Abstract
In the postantibiotic era, available treatment options for severe bacterial infections caused by methicillin-resistant Staphylococcus aureus have become limited. Therefore, new and innovative approaches are needed to combat such life-threatening infections. Virulence factor expression in S. aureus is regulated in a cell density-dependent manner using “quorum sensing,” which involves generation and secretion of autoinducing peptides (AIPs) into the surrounding environment to activate a bacterial sensor kinase at a particular threshold concentration. Mouse monoclonal antibody AP4-24H11 was shown previously to blunt quorum sensing-mediated changes in gene expression in vitro and protect mice from a lethal dose of S. aureus by sequestering the AIP signal. We have elucidated the crystal structure of the AP4-24H11 Fab in complex with AIP-4 at 2.5 Å resolution to determine its mechanism of ligand recognition. A key GluH95 provides much of the binding specificity through formation of hydrogen bonds with each of the four amide nitrogens in the AIP-4 macrocyclic ring. Importantly, these structural data give clues as to the interactions between the cognate staphylococcal AIP receptors AgrC and the AIPs, as AP4-24H11·AIP-4 binding recapitulates features that have been proposed for AgrC-AIP recognition. Additionally, these structural insights may enable the engineering of AIP cross-reactive antibodies or quorum quenching vaccines for use in active or passive immunotherapy for prevention or treatment of S. aureus infections.
Keywords: Antibodies, Bacteria, Bacterial Signal Transduction, Crystal Structure, Protein-Protein Interactions, Autoinducing Peptide, Quorum Sensing, Staphylococcus aureus
Introduction
Prokaryotic and eukaryotic single-cell organisms use cell-to-cell communication to coordinate their gene expression as they adapt to changing environmental conditions and compete with multicellular organisms. This chemical exchange of information among microorganisms has been termed “quorum sensing” (QS)3 (1, 2). With the emergence of highly antibiotic-resistant bacterial strains, including methicillin-resistant Staphylococcus aureus, new approaches for combating microbial infections are needed desperately (3–5). Although most antibiotics target essential metabolic pathways, inhibition of virulence-associated processes, such as QS signaling, attenuates the bacteria without exerting as much selective pressure, thereby decreasing development of resistance. Thus, this approach represents a new and innovative concept for antimicrobial drug discovery and might hold promise for the design of the elusive S. aureus vaccine (6–9).
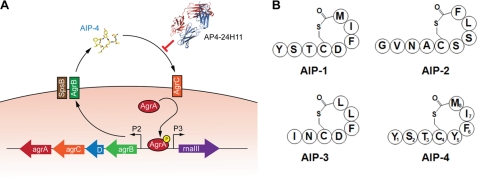
The agr (accessory gene regulator) QS system in S. aureus contributes to pathogenesis by orchestrating the temporal cell density-dependent expression of virulence genes. During exponential growth, the bacterial cell surface and adhesion molecules are expressed, whereas upon entering stationary phase, the expression pattern changes and results in the down-regulation of surface proteins and activation of genes encoding exoproteins and toxins (10–12). The agr locus is composed of two transcriptional units: the agr (or P2) operon under control of the P2 promoter and RNAIII, the de facto effector of agr QS, regulated by the P3 promoter (see Fig. 1A) (13, 14). The P2 operon consists of four genes, agrBDCA, which encode the proteins responsible for the synthesis of and response to the QS peptides. The agrD gene encodes a 46-amino acid AIP precursor peptide that is processed and cyclized by SpsB and AgrB (15) via formation of a thiolactone bond between a cysteine and the carboxyl group of the C-terminal residue. The resulting AIP is then secreted into the extracellular environment (16). The cognate receptor for the AIPs is the transmembrane sensor kinase AgrC. The N-terminal receptor domain of AgrC is predicted to consist of six membrane-spanning helices with three extracellular loops that constitute the AIP binding site (17–19). Upon AIP binding, the C-terminal cytoplasmic kinase domain relays the signal to AgrA and phosphorylated AgrA binds to the P2 and P3 promoters to activate AIP-controlled gene expression (20). Interestingly, S. aureus strains can be divided into four distinct agr subgroups, generally referred to as groups I, II, III, and IV. In each agr group, the AgrC receptor recognizes a specific AIP structure (i.e. AIP-1 through AIP-4, Fig. 1B) that promotes RNAIII transcription. Through so-called “bacterial interference,” the AIP signal of one agr group can compete for the AgrC receptor of another group and inhibit RNAIII transcription (21). Based on these observations, the AIPs are often classified into three cross-inhibitory groups: (i) AIP-1 and AIP-4, (ii) AIP-2, and (iii) AIP-3. AIP-1 and AIP-4 are grouped together because these structures differ by only one amino acid (Asp5 or Tyr5, respectively) (17, 19).
