Abstract
The objective of this work was to develop an antibody-specific immunoglobulin G (IgG) avidity assay to discriminate between acute and latent phases of Toxoplasma gondii infection by using recombinant antigens. One hundred twenty-one serum samples from women who developed IgG antibodies against Toxoplasma during pregnancy were used. The IgG avidities of antibodies directed against epitopes carried by fragments of GRA3, GRA7, MIC3, and SAG1 antigens were measured by performing parallel enzyme immunoassays. The avidity index for Toxoplasma-specific antibodies against a homogeneous mixture of recombinant GRA3, GRA7, MIC3, and SAG1 antigens correlated closely with the IgG avidity of antibodies against lysed whole-cell T. gondii antigen. The avidity assay performed with the recombinant MIC3 antigen highlighted the presence of avidity low-antibodies IgG exclusively in sera collected within 2 months after primary infection. The presence of T. gondii-specific, low-avidity IgG antibodies against recombinant MIC3 antigen can be used to determine the point of infection with T. gondii within a 2-month time frame after infection.
Toxoplasma gondii is an obligate intracellular parasite that is distributed worldwide and responsible for the anthropozoonosis toxoplasmosis. T. gondii infection in humans is generally asymptomatic and induces a self-limiting disease. In contrast, primary infection during pregnancy can be transmitted to the fetus throughout the placenta, causing severe disease, such as premature birth, permanent neurological damage, and visual impairment (24). Identifying the gestational age of primary infection is crucial for the clinical management of pregnant women, since the severity of toxoplasmosis for the fetus decreases and transmission rate increases as gestational age progresses (9, 15). Prenatal therapy of gestational toxoplasmosis is generally considered effective for reducing both the incidence of clinical manifestations in infected infants (11) and the vertical transmission rate (8, 26). Thus, prenatal surveillance of T. gondii infection in pregnancy is widespread in Europe, enabling the appropriate management of gestational toxoplasmosis as soon as primary infection is diagnosed (5, 11, 27).
Diagnosis of acute infection depends on detection of Toxoplasma-specific immunoglobulin G (IgG) and IgM antibodies (1, 24) but does not make it possible to estimate the time of infection with accuracy (12, 17). Measurement of specific IgG avidity has been shown to be the best tool, in combination with IgM analysis, for determining when the infection took place (14, 16, 18, 19, 21, 23, 25). The functional affinity of specific IgG antibodies is initially low after primary antigenic challenge and increases during subsequent weeks and months by antigen-driven B-cell selection. One problem with this method is that some patients have persistent, low-avidity IgG antibodies for many months against lysed whole-cell Toxoplasma antigen, emphasizing the need for improvement of the IgG avidity assay (7, 21, 23).
Our strategy was to study the affinity maturation of IgG antibodies against specific Toxoplasma recombinant antigens compared to the IgG avidity assay with lysed whole-cell antigen. The IgG avidities of antibodies were analyzed by using sera collected from women for whom the time of infection was known. We show here that a recombinant antigen containing a region of the MIC3 protein represents a promising marker for an avidity assay to improve diagnosis of T. gondii infection.
MATERIALS AND METHODS
Serum samples.
One hundred twenty-one serum samples from 80 women who were seronegative for T. gondii at the beginning of pregnancy but developed IgG antibodies (seroconverted) during gestation were included in the study. The time of infection was defined as the midpoint of the interval of time between the last IgG-negative and the first IgG-positive sample (sampling intervals, usually 4 to 5 weeks).
Sera were collected at different intervals after initial seroconversion as follows: within the 1st month of infection (n = 11) and between the 1st and 2nd (n = 9), 2nd and 3rd (n = 9), 3rd and 4th (n = 9), 4th and 5th (n = 8), 5th and 6th (n = 8), 6th and 12th (n = 44), and 12th and 24th (n = 23) months of infection.
All samples were collected at the Referral Centre for Perinatal Infections of the Campania Region, Southern Italy (University Federico II of Naples). Sera taken from 30 Toxoplasma IgG-negative women referred to the Centre for other infectious diseases (cytomegalovirus, hepatitis C virus, or rubella infections) were used as controls. The serum samples were analyzed in blind fashion.
Clinical management of infected pregnant women.
