Abstract
The Mac-1 integrin is expressed mainly on myeloid cells and binds several ligands, including members of the ICAM family and the complement factor iC3b. It is involved in essential immunological processes, such as leukocyte extravasation and phagocytosis. In addition, Mac-1 has been described to negatively regulate immune cell signaling. Recently, a single nucleotide polymorphism conferring an amino acid change in the Mac-1 integrin extracellular domain, R77H, was shown to be associated with systemic lupus erythematosus. Here, we demonstrate that the R77H-substituted Mac-1 can be expressed on the cell surface in transfected cells and can undergo conformational changes in response to integrin activation. The affinity of the integrin for ICAMs is only partially reduced, but cell adhesion to ICAM-1 and ICAM-2 is severely compromised, and Jβ2.7 cells expressing R77H substituted integrins are deficient in adhesion to ICAM-1 under shear flow conditions. Importantly, cell adhesion to the complement factor iC3b is also diminished, and COS cells expressing R77H-substituted integrins display reduced iC3b-dependent phagocytosis. In addition, U937 cells expressing R77H-CD11b display increased IL-6 production as compared with WT-CD11b-expressing cells. These results suggest that the R77H substitution results in the deficiency of the mutated integrin to mediate cell adhesion to ligands such as ICAMs and iC3b. These deficiencies may ultimately lead to detrimental effects on the immune system and contribute to the development of systemic lupus erythematosus.
Keywords: Cell Adhesion, Cell Surface Receptor, Genetic Diseases, Immunology, Integrin, Leukocyte, Receptor Structure Function, Phagocytosis, Systemic Lupus Erythematosus
Introduction
Systemic lupus erythematosus (SLE)3 is a multisystem autoinflammatory disorder characterized by immune complex-mediated tissue injury. Recently, SLE has been found to be associated with single nucleotide polymorphisms located in several genes, one of which encodes the β2 integrin, Mac-1 (CD11b/CD18, αMβ2, CR3) (1, 2). The association has been attributed to single nucleotide polymorphism rs1143679, which results in an arginine-to-histidine substitution at position 77 of the CD11b/αM coding region (R77H) (3).
Mac-1 is a member of the β2 integrin family of heterodimeric receptors expressed on leukocytes. Mac-1 is predominantly expressed on myeloid cells but is also found on lymphocyte subsets and binds a wide variety of ligands, including the intercellular adhesion molecule (ICAM) family and the complement protein iC3b. Mac-1 mediates essential immunological processes such as leukocyte migration and phagocytosis (4, 5). Recently, Mac-1 has also been described to negatively regulate immune cell signaling following ligand binding. It is involved in suppression of T-cell activation (6), negative regulation of Th17 cell development (7), suppression of dendritic cell production of inflammatory cytokines (8), and negative regulation of Toll-like receptor-mediated inflammatory responses (9). Perhaps because of these suppressive roles in immune system regulation, CD11b−/− animals display exacerbated disease progression in several autoimmune disease models, including SLE (10).
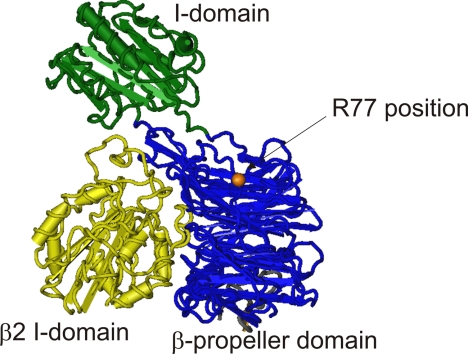
The Arg-77 residue lies in the CD11b β-propeller domain proximal to the ligand binding I domain and may thus influence ligand binding (Fig. 1). However, no molecular or functional mechanisms that explain the association of the R77H substitution with the development of SLE have been reported so far.
FIGURE 1.
The position of the R77H substitution in an integrin “head” domain. The αX β2 integrin is the most closely related integrin to Mac-1 with full head domain structural data available (33). It is 66% identical and 77% homologous to Mac-1 over the β-propeller (blue) and ligand-binding (green) I domains. The β2 integrin I-like domain is shown in yellow. The approximate position of the Arg-77 residue in this structure is highlighted in orange (this is a glycine residue in αX). The αX head domain structure was downloaded from the Molecular Modeling Database (accession number 3K6S) and dapted for this figure using Cn3D software.
Here, we have expressed R77H and wild-type integrins in Jβ2.7, U937, and COS cells to examine the functional properties of the R77H-substituted receptor. We demonstrate that the R77H substitution dramatically reduces Mac-1-mediated leukocyte adhesion to ligands ICAM-1 and ICAM-2 under static as well as under shear flow conditions. Importantly, leukocyte adhesion to the complement protein iC3b and phagocytosis of iC3b-coated particles is also significantly reduced, and IL-6 production is increased in R77H-CD11b-expressing cells. We suggest that the contribution of the Mac-1 R77H substitution to SLE pathology may occur through its detrimental effect on processes involving ICAM and/or iC3b binding, perhaps resulting in an attenuated ability of the receptor to negatively regulate immunological or inflammatory processes.
