocardium. Deletion of either ErbB2 (28) or ErbB4 (29) in the mouse results in embryonic lethality at E10.5 with impaired myocardial trabeculation. Activation of the ErbB receptors leads to activation of multiple signaling pathways (30–32). Other proteins required for proper trabeculation include FKBP12 (33) and Bmp10 (bone morphogenetic protein 10) (34). Although multiple genes have been implicated in the trabeculation process, the precise molecular pathways involved are unknown.
We set out to identify Jarid2-dependent molecular pathways critical for cardiac chamber development focusing on the formation of the trabecular and compact layers of the ventricle. To delineate lineage-specific roles of Jarid2, we generated lineage-specific knock-outs of Jarid2 in the myocardium, endothelium, epicardium, and cardiac neural crest cells. Interestingly, only an endothelial deletion of Jarid2 recapitulated cardiac defects observed in the whole body mutant. In this study, we demonstrate that Notch1 is a potential target of Jarid2 in the endocardium of the developing ventricle. Deletion of Jarid2 dysregulates Notch1 expression at crucial developmental times, which in part leads to a hypertrabecular phenotype accompanied by noncompaction of the ventricular wall.
EXPERIMENTAL PROCEDURES
Microarray Analysis
Microarray was performed using a Mus musculus whole mouse genome array (4X44K; Agilent Technologies). RNA was isolated from two pooled wild type or Jarid2 KO E17 ventricles, and microarray analyses were performed three times. The data from the scan file were analyzed using Edge3 software (35). Following analyses, the up-regulated genes were sorted into biological pathways using DAVID functional analyses (36, 37).
Quantitative Real Time PCR
To perform quantitative real time PCR (qRT-PCR), hearts were snap frozen in TRIzol® reagent (Invitrogen), while genotyping was performed as described (3, 38). Each heart was treated as an individual sample. Following RNA isolation, reverse transcription was performed using oligo(dT)20 and SuperScript® III reverse transcriptase (Invitrogen). cDNA was used to perform qRT-PCR using Power SYBR® Green on an Applied Biosystems 7300 real time PCR system. All of the experiments were performed in duplicate, and an n > 4 was used to generate all data using the standard curve method. All of the samples were standardized to RNA polymerase II, except E15.5 hearts that were standardized to hypoxanthine guanine phosphoribosyl transferase (HPRT). The following primers from Integrated DNA Technologies were used for qRT-PCR: RNA polymerase II forward, 5′-CGAATCCGCATCATGAACAG-3′; RNA polymerase II reverse, 5′-TGATGTCTTCCTGCGATGCA-3′; HPRT forward, 5′-ATTGGTGGAGATGATCTCTCAACTTT-3′; HPRT reverse, 5′-TTTCCCTGGTTAAGCAGTACAGC-3′; Notch1 forward, 5′-GCCTTCGTGCTCCTGTTCTT-3′; Notch1 reverse, 5′-AGCACCATCTGAGGCATTCT-3′; Notch4 forward, 5′-GAGGACCTGGTTGAAGAATTGATC-3′; Notch4 reverse, 5′-GGGATAAAAGGGGAAAAACTGCA-3′; Delta4 forward, 5′-GACCTGCGGCCAGAGACTT-3′; Delta4 reverse, 5′-AAACTCTCTCATCAGCCAAATCATC-3′; Nrg1 forward, 5′-TGTCACCCAGACTCCTAGTCACA-3′; Nrg1 reverse, 5′-ACGTCATCGGTAGAGAACAGCA-3′; Nrg4 forward, 5′-AATCTGTCGGCAGCTTTCGT-3′; Nrg4 reverse, 5′-ACCTTCAGAGGGCCAGTTCA-3′; Jagged1 forward, 5′-TCAGAGGCGTCCTCTGAAAAA-3′; Jagged1 reverse, 5′-AGTGGCTTGGGTCTGTTGCT-3′; ErbB2 forward, 5′-GTACAGTGAGGATCCCACATTACCT-3′; ErbB2 reverse, 5′-CAGAGGTTCGGCCTCAGTCT-3′; ErbB4 forward, 5′-CAATGCATGACAAGCCCAAA-3′; ErbB4 reverse, 5′-AGAGAACCCTTTTGTGTCCCG-3′; Hey1 forward, 5′-ATCACCTGAAAATGCTGCACAC-3′; Hey1 reverse, 5′-GGAAAGGTTATTTTGACGCGC-3′; Hey2 forward, 5′-GGACGAGACCATCGACGTG-3′; and Hey2 reverse, 5′-CCTGGGCACGCTACAAGC-3′.
Section in Situ Hybridization, Histology, and Immunodetection
Section in situ hybridization was performed as described previously (39). The sections were imaged on a Zeiss Axiovert 200, and images were captured using a Zeiss AxioCam.
cDNAs used for generation of the digoxigenin-labeled riboprobes were prepared using the DIG RNA labeling kit (Roche Applied Science). The probes used were from mouse sequences and were Notch1 (nucleotides 3776–4639 (40), GenBankTM accession number NM_008714), Nrg1 (nucleotides 1185–1570, GenBankTM accession number NM_178591), and for ANF and αMHC as described (3).
Hematoxylin and eosin staining was done as described (3). Fluorescent immunostaining was performed on paraffin-embedded sections as per the protocol on the Cell Signaling Technology website. Antibodies used for fluorescent immunostaining were anti-NIC-1 (41) and anti-ErbB2/4 (Santa Cruz, sc-284). Jarid2 staining was performed using the Jarid2 P-100 antibody (38, 42) with the TSATM-Plus cyanine 3/cyanine 5 system (PerkinElmer Life Sciences).
Western blotting was performed on heart tissue as described previously (38). Images were scanned and densitometry was performed using National Institutes of Health ImageJ. Primary antibodies used were anti-Cleaved Notch1 (Val-1744) (2421S; Cell Signaling Technology), anti-ErbB4 (sc-283; Santa Cruz), and anti-β-actin (ab6276; Abcam).
Quantitative ChIP (qChIP)
qChIP experiments were performed as described previously (43). Primers were designed to amplify 50–150-bp amplicons. The products were measured using SYBR green fluorescence (ABI Prism 7300; Power SYBR® Green Master Mix; Applied Biosystems) in 15-μl reactions. The amount of product was determined relative to a standard curve of input chromatin. Dissociation curves showed that PCRs yielded a single product. All of the experiments were repeated in duplicate at least three times. qChIP was performed using 10 pooled E17 wild type mouse hearts with preimmune serum or Jarid2 P-100 specific antibody and on three pooled control or Jarid2en E17 hearts using Jarid2 P-100 antibody. For amplification of the Notch1 locus, the following primers from Integrated DNA Technologies were used: −4.5 kb forward, 5′-TTTAAATGGCCCTGAGCAAGA-3′; −4.5 kb reverse, 5′-AAATTGCCCAAACCAGAGACA-3′; −500 bp forward, 5′-GCAGCTTTCCTTTCCCACAA-3′; −500 bp reverse, 5′-TTTGGCCAGAATTTGCATTTC-3′; +350 bp forward, 5′-GAGGAGGACCTTTCTCTTTCCA-3′; +350 bp reverse, 5′-TTGGGCGCTATGAGAAAAGTG-3′; +1150 bp forward, 5′-CTGGAGATGCCTGCGAACA-3′; +1150 bp reverse, 5′-AGACCTGCCCCGCCTACT-3′; +1750 bp forward, AACCAAGCCTGACCTCTCTCTTC-3′; and +1750 bp reverse, 5′-CACTTGGCTGGGAGCATCTC-3′.