As soon as diagnoses were confirmed, all women were treated up to delivery with an alternate regimen of pyrimethamine-sulfadiazine, folinic acid, and spiramycin.
Recombinant antigens.
Four T. gondii antigens, GRA3 (amino acids 35 to 133) (4), GRA7 (amino acids 24 to 103) (10), MIC3 (amino acids 233 to 307) (13), and SAG1 (amino acids 182 to 303) (6), were used. Recombinant antigens were expressed in bacterial cells as fusion proteins either with glutathione S-transferase (GST) or with Lambda protein D (3) and were purified by affinity chromatography as previously described (3). Briefly, recombinant Escherichia coli was induced with isopropyl-β-d-thiogalactopyranoside (IPTG), centrifuged, and suspended in STE buffer (10 mM Tris-HCl [pH 8], 150 mM NaCl) containing 100 μg of lysozyme per ml and protease inhibitor cocktail (Boehringer, Mannheim, Germany). The mixture was sonicated at 4°C and Triton X-100 was added to a final concentration of 1%. After centrifugation at 10,000 × g for 30 min at 4°C, the supernatant was recovered and incubated with either glutathione-Sepharose (for GST fusion proteins; Amersham Pharmacia Biotech, Uppsala, Sweden) or Ni-nitrilotriacetic acid resin (D fusion protein; Qiagen). Fusion proteins were eluted by following the manufacturer's instructions and were finally stored at −20°C.
Lysed whole-cell enzyme immunoassays.
Lysed whole-cell T. gondii antigen was used for determining Toxoplasma IgG, IgM, and IgG avidity indexes (Vidas system; bioMerièux, Marcy l'Etoile, France) and IgA (Platelia Toxo-IgA test; Pasteur, Paris, France).
Recombinant protein enzyme immunoassays.
Maxisorb multiwell plates (Nunc, Roskilde, Denmark) were coated with recombinant proteins at a final concentration of 5 μg/ml in coating buffer (50 mM NaHCO3, pH 9.6). After overnight incubation at 4°C, the plates were blocked with 200 μl of blocking solution (5% nonfat dry milk and 0.05% Tween 20 in phosphate-buffered saline [PBS]) per well and subsequently incubated with human sera diluted 1:50 in blocking buffer (100 μl/well). Plates were incubated for 1 h at 37°C and then washed with washing solution (0.05% Tween 20 in PBS). One hundred microliters of alkaline phosphatase-conjugated anti-human IgG antibody (Sigma) diluted 1:7,500 in blocking solution was added to each well. After 30 min at 37°C, the plates were washed and then incubated for 30 min at room temperature with the chromogenic substrate p-nitrophenyl phosphate (Sigma) in developing solution (10% diethanolamine [pH 9.8], 0.5 mM MgCl2, 0.05% NaN3). The results were recorded as the differences between the optical densities (ODs) at 405 nm and at 620 nm, measured with an automated enzyme-linked immunosorbent assay reader (Multiskan Labsystems, Helsinki, Finland).
IgG avidity assay.
Maxisorb plates (Nunc) were coated with recombinant proteins as described above. Samples were tested in two fourfold titration rows, starting at a dilution of 1:12.5. After a 1-h incubation at 37°C, plates were washed three times with washing buffer. Row A was then incubated with 200 μl of dissociation buffer (6 M urea and 0.05% Tween 20 in PBS) per well, and row B was incubated with 200 μl of washing buffer per well. After 30 min of incubation at 37°C with vigorous shaking, plates were washed once with washing buffer and processed as described above. Endpoint titers, one for the dissociation buffer and the other for the control, were calculated by using the following formula: endpoint titer = dilution factor × (ODdilution factor − ODcutoff).
Cutoff values were calculated for each dilution as the means plus 2 standard deviations (SD) of the Toxoplasma-negative sample reactivities, with or without urea treatment. Dilution factor values corresponded to the highest dilutions with ODs above the cutoff in assays with and without urea. IgG avidity was calculated as the ratio between the endpoint titer obtained for the reaction including urea and that obtained for the control, expressed as a percentage.
Statistical analysis.
Means, medians, ranges, and SD of the avidity measurements for the sera from the different groups are given. Spearman's rank correlation coefficient (r) was used to compare the results obtained with lysed whole-cell T. gondii antigen and those obtained with the mixture of recombinant antigens. Results obtained by using single MIC3 antigen were compared by the nonparametric unpaired two-group Mann-Whitney test.