EXPERIMENTAL PROCEDURES
Plasmids, Antibodies, and Peptides
The cDNAs for CD11b and CD18 were cloned into pcDNA3 (11). Site-directed mutagenesis was used to create pcDNA-CD11bR77H using the oligonucleotides 5′-cgagcccatccacctgcaggtcccc-3′ and 5′-ggggacctgcaggtggatgggctcg-3′, and plasmids were verified through sequencing. Antibodies MEM170 (anti-CD11b), Mab24, and fluorescently conjugated secondary antibodies were purchased from Abcam. The CBRM1/5 antibody was from Ebioscience. The CBRM1/32 antibody was a kind gift from T. Springer (Harvard Medical School). FITC-conjugated C3c antibody was purchased from Dako. The R7E4 and R2E7B antibodies and pCD11b serum (11) were kind gifts from C. Gahmberg (University of Helsinki). FITC- and phycoerythrin-conjugated antibodies to CD11b (clone D12) and CD18 (Clone L130) were purchased from BD Biosciences. The peptides PIRLQVPVEAVN (R77-loop peptide) and PIHLQVPVEAVN (H77-loop peptide) were from Peptide 2.0, Inc. (Chantilly, VA).
Cell Lines and Transfection
The human T-cell lymphoma cell line clone J-β2.7 (12), which lacks CD11 chains, was a kind gift from N. Hogg (London, UK). Cells were transfected using Fugene 6 reagent (Roche) according to the manufacturer's instructions. CD11b-expressing Jβ2.7 cells were purified using magnetic cell sorting columns and CD11b-specific microbeads (Miltenyi Biotech) according to the manufacturer's instructions. U937 cells were transfected by electroporation with pcDNA3-WTCD11b and R77HCD11b. G418 (0.4 μg/ml) was used to maintain stably transfected cell populations. For phagocytosis assays, COS cells were transiently transfected using Fugene 6 reagent with the pcDNA3-CD11b and pcDNA3-CD18 constructs according to the manufacturer's protocol. Phagocytosis was analyzed 24–36 h post-transfection.
Immunoprecipitation and Western Blotting
Cells were lysed in IP buffer (50 mm Tris HCl (pH7.4), 150 mm NaCl, 10 mm EDTA, 50 mm NaF, 1% Triton X-100, protease inhibitor mixture (Roche)). Mac-1 integrin was immunoprecipitated from cell lysates using 1 μg of R7E4-antibody, which recognizes the integrin CD18 chain. Western blotting was performed using the pCD11b antiserum (against CD11b) and R2E7B (against CD18), as described previously (11).
Cell Adhesion Assays, Soluble ICAM-2/Fc, and mAb24 Reporter Binding Assays
Cellular adhesion assays were performed as described previously (13). Soluble ICAM-2, mAb24, and CBRM1/5 reporter binding assays were performed as described previously (11, 14). Additionally, some reporter assays were performed using low-chloride buffer (150 mm sodium glutamate, 5 mm KCl, 5.5 mm d-glucose, 10 mm Hepes (pH7.4), 1 mm CaSO4, 1 mm MgSO4) (14).
Shear Flow Cell Adhesion Assay
Cells (1 × 106 cells/ml) were preincubated with 200 nm PDBu or 5 mm MgCl2/1 mm EGTA for 3 min in RPMI with 0.1% BSA, 40 mm Hepes, and 2 mm MgCl2. The cell suspension was then injected into a flow system using a silicone tubing loop connected to a multiphaser NE-1000 syringe pump (New Era Pump Systems, Inc.), allowing cells to flow over human ICAM1-coated Ibidi microslides VI 0.4. An initial continuous shear flow rate of 0.3 dynes/cm2 was used for 30 s. The flow rate was then reduced to 0.15 dynes/cm2 for PDBu-treated cells and to 0.1dynes/cm2 for 5 mm MgCl2/1 mm EGTA-treated cells. Adhering cells were monitored over time by microscopy.
Phagocytosis Assay
Phagocytosis assays for Mac-1-mediated phagocytosis were performed as described in Ref. 15. Briefly, transfected COS-1 cells were stimulated with 200 nm PDBu and challenged with C3bi opsonized sheep RBCs for 45 min. Cells were washed with PBS, and non-internalized RBCs were lysed in H2O for 2 min. Transfected cells were labeled using RE74 antibody and rhodamine-conjugated rabbit anti-mouse secondary antibody, and iC3b-opsonized RBCs were labeled using FITC-conjugated C3c antibody (Dako). Cells were analyzed by fluorescence microscopy, and only cells exhibiting strong expression of integrins on the cell surface were assessed for phagocytosis of RBCs.
Cytokine Assays
IL-6 production in phorbol 12-myristate 13-acetate-differentiated U937 cells was assessed by ELISA (Ready-Set-Go human IL-6 ELISA, Ebiosciences) according to the manufacturer's instructions.
RESULTS
The R77H Mutation Does Not Compromise Expression of the Mac-1 Integrin on the Surface of Transfected Cells
To examine the function of CD11bR77H integrins in leukocytes, we transfected Jβ2.7 cells with pcDNA-CD11bWT or pcDNA-CD11bR77H. Jβ2.7 cells contain the CD18 (β2) integrin polypeptide but lack CD11a (αL) and CD11b (αM) chains. The β2-integrin polypeptide remains intracellular until transfection with CD11a or CD11b, as the integrin can only be transported to the cell surface as a heterodimer in these cells (11–13, 16). This cell line is useful for investigation of Mac-1 integrin function, as Mac-1 can be investigated in isolation without other interfering β2-integrins. Also, the Mac-1 integrin can be activated by inside-out signaling in this cell type (11, 16). Following transfection, cells were selected for CD11b expression using CD11b-specific microbeads, and stable cell populations were maintained using 0.4 mg/ml G418, leading to the production of the stable cell populations Jβ2.7-CD11bWT and Jβ2.7-CD11bR77H.