FIGURE 1.
The agr operon. A, the agrBDCA genes encode the AgrB, AgrD, AgrC, and AgrA proteins, which are all involved in the biosynthesis of AIPs. The propeptide AgrD is processed by AgrB and SpsB into AIP-4, which is sensed by the two-component regulatory system AgrC and AgrA. Phosphorylated AgrA activates transcription at the P2 and P3 promoters. Autoinduction of the agr operon leads to induction the response regulator, RNA III, and changes in gene expression. AP4–24H11 sequesters AIP-4 and prevents the activation of AgrC. B, schematic representation of the four AIPs encoded by agr groups I–IV. The residues of AIP-4 are numbered to illustrate and clarify the nomenclature.
Recently, blockade of quorum sensing has been shown to attenuate the expression of virulence factors in Gram-positive bacteria (22–24). Park et al. (24) reported the generation of the murine monoclonal antibody AP4-24H11 that sequesters AIP-4 utilized by S. aureus agr group IV strains as a QS signaling molecule. Treatment of S. aureus cultures with AP4-24H11 caused an increase in protein A expression and decreases in α-hemolysin expression and RNA III transcription, consistent with suppression of QS signaling (24). Most impressive, however, was the ability of this antibody to protect mice from S. aureus infections in vivo, including abscess formation in a skin infection model and lethality in a peritonitis model using a S. aureus group IV strain.
Here, we present the structure of the AP4-24H11 Fab in complex with AIP-4, which is the first structure of a quorum-sensing peptide bound to a receptor protein, in this case, an antibody. These structural data provide some possible insights into the interactions between the cognate staphylococcal AIP receptors AgrC and the AIPs, as the antibody binding to AIP-4 shares many features that have been hypothesized for recognition of AIP by AgrC.
EXPERIMENTAL PROCEDURES
Fab Production, Sequencing, and Purification
IgG was produced (25) and sequenced (26) using established methods. N-terminal sequencing of the AP4-24H11 heavy and light protein chains was performed at The University of Texas Biomedical Branch, Biomolecular Resource Facility. AP4-24H11 IgG was digested to Fab and Fc using 4% (w/w) papain for 4 h (27). The Fab was purified by successive protein A and protein G chromatography. Fab was further purified by MonoS where the Fab eluted at concentrations of 220–240 mm sodium chloride. The purified protein was concentrated to 19 mg/ml in 100 mm sodium acetate (pH 5.5), 1.25 mm EDTA, and 0.02% (w/v) sodium azide.
Crystallization and Data Collection
The AP4-24H11 Fab alone was crystallized by vapor diffusion using 1.0-μl sitting drops. Drops contained equal volumes (0.5 μl) of purified protein and the precipitant (20% PEG 4000, 0.2 m diammonium hydrogen phosphate (pH 8.0)). Crystals appeared after 1 month and grew as clusters of needles. The diffraction data were indexed in space group H3 with one molecule in the asymmetric unit.
AIP-4 was synthesized as described previously (24). AIP-4 at a final concentration of 5 mm was mixed with the AP4-24H11 Fab (13-fold excess of AIP-4 to Fab) immediately before setting up for crystallization by vapor diffusion using 1.0 μl sitting drops. The AIP-4·AP4-24H11 Fab complex was co-crystallized using equal volumes (0.5 μl) of the protein·ligand complex and precipitant (29 mm zinc acetate, 20% PEG 4000, 100 mm sodium cacodylate (pH 6.5)). Crystals appeared after 1 day and grew as clusters of long needles or plates and were cryoprotected in 20% glycerol prior to flash freezing in liquid nitrogen. The crystals were highly anisotropic with almost no diffraction observable when the beam was parallel to the plane of the very thin, plate-like crystals. The data were indexed in monoclinic space group P21 with two Fab molecules in the asymmetric unit. All data were collected at the Advanced Photon Source beamline 23ID-B (Argonne National Laboratory) and were processed with HKL2000 (28).
Structure Determination, Refinement, and Analysis
The AP4-24H11 Fab structure was solved by molecular replacement using Phaser (29). Domains of Protein Data Bank code 15C8 (30) were used as a search model based on homology to the AP4-24H11 Fab framework regions. Coot (31) was used for model building, and Refmac (32) was used for refinement. Only LeuL51 and ProH149 are designated as Ramachandran “outliers,” but both have a good fit to the corresponding electron density. LeuL51 is in a γ-turn commonly found in almost all Fab structures (33). Additional density in the binding site was modeled as 10 atoms of PEG. There was weak or missing 2Fo − Fc density for heavy chain residues 128–130, 133, and 134, that is also absent or weak in many other Fab structures, and these residues were omitted from the refined structure.