Reporter Gene Assay
A Notch reporter plasmid containing the Jarid2 interaction region based on ChIP assays was constructed (Fig. 6D, N+1750) by subcloning a region of the Notch1 locus from −500 bp of the transcriptional start site to +1750 bp into the pGL3 basic vector (Promega). A reporter gene without the Jarid2 interaction region was constructed by subcloning a region from −500 to +350 bp into the pGL3 basic vector (Fig. 6D, N+350). HEK293 cells in a 24-well plate were transfected with 25 ng of a reporter gene without or with 50 or 100 ng of pCMV2-FLAG-Jarid2 (44) along with 10 ng of β-galactosidase-CMV vector using Lipofectamine and Plus reagent (Invitrogen). Luciferase assays were performed 24 h post-transfection using the luciferase assay system (Promega) and normalized to β-galactosidase activity. The assays were repeated four times in duplicate.
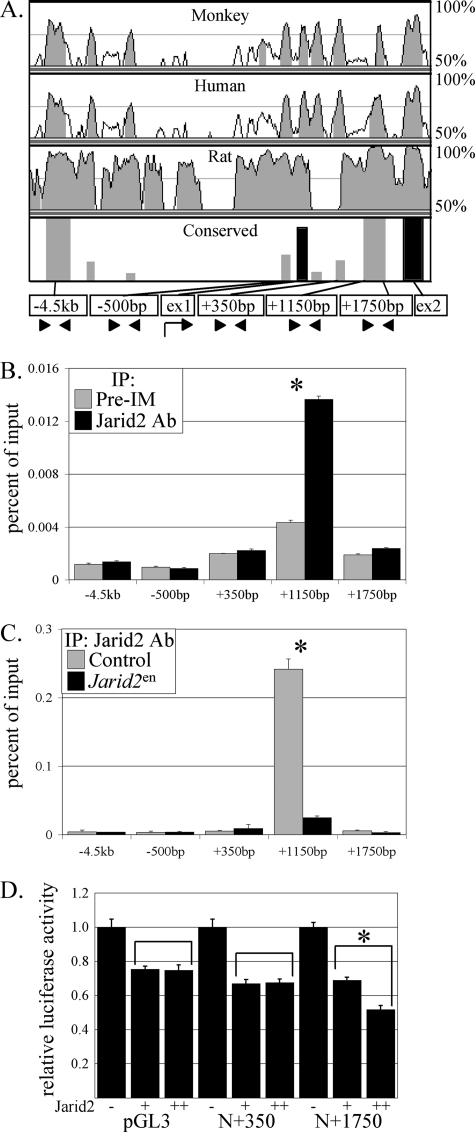
FIGURE 6.
Jarid2 occupies a specific site at the Notch1 locus. A, a VISTA alignment was performed on the Notch1 locus for mouse, monkey, human, and rat to determine conserved regions. In the bottom panel, gray bars indicate regions with greater than 75% sequence conservation, whereas black bars indicate highly conserved exon regions. Arrowheads indicate primer sites in conserved regions. B, Jarid2 occupies a specific region at +1150 bp of the Notch1 locus. qChIP was performed four times on 10 pooled E17 wild type mouse hearts using either preimmune (Pre-IM, gray bar) or a Jarid2 specific antibody (Jarid2 antibody, black bar). IP, immunoprecipitation. C, Jarid2 does not accumulate at the +1150-bp region of the Notch1 locus when Jarid2 is deleted in the endocardium. qChIP was performed three times on three pooled E17 control (gray bar) or Jarid2en (black bar) mouse hearts using a Jarid2 antibody. D, luciferase reporter gene assay. pGL3, pGL3-Notch1 −500 to +350 (N+350), and pGL3-Notch1 −500 to +1750 (N+1750) were transfected into HEK293 cells without or with two doses of Jarid2. The asterisk indicates a significant difference when p values are less than 0.01 from a Student's paired t test, and the error bars represent the standard error of the mean.
Animal Husbandry and Genotyping
All of the mice were housed in accordance with University of Wisconsin Research Animal Resource Center policies. To generate Jarid2 KO mice, Jarid2 male and female heterozygous (Jarid2 +/−) mice were mated (3). For all conditional deletion of Jarid2, Jarid2F/+; Cre/+ males were mated with Jarid2F/F females. All of the mice employed for this study were bred to a mixed 129/Svj and C57BL/6 background, and genotyping was performed as described previously (3, 38).
RESULTS
Tissue-specific Deletion of Jarid2
Jarid2 KO mice display cardiac defects including VSD, DORV, and hypertrabeculation associated with noncompaction of the ventricular wall, resulting in a thin compact layer (3, 4, 45). However, genetic ablation in the whole body is insufficient for determining protein function in specific cell type(s). Jarid2 is expressed in multiple cell types in the heart including the cardiac neural crest cells, epicardium, myocardium, and endocardium (3, 46, 47). Therefore, Jarid2 was deleted in multiple cell lineages using Cre-loxP technology to delineate the lineage-specific roles of Jarid2 in the developing heart. Our Jarid2 floxed mice (Jarid2F/F) that exhibited a functional loxP allele (38) were crossed with various Cre mice.
We used Tie2-Cre mice (Jackson Laboratory), an endothelium-specific Cre, to study the function of Jarid2 specifically in the endocardium of the heart (Jarid2en). Tie2 is expressed as the first endothelial cells arise, and expression continues in all endothelial cells throughout development and life (48–50). Tie2-Cre mice have been widely employed to study endocardium-specific functions of genes by many groups (17, 18, 51). To delete Jarid2 in the epicardium, the Wt1-Cre mice (Jarid2epi) were used. Wt1 (Wilms' tumor 1) is a tumor suppressor protein that has an epicardial specific expression pattern in the heart (52, 53). Wt1-Cre mice were generated using a recombineering strategy to insert an IRES/EGFP-CRE cassette immediately downstream of the Wt1 stop codon in a BAC clone to express Cre in the epicardial lineage beginning at the proepicardial stage.3 Both αMHC-Cre mice (54) and Nkx2.5-Cre mice (55) (Jarid2MHC-myo or Jarid2Nkx-myo) were used to delete Jarid2 specifically in the myocardium. Nkx2.5 is expressed earlier than αMHC, and αMHC becomes more active later in embryogenesis and in the adult mouse heart. Finally, the cardiac neural crest-specific Wnt1-Cre mice (Jarid2cnc) (56) were used to generate a neural crest deletion of Jarid2.
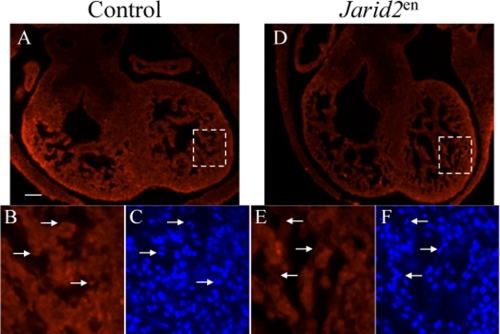
To generate various conditional knock-out mice, female Jarid2F/F mice were mated with male Jarid2F/+; Cre/+ mice. We examined mice from E8.5 through postnatal day 21 to determine the Mendelian ratio (Table 1). Only an endothelium-specific deletion of Jarid2 resulted in a significantly lower than expected Mendelian ratio at all of the stages examined (6.9% instead of the expected 25%). In all of the other mutants generated, there was no significant deviation from the expected Mendelian ratio, and no gross morphological defects were observed by hematoxylin and eosin staining (data not shown). These mice were able to reproduce normally and showed no increased lethality under normal living conditions. We have previously shown that Jarid2 is efficiently deleted in Jarid2MHC-myo mice (38). To ensure that Jarid2 was efficiently deleted in the endocardium of Jarid2en mice, immunostaining was performed using a Jarid2 antibody. Although Jarid2 is expressed in the myocardium, Jarid2 is efficiently deleted in the endocardium of Jarid2en (Fig. 1, D and E) mice as compared with control (Fig. 1, A and B). These results suggest that Jarid2 is functionally important in the endocardium of the heart.