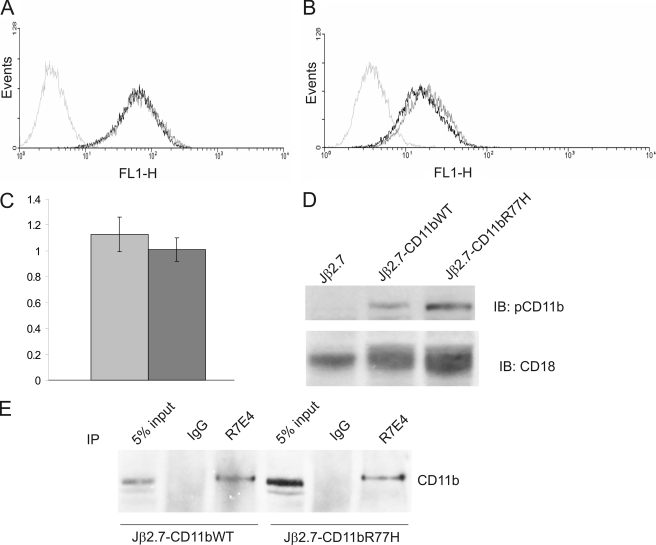
Integrin expression was quantified through flow cytometric analysis using MEM170 and R7E4 antibodies specific for CD11b and CD18, respectively (Fig. 2, A and B). Untransfected Jβ2.7 cells did not express CD11b or CD18 chains on their surface (Fig. 2). Both Jβ2.7-CD11bWT and Jβ2.7-CD11bR77H cells expressed comparable quantities of CD11b/CD18 integrin. Similar results were obtained using PE- and FITC-conjugated antibodies to CD11b and CD18 (BD Biosciences, results not shown). Also, the R77H substitution did not appear to grossly perturb the β-propeller domain, as the β-propeller antibody CBRM1/32 recognized both the CD11bWT- and CD11bR77H-transfected cells (Fig. 2C). To confirm these results, we examined CD11b and CD18 expression in the transfected cells by immunoblotting. As expected, untransfected Jβ2.7 cells only contained CD18 protein and lacked CD11b (Fig. 2D). Both Jβ2.7-CD11bWT and Jβ2.7-CD11bR77H cells expressed the CD11b polypeptide in the cells, with the expression similar in both cell types (Fig. 2D). In Jβ2.7-CD11bWT cells, the CD18 band underwent an electrophoretic band shift (Fig. 2D), presumably because it became paired with the CD11b chain. We have shown previously that there are two forms of CD18 in Jurkat cells, the lower molecular weight band corresponding to free CD18, which is immaturely glycosylated (16). Pairing with a CD11 chain results in processing of oligosaccharides attached to the CD18 chain and transport to the cell surface, which can be seen as an electrophoretic band shift (17). Importantly, a similar band shift of the CD18 band was seen in Jβ2.7-CD11bR77H cells. Therefore, this result indicated that both CD11bWT and CD11bR77H can form heterodimers with the CD18 polypeptide, leading to similar surface expression. To confirm this, we immunoprecipitated the CD18 polypeptide with a CD18-specific antibody from WT-CD11b- and R77H-CD11b-expressing cells (Fig. 2E). Importantly, the CD18 antibody pulled down similar amounts of CD11b from both Jβ2.7-CD11bWT and Jβ2.7-CD11bR77H cells, indicating that the CD11bR77H polypeptide can indeed form a heterodimer with CD18 in transfected cells. These results indicate that the R77H substitution does not affect Mac-1 integrin expression or the ability to form heterodimers.
FIGURE 2.
R77H CD11b can be expressed on transfected Jβ2. 7 cells. A and B, flow cytometric analysis of stable cell populations of pcDNA3-CD11b-transfected (black) and pcDNA-CD11bR77H-transfected (dark gray) cells and untransfected Jβ2.7 cells (light gray). Cells were incubated with CD11b-specific antibody (MEM170) (A), a CD18-specific antibody (RE74) (B), or an antibody against the β-propeller domain in CD11b (CBRM1/32) (C). In C, the result is expressed as fold of CD11b expression (MEM170 binding). Gray, WT-CD11b; dark gray, R77H-CD11b. D, CD11b and CD18 protein levels in transfected Jβ2.7 cells were examined by Western blot analysis with antibodies specific for CD11b (pCD11b serum) and CD18 (R2E7B). IB, immunoblotting. E, immunoprecipitations (IP) with RE74 (CD18 antibody) or control IgG from Jβ2.7-CD11bWT and Jβ2.7-CD11bR77H cell lysates were analyzed by Western blotting using an antibody specific for CD11b (pCD11b serum).
Adhesion of Cells Expressing CD11bR77H to ICAMs Is Severely Reduced as Compared with Cells Expressing Wild-type CD11b
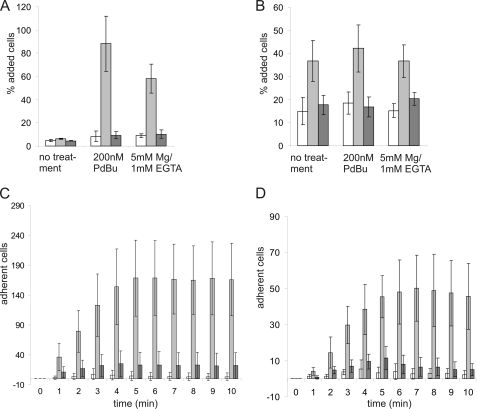
Mac-1 is an important receptor for ICAM ligands on the endothelium and other leukocytes, and ICAM binding has been hypothesized to be influenced by the CD11b R77H substitution (3). Therefore, we examined adhesion of the integrin-expressing Jβ2.7 cells to ICAM-1 and ICAM-2 in static cell adhesion assays. Jβ2.7-CD11b cells adhered to coated ICAM-1 and ICAM-2 when cells were stimulated with phorbol ester, an activating inside-out stimulus for integrins (Fig. 3, A and B). In addition, adhesion of Jβ2.7-CD11bWT cells was stimulated by Mg/EGTA, which bypasses intracellular signaling events and directly influences integrin extracellular domains (Fig. 3, A and B). In contrast, the adhesion of Jβ2.7-CD11bR77H cells to both ICAM-1 and ICAM-2 ligands when stimulated with phorbol ester or divalent cations was effectively abolished as compared with Jβ2.7-CD11bWT cells (Fig. 3, A and B). Increasing the ligand concentration in the adhesion assay did not result in increased binding of Jβ2.7-CD11bR77H cells to coated ICAM ligands (data not shown).