The AIP-4·AP4-24H11 Fab complex structure was determined by molecular replacement using the AP4-24H11 Fab solution. The AIP-4 ligand was initially built using Coot. AIP-4 geometric restraints were created with the PRODRG online server (34) and Sketcher (35). No density in 2Fo − Fc maps at the 1.0σ level could be seen for AIP-4 Tyr1, and this residue was omitted from the model. Coot was used for model building, and Buster-TNT (36) was used for refinement. Final rounds of refinement were performed in Refmac. Five zinc ions in the asymmetric unit mediate contacts between Fab molecules and aid in crystal packing interactions. Weak or missing 2Fo − Fc map density was again noted for heavy chain residues 129, 130, 133, and 134, and these residues were omitted from the structure.
Both Fab structures were numbered in the Kabat convention using the Abnum online server (37) and Pdbset (35). MolProbity (38) was used to calculate Ramachandran statistics. The 24H11-AIP-4 Fab structure was analyzed using Contacsym and MS (39, 40) to calculate buried surface area and enumerate van der Waals interactions. HBPLUS (41) was used to identify hydrogen bonds between AIP-4 and AP4-24H11. One of the Fab complexes (Fab chains A (light chain) and B (heavy chain) and AIP-4 chain D) was used for all calculations and measurements reported here.
Assessing Quorum Quenching Using S. aureus YFP Reporter Strains
Plasmid pDB59 (42) was transformed into four S. aureus strains representing agr group I (USA300 LAC), agr group II (SA502A), agr group III (MW2), and agr group IV (MN EV). All S. aureus reporter strains were grown overnight in tryptic soy broth supplemented with chloramphenicol at 10 μg/ml at 37 °C with shaking. For antibody inhibition testing, cultures were diluted 100-fold into fresh media, incubated for 1 h at 37 °C, and 180 μl was dispensed into wells of a microtiter plate (Costar 3603). AP4-24H11 was diluted to a working concentration of 2 mg/ml in PBS, and 2-fold serial dilutions were generated in PBS to a final concentration of 9.8 × 10−4 mg/ml. 20 μl of each antibody dilution was added to the reporter cultures in the microtiter plates in quadruplicate, resulting in an additional 10-fold dilution. As controls, PBS was used as a mock sample (no antibody), and an unrelated mouse IgG2a isotype control antibody was added at 2 mg/ml. The filtered spent medium containing AIP-1 from LAC or AIP-3 from MW2 was used as inhibition controls. Plates were incubated for 24 h at 37 °C with shaking at 200 rpm. Absorbance and fluorescence was measured in a Tecan Infinite M200 plate reader using an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
RESULTS
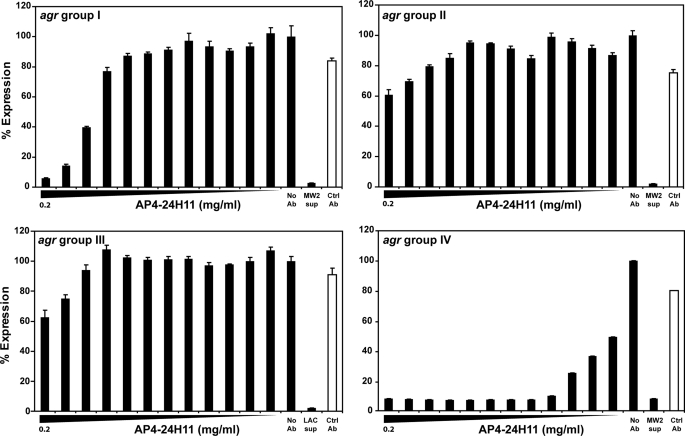
Park et al. (24) demonstrated that AP4-24H11 cross-reacts with AIP-1 and AIP-4, resulting in the complete suppression of QS-regulated virulence factor α-hemolysin expression in an agr IV S. aureus strain and partial inhibition in a group I isolate. To clearly delineate the extent of AIP cross-reactivity of AP4-24H11, four S. aureus reporter strains with the agr P3 promoter driving expression of YFP were constructed to represent each agr group. A range of AP4-24H11 concentrations was mixed with early growth phase cultures to assess antibody impact on actively growing S. aureus (Fig. 2). Notably, co-incubation of AP4-24H11 with S. aureus reporter strains belonging to agr group IV (IC50 = 0.00011 mg/ml [0.73 nm]) and to a lesser extent group I (IC50 = 0.040 mg/ml [27 nm]) resulted in significant decreases in YFP production in a dose-dependent manner, whereas QS signaling in agr groups II and III strains was not affected significantly by the antibody (IC50 >1 μm; Fig. 2). These data demonstrate that AP4-24H11 is greater than 350 times more potent in quenching agr signaling in a cognate group IV strain than in an S. aureus group I isolate that produces the nearly sequence-identical AIP-1.