TABLE 1.
Mendelian percentage of mutants using various Cre lines
Jarid2F/F female mice were mated with different Jarid2F/+; Cre/+ male mice to generate endothelial, epicardial, myocardial, and neural crest-specific deletions of Jarid2F/F; Cre/+. The expected Mendelian ratio of Jarid2F/F; Cre/+ mice is 25% according to our breeding scheme. Only mice with an endothelial specific deletion of Jarid2 showed a significant reduction in the expected Mendelian ratio.
| Cre mouse | Number of pups examined | Percentage of mutants |
|---|---|---|
| Endothelial | ||
| Tie2 | 1006 | 6.9 |
| Epicardial | ||
| WT1 | 34 | 26 |
| Myocardial | ||
| αMHC | 66 | 21.2 |
| Nkx2.5 | 48 | 29.8 |
| Neural crest | ||
| Wnt1 | 65 | 23.1 |
FIGURE 1.
Jarid2 is deleted in the endocardium of Jarid2en mice. A and B, E12.5 Jarid2F/F mouse heart displays Jarid2 expression in the myocardium and endocardium (arrows) as indicated by red fluorescent staining. C, Hoechst staining to indicate nuclei. B and C, the arrows indicate the nuclei of endocardial cells positive for Jarid2 staining. D and E, Jarid2en mice display a lack of red fluorescent staining in the endocardium, indicating a lack of Jarid2 expression. F, Hoechst staining to indicate nuclei. E and F, the arrows indicate the nuclei of endocardial cells negative for Jarid2 staining. The scale bar in A represents 100 μm.
Endothelium-specific Deletion of Jarid2 Partially Recapitulates Cardiac Defects of Jarid2 KO Mice
Because only the Jarid2en mice showed a significant decrease in Mendelian ratio, we set out to determine whether they displayed any of the cardiac defects that characterize the Jarid2 KO mice. As previously reported, Jarid2 KO mice display cardiac defects including VSD, DORV, hypertrabeculation associated with noncompaction of the ventricular wall where endocardial cells invaginate deeper into the compact layer.
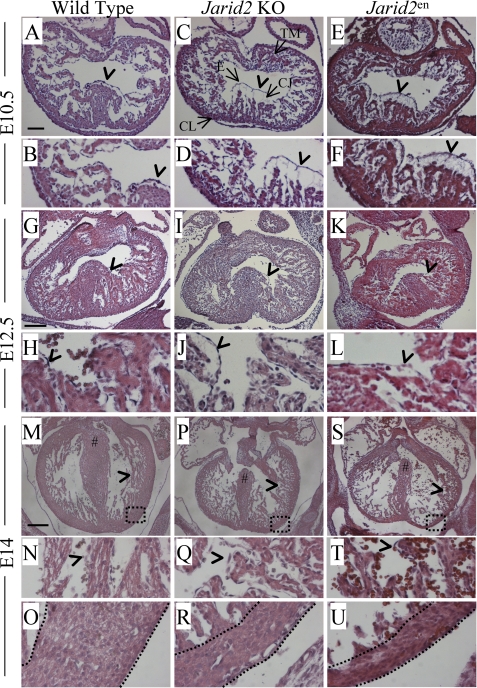
At E10.5, Jarid2 KO and Jarid2en mice exhibit similar defects (Fig. 2). In wild type E10.5 embryos, trabeculation that begins approximately at E9.5 progresses, and the endocardium is normally separated from the underlying myocardium by cardiac jelly. However, both the Jarid2 KO and Jarid2en hearts display abnormal phenotypes. Both Jarid2 KO and Jarid2en hearts have an increase in the number of trabecular projections (Fig. 2, C and E) as compared with the wild type (Fig. 2A). Further, the Jarid2 KO and Jarid2en hearts show increased distance between the endocardium and myocardium (Fig. 2, D and F, arrowheads) as compared with the wild type (Fig. 2B, arrowhead).
FIGURE 2.
Morphological analysis of Jarid2 mutants. Cardiac transverse sections were hematoxylin and eosin stained at various stages of development. A–F, E10.5 wild type (A and B), Jarid2 KO (C and D), and Jarid2en (E and F) sections. B, D, and F are higher magnifications of A, C, and E, respectively. G–L, E12.5 wild type (G and H), Jarid2 KO (I and J), and Jarid2en (K and L) sections. H, J, and L are higher magnifications of G, I, and K, respectively. M–U, E14 wild type (M–O), Jarid2 KO (P–R), and Jarid2en (S–U) sections. N, Q, and T are higher magnifications of M, P, and S, respectively. O, R, and U show the boxed regions in M, P, and S at a higher magnification. # sign indicates fused septum (M) or VSD (P and S). The spaces between dotted lines indicate compact layers (O, R, and U). The arrowheads indicate endocardium. In C, TM, trabecular myocardium; E, endocardium; CJ, cardiac jelly; CL, compact layer. The scale bars in A, G, and M represent 100, 200, and 300 μm, respectively.
In the wild type mouse heart at E12.5, trabeculation continues but slows, and the space between the myocardium and endocardium decreases. However, in both the Jarid2 KO and Jarid2en hearts, the trabecular projections appear to be increased (Fig. 2, I and K) as compared with the wild type (Fig. 2G). Finally, the space between the endocardium and myocardium is increased in the Jarid2 KO and Jarid2en hearts (Fig. 2, J and L, arrowheads) as compared with wild type (Fig. 2H, arrowhead).
At E14 in the wild type heart, the interventricular septum has fused to the endocardial cushion, and the right ventricle is separated from the left ventricle (Fig. 2M, indicated by #). Also, the cardiac jelly has been degraded, and the endocardium is in direct contact with the myocardium (Fig. 2, M and N, arrowheads) signaling the end of trabeculation. However, in both Jarid2 KO and Jarid2en hearts, the interventricular septum fails to fuse with the endocardial cushion leading to ventricular septal defects (Fig. 2, P and S, indicated by #). Also in Jarid2 KO and Jarid2en hearts, the endocardium is not in direct contact with the myocardium, and trabeculation continues (Fig. 2, P, Q, S, and T, arrowheads). In the wild type heart at E14, a thickened compact layer was observed (Fig. 2, M and O), but in both Jarid2 KO and Jarid2en hearts, the compact layer fails to thicken (Fig. 2, P, R, S, and U), and the endocardium is deeply invaginated into what little compact layer is present as indicated by the space between dotted lines in Fig. 2 (O, R, and U; see also Fig. 4, J and K).
FIGURE 4.