FIGURE 3.
CD11b integrin-mediated binding to ICAMs is reduced by the R77H substitution. A and B, CD11bWT- (gray) and CD11bR77H-expressing (dark gray) cell populations and untransfected Jβ2.7 cells (white) were left untreated, stimulated with 200 nm PDBu or with 5 mm Mg2+/1 mm EGTA and allowed to adhere to wells coated with ICAM-1 (A) or ICAM-2 (B). Adhesion to 1% w/v PVP was used as a specificity control and was found to be less than 10% in all cell types, whether untreated or stimulated (not shown). The experiments were repeated at least three times. Error bars represent mean ± S.D. Significant differences (two-tailed t test) were found between WT-CD11b- and R77H-CD11b-transfected cells adhering to ICAM-1 after PDBu and Mg stimulation (p ≤ 0.001) and for ICAM-2 under all conditions (p ≤ 0.05). C and D, cells were stimulated with 200 nm PDBu (C) or 5 mm Mg2+/1 mm EGTA (D) and passed over ICAM-1-coated flow cells as described under “Experimental Procedures.” The number of adherent cells was determined at 1-min intervals by manual counting. White, Jβ2.7; gray, Jβ2.7-CD11bWT; dark gray, Jβ2.7-CD11bR77H. Experiments were repeated at least three times. Error bars represent mean ± S.D. There was a significant difference between WT-CD11b and R77H-CD11b expressing cells at each time point (PDBu stimulation: p < 0.01, two-tailed t test) and for time points between 3 and 10 min for Mg-stimulated cells (p < 0.05, two-tailed t test). PVP, polyvinylpyrrolidone.
Cell adhesion to ICAMs by β2-integrins is especially important under flow conditions, when leukocytes bind to and crawl along endothelial cells prior to extravasation (5). Therefore, we next assessed ICAM binding under shear flow conditions. Indeed, ICAM ligand binding under flow conditions was abolished for the Jβ2.7-CD11bR77H cells (Fig. 3, C and D). Together, these results indicate that the R77H Mac-1 integrin cannot mediate cell adhesion to ICAM ligands and therefore may be unable to mediate immune processes that depend on these interactions.
R77H Mac-1 Integrin Affinity for ICAM Is Partially Reduced, but Conformational Changes in Response to Activation Can Still Occur
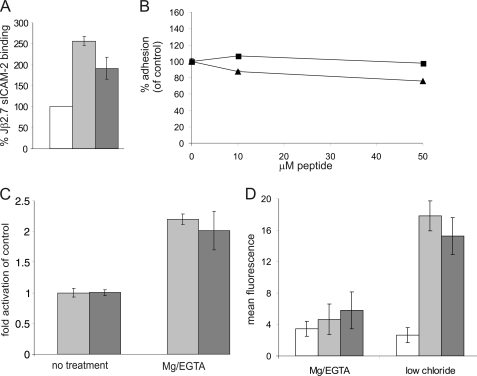
To further examine the mechanism by which the R77H substitution affected cell adhesion, we employed a soluble ligand binding assay to assess integrin affinity for ICAM (11, 13). sICAM-2Fc bound to CD11bWT-expressing cells, as reported previously (11). We found that the binding of soluble ICAM-2 was partially reduced (although not abolished) in Jβ2.7-CD11bR77H cells as compared with Jβ2.7-CD11bWT cells (Fig. 4A).
FIGURE 4.
The R77H-substituted CD11b integrin has slightly reduced affinity for ICAM but can undergo conformational changes in response to integrin activation. A, soluble ICAM-2 binding assay. Cell populations were stimulated with 200 nm PDBu and then incubated with 200 μg/ml soluble ICAM-2/Fc for 10 min. Following incubation with FITC-conjugated anti-human IgG-Fc-specific antiserum, ICAM-2/Fc binding was analyzed by flow cytometry. Results are expressed normalized to untransfected Jβ2.7 cell-ligand binding. White, Jβ2.7; gray, Jβ2.7-CD11bWT; dark gray, Jβ2.7-CD11bR77H. The R77H-CD11b still retained ICAM-binding activity, but there was a statistically significant difference between WT-CD11b and R77H-CD11b-transfected cells (two-tailed t test, p = 0.0295). B, WT-CD11b/Jβ2.7 PDBu-activated cell adhesion to ICAM-1 was assessed in the presence of Arg-77 loop peptide (■) or His-77-loop peptide (▴) at different concentrations. The Arg-77 peptide did not affect cell adhesion to ICAM-1. C, flow cytometric analysis of activation-specific epitope reporter Mab24. CD11bWT (gray) and CD11bR77H-expressing (dark gray) cell populations were incubated with Mab24 (5 μg/ml) to analyze integrin activation following treatment with 5 mm Mg2+/1 mm EGTA or control (no treatment). Mab24 labeling is expressed normalized to untreated cell populations. No significant difference in activation was observed between the two cell populations. D, flow cytometric analysis of activation-specific epitope reporter CBRM1/5. Jβ2.7 (white), CD11bWT (gray), and CD11bR77-expressing (dark gray) cell populations were incubated with CBRM1/5 (5 μg/ml) to analyze I domain opening in the presence of divalent cations or in low-chloride buffer. No significant differences was detected between the two cell populations.