RESULTS
Immunoreactivities of fusion proteins.
The immunoreactivities of GST-GRA3, GST-GRA7, GST-MIC3, and D-SAG1 (3) were determined for 60 Toxoplasma IgG-positive sera. Thirty Toxoplasma IgG-negative sera were assayed as controls. Specific levels of anti-Toxoplasma IgG, as determined by the whole-cell Toxoplasma immunoassay, ranged from 30 to 3,000 international units (IU)/ml for the positive samples and from 0 to 6 IU/ml for the negative controls (22).
The enzyme-linked immunosorbent assay performance of the four fusion proteins was assessed with the 60 IgG-positive samples, and for each recombinant product the cutoff value was determined as the mean plus 2 SD of the absorbency readings obtained for the 30 Toxoplasma IgG-negative sera. D-SAG1b and GST-MIC3 fusion proteins reacted with 92 and 95% of the positive sera, respectively, and both GST-GRA3 and GST-GRA7 reacted with 85% of the positive sera. One of the 30 Toxoplasma-negative sera did react with the D-SAG1 antigen and none recognized the GST-GRA3, GST-GRA7, and GST-MIC3 antigens.
The avidity assay with recombinant antigen fragments.
The avidities of the immunoglobulins directed against the epitopes carried by the GST-GRA3, GST-GRA7, GST-MIC3, and D-SAG1b fusion proteins were compared with the avidities measured by the lysed whole-cell assay (Vidas; bioMérieux). Serum samples were arbitrarily divided into two groups as follows: sera collected within 2 months of infection and sera collected >2 months after infection. Table 1 shows the IgG avidity indexes for individual recombinant antigens tested as single protein products and as a homogeneous mixture. A similar trend in affinity maturation of antibodies was found when the results obtained with lysed whole-cell Toxoplasma antigen and those obtained with a mixture of recombinant antigens were compared (r = 0.83; P < 0.001; confidence level, 95%). Avidities of antibodies against single antigen fragments were variable, especially for serum samples collected >2 months after infection. In particular, the recombinant MIC3 antigen gave fewer low-avidity results than the other antigens, with a mean avidity of 63.9%. GRA7 and SAG1 recombinant products exhibited the lowest avidities, with respective means of 36.5 and 33.6%. Interestingly, when we used the MIC3 antigen fragment, no overlapping between IgG avidity values for samples collected before and after 2 months of infection was observed.
TABLE 1.
Toxoplasma-specific IgG avidity of 60 serum samples from 49 women with primary infection during gestation
| Group or antigen | No. of reactive sera | % Avidity
|
|||
|---|---|---|---|---|---|
| Mean | Median | Range | SD | ||
| Aa | |||||
| Whole-cell Toxoplasma antigen (Vidas) | 13 | 8.5 | 6.0 | 3-28 | 7.7 |
| Recombinant antigen mix | 13 | 11.6 | 11.2 | 6-20 | 4.8 |
| GST-GRA3 | 12 | 9.1 | 7.9 | 2-20 | 5.3 |
| GST-GRA7 | 12 | 7.8 | 6.2 | 3-17 | 4.7 |
| GST-MIC3 | 13 | 13.0 | 11.1 | 3-24 | 7.4 |
| D-SAG1b | 12 | 12.1 | 7.8 | 4-33 | 10.0 |
| Bb | |||||
| Whole-cell Toxoplasma antigen (Vidas) | 47 | 44.3 | 47.0 | 2-74 | 17.4 |
| Recombinant antigen mix | 46 | 44.9 | 47.2 | 12-70 | 13.9 |
| GST-GRA3 | 39 | 44.4 | 41.3 | 5-91 | 21.6 |
| GST-GRA7 | 39 | 36.5 | 33.9 | 5-76 | 15.8 |
| GST-MIC3 | 44 | 63.9 | 67.4 | 30-88 | 16.1 |
| D-SAG1b | 43 | 33.6 | 31.7 | 4-90 | 18.2 |
Sera were collected within 2 months after acquisition of infection.
Sera were collected after 2 months and before 24 months after acquisition of infection.
IgG avidity for single recombinant MIC3 antigen.