Identification of genes dysregulated in Jarid2 mutant mice. A–C, qRT-PCR was performed on wild type and Jarid2 KO hearts at E10.5 (A), E12.5 (B), and E15.5 (C) for Notch1, Nrg1, Nrg4, Jagged1, ErbB2, ErbB4, Notch4, Hey1, Hey2, and Delta4. For all qRT-PCR, at least three hearts for each stage were used, and qRT-PCR was performed in duplicate. An asterisk indicates a significant difference when p values are less than 0.05 from a Student's paired t test, and error bars represent the standard error of the mean. D–K, section in situ hybridization was performed using DIG-labeled Notch1 riboprobes on wild type and Jarid2 KO mice at E10.5 (D–G) and E12.5 (H–K). Jarid2 KO mice exhibit higher levels of Notch1, and arrows indicate deeper invagination of endocardium into compact layer in Jarid2 KO heart (K) as compared with wild type (J). rv, right ventricle; lv, left ventricle. L–O, section in situ hybridization was performed using DIG-labeled Nrg1 riboprobes on wild type (L and N) and Jarid2 KO (M and O) at E13.5. Jarid2 KO mice exhibit higher levels of Nrg1. rv, right ventricle; lv, left ventricle. P–S, section in situ hybridization was performed on E13 control (P and R) and Jarid2en (Q and S) mice using a DIG-labeled Notch1 riboprobe. Jarid2en mice exhibit higher levels of Notch1 than a littermate control. The scale bars in D, H, L, and P represent 200 μm.
Analysis of the cardiac defects observed in the Jarid2en mice revealed that not all phenotypes are fully penetrant (Table 2). This could be due to contribution from other cell lineages where Jarid2 functions normally such as the myocardium or epicardium. We cannot exclude the possibility that there is an incomplete Cre-mediated deletion of Jarid2 at the floxed locus. However, this is unlikely because we have observed efficient deletion of Jarid2 in Jarid2MHC-myo mice (38) and Jarid2en (Fig. 1, D and E) mice. Phenotypes for Jarid2 KO mice are not listed, because all Jarid2 KO mice display complete penetrance of the phenotypes discussed (3). These results strongly suggest that the defects observed in Jarid2 KO and Jarid2en mice are likely due to the lack of Jarid2 in the endocardium. Because Jarid2en mice partially recapitulate the phenotypic defects observed in Jarid2 KO mice, the myocardial defects observed, such as hypertrabeculation and noncompaction of the ventricular wall, are unlikely to be cardiomyocyte-autonomous. Because the myocardial defects are likely due to improper signaling from the endocardium to the myocardium, we investigated the molecular pathways that are dysregulated in the endocardium of Jarid2 KO mice.
TABLE 2.
Cardiac phenotypic defects observed in Jarid2en mice
Jarid2en mice at various stages were examined by H&E staining on transverse sections. Jarid2en mice exhibited cardiac defects including VSD, noncompaction of the ventricular compact layer and hypertrabeculation, which recapitulated defects observed in Jarid2 KO mice.
| Genotype | Number analyzed | VSD | Noncompaction | Hypertrabeculation |
|---|---|---|---|---|
| Wild type | ||||
| E9.5–E12.5 | 7 | N/A | N/A | 0 |
| E13.5–E15.5 | 9 | 0 | 0 | 0 |
| Mutant | ||||
| E9.5–E12.5 | 11 | N/A | N/A | 10 |
| E13.5–E15.5 | 11 | 7 | 8 | 9 |
Jarid2en Mice Recapitulate the Abnormal Gene Expression Observed in Jarid2 KO Mice
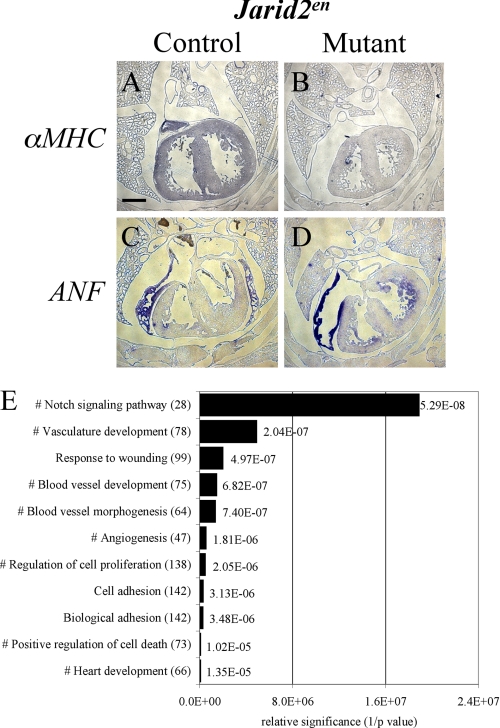
The observation that only an endocardial deletion of Jarid2 recapitulates the cardiac defects in whole body jarid2 KO mice led us to investigate the dysregulation of endocardium-specific factors as the cause of the phenotypic defects. First, we examined whether Jarid2en mice showed similar defects in cardiac gene expression as observed in Jarid2 KO mice (3). We have previously demonstrated that Jarid2 KO mice failed to up-regulate αMHC in the ventricle and failed to repress ANF in the trabecular myocardium at E17 (3). Jarid2en mice also showed a similar dysregulation of both αMHC (Fig. 3, A and B) and ANF (Fig. 3, C and D) as compared with littermate control, suggesting that Jarid2en mice not only recapitulate morphological defects but also display the same defects in myocardial gene expression present in Jarid2 KO mice.
FIGURE 3.
Dysregulated pathways in Jarid2 mutant hearts. A–D, in situ hybridization was performed using DIG-labeled αMHC (A and B) and ANF (C and D) riboprobes on E17.5 control (A and C) and mutant Jarid2en (B and D) mice. The scale bar in A represents 500 μm. E, analyses of all up-regulated genes by DAVID indicate that the Notch signaling pathway is the most significantly up-regulated pathway in Jarid2 KO versus wild type hearts at E17. The numbers in parentheses represent the number of up-regulated genes in the pathway, and # indicates pathways where Notch1 is up-regulated. The p values from DAVID are listed next to the bars. The x axis is relative significance based on inverse p values (1/p).
Jarid2 KO Mice Display Higher Expression of Notch1 and Downstream Targets in the Heart
To delineate the pathways affected in Jarid2 mutants, we employed a candidate gene approach evaluating genes associated with defective trabecular formation. Because Jarid2en mice partially recapitulate the defects observed in Jarid2 KO mice including hypertrabeculation and thin myocardium and because Jarid2 acts as a transcriptional repressor (42, 44, 57), we hypothesized that critical endocardial genes would be up-regulated in the endocardium of the Jarid2 KO heart. Further, myocardial genes would be dysregulated because of the increased endocardial signaling. A large number of control and mutant mice are required to screen candidates using in situ hybridization, qRT-PCR, and immunostaining. Because of the limited number of Jarid2en mice recovered and partial penetrance of cardiac defects, we employed the Jarid2 KO mice that are more efficiently obtained (25% versus 6.9%) and display full penetrance. We examined members of many pathways known to be important in cardiac chamber development. These candidates included members of the Notch pathway that are crucial for trabeculation (58).
Simultaneously, to determine possible targets of Jarid2 critical for ventricular myocardium development at late stages, microarray was performed on wild type or Jarid2 KO ventricles at E17. Because Jarid2 acts as a transcriptional repressor (4, 42, 44, 57, 59), we chose to focus on genes that were consistently up-regulated in Jarid2 KO ventricles from all three arrays performed. All of the up-regulated genes were analyzed using DAVID functional analysis software online (36, 37). Interestingly, the most significantly up-regulated pathway based on gene ontology of biological pathways (GOTERM_BP_FAT) was the Notch signaling pathway (Fig. 3E; see supplemental Table S1 for a complete list of genes). Further, Notch1 is included in other significantly up-regulated pathways involved in heart and blood vessel development. Notch1 mutant mice display little or no trabeculation (18), whereas Jarid2 KO mice have a hypertrabecular phenotype. Therefore, we hypothesized that the hypertrabeculation could be due to an increase in the Notch signaling pathway in Jarid2 KO mice.