To investigate the role of the Arg-77/His-77 amino acid in ICAM-1 binding directly, peptides corresponding to the surface-exposed β-propeller loop (residues 75–86 in CD11b) containing either Arg-77 or His-77 were synthesized, and their effect on integrin-mediated adhesion to coated ICAM-1 was assessed. The Arg-77-loop peptide did not inhibit adhesion to ICAM-1 (Fig. 4B), indicating that there is not a direct interaction between this region in the CD11b β-propeller and ICAM-1.
To determine whether the R77H substitution affected the ability of Mac-1 to be activated (i.e. undergo conformational changes in response to activating stimulus), we examined the binding of Mab24, an epitope-specific antibody for the active form of the CD18 integrin subunit. We found that in Jβ2.7-CD11bWT and Jβ2.7-CD11bR77H cells, treatment with Mg/EGTA resulted in similar increases in presentation of the mAb24 activation-specific epitope (Fig. 4C). We also measured binding of the open I-domain-specific CBRM1/5 antibody to the cells (Fig. 4D). There was no binding of CBRM1/5 to either Jβ2.7-CD11bWT or Jβ2.7-CD11bR77H in the presence of Mg/EGTA, but low-chloride/glutamate buffer(14) could induce CBRM1/5 expression in both cell types (Fig. 4D). This suggests that the I domain is not affected by the R77H substitution and that the R77H substituted integrin can still undergo conformational changes upon activation.
Integrin-mediated iC3b-dependent Adhesion Is Compromised for the R77H-substituted Receptor
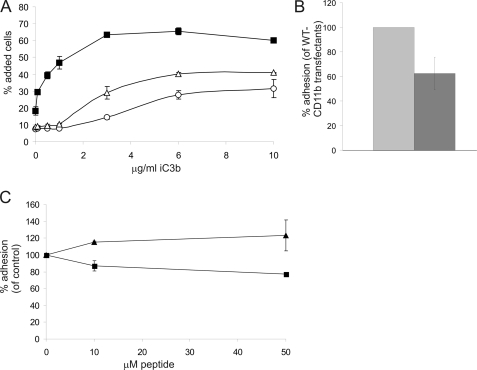
In addition to mediating leukocyte adhesion to ICAM-bearing cells, Mac-1 also functions as a complement receptor and mediates phagocytosis of iC3b-bearing particles and apoptotic cells (4). Therefore, we examined the binding of Jβ2.7-CD11bWT and Jβ2.7-CD11bR77H cells to the complement protein iC3b. Unstimulated cells were used in these experiments, as iC3b binding in CD11b-transfected Jβ2.7 cells is constitutive (11). Importantly, the binding of Jβ2.7-CD11bR77H cells to iC3b was significantly reduced compared with that of Jβ2.7-CD11bWT cells (Fig. 5A) and, despite increasing at higher concentrations of iC3b, always remained well below that of Jβ2.7-CD11bWT cells. To assess the role of the R77H substitution in iC3b binding in a more physiological cell model, we transfected U937 monocyte-like cells with CD11bWT and CD11bR77H constructs. These cells contain endogenous CD11b, but transfection of CD11b increased the surface expression of Mac-1 (not shown). Both cell types expressed similar amounts of CD11b/CD18 heterodimers, as assessed by flow cytometry and immunoprecipitation with a CD18-antibody (not shown). As in Jβ2.7-cells, a decrease in binding to iC3b was seen for the R77H-substituted integrin in stably transfected U937 cells differentiated with phorbol 12-myristate 13-acetate (Fig. 5B). Interestingly, upon long-term phorbol 12-myristate 13-acetate treatment (3 days) to induce macrophage differentiation of U937 cells, the R77H-CD11b-transfected U937 cells displayed reduced adhesion and spreading on plastic compared with WT-CD11b-transfected cells (not shown). These results indicate that the effect of the R77H substitution is not ligand-specific but rather affects many Mac-1-mediated adhesive properties.
FIGURE 5.
CD11b integrin-mediated binding to iC3b is reduced by the R77H substitution. A, untransfected Jβ2.7 cells (○), CD11bWT (■), and CD11bR77H-expressing (△) cell populations were allowed to adhere to wells coated in varying concentrations of iC3b. Results are representative of three separate experiments. B, U937 macrophage-like cells were stably transfected with WT-CD11b and R77H-CD11b. Cells were differentiated for 3 days with 162 nm phorbol 12-myristate 13-acetate to induce integrin expression, and cell adhesion to iC3b was assessed. Gray, U937-CD11bWT; dark gray, U937-CD11bR77H. The difference between the cell populations was statistically significant (two-tailed t test, p = 0.003). C, WT-CD11b/Jβ2.7 cell adhesion to iC3b was assessed in the presence of the Arg-77 loop peptide (■) or His-77 loop peptide (▴) at different concentrations. The difference in adhesion between Arg-77 loop peptide- and His-77 loop peptide-treated samples was statistically significant (p < 0.01, two-tailed t test).
The β-propeller domain has been described to contain binding sites for iC3b (18). Therefore, we assessed whether the Arg-77-containing surface-exposed loop in the CD11b β-propeller is a direct binding site for iC3b. The Arg-77-containing β-propeller loop peptide reduced adhesion to iC3b, although the reduction was not dramatic, whereas the His-77-containing peptide slightly increased adhesion (Fig. 5C). Therefore, it is possible that iC3b binds directly to this site in the CD11b β-propeller domain.