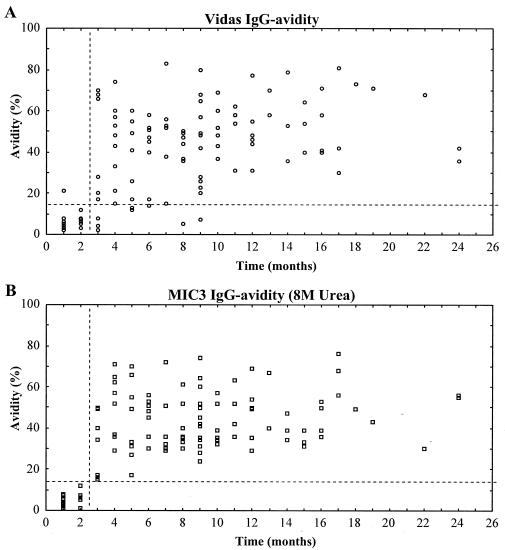
The clinical usefulness of recombinant antigens in the IgG avidity assay was further investigated by using the GST-MIC3 product. One hundred twenty-one sera were analyzed with the MIC3 antigen, and the assay was performed with various concentrations of the dissociation agent. As is clearly shown in Fig. 1, when we used the 8 M urea concentration, no overlapping between IgG avidity values of samples collected after 2 and 3 months of infection was observed. The MIC3 IgG avidity index showed no statistical difference between the first and second months after seroconversion (P = 0.8) but was significantly different between months 2 and 3 after seroconversion (P = 0.012). The MIC3 IgG avidity indexes for months 1 and 2 and for months 3 and 4 after seroconversion were highly significantly different (P = 0.0002).
FIG. 1.
IgG avidity assay with MIC3 antigen. (A) Toxoplasma-specific IgG avidity index for lysed whole-cell T. gondii antigen (Vidas system; bioMérieux). (B) Toxoplasma-specific IgG avidity index for recombinant GST-MIC3 and elution buffer with 8 M urea.
Table 2 shows the results of the MIC3 IgG avidity assay compared to the IgG avidity index obtained with lysed whole-cell antigen. The increase of urea concentration from 6 to 8 M resulted in a marked reduction of avidity index values for both groups, with a mean decrease of 10.3 to 5.3% for group A and 60.9 to 44.4% for group B.
TABLE 2.
Toxoplasma-specific IgG avidity of 121 serum samples from 80 women with primary infection during gestation
| Group or antigen | n | % Avidity
|
|||
|---|---|---|---|---|---|
| Mean | Median | Range | SD | ||
| Aa | |||||
| Whole-cell Toxoplasma antigen (Vidas) | 20 | 5.7 | 4.6 | 2.3-28 | 4.2 |
| MIC3 6 M urea | 20 | 10.3 | 9.3 | 2.3-23.9 | 5.6 |
| MIC3 8 M urea | 20 | 5.3 | 5.3 | 1.0-12.4 | 3.0 |
| Bb | |||||
| Whole-cell Toxoplasma antigen (Vidas) | 101 | 45.5 | 46.8 | 1.8-85.6 | 19.7 |
| MIC3 6 M urea | 97 | 60.9 | 61.8 | 21.6-95.1 | 16.3 |
| MIC3 8 M urea | 97 | 44.4 | 42.1 | 14.9-76.1 | 14.6 |
Sera were collected within 2 months after acquisition of infection.
Sera were collected after 2 months and before 24 months after acquisition of infection.
The proportions of sera that were correctly identified as having been collected within 2 months or after 2 months of infection by using different diagnostic criteria are shown in Table 3. All serum samples collected >2 months after seroconversion had IgG avidity indexes of >14%, while 92% of the sera analyzed with the whole-cell Toxoplasma antigen showed comparable results. This means that the low-avidity result (<14%) obtained with the MIC3 assay might determine, with great accuracy, whether the infection took place within the previous 2 months. In contrast, a high-avidity result (>14%) means that the primary infection occurred >2 months before the test.
TABLE 3.