To determine dysregulated genes in Jarid2 KO mouse hearts, qRT-PCR was performed on control or Jarid2 KO hearts at various stages of development. We examined whether components of the Notch pathway were dysregulated in Jarid2 KO mice during development, including Notch1, Notch4, Delta4, Jagged1, Nrg1, Nrg4 (Neuregulin 4), ErbB2, ErbB4, Hey1, and Hey2. Notch1 and its ligand Delta4 are significantly elevated in Jarid2 KO mouse hearts at E10.5 (Fig. 4A). In Jarid2 KO mouse hearts at E12.5, Notch1 continues to be significantly elevated, and the levels of Nrg1 are also significantly elevated (Fig. 4B). At E15.5 in Jarid2 KO mouse hearts, Notch1, Delta4, Nrg1, Nrg4, ErbB4, and Hey1 are all significantly up-regulated (Fig. 4C), which likely represents a failure of Notch1 down-regulation in mutant hearts. Based on both candidate approach and microarray analyses, our data demonstrate that Notch1 is consistently up-regulated at all stages examined in Jarid2 KO mice, indicating that Notch1 is a potential target gene of Jarid2.
Because Jarid2 is expressed in both the endocardium and myocardium, we investigated whether the elevation in Notch1 was endocardium-specific. In situ hybridization on sections of wild type and Jarid2 KO mice showed that Notch1 levels are elevated specifically in the endocardium in the Jarid2 KO mouse. Notch1 is normally expressed in all endocardial cells at E10.5 in the wild type heart (Fig. 4, D and F). However, in the Jarid2 KO heart (Fig. 4, E and G), Notch1 expression is higher, and trabeculation is increased as compared with the wild type.
Notch1 continues to be expressed in the normal developing ventricular chamber at E12.5 and is restricted to the endocardium (Fig. 4, H and J). However, levels of Notch1 are elevated in the Jarid2 KO mouse (Fig. 4, I and K). Again, there is more trabeculation in the mutant hearts, and the invagination of the endocardial cells is deeper in the Jarid2 KO mouse (Fig. 4, J and K, arrows). The dashed lines in Fig. 4 (J and K) represent the epicardium of the heart, which is difficult to see because of the lack of a counterstain.
Notch1 signaling is required for the expression of the Ephrins (Ephrin B2/B4), which in turn are necessary for Nrg1 expression (18). Nrg1 is a secreted endocardial molecule that binds to myocardial cells expressing the receptors ErbB2/ErbB4, which are involved in normal cardiomyocyte proliferation and differentiation (60). Overexpression of ligands of ErbB2/ErbB4 results in an increase in proliferation of the cardiomyocytes (61, 62). We therefore examined whether Nrg1 is up-regulated in Jarid2 KO mice. Nrg1 is up-regulated in the endocardium of Jarid2 KO mice (Fig. 4, M and O) as compared with wild type mice (Fig. 4, L and N) at all of the stages examined (Fig. 4, A–C).
Our data suggest that elevated endocardial Notch1 and Nrg1 lead to increased signaling to the myocardium possibly via ErbB receptors, thereby yielding a hypertrabecular phenotype. To confirm that the elevation in Notch1 was in fact due to endothelial deletion of Jarid2, in situ hybridization was performed on Jarid2en mice and a littermate control. Indeed, Jarid2en mice displayed higher levels of Notch1 in the endocardium (Fig. 4, Q and S) as compared with a littermate control (Fig. 4, P and R). This directly links the deletion of Jarid2 in the endocardium to the morphological defects observed in the myocardium.
To confirm that the increases in mRNA levels correlate to increases in protein expression, immunohistochemistry and Western blotting experiments were performed. Notch1 protein in the endocardium was also elevated in the Jarid2 KO mouse heart at both E10.5 (Fig. 5D) and E12.5 (Fig. 5H) as compared with the wild type (Fig. 5, C and G). N1ICD protein levels were also examined by Western blotting to examine activated Notch1. N1ICD levels are significantly higher in the hearts of Jarid2 KO mice versus wild type mice where N1ICD is expressed at a very low level (Fig. 5, M and N). Finally, we examined whether the elevation in endocardial proteins of Notch signaling resulted in an increase in the myocardial protein expression downstream of Notch1 signaling. Immunostaining showed increased ErbB2/4 levels in Jarid2 KO hearts as compared with wild type (Fig. 5, L and K). Also, levels of ErbB4 were significantly increased in Jarid2 KO hearts versus wild type by Western blotting (Fig. 5, O and P).
FIGURE 5.
Notch1 and ErbB2/4 are elevated in Jarid2 KO hearts. A–H, immunostaining for Notch1 was performed on wild type and Jarid2 KO mice at both E10.5 (C and D) and E12.5 (G and H). The arrows indicate higher levels of Notch1 in the endocardium of Jarid2 KO mice (D and H) as compared with wild type mice (C and G). Hoechst staining was done to stain for nuclei (A, B, E, and F). I–L, immunostaining for ErbB2/4 was performed on Jarid2 KO (L) and wild type mice (K) at E15.5. Jarid2 KO mice display higher levels of ErbB2/4 in the myocardium versus wild type. Hoechst staining was performed to stain nuclei (I and J). M–P, Western blotting was performed on E14.5 wild type or Jarid2 KO hearts for N1ICD (M and N; n = 3) or ErbB4 (O and P; n = 5). Protein levels were standardized to β-actin. An asterisk indicates a significant difference when p values are less than 0.05 from a Student's paired t test, and error bars represent the standard error of the mean.
Our results suggest that elevated levels of the endocardium-specific Notch1 and Nrg1 genes induce up-regulation of myocardial factors including the ErbB receptors. Our data demonstrate Notch1 as an early endocardium-specific gene to be consistently up-regulated at all stages examined in Jarid2 KO mice, indicating that Notch1 is a potential direct target gene of Jarid2 in the endocardium of the developing ventricle. This is significant because our data reveal a critical role of Jarid2 in the ventricular endocardium. Endocardial expression of Jarid2 is crucial for normal regulation of the Notch pathway and for normal myocardial trabeculation. This also provides further supporting evidence that proper cardiac development is dependent on cross-talk between the endocardial and myocardial layers.
Jarid2 Occupies a Specific Region of the Notch1 Locus
To determine whether Jarid2 controls Notch1 expression, qChIP analysis was performed to examine Jarid2 occupancy at the endogenous Notch1 locus. VISTA alignments compare nucleotide sequences of various species and score them based on sequence conservation. High sequence conservation is often indicative of important functions. To determine conserved regions of the Notch1 locus among different species, a VISTA alignment was performed using the Notch1 genomic sequences of mouse, human, monkey, and rat (Fig. 6A) specifically from 10 kilobases upstream of the Notch1 transcriptional start site to 10 kilobases downstream of the Notch1 transcriptional stop site (∼70 kilobases). The alignment revealed more than 20 conserved regions throughout the Notch1 locus. To determine whether Jarid2 occupies these regions, qChIP was performed using mouse embryonic heart tissue and a Jarid2 antibody on a broad region (data not shown). Jarid2 occupancy was only detected at one highly conserved region at +1150 bp relative to the transcriptional start site (Fig. 6B). This region is in intron 1 of the Notch1 gene, and exon 1 contains both the transcription and translation start sites. Thus, Jarid2 occupies a previously unknown regulatory region at the endogenous Notch1 locus, suggesting that Jarid2 directly controls Notch1 expression. To confirm that deletion of Jarid2 in the endocardium results in a loss of accumulation of Jarid2 at the Notch1 locus, qChIP was performed using hearts from Jarid2en hearts or littermate controls at E17. Jarid2 only accumulates at the +1150-bp region of the Notch1 locus when Jarid2 is expressed in the endocardium (Fig. 6C).