Phagocytosis and Modulation of Cytokine Production Is Compromised in R77H-CD11b-expressing Cells
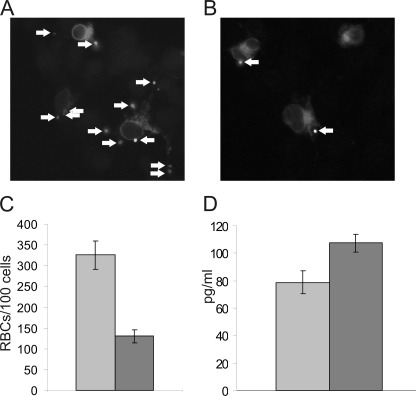
COS cells have been widely used as a model system to investigate the role and regulation of Mac-1 and other phagocytic receptors, as phagocytosis can be induced in these cells through expression of Mac-1 or other phagocytic receptors (15, 19–21). Unlike U937 cells, which express endogenous CD11b, CD11c, and CD18 molecules, COS cells do not express endogenous CD18 integrins, and therefore, phagocytosis by WT-Mac-1 or R77H-Mac-1 can be investigated without interfering endogenous receptors. We transfected COS cells with WT or R77H CD11b together with the integrin CD18 chain. Integrin expression in transfected COS cells was assessed by flow cytometry and was similar for both WT-CD11b- and R77H-CD11b-transfected cells (not shown). Expression of single integrin chains (CD11b or CD18) did not induce phagocytosis (not shown), but phagocytosis was readily detected in CD11bWT/CD18-transfected cells, as reported previously (Fig. 6, A and C) (19–21). We next compared the ability of the WT and R77H-mutated receptor to mediate phagocytosis of iC3b-coated particles. Importantly, phagocytosis of iC3b-coated sheep red blood cells was reduced (though not abolished) for CD11bR77H-transfected COS cells as compared with CD11bWT-transfected cells (Fig. 6, A–C), indicating that the reduced binding of iC3b for the mutated receptor translates as a deficiency of integrin-mediated phagocytosis.
FIGURE 6.
CD11b-mediated phagocytosis is reduced by the R77H substitution, and IL-6 production is up-regulated in R77H-CD11b-expressing cells. A–C, COS-1 cells transfected with CD18 and CD11b were stimulated with 200 nm PDBu and challenged with iC3b-opsonized red blood cells for 45 min. Following extensive washing, phagocytosis was analyzed by fluorescence microscopy. A and B, representative photographs of CD11bWT- (A) and CD11bR77H-espressing (B) COS-1 cells. Arrows indicate iC3b-opsonized sheep RBCs internalized by the cells. C, the number of RBCs internalized in 100 separate CD11bWT- (gray) or CD11bR77H-expressing (dark gray) COS-1 cells were counted. The experiments were repeated at least three times. Error bars represent mean ±S.D. The difference in phagocytosis between WT-CD11b- and R77H-CD11b-transfected cells was statistically significant (two-tailed t test, p = 0.0026). D, IL-6 production in transfected U937 cells was measured in triplicate as described under “Experimental Procedures.” Gray, U937-CD11bWT; dark gray, U937-CD11bR77H. One representative result of three is shown. Error bars represent mean ± S.D.
Inflammatory cytokines such as IL-6, type I IFN, and TNF-α play a major role in SLE, and the Mac-1 integrin has been reported to down-regulate macrophage and dendritic cell cytokine expression in a variety of settings (9, 22). Therefore, we assessed the ability of the WT and R77H Mac-1 receptor to modulate cytokine production in U937 cells. TNF-α and IL-1β production in these cells was very low, but IL-6 was readily detected. WT-CD11b-expressing U937 cells indeed produced less IL-6 than R77H-CD11b-expressing cells (Fig. 6D), indicating that the non-functional R77H integrin may influence inflammatory cytokine production by macrophages and/or other cells expressing Mac-1 integrins.
DISCUSSION
SLE is a multisystem autoinflammatory disorder. Symptoms range from skin rashes and mild arthritis to life-threatening nephritis and kidney failure. Although the underlying causes of SLE remain incompletely understood, the disease appears to be caused by a combination of environmental and genetic factors resulting in T and B lymphocyte activation, production of autoantibodies to nuclear antigens, abnormalities in apoptosis, and failure to clear immunocomplexes and apoptotic cells.
The single nucleotide polymorphism which confers the R77H amino acid substitution in CD11b has been convincingly shown to be associated with increased risk of developing SLE, especially renal disease (1–3, 23). However, no molecular or functional mechanisms that explain the association of the R77H substitution with the development of SLE have been reported so far. Therefore, we examined the functional properties of R77H-substituted Mac-1 in transfected cells. We have now shown that the R77H-substituted integrin can still be expressed at levels comparable with the wild-type receptor on the surface of transfected cells, as detected by flow cytometry with several different antibodies to CD11b or CD18-chains, as well as by immunoblotting and immunoprecipitation. The R77H-substituted integrin can still undergo conformational changes upon activation (as shown by its ability to bind mAb24/CBRM1/5 after stimulation with divalent cations or in low-chloride buffer). Additionally, the R77H-substituted receptor displays only slightly reduced affinity for ICAM ligands, and the Arg-77-containing loop peptide did not affect ICAM-1 adhesion (although it did reduce cell adhesion to iC3b somewhat). However, the R77H amino acid substitution effectively abolished integrin-mediated cell adhesion to ICAM ligands both under static and shear flow conditions, and also reduced cell adhesion and spreading on plastic, cell adhesion to complement iC3b, and iC3b-mediated phagocytosis. The R77H substitution therefore appears not to detrimentally affect integrin activation or ligand binding, whereas it severely affects cell adhesion to several Mac-1 ligands. Interestingly, two antibodies against the CD11b β-propeller domain (CBRM1/32 and anti-MART) reduce cell adhesion to many Mac-1 ligands, including ICAM-1 (24, 25). ICAM-1 has not been reported to bind directly to the CD11b β-propeller, indicating that the β-propeller domain plays an indirect role in regulating cell adhesion. It is possible that the R77H substitution regulates the conformation of the integrin ligand-binding head domain, although we have not been able to detect any such changes. Alternatively, it may be that the R77H substitution negatively affects integrin outside-in signaling and adhesion strengthening in response to ligand binding. This hypothesis is supported by the fact that the effect of the R77H substitution is not ligand-specific, i.e. adhesion to ICAMs, iC3b, and plastic are all affected, although these ligands have different binding sites in Mac-1. Further structural and functional studies of the R77H-substituted Mac-1 integrin are required to understand how this amino acid substitution affects cell adhesion.