Proportion of sera correctly identified as having been collected within or after 2 months of primary infection, using either lysed whole-cell Toxoplasma antigen (standard assay) or MIC3 avidity assays
| Time of serum collection (n) | Diagnostic criterion (% IgG avidity) | % of sera for which collection time was correctly identified
|
|
|---|---|---|---|
| Standard assay | MIC3 (8 M urea) | ||
| Within 2 mos of infection (20) | <10 | 90 | 90 |
| <14 | 95 | 100 | |
| <20 | 95 | 100 | |
| More than 2 mos after infection (97) | >10 | 95 | 100 |
| <14 | 92 | 100 | |
| <20 | 85 | 86 | |
Using an avidity ratio cutoff of 14%, the MIC3 IgG avidity index could separate all samples according to infection within 2 months of or >2 months after infection, while the assay using whole-cell Toxoplasma antigen showed low avidity for some samples for up to 9 months after infection (Fig. 1). Table 4 shows results for individual patients, and it is noteworthy that all these sera had specific IgM antibodies, even many months after primary infection.
TABLE 4.
Comparison of whole-cell Toxoplasma antigen (standard assay) and MIC3 avidity determinations for serum samples from patients in which the lysed whole-cell assay inaccurately estimated the time of infection
| Patient no. | Time after infection (mos) | IgG concn (IU/ml) (cutoff = 10) | IgM concn (IU/ml) (cutoff = 0.65) | Lysed whole-cell assay
|
MIC3 assay (8 M urea)
|
||
|---|---|---|---|---|---|---|---|
| % Avidity | Interpretationa | % Avidity | Interpretationa | ||||
| T20 | 1 | 83 | 1.0 | 20.7 | H | 7.8 | L |
| T94 | 3 | 1,152 | 0.79 | 8.3 | L | 14.9 | H |
| T33 | 3 | 300 | 0.67 | 3.9 | L | 17.0 | H |
| T58 | 3 | 300 | 2.34 | 1.8 | L | 34.0 | H |
| T87 | 5 | 60 | 1.17 | 12.0 | L | 33.2 | H |
| T46 | 5 | 175 | 1.04 | 12.8 | L | 31.2 | H |
| T55 | 6 | 870 | 0.92 | 13.9 | L | 35.8 | H |
| T37 | 8 | 78 | 1.41 | 5.3 | L | 51.5 | H |
| T54 | 9 | 137 | 2.43 | 6.5 | L | 28.3 | H |
Diagnostic criteria were as follows: if avidity was <14% (L, low avidity), infection was acquired within 2 months previous; if avidity was >14% (H, high avidity), infection was acquired more than 2 months previous.
DISCUSSION
Most assays for T. gondii IgG avidity routinely detect antibodies against whole tachyzoite cell extracts. Consequently, the avidity index is a measure of polyclonal IgG avidity that includes the contribution of antibody avidity against single antigens. Our starting hypothesis was that affinity maturation of antibodies against single epitopes represented by recombinant Toxoplasma antigens might follow a different pattern. Few studies have investigated this hypothesis (20, 28), and little is known about the use of single antigens in the avidity assay.
In a previous study, we identified and characterized a panel of immunodominant regions of T. gondii proteins (2, 3). In this study, we investigated further the IgG avidities of specific Toxoplasma antibodies directed against four distinct antigenic regions. There was a close correlation between the avidity indexes for lysed whole-cell Toxoplasma antigen and those for a recombinant antigen mixture. However, the assays performed with whole parasite extract and with a recombinant antigen mixture detected low-avidity antibodies in the sera of patients who seroconverted >2 months before sampling, which is in accordance with other studies which have found that low-avidity IgG antibodies may persist for many months beyond the acute infection (7, 21, 23). Therefore, the use of this mixture of recombinant antigens would have the same limitation as tests based on whole-cell lysate antigen.
However, by using the GST-MIC3 antigen, we could detect low-avidity IgG antibodies exclusively in serum samples from pregnant women infected within 2 months prior to the test. The presence of low-avidity antibodies against the GST-MIC3 antigen therefore reduces the time frame in which the acute T. gondii infection may have occurred to a 2-month window. The whole-cell lysate assay detected low-avidity antibodies in sera from patients who had seroconverted up to 9 months previously, in practice making a low-avidity index of almost no use for the clinician in the timing of infection. Previous studies have also found that low-avidity IgG antibodies may persist for many months beyond the acute infection (7, 21, 23).