To determine the functional consequence of Jarid2 binding to the Notch1 locus, the Notch1 reporter genes were transfected into HEK293 cells. Jarid2 significantly inhibits the Notch1 reporter gene in a dose-dependent manner when the site of Jarid2 occupancy is present (Fig. 6D, N+1750). Jarid2 displays a nonspecific, non-dose-dependent repression of the pGL3 basic reporter and Notch1 deletion reporter that does not contain the site of Jarid2 occupancy (Fig. 6D, N+350). These data suggest that Jarid2 occupancy at the Notch1 locus is critical for transcriptional repression of Notch1.
DISCUSSION
Jarid2 KO mice display cardiac defects including DORV, VSD, hypertrabeculation, and noncompaction of the trabecular layer associated with a thin compact layer (3, 4, 45). However, the cell lineage where Jarid2 is important for function has not been elucidated. Using Cre-loxP technology, we conditionally deleted Jarid2 in the endothelium, myocardium, epicardium, and cardiac neural crest cells. Only those mice with an endothelium-specific deletion of Jarid2 displayed a decrease in expected Mendelian ratio as well as a partial recapitulation of cardiac defects observed in the Jarid2 KO mouse. Therefore, we dissected the endocardium-specific pathways that Jarid2 regulates during embryonic development.
The Notch1 pathway controls many important processes including normal organ development and tumor formation (63). Deletion of Notch1 in the mouse results in embryonic lethality because of cardiac insufficiency (18, 64). The hearts of Notch1 mutants have impaired trabeculation and myocardial proliferation. However, little is known about how Notch1 is regulated in the developing embryo. We demonstrate for the first time that Jarid2 directly controls Notch1 expression through occupancy of a previously unidentified region within intron 1 of the Notch1 locus. Failure to regulate Notch1 expression as is the case in Jarid2 KO mice leads to proliferation and differentiation defects in the developing heart. These defects are manifested in the form of hypertrabeculation and noncompaction of the myocardium associated with a thin ventricular wall. Noncompaction cardiomyopathy has been recently considered to be a genetic cardiomyopathy in humans (16).
Based on the data presented along with published data, our working model is that Jarid2 normally regulates Notch1 during the trabeculation process in the heart. Jarid2 represses the Notch signaling pathway in the wild type mouse heart at later stages of cardiac development (Fig. 7A). However, the absence of Jarid2 causes the dysregulation of Notch1, leading to both increased and prolonged Notch1 expression (Fig. 7B). The increased Notch1 expression leads to elevated Nrg1 possibly via EphrinB2 and EphrinB4 as previously reported (18). The increased Nrg1 signaling causes an increase in the ErbB2/B4 signaling in the myocardium that leads to increased trabeculation. Therefore, failure to terminate Notch1 expression is likely involved in increased trabeculae and defective compaction in the absence of Jarid2.
FIGURE 7.
Proposed model of how endocardial Jarid2 expression regulates myocardial development. A, in the wild type mouse at later stages of development, Jarid2 represses Notch1 by binding to the Notch1 +1150-bp locus. Notch1 signaling to the myocardium ceases, and there is a decrease in trabecular proliferation and normal terminal differentiation. B, in the Jarid2 KO mouse at later stages of development, Jarid2 does not bind to the +1150-bp Notch1 locus, and Notch1 remains active. The prolonged activation of the Notch1 pathway results in increased trabecular proliferation and defective terminal differentiation.
However, other potential mechanisms might also be relevant. Proper regulation of the amount of cardiac jelly is required for proper trabeculation (17). The amount of cardiac jelly is controlled in part by a chromatin modifying complex, the Brg1-associated factor complex in the endocardium. It is interesting that we observe an increased space between the endocardium and myocardium in Jarid2 KO hearts, possibly suggesting the persistent presence of cardiac jelly. Thus, Jarid2 might have a role in the regulation of Brg1-associated factor or as yet unidentified endocardial factors.
Deletion of Notch1 or members of the Notch1 pathway including RBPJk (65), Nrg1 (26), ErbB2 (28), and ErbB4 (29) results in mice that die at E10.5. These embryos display little to no trabeculation. Notch1 and RBPJk mutants also exhibit a reduction in Bmp10 expression, which is required for proper trabeculation. Bmp10 knock-out mice also die at E10.5 with a lack of trabeculation (34). FKBP12-deficient mice display a hypertrabeculation and noncompaction phenotype along with elevated Bmp10 in the myocardial layer of the heart (34).
Jarid2 is a transcriptional repressor that regulates cardiac factors including ANF (44) and αMHC (57). Jarid2 represses cyclinD1 expression, and the deletion of Jarid2 leads to an increase in cyclinD1 in the myocardium (4, 59). However, the up-regulation of cyclinD1 in Jarid2 KO mice could also be linked to Notch1 overexpression. Notch1 activates Nrg1 in the endocardium, which is then secreted, and binds ErbB2/4 in the myocardium leading to an increase in cyclinD1 activation (66). Therefore, the up-regulation of cyclinD1 is unlikely a cardiomyocyte cell autonomous phenomenon because myocardial deletion of Jarid2 does not recapitulate a hypertrabecular phenotype.
The result that Jarid2 directly regulates Notch signaling has significance beyond cardiac development. Jarid2 is required for balancing maintenance and differentiation of stem cells including embryonic and induced pluripotent stem cells (67, 68). Reactivation of Notch signaling causes embryonic stem cell-derived and neonatal ventricular cardiomyocytes to re-enter the cell cycle (69, 70). Notch1 is also re-expressed in a number of types of diseases including cancer (71). However, the mechanisms by which Notch expression is regulated remain to be elucidated.
Transcriptional regulation involves the modification of numerous residues within the histone tails. Because lysine residues can be un-, mono-, di-, or tri-methylated, there can be multiple functional consequences resulting from the methylation status of a single lysine residue. The discovery of the Jumonji factor family, all of which contain a JmjC domain, revealed a novel class of enzymes that catalyze demethylation. Although Jarid2 is enzymatically inactive (9–11), increasing evidence suggest that Jarid2 plays critical roles in cellular processes via histone modification. We have shown that Jarid2 interacts with Zkscan17 (Nizp1 and Zfp496) (14), which interacts with Nsd1 (nuclear receptor-binding SET domain protein 1), a histone methyltransferase (72). Jarid2 has recently been shown to interact with members of the polycomb repressor complex to modulate the methylation of histone H3K27 (H3 lysine 27) in stem cells (9–11, 13, 73). Recruitment of G9a and GLP by Jarid2 results in accumulation of H3K9me1 and H3K9me2 at the cyclin D1 promoter in cultured cells (74). Methylation of H3K9 and H3 lysine 27 is generally associated with heterochromatin formation and gene silencing (75–77). It would be interesting to hypothesize that Jarid2 regulates Notch1 expression through histone lysine methylation. Therefore, further investigation into the methylation status at the Notch1 locus as well as the molecular partners of Jarid2 is required.
It is not clear why a small percentage of Jarid2en mice survive to adulthood. Because an endothelial deletion of Jarid2 is not sufficient for complete penetrance and recapitulation of all of the phenotypic defects observed in Jarid2 KO mice, these mice survive. To determine whether cardiac defects observed in Jarid2 KO mice resulted from a combination of defective signals from multiple cell lineages, it would be interesting to generate and characterize compound lineage-specific Jarid2 KO mice.