The reduced Mac-1-ICAM binding may contribute to SLE development in several ways, for example by affecting T cell, dendritic cell, or Th17 cell activation, as Mac-1-ICAM binding has been reported to influence these immunological processes(6–8). The R77H Mac-1 integrin expressed on dendritic or other antigen-presenting cells may be unable to contribute to immunosuppressive effects because it is unable to bind ICAM ligands expressed on T lymphocytes and lymphatic endothelium. Therefore, it is possible that defects in these processes contribute to excessive T cell/dendritic cell activation in SLE (26–28). These possibilities require further investigation in the future, with primary antigen-presenting cell populations isolated from human subjects.
Interestingly, the R77H-substituted Mac-1 displays reduced phagocytosis of iC3b-coated particles. Phagocytic clearance of apoptotic cells is important for maintenance of immunological tolerance, as this process is immunologically “silent” and does not cause inflammation. Mac-1 is an essential receptor mediating phagocytosis of iC3b-coated apoptotic cells (22). Disturbed apoptosis and/or defects in clearance of apoptotic cells plays an important role in the pathogenesis of SLE, as these defects result in activation of dendritic cells, production of inflammatory cytokines, and presentation of autoantigens to autoreactive T cells (29).
Active Mac-1 has been described to suppress cytokine production in both macrophages and dendritic cells(9, 22). Interestingly, IL-6 production was higher in R77H-CD11b transfectants than in WT-CD11b-transfected cells, indicating that this nonfunctional integrin may fail to suppress signaling and cytokine production in cells (as has been reported in primary cells lacking Mac-1 integrins (9)). Therefore, disturbances in Mac-1-iC3b binding, phagocytosis, and cytokine production such as the ones described in this report may contribute to the clearance deficiency and cytokine profiles reported to contribute to development of SLE (29–32).
The single nucleotide polymorphism conferring the R77H substitution in the Mac-1 integrin extracellular domain is robustly associated with SLE (3). We have now shown that the SLE-associated R77H substitution in Mac-1 severely affects adhesion of Mac-1 expressing cells to ICAM ligands and iC3b as well as leukocyte adhesion under flow, phagocytosis, and the production of inflammatory cytokines. As these Mac-1-dependent processes are central for the immune response, these mechanistic findings may help explain how the mutation has detrimental effects on the immune system and ultimately contributes to the development of SLE. The physiological significance of these mechanistic findings remains to be examined. Therefore, future studies with primary cells isolated from individuals expressing R77H substituted Mac-1 will be important to confirm and expand the data described in this report.
This work is supported by Cancer Research UK, Biotechnology and Biological Sciences Research Council, and Tenovus Scotland.
- SLE
- systemic lupus erythematosus
- ICAM
- intercellular adhesion molecule
- PDBu
- phorbol 12, 13-dibutyrate.
REFERENCES
- 1. Harley J. B., Alarcón-Riquelme M. E., Criswell L. A., Jacob C. O., Kimberly R. P., Moser K. L., Tsao B. P., Vyse T. J., Langefeld C. D., Nath S. K., Guthridge J. M., Cobb B. L., Mirel D. B., Marion M. C., Williams A. H., Divers J., Wang W., Frank S. G., Namjou B., Gabriel S. B., Lee A. T., Gregersen P. K., Behrens T. W., Taylor K. E., Fernando M., Zidovetzki R., Gaffney P. M., Edberg J. C., Rioux J. D., Ojwang J. O., James J. A., Merrill J. T., Gilkeson G. S., Seldin M. F., Yin H., Baechler E. C., Li Q. Z., Wakeland E. K., Bruner G. R., Kaufman K. M., Kelly J. A. (2008) Nat. Genet. 40, 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hom G., Graham R. R., Modrek B., Taylor K. E., Ortmann W., Garnier S., Lee A. T., Chung S. A., Ferreira R. C., Pant P. V., Ballinger D. G., Kosoy R., Demirci F. Y., Kamboh M. I., Kao A. H., Tian C., Gunnarsson I., Bengtsson A. A., Rantapää-Dahlqvist S., Petri M., Manzi S., Seldin M. F., Rönnblom L., Syvänen A. C., Criswell L. A., Gregersen P. K., Behrens T. W. (2008) N. Engl. J. Med. 358, 900–909 [DOI] [PubMed] [Google Scholar]
- 3. Nath S. K., Han S., Kim-Howard X., Kelly J. A., Viswanathan P., Gilkeson G. S., Chen W., Zhu C., McEver R. P., Kimberly R. P., Alarcón-Riquelme M. E., Vyse T. J., Li Q. Z., Wakeland E. K., Merrill J. T., James J. A., Kaufman K. M., Guthridge J. M., Harley J. B. (2008) Nat. Genet. 40, 152–154 [DOI] [PubMed] [Google Scholar]