FIGURE 2.
High agr specificity of quorum-sensing signaling inhibition mediated by AP4-24H11 in S. aureus. S. aureus strains containing a plasmid with an agr P3-YFP promoter fusion (pDB59) were used to examine the quorum-quenching activity of AP4-24H11. Reporter strains representing each of the four agr groups were constructed and incubated with AP4-24H11 in concentrations ranging from 0.2 to 9.8 × 10−5 mg/ml. As controls (Ctrl), assays containing no antibody or an isotype control antibody (0.2 mg/ml) were included. For agr inhibition controls, supernatants (sup) from MW2 containing AIP-3 or LAC containing AIP-1 were included as indicated. Using AP4-24H11, strong inhibition of agr group IV and modest inhibition of agr group I was observed, whereas minimal inhibition of agr group II and III was evident only at high AP4-24H11 doses. Error bars represent S.D. of quadruplicate samples, and each experiment was repeated.
To elucidate the molecular interactions that allow for antibody discrimination between similar quorum-sensing peptides, we determined the crystal structure of the AIP-4·AP4-24H11 Fab complex at 2.5 Å resolution (Table 1). Although electron density in 2Fo − Fc maps (contoured at 1.0σ) for the thiolactone ring of AIP-4 is well defined, the three residue N-terminal tail generally is less ordered. However, in one of the two Fab copies in the asymmetric unit, electron density was present for AIP-4 Ser2 and Thr3. The peptide bonds in the thiolactone ring are oriented such that most of the backbone carbonyl oxygens point in the general direction of the light chain, and the amide nitrogens are oriented toward the heavy chain. The side chains of the AIP-4 residues splay outwards from the ring.
TABLE 1.
Data collection and refinement statistics
| AIP-4·AP4–24H11 | AP4–24H11·PEG | |
|---|---|---|
| Data collection | ||
| Wavelength (Å) | 0.980 | 1.033 |
| Resolution (Å) | 2.50 (2.50-2.59)a | 2.80 (2.80-2.90) |
| Space group | P21 | H3 |
| Cell dimensions | a = 68.4, b = 98.5, c = 73.7 Å; α = 90, β = 114.0, γ = 90° | a = 183.2, b = 183.2, c = 42.6 Å; α = 90, β = 90, γ = 120° |
| No. of observations | 83,591 | 29,584 |
| Unique reflections | 29,017 | 13,033 |
| Redundancy | 2.9 (2.4)a | 2.3 (2.1) |
| Completeness (%) | 94.3 (90.7) | 97.9 (94.0) |
| Rsym (%)b | 16.8 (43.1) | 10.8 (40.8) |
| I/σ | 6.4 (1.6) | 7.7 (1.9) |
| Refinement statistics | ||
| Resolution | 39.7-2.50 (2.57-2.50) | 90-2.78 (2.85-2.78) |
| No. of reflections (working) | 25,883 | 11,734 |
| No. of reflections (test) | 1,477 | 644 |
| Rcryst (%)c | 18.3 (26.4) | 19.5 (29.2) |
| Rfree (%)d | 23.8 (34.0) | 24.0 (36.3) |
| No. of moles in asug | 2 | 1 |
| No. of Fab atoms | 6,586 | 3,272 |
| No. of ligand atoms | 110 (AIP4) | 27 (PEG) |
| No. of water molecules | 294 | 51 |
| No. of metal ions | 11 | 1 |
| Overall B values (Å2) | ||
| Antibody | 27.1 | 53.5 |
| Ligands | 26.6 (AIP4) | 59.5 (PEG) |
| Water | 19.8 | 38.4 |
| Ions | 31.1/50.5 (Na+1/Zn+2) | 53.4 (Na+1) |
| Wilson B-value (Å2) | 40.6 | 41.2 |
| Ramachandran plot (%)e | ||
| Favored | 96.9 | 93.1 |
| Allowed | 3.1 | 6.4 |
| Disallowed | 0.0 | 0.5 |
| r.m.s.d.f | ||
| Bond length (Å) | 0.010 | 0.008 |
| Angle | 1.46° | 1.21° |
a Outer shell.
b Rsym = Σhkl|I − 〈I〉|/Σhkl|I|.
c Rcryst = Σhkl|Fobs − Fcalc|/ΣhklFobs.
d Rfree is the same as Rcryst except for 5% of the data excluded from refinement.
e Ramachandran statistics were calculated with MolProbity (39).
f r.m.s.d., root mean square deviation.
g asu, asymmetric unit.