The MIC3 antigen fragment was found to be a good marker for diagnosing recently acquired infections, and in contrast to the lysed whole-cell assay, both high- and low-avidity results may be used to determine the time of infection. This is important to know when pregnant women ask for a test of immunity, which in most countries, including Italy, is usually performed between gestational weeks 8 and 14.
In conclusion, our results show that the avidity assay based on recombinant antigens has potential clinical usefulness for diagnosing the acute phase of T. gondii infection during pregnancy. It will be interesting to investigate further the presence of other antigens that might be useful, in combination with the MIC3 fragment, to further enhance the power of the IgG avidity assay for the diagnosis of T. gondii infection during pregnancy.
Acknowledgments
We are deeply grateful to Klaus Hedman for critical reading of the manuscript and for very helpful suggestions and advice during this work. We also acknowledge Luca Bruno for technical assistance and Annalisa De Vita for secretarial assistance. We thank Ashraf Virmani for linguistic revision of the text.
REFERENCES
- 1.Beaman, M. H., R. E. McCabe, S. Y. Wong, and J. S. Remington. 1995. Toxoplasma gondii, p. 2455-2475. In G. L. Mandel, J. E. Bennett, and R. Dolin (ed.), Principles and practices of infectious diseases, 4th ed. Churchill Livingstone, Inc., New York, N.Y.
- 2.Beghetto, E., A. Pucci, O. Minenkova, A. Spadoni, L. Bruno, W. Buffolano, D. Soldati, F. Felici, and N. Gargano. 2001. Identification of a human immunodominant B-cell epitope within the GRA1 antigen of Toxoplasma gondii by phage display of cDNA libraries. Int. J. Parasitol. 31:1659-1668. [DOI] [PubMed] [Google Scholar]
- 3.Beghetto, E., A. Spadoni, W. Buffolano, M. Del Pezzo, O. Minenkova, E. Pavoni, A. Pucci, R. Cortese, F. Felici, and N. Gargano. 2003. Molecular dissection of the human B-cell response against Toxoplasma gondii infection by lambda display of cDNA libraries. Int. J. Parasitol. 33:163-173. [DOI] [PubMed] [Google Scholar]
- 4.Bermudes, D., J. F. Dubremetz, A. Achbarou, and K. A. Joiner. 1994. Cloning of a cDNA encoding the dense granule protein GRA3 from Toxoplasma gondii. Mol. Biochem. Parasitol. 68:247-257. [DOI] [PubMed] [Google Scholar]
- 5.Buffolano, W., L. Sagliocca, D. Fratta, A. Tozzi, A. Cardone, and N. Binkin. 1994. Prenatal toxoplasmosis screening in Campania region, Italy. It. J. Gynecol. Obstet. 6:70-74. [Google Scholar]
- 6.Burg, J. L., D. Perelman, L. H. Kasper, P. L. Ware, and J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141:3584-3591. [PubMed] [Google Scholar]
- 7.Cozon, G. J., J. Ferrandiz, H. Nebhi, M. Wallon, and F. Peyron. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 17:32-36. [DOI] [PubMed] [Google Scholar]
- 8.Desmonts, G. D., and J. Couvreur. 1974. Congenital toxoplasmosis: a prospective study of 378 pregnancies. N. Engl. J. Med. 290:1110-1116. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, D., M. Wallon, F. Peyron, E. Petersen, C. Peckham, and R. Gilbert. 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353:1829-1833. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, H. G., S. Stachelhaus, M. Sahm, H. M. Meyer, and G. Reichmann. 1998. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol. Biochem. Parasitol. 91:251-262. [DOI] [PubMed] [Google Scholar]
- 11.Foulon, W., I. Villena, B. Stray-Pedersen, A. Decoster, M. Lappalainen, J. M. Pinon, P. A. Jenum, K. Hedman, and A. Naessens. 1999. Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children's sequelae at age 1 year. Am. J. Obstet. Gynecol. 180:410-415. [DOI] [PubMed] [Google Scholar]
- 12.Francis, J. M., and D. H. Joynson. 1993. Duration of specific immunoglobulin A antibody following acute toxoplasmosis as determined by enzyme immunoassay and immunosorbent agglutination assay. Eur. J. Clin. Microbiol. Infect. Dis. 12:556-559. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Règuet, N., M. Lebrun, M. N. Fourmaux, O. Mercereau-Puijalon, T. Mann, J. M. Beckers, B. Samin, J. Van Beeumen, D. Bout, and J. F. Dubremetz. 2000. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface on the host cells and the surface of the parasite. Cell. Microbiol. 2:353-364. [DOI] [PubMed] [Google Scholar]
- 14.Hedman, K., M. Lappalainen, I. Seppala, and O. Makela. 1989. Recent primary Toxoplasma gondii infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159:736-740. [DOI] [PubMed] [Google Scholar]
- 15.Hohlfeld, P., F. Daffos, J. M. Costa, P. H. Thuillez, F. Forestier, and M. Vidaud. 1994. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain reaction test on amniotic fluid. N. Engl. J. Med. 331:695-699. [DOI] [PubMed] [Google Scholar]
- 16.Jenum, P. A., B. Stray-Pedersen, and A. G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of anti-toxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joss, A. W. L. 1992. Diagnosis, p. 79-118. In D. O. Ho-Yen and A. W. L. Joss (ed.), Human toxoplasmosis. Oxford Medical Publications, Oxford, United Kingdom.