It is largely unknown how trabeculation and compaction of the ventricular wall normally take place in the developing mouse heart. However, we present strong evidence that the interplay between the endocardium and myocardium is extremely important for proper trabecular formation in the ventricle. The deletion of Jarid2 in the endocardium causes initial dysregulation of endocardial genes that eventually leads to defective expression of myocardial genes. The finding that Jarid2 directly regulates the Notch1 pathway holds far-reaching implications for many developmental and disease pathways.
Supplementary Material
Acknowledgments
We deeply thank Drs. John Burch and Andrew Wessels for generously providing the Wilms' Tumor Cre mice, Michael Schneider for the αMHC Cre mice, David Rowitch for the Wnt1 Cre mice, and Eric Olson for the Nkx2.5 Cre mice. We thank Drs. Michael Chin, Nobutaka Koibuchi, and Kirby Johnson for technical advice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL67050 (to Y. L.) and DK068634 (to E. H. B.). This work was also supported by American Heart Association Grants 0815663G and 0615633Z (to M. R. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
J. Burch, unpublished observations.
- VSD
- ventricular septal defect
- DORV
- double outlet right ventricle
- En
- embryonic day n
- N1ICD
- Notch1 intracellular domain
- qRT-PCR
- quantitative real time PCR
- DIG
- digoxigenin
- qChIP
- quantitative ChIP.
REFERENCES
- 1. Olson E. N. (2004) Nat. Med. 10, 467–474 [DOI] [PubMed] [Google Scholar]
- 2. Olson E. N. (2006) Science 313, 1922–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee Y., Song A. J., Baker R., Micales B., Conway S. J., Lyons G. E. (2000) Circ. Res. 86, 932–938 [DOI] [PubMed] [Google Scholar]
- 4. Jung J., Kim T. G., Lyons G. E., Kim H. R., Lee Y. (2005) J. Biol. Chem. 280, 30916–30923 [DOI] [PubMed] [Google Scholar]
- 5. Balciunas D., Ronne H. (2000) Trends Biochem. Sci. 25, 274–276 [DOI] [PubMed] [Google Scholar]
- 6. Clissold P. M., Ponting C. P. (2001) Trends Biochem. Sci. 26, 7–9 [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi T., Yamazaki Y., Katoh-Fukui Y., Tsuchiya R., Kondo S., Motoyama J., Higashinakagawa T. (1995) Genes Dev. 9, 1211–1222 [DOI] [PubMed] [Google Scholar]
- 8. Whetstine J. R., Nottke A., Lan F., Huarte M., Smolikov S., Chen Z., Spooner E., Li E., Zhang G., Colaiacovo M., Shi Y. (2006) Cell 125, 467–481 [DOI] [PubMed] [Google Scholar]
- 9. Shen X., Kim W., Fujiwara Y., Simon M. D., Liu Y., Mysliwiec M. R., Yuan G. C., Lee Y., Orkin S. H. (2009) Cell 139, 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li G., Margueron R., Ku M., Chambon P., Bernstein B. E., Reinberg D. (2010) Genes Dev. 24, 368–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landeira D., Sauer S., Poot R., Dvorkina M., Mazzarella L., Jørgensen H. F., Pereira C. F., Leleu M., Piccolo F. M., Spivakov M., Brookes E., Pombo A., Fisher C., Skarnes W. C., Snoek T., Bezstarosti K., Demmers J., Klose R. J., Casanova M., Tavares L., Brockdorff N., Merkenschlager M., Fisher A. G. (2010) Nat. Cell Biol. 12, 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeuchi T., Watanabe Y., Takano-Shimizu T., Kondo S. (2006) Dev. Dyn. 235, 2449–2459 [DOI] [PubMed] [Google Scholar]
- 13. Peng J. C., Valouev A., Swigut T., Zhang J., Zhao Y., Sidow A., Wysocka J. (2009) Cell 139, 1290–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mysliwiec M. R., Kim T. G., Lee Y. (2007) FEBS Lett. 581, 2633–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finsterer J., Stollberger C., Bonner E. (2009) Int. J. Cardiol. [Google Scholar]
- 16. Maron B. J., Towbin J. A., Thiene G., Antzelevitch C., Corrado D., Arnett D., Moss A. J., Seidman C. E., Young J. B. (2006) Circulation 113, 1807–1816 [DOI] [PubMed] [Google Scholar]
- 17. Stankunas K., Hang C. T., Tsun Z. Y., Chen H., Lee N. V., Wu J. I., Shang C., Bayle J. H., Shou W., Iruela-Arispe M. L., Chang C. P. (2008) Dev. Cell 14, 298–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grego-Bessa J., Luna-Zurita L., del Monte G., Bolós V., Melgar P., Arandilla A., Garratt A. N., Zang H., Mukouyama Y. S., Chen H., Shou W., Ballestar E., Esteller M., Rojas A., Pérez-Pomares J. M., de la Pompa J. L. (2007) Dev. Cell 12, 415–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fortini M. E., Artavanis-Tsakonas S. (1994) Cell 79, 273–282 [DOI] [PubMed] [Google Scholar]
- 20. Iso T., Kedes L., Hamamori Y. (2003) J. Cell. Physiol. 194, 237–255 [DOI] [PubMed] [Google Scholar]
- 21. Ronchini C., Capobianco A. J. (2001) Mol. Cell. Biol. 21, 5925–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stahl M., Ge C., Shi S., Pestell R. G., Stanley P. (2006) Cancer Res. 66, 7562–7570 [DOI] [PubMed] [Google Scholar]
- 23. Iso T., Maeno T., Oike Y., Yamazaki M., Doi H., Arai M., Kurabayashi M. (2006) Biochem. Biophys. Res. Commun. 341, 708–714 [DOI] [PubMed] [Google Scholar]
- 24. Wang H. U., Chen Z. F., Anderson D. J. (1998) Cell 93, 741–753 [DOI] [PubMed] [Google Scholar]
- 25. Gerety S. S., Anderson D. J. (2002) Development 129, 1397–1410 [DOI] [PubMed] [Google Scholar]
- 26. Meyer D., Birchmeier C. (1995) Nature 378, 386–390 [DOI] [PubMed] [Google Scholar]
- 27. Liu X., Hwang H., Cao L., Buckland M., Cunningham A., Chen J., Chien K. R., Graham R. M., Zhou M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13024–13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee K. F., Simon H., Chen H., Bates B., Hung M. C., Hauser C. (1995) Nature 378, 394–398 [DOI] [PubMed] [Google Scholar]
- 29. Gassmann M., Casagranda F., Orioli D., Simon H., Lai C., Klein R., Lemke G. (1995) Nature 378, 390–394 [DOI] [PubMed] [Google Scholar]
- 30. Fiddes R. J., Janes P. W., Sivertsen S. P., Sutherland R. L., Musgrove E. A., Daly R. J. (1998) Oncogene 16, 2803–2813 [DOI] [PubMed] [Google Scholar]
- 31. Neve R. M., Holbro T., Hynes N. E. (2002) Oncogene 21, 4567–4576 [DOI] [PubMed] [Google Scholar]
- 32. Vijapurkar U., Kim M. S., Koland J. G. (2003) Exp. Cell Res. 284, 291–302 [DOI] [PubMed] [Google Scholar]
- 33. Shou W., Aghdasi B., Armstrong D. L., Guo Q., Bao S., Charng M. J., Mathews L. M., Schneider M. D., Hamilton S. L., Matzuk M. M. (1998) Nature 391, 489–492 [DOI] [PubMed] [Google Scholar]