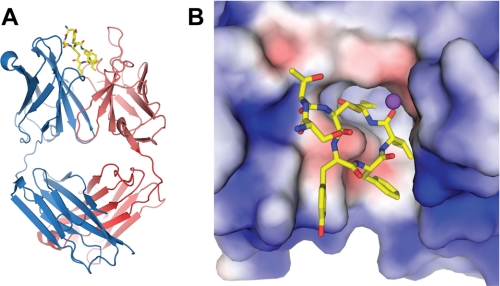
The AIP-4 macrocycle is intercalated into the antibody combining site (Fig. 3). This disposition directs the AIP-4 Phe6, Ile7, and Met8 side chains into the binding site and accounts for the majority of its buried surface area (Table 2). The AIP-4 ligand is recognized by AP4-24H11 primarily through the heavy chain (60%) (Table 3), although all complementarity determining regions (CDRs) make contributions, as well as some framework regions (4.8%). Although CDR1 of the heavy chain (CDRH1) buries the most ligand surface area of any CDR, the most striking features with respect to ligand recognition involve CDRH3.
FIGURE 3.
Crystal structure of AIP-4·AP4-24H11. A, structure of AP4-24H11 Fab with AIP-4 inserted between the heavy chain (blue) and light chain (red) in the combining site. B, electrostatic surface of the 24H11 binding site (+3.0 kV (blue) to −3.0 kV (red)). The hydrophobic residues AIP-4 Phe6, Ile7, and Met8 are buried in a hydrophobic pocket. The negative potential beneath the ring arises from GluH95. A sodium ion is modeled as a purple sphere.
TABLE 2.
AIP-4 contacts with AP4-24H11
| AIP-4 Residue | Main chaina | Side chaina | Buried surface (Å2) |
|---|---|---|---|
| Ser2 | 0/0 | 0/0 | 14.5 |
| Thr3 | 1/4 | 0/6 | 56.6 |
| Cys4 | 0/2 | 0/4 | 22.1 |
| Tyr5 | 1/3 | 0/23 | 72.6 |
| Phe6 | 1/5 | 0/9 | 98.1 |
| Ile7 | 2/4 | 0/13 | 88.7 |
| Met8 | 1/17 | 0/20 | 122.3 |
| Total | 6/35 | 0/65 | 474.9 |
a Hydrogen bonds/van der Waals contacts.
TABLE 3.
Contribution of AP4-24H11 regions to AIP-4 buried surface
Regions were defined using the contact numbering scheme (37).
| Region | Buried surface contribution (%) |
|---|---|
| CDRL1 | 10.5 |
| CDRL2 | 14.4 |
| CDRL3 | 12.7 |
| FRL4 | 2.2 |
| FRH1 | 0.2 |
| CDRH1 | 21.6 |
| FRH2 | 2.4 |
| CDRH2 | 17.1 |
| CDRH3 | 18.7 |
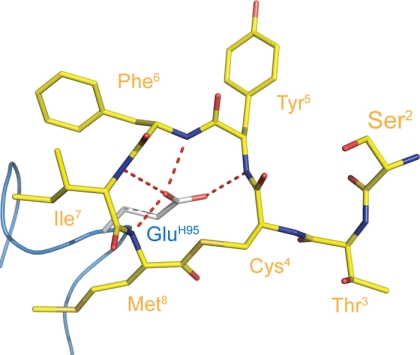
Despite its short length of only four residues, the CDRH3 loop is responsible for a large portion of the ligand buried surface area, which primarily results from a glutamic acid (GluH95; according to Kabat numbering (37)) that is positioned at the apex of the loop. The GluH95 carboxyl is oriented toward the center of the AIP-4 thiolactone ring and hydrogen bonds with each of the four amide nitrogens in the ring (Fig. 4). Additionally, the aliphatic portion of the GluH95 side chain runs parallel to the AIP-4 Met8 contributing to the burial of hydrophobic surface area. Thus, GluH95 accounts for 13.1% of the total ligand buried surface, greater than twice that of any other single residue.