- 18.Lappalainen, M., P. Koskela, M. Koskiniemi, P. Ammala, V. Hiilesma, K. Teramo, K. O. Raivio, J. S. Remington, and K. Hedman. 1993. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J. Infect. Dis. 167:691-697. [DOI] [PubMed] [Google Scholar]
- 19.Liesenfeld, O., J. G. Montoya, S. Kinney, C. Press, and J. S. Remington. 2001. Effect of testing for IgG avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: experience in a U.S. reference laboratory. J. Infect. Dis. 183:1248-1253. [DOI] [PubMed] [Google Scholar]
- 20.Marcolino, P. T., D. A. Silva, P. G. Leser, M. E. Camargo, and J. R. Mineo. 2000. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by western blotting. Clin. Diagn. Lab. Immunol. 7:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montoya, J. G., O. Liesenfeld, S. Kinney, C. Press, and J. S. Remington. 2002. VIDAS test for avidity of Toxoplasma-specific immunoglobulin G for confirmatory testing of pregnant women. J. Clin. Microbiol. 40:2504-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelloux, H., P. Ciapa, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1993. Evaluation du systeme VIDAS pour le diagnostic serologique de la toxoplasmose. Ann. Biol. Clin. 50:875-878. [PubMed] [Google Scholar]
- 23.Pelloux, H., E. Brun, G. Vernet, S. Marcillat, M. Jolivet, D. Guergour, H. Hidalgo, A. Fleuret, and P. Ambroise-Thomas. 1998. Determination of anti-Toxoplasma gondii immunoglobulin G avidity: adaptation to the VIDAS System (BioMerieux). Diagn. Microbiol. Infect. Dis. 32:69-73. [DOI] [PubMed] [Google Scholar]
- 24.Remington, J. S., R. McLeod, and G. Desmonts. 2000. Toxoplasmosis, p. 205-346. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the foetus and new born infant, 5th ed. The W. B. Saunders Company, Philadelphia, Pa.
- 25.Roberts, A., K. Hedman, V. Luyasu, J. Zufferey, M. H. Bessieres, R. M. Blatz, E. Candolfi, A. Decoster, G. Enders, U. Gross, E. Guy, M. Hayde, D. Ho-Yen, J. Johnson, B. Lecolier, A. Naessens, H. Pelloux, P. Thulliez, and E. Petersen. 2001. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 20:457-474. [DOI] [PubMed] [Google Scholar]
- 26.Stray-Pedersen, B. 1992. Treatment of toxoplasmosis in the pregnant mother and newborn child. Scand. J. Infect. Dis. 84(Suppl.):23-31. [PubMed] [Google Scholar]
- 27.Thuillez, P., and S. Romand. 2000. Prenatal screening and prevention of congenital toxoplasmosis in France, p. 293-298. In P. Ambroise-Thomas and E. Petersen (ed.), Congenital toxoplasmosis, Springer Verlag, New York, N.Y.
- 28.Villavedra, M., J. J. Battistoni, S. Rossi, H. Carol, and A. Nieto. 2001. Avidity analysis of the human immune response to a chitin binding protein of Toxoplasma gondii. Int. J. Parasitol. 31:1087-1092. [DOI] [PubMed] [Google Scholar]