- 34. Chen H., Shi S., Acosta L., Li W., Lu J., Bao S., Chen Z., Yang Z., Schneider M. D., Chien K. R., Conway S. J., Yoder M. C., Haneline L. S., Franco D., Shou W. (2004) Development 131, 2219–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vollrath A. L., Smith A. A., Craven M., Bradfield C. A. (2009) BMC Bioinformatics 10, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang da W., Sherman B. T., Lempicki R. A. (2009) Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 37. Dennis G., Jr., Sherman B. T., Hosack D. A., Yang J., Gao W., Lane H. C., Lempicki R. A. (2003) Genome Biol. 4, P3. [PubMed] [Google Scholar]
- 38. Mysliwiec M. R., Chen J., Powers P. A., Bartley C. R., Schneider M. D., Lee Y. (2006) Genesis 44, 407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koibuchi N., Chin M. T. (2007) Circ. Res. 100, 850–855 [DOI] [PubMed] [Google Scholar]
- 40. Stump G., Durrer A., Klein A. L., Lütolf S., Suter U., Taylor V. (2002) Mech. Dev. 114, 153–159 [DOI] [PubMed] [Google Scholar]
- 41. Chu J., Jeffries S., Norton J. E., Capobianco A. J., Bresnick E. H. (2002) J. Biol. Chem. 277, 7587–7597 [DOI] [PubMed] [Google Scholar]
- 42. Kim T. G., Kraus J. C., Chen J., Lee Y. (2003) J. Biol. Chem. 278, 42247–42255 [DOI] [PubMed] [Google Scholar]
- 43. Johnson K. D., Kim S. I., Bresnick E. H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15939–15944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim T. G., Chen J., Sadoshima J., Lee Y. (2004) Mol. Cell. Biol. 24, 10151–10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jung J., Mysliwiec M. R., Lee Y. (2005) Dev. Dyn. 232, 21–32 [DOI] [PubMed] [Google Scholar]
- 46. Takeuchi T., Kojima M., Nakajima K., Kondo S. (1999) Mech. Dev. 86, 29–38 [DOI] [PubMed] [Google Scholar]
- 47. Barth J. L., Clark C. D., Fresco V. M., Knoll E. P., Lee B., Argraves W. S., Lee K. H. (2010) Dev. Dyn. 239, 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato T. N., Qin Y., Kozak C. A., Audus K. L. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 9355–9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schnürch H., Risau W. (1993) Development 119, 957–968 [DOI] [PubMed] [Google Scholar]
- 50. Wong A. L., Haroon Z. A., Werner S., Dewhirst M. W., Greenberg C. S., Peters K. G. (1997) Circ. Res. 81, 567–574 [DOI] [PubMed] [Google Scholar]
- 51. Gitler A. D., Zhu Y., Ismat F. A., Lu M. M., Yamauchi Y., Parada L. F., Epstein J. A. (2003) Nat. Genet. 33, 75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moore A. W., Schedl A., McInnes L., Doyle M., Hecksher-Sorensen J., Hastie N. D. (1998) Mech. Dev. 79, 169–184 [DOI] [PubMed] [Google Scholar]
- 53. Watt A. J., Battle M. A., Li J., Duncan S. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12573–12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agah R., Frenkel P. A., French B. A., Michael L. H., Overbeek P. A., Schneider M. D. (1997) J. Clin. Invest. 100, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McFadden D. G., Barbosa A. C., Richardson J. A., Schneider M. D., Srivastava D., Olson E. N. (2005) Development 132, 189–201 [DOI] [PubMed] [Google Scholar]
- 56. Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998) Curr. Biol. 8, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 57. Kim T. G., Jung J., Mysliwiec M. R., Kang S., Lee Y. (2005) Biochem. Biophys. Res. Commun. 329, 544–553 [DOI] [PubMed] [Google Scholar]
- 58. Niessen K., Karsan A. (2008) Circ. Res. 102, 1169–1181 [DOI] [PubMed] [Google Scholar]
- 59. Toyoda M., Shirato H., Nakajima K., Kojima M., Takahashi M., Kubota M., Suzuki-Migishima R., Motegi Y., Yokoyama M., Takeuchi T. (2003) Dev. Cell 5, 85–97 [DOI] [PubMed] [Google Scholar]
- 60. Iwamoto R., Mekada E. (2006) Cell. Struct. Funct. 31, 1–14 [DOI] [PubMed] [Google Scholar]
- 61. Zhao Y. Y., Sawyer D. R., Baliga R. R., Opel D. J., Han X., Marchionni M. A., Kelly R. A. (1998) J. Biol. Chem. 273, 10261–10269 [DOI] [PubMed] [Google Scholar]
- 62. Bersell K., Arab S., Haring B., Kühn B. (2009) Cell 138, 257–270 [DOI] [PubMed] [Google Scholar]
- 63. Kopan R., Ilagan M. X. (2009) Cell 137, 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Conlon R. A., Reaume A. G., Rossant J. (1995) Development 121, 1533–1545 [DOI] [PubMed] [Google Scholar]
- 65. Oka C., Nakano T., Wakeham A., de la Pompa J. L., Mori C., Sakai T., Okazaki S., Kawaichi M., Shiota K., Mak T. W., Honjo T. (1995) Development 121, 3291–3301 [DOI] [PubMed] [Google Scholar]
- 66. Timms J. F., White S. L., O'Hare M. J., Waterfield M. D. (2002) Oncogene. 21, 6573–6586 [DOI] [PubMed] [Google Scholar]
- 67. Assou S., Cerecedo D., Tondeur S., Pantesco V., Hovatta O., Klein B., Hamamah S., De Vos J. (2009) BMC Genomics 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sun Y., Li H., Liu Y., Mattson M. P., Rao M. S., Zhan M. (2008) PLoS One 3, e3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Campa V. M., Gutiérrez-Lanza R., Cerignoli F., Díaz-Trelles R., Nelson B., Tsuji T., Barcova M., Jiang W., Mercola M. (2008) J. Cell Biol. 183, 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Collesi C., Zentilin L., Sinagra G., Giacca M. (2008) J. Cell Biol. 183, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Talora C., Campese A. F., Bellavia D., Felli M. P., Vacca A., Gulino A., Screpanti I. (2008) Biochim. Biophys. Acta 1782, 489–497 [DOI] [PubMed] [Google Scholar]
- 72. Nielsen A. L., Jørgensen P., Lerouge T., Cerviño M., Chambon P., Losson R. (2004) Mol. Cell. Biol. 24, 5184–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pasini D., Cloos P. A., Walfridsson J., Olsson L., Bukowski J. P., Johansen J. V., Bak M., Tommerup N., Rappsilber J., Helin K. (2010) Nature 464, 306–310 [DOI] [PubMed] [Google Scholar]
- 74. Shirato H., Ogawa S., Nakajima K., Inagawa M., Kojima M., Tachibana M., Shinkai Y., Takeuchi T. (2009) J. Biol. Chem. 284, 733–739 [DOI] [PubMed] [Google Scholar]
- 75. Nakayama J., Rice J. C., Strahl B. D., Allis C. D., Grewal S. I. (2001) Science 292, 110–113 [DOI] [PubMed] [Google Scholar]
- 76. Peters A. H., O'Carroll D., Scherthan H., Mechtler K., Sauer S., Schöfer C., Weipoltshammer K., Pagani M., Lachner M., Kohlmaier A., Opravil S., Doyle M., Sibilia M., Jenuwein T. (2001) Cell 107, 323–337 [DOI] [PubMed] [Google Scholar]
- 77. Rea S., Eisenhaber F., O'Carroll D., Strahl B. D., Sun Z. W., Schmid M., Opravil S., Mechtler K., Ponting C. P., Allis C. D., Jenuwein T. (2000) Nature 406, 593–599 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.