FIGURE 4.
Antibody recognition of the AIP-4 main chain conformation. GluH95 in CDRH3 extends toward the center of the AIP-4 macrocyclic ring. The GluH95 side chain makes hydrogen bonds to each of the amide nitrogens of AIP-4 residues 5–8.
During model building and refinement, we observed strong, spherical density at a distance of 4–5 Å from three of the AIP-4 main chain carbonyl oxygens. Furthermore, many oxygen-containing groups in the nearby light chain are oriented in the direction of this unaccounted for density. Additionally, the plane of the aromatic ring of TyrL32 is oriented such that the ζ-carbon is centered 4.5 Å from the center of this site (43, 44). These data suggest the presence of a monovalent cation that forms a cation-π interaction with TyrL32, as well as electrostatic interactions with neighboring electron-donating groups. We have, therefore, modeled this additional density as a monovalent sodium cation. Subsequent refinement showed that it has similar B-values to the side chain of TyrL32 and provides some validation for this assignment. Nevertheless, although we cannot be absolutely certain of the identity of this presumed ion, the positive charge of the sodium would provide stabilizing interactions with the carbonyl oxygens of the thiolactone ring. This interaction, together with the GluH95 hydrogen bonding to the amide nitrogens of the macrocycle, would facilitate antibody recognition of both faces of the AIP-4 ring.
Thus far, we have described the AIP-4-AP4-24H11 interaction and specificity as resulting from burial of the C-terminal hydrophobic side chains, recognition of the AIP-4 peptide backbone by hydrogen bonding to GluH95 and proposed electrostatic interactions with a metal ion. However, Park et al. (24), as well as the S. aureus reporter assay data (Fig. 2), demonstrated that AP4-24H11 is able to discriminate finely between the AIP molecules of different S. aureus groups. In fact, AIP-1 and AIP-4 differ only at position 5 where AIP-1 contains an aspartic acid rather than tyrosine in AIP-4 (Fig. 1B), which might account for the reduced inhibition of toxin production (24) and YFP expression in the reporter assay (Fig. 2A) in an agr group I strain by AP4-24H11. Structurally, AIP-4 Tyr5 makes a CH-π bond with TyrH33 where one of the AIP-4 Tyr5 ϵ-carbons is positioned 3.5–4.0 Å from the TyrH33 aromatic ring (Fig. 5A). Similarly, AIP-4 Phe6 makes a CH-π bond with TyrL49 with its ζ-carbon positioned 4.1–4.5Å from the TyrL49 aromatic ring (Fig. 5B). These are the only interactions where the AIP-4 side chains are recognized specifically by the antibody and may account for the observed discrimination of AIP-1 and AIP-4 by AP4-24H11. Although CH-π, or edge-to-face, interactions are typically regarded as weak, they can contribute 0.6–1.3 kcal/mol in stabilization energy (45, 46). Thus, although recognition of the main chain conformation and burial of hydrophobic surfaces in AIP-4 likely are the major modes of interaction with AP4-24H11, these additional aromatic-aromatic interactions aid in the specificity of ligand recognition. For AIP-2 and AIP-3, although AIP-4 Ile7 does not engage in any specific interactions, the Leu7 side chain in other AIPs might create steric clashes within the binding site (Fig. 5B). Additionally, the long unbranched AIP-4 Met8 packs parallel to the GluH95 side chain and part of the main chain, in contrast to the branched side chains of the other AIPs at this position which would likely have steric clashes with the Fab in this region (Fig. 5C).
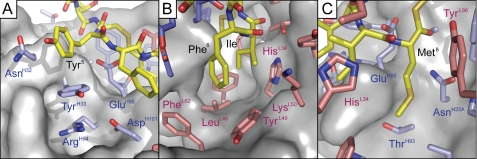
FIGURE 5.
Molecular interactions between AP4-24H11 and AIP-4 side chains. Close-up illustration of the interactions of AIP-4 macrocycle side chains with the antibody combining site of AP4-24H11. A, the aromatic ring of AIP-4 Tyr5 is positioned 3.5–4.0Å from TyrH33, creating a CH-π bond. B, Phe6 of AIP-4 makes a CH-π bond with TyrL49 with the ζ-carbon of AIP-4 Phe6 positioned 4.1–4.5Å from the TyrL49 aromatic ring. Ile7 does not engage in any specific interactions, but the leucine side chain found in other AIPs might create steric clashes within the binding site. C, Met8 of AIP-4 packs along the GluH95 side chain and part of the main chain, which might prevent interactions with the branched side chains found in other AIPs at this position.