- 4. Dupuy A. G., Caron E. (2008) J. Cell Sci. 121, 1773–1783 [DOI] [PubMed] [Google Scholar]
- 5. Phillipson M., Heit B., Colarusso P., Liu L., Ballantyne C. M., Kubes P. (2006) J. Exp. Med. 203, 2569–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varga G., Balkow S., Wild M. K., Stadtbaeumer A., Krummen M., Rothoeft T., Higuchi T., Beissert S., Wethmar K., Scharffetter-Kochanek K., Vestweber D., Grabbe S. (2007) Blood 109, 661–669 [DOI] [PubMed] [Google Scholar]
- 7. Ehirchiou D., Xiong Y., Xu G., Chen W., Shi Y., Zhang L. (2007) J. Exp. Med. 204, 1519–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Podgrabinska S., Kamalu O., Mayer L., Shimaoka M., Snoeck H., Randolph G. J., Skobe M. (2009) J. Immunol. 183, 1767–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han C., Jin J., Xu S., Liu H., Li N., Cao X. (2010) Nat. Immunol. 11, 734–742 [DOI] [PubMed] [Google Scholar]
- 10. Kevil C. G., Hicks M. J., He X., Zhang J., Ballantyne C. M., Raman C., Schoeb T. R., Bullard D. C. (2004) Am. J. Pathol. 165, 609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fagerholm S. C., Varis M., Stefanidakis M., Hilden T. J., Gahmberg C. G. (2006) Blood 108, 3379–3386 [DOI] [PubMed] [Google Scholar]
- 12. Weber K. S., York M. R., Springer T. A., Klickstein L. B. (1997) J. Immunol. 158, 273–279 [PubMed] [Google Scholar]
- 13. Fagerholm S. C., Hilden T. J., Nurmi S. M., Gahmberg C. G. (2005) J. Cell Biol. 171, 705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lomakina E., Knauf P. A., Schultz J. B., Law F. Y., McGraw M. D., Waugh R. E. (2009) Blood Cells Mol. Dis. 42, 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chow C. W., Downey G. P., Grinstein S. (2004) Curr. Protoc. Cell Biol. 15, 15–17 [DOI] [PubMed] [Google Scholar]
- 16. Weber K. S., Klickstein L. B., Weber C. (1999) Mol. Biol. Cell 10, 861–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fagerholm S., Hilden T. J., Gahmberg C. G. (2002) Eur. J. Immunol. 32, 1670–1678 [DOI] [PubMed] [Google Scholar]
- 18. Li Y., Zhang L. (2003) J. Biol. Chem. 278, 34395–34402 [DOI] [PubMed] [Google Scholar]
- 19. Caron E., Hall A. (1998) Science 282, 1717–1721 [DOI] [PubMed] [Google Scholar]
- 20. Lim J., Wiedemann A., Tzircotis G., Monkley S. J., Critchley D. R., Caron E. (2007) Mol. Biol. Cell 18, 976–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim J., Dupuy A. G., Critchley D. R., Caron E. (2010) J. Cell. Biochem. 111, 99–1009 [DOI] [PubMed] [Google Scholar]
- 22. Morelli A. E., Larregina A. T., Shufesky W. J., Zahorchak A. F., Logar A. J., Papworth G. D., Wang Z., Watkins S. C., Falo L. D., Jr., Thomson A. W. (2003) Blood 101, 611–620 [DOI] [PubMed] [Google Scholar]
- 23. Kim-Howard X., Maiti A. K., Anaya J. M., Bruner G. R., Brown E., Merrill J. T., Edberg J. C., Petri M. A., Reveille J. D., Ramsey-Goldman R., Alarcon G. S., Vyse T. J., Gilkeson G., Kimberly R. P., James J. A., Guthridge J. M., Harley J. B., Nath S. K. (2010) Ann. Rheum. Dis. 69, 1329–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sachs U. J., Chavakis T., Fung L., Lohrenz A., Bux J., Reil A., Ruf A., Santoso S. (2004) Blood 104, 727–734 [DOI] [PubMed] [Google Scholar]
- 25. Yalamanchili P., Lu C., Oxvig C., Springer T. A. (2000) J. Biol. Chem. 275, 21877–21882 [DOI] [PubMed] [Google Scholar]
- 26. Palucka A. K., Banchereau J., Blanco P., Pascual V. (2002) Immunol. Cell Biol. 80, 484–488 [DOI] [PubMed] [Google Scholar]
- 27. Nalbandian A., Crispín J. C., Tsokos G. C. (2009) Clin. Exp. Immunol. 157, 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blanco P., Palucka A. K., Pascual V., Banchereau J. (2008) Cytokine Growth Factor Rev. 19, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaipl U. S., Voll R. E., Sheriff A., Franz S., Kalden J. R., Herrmann M. (2005) Autoimmun. Rev. 4, 189–194 [DOI] [PubMed] [Google Scholar]
- 30. Kelley J. M., Edberg J. C., Kimberly R. P. (2010) Autoimmun. Rev. 9, 473–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manderson A. P., Botto M., Walport M. J. (2004) Annu. Rev. Immunol. 22, 431–456 [DOI] [PubMed] [Google Scholar]
- 32. Tackey E., Lipsky P. E., Illei G. G. (2004) Lupus 13, 339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie C., Zhu J., Chen X., Mi L., Nishida N., Springer T. A. (2010) EMBO J. 29, 666–679 [DOI] [PMC free article] [PubMed] [Google Scholar]