To gain additional insight into the structural dynamics of the AIP-4-AP4-24H11 interactions, we also determined the structure of the AP4-24H11 Fab alone (2.8 Å resolution) to identify any changes that might take place upon antigen binding (Table 1). It is important to note that this AP4-24H11 Fab structure is not truly an apo-Fab structure. Extra density at the bottom of the antibody-combining site was modeled as PEG, which was present in the crystallization solution. This finding is reminiscent of the crystal structure of the quorum quenching antibody RS2–1G9, an anti-acyl homoserine lactone mAb, solved previously by our laboratory that also harbored an ethylene glycol molecule in the absence of the natural acyl homoserine lactone ligand (47). The PEG molecule occupies the location that is normally filled by the AIP-4 Ile7 and AIP-4 Met8 side chains. Superimposition of the variable regions revealed that, although no major rearrangements or loop movements occur upon ligand binding, the heavy chain shows small, but important, conformational changes in the CDR loops in the absence of AIP-4. The largest changes are observed in CDRH1 and CDRH3. In CDRH1, TyrH33 reorients to form one of the CH-π interactions with AIP-4 Tyr5. Interestingly, this reorientation also allows TyrH33 to form a cation-π interaction with ArgH94. These changes in CDRH1 also rotate and reposition AsnH32 to avoid a steric clash with AIP-4. In CDRH3, the backbone torsional angles of GluH95 also change to avoid a clash with AIP-4 and enable it to pack parallel to the AIP-4 Met8 side chain. In CDRH2, a tyrosine side chain has to change its rotamer to avoid a clash upon AIP-4 binding. On the other hand, the light chain shows almost no structural change induced by ligand binding, even at the side chain level. Notably, we again found spherical density positioned above TyrL32 implying that the proposed monovalent cation is prebound to the antibody.
DISCUSSION
Crystal structures of AP4-24H11 have revealed how the antibody interacts with the AIP-4 peptide, the QS signaling molecule of S. aureus agr group IV. Recognition of AIP-4 by AP4-24H11 is accomplished primarily through polar contacts with the AIP-4 macrocyclic peptide backbone and nonpolar interactions with the side chains of the C-terminal hydrophobic residues. GluH95 is a key residue as it contributes four of the six hydrogen bonds from the antibody to AIP-4 and thus buries a large portion of the AIP-4 hydrophobic surface area. These hydrogen bonds specifically recognize the macrocyclic configuration of the AIP-4 backbone. Analysis of the Fab structures clearly reveals how AP4-24H11 can discriminate among the different AIP molecules. We hypothesize that AIP-1 Asp5 would be unable to participate in the same CH-π interaction with the mAb as the corresponding AIP-4 Tyr5, thus conferring the ability to discriminate between AIP-1 and AIP-4. With regard to AIP-2 and -3, each has a leucine in lieu of AIP-4 Ile7 and a phenyalanine and a leucine, respectively, in place of AIP-4 Met8, none of which can be accommodated as well as the AIP-4 residues.
It seems prudent to compare this structural information on the interactions of AIP-4 and AP4-24H11 with those proposed for AgrC, the native S. aureus AIP receptor, and, in particular, with AgrC from group IV. Novick and co-workers (48) hypothesized that positions 7 and 8 of the AIPs need to be nonpolar, which led to the hypothesis that AgrC buries these residues in a hydrophobic pocket. Also, the requirement of a specificity-determining interaction, e.g. AIP-1 Asp5 and AIP-4 Tyr5, was proposed (48). Lastly, it has been demonstrated the N-terminal AIP tail is essential for receptor activation and signaling of AgrC (22). Remarkably, the first two of these three postulated key features are recapitulated in the antibody recognition and binding to AIP-4, namely the energetically favorable burial of hydrophobic AIP-4 residues and discrimination between AIP-4 Tyr5 and AIP-1 Asp5. However, the tail region of AIP-4 makes only limited contact with AP4-24H11. The hallmark feature of AP4-24H11, i.e. the prominent role of GluH95, might even be reflected in the AgrC receptors of all groups, as the extracellular loops contain several candidate acidic residues.
Notably, AP4-24H11 recapitulates the recognition patterns of group IV AgrC, in that the native receptor recognizes and is activated by AIP-4, and to a lesser extent by AIP-1 (19). Recently, Novick and colleagues detailed the evolution of agr genes, proposing agr groups I and II diverged from a common ancestor early on, and groups III and IV split from group I more recently. In fact, the agr group IV appears to be the most recent evolution of an agr group (49). In this context, it is important to note that evolution affecting agr diversification absolutely requires the coordination of mutations within all three key genes encoding agr group specificity, namely the pro-AIP (AgrD), the AIP receptor (AgrC) and the pro-AIP processing enzyme (AgrB), thus severely restricting the possible mutational space. However, this mutational restriction might explain the somewhat relaxed ligand specificity as cross-recognition of an existing AIP, as well as a newly evolved AIP, would most likely be a key intermediate step in the evolution of new agr groups (49). The antibody structure then might provide hints for how group IV AgrC is able to still maintain AIP-1 recognition while developing a strong preference for AIP-4.
The structures reported here also suggest how to engineer AP4-24H11 for new or enhanced recognition of the other known AIP molecules for broad spectrum neutralization. For example, directed mutagenesis of the region responsible for the AIP-4 Tyr5 and AIP-1 Asp5 discrimination might switch the antibody preference from AIP-4 to AIP-1. Alternatively, modification of the antibody combining site to more readily accept the different hydrophobic residues of the other AIPs might result in a more efficient AIP-2 binder. Lastly, a combination of both approaches might yield an AIP-3-specific antibody. Obviously, antibodies with high affinity for two or more different AIP molecules could be useful for passive immunotherapy to prevent or treat infections caused by the different groups of S. aureus. AP4-24H11 recognition of the thiolactone ring backbone, which might be a general feature of all staphylococcal AIPs, and the absence of interactions with the N-terminal hydrophobic residues make engineering of AP4-24H11 more feasible. With regard to the use of the AP4 hapten as a scaffold for an active S. aureus vaccine, the crystallographic data might be used to delineate a path for further improvement of the AIP haptens to elicit a more AIP cross-reactive immune response. For example, the construction of hybrid macrocyclic AIP haptens incorporating many of the critical features described above, as well an N-terminal tail truncation, might indeed result in a broadly AIP cross-reactive antibody response.
Acknowledgments
We thank Sharon Ferguson for optimizing the IgG digestion conditions, Erik Debler for help with antibody crystallization and diffraction experiments, Jeffrey S. Kavanaugh (University of Iowa) for a critical reading of the manuscript, the Joint Center for Structural Genomics for use of the structure quality control server, and the Advanced Photon Source at Argonne National Laboratory for data collection at beamline 23ID-B.
This work was supported in part by National Institutes of Health Grants AI078921 (to A. R. H.), AI080715 (to G. F. K.), and AI042266 and CA058896 (to I. A. W.). This work was also supported by The Skaggs Institute (to I. A. W. and K. D. J.). The Advanced Photon Source at Argonne National Laboratory is supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences Contract DE-AC02-06CH11357. This is Paper 20569 from The Scripps Research Institute.
The atomic coordinates and structure factors (codes 3QG6 and 3QG7) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- QS
- quorum sensing
- AIP
- autoinducing peptide
- CDR
- complementarity determining region.
REFERENCES
- 1. Fuqua W. C., Winans S. C., Greenberg E. P. (1994) J. Bacteriol. 176, 269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reading N. C., Sperandio V. (2006) FEMS. Microbiol. Lett. 254, 1–11 [DOI] [PubMed] [Google Scholar]
- 3. David M. Z., Daum R. S. (2010) Clin. Microbiol. Rev. 23, 616–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deleo F. R., Otto M., Kreiswirth B. N., Chambers H. F. (2010) Lancet 375, 1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischbach M. A., Walsh C. T. (2009) Science 325, 1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clatworthy A. E., Pierson E., Hung D. T. (2007) Nat. Chem. Biol. 3, 541–548 [DOI] [PubMed] [Google Scholar]
- 7. Kaufmann G. F., Park J., Janda K. D. (2008) Expert. Opin. Biol. Ther. 8, 719–724 [DOI] [PubMed] [Google Scholar]
- 8. Projan S. J., Nesin M., Dunman P. M. (2006) Curr. Opin. Pharmacol. 6, 473–479 [DOI] [PubMed] [Google Scholar]
- 9. Suga H., Smith K. M. (2003) Curr. Opin. Chem. Biol. 7, 586–591 [DOI] [PubMed] [Google Scholar]
- 10. Lebeau C., Vandenesch F., Greenland T., Novick R. P., Etienne J. (1994) J. Bacteriol. 176, 5534–5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MDowell P., Affas Z., Reynolds C., Holden M. T., Wood S. J., Saint S., Cockayne A., Hill P. J., Dodd C. E., Bycroft B. W., Chan W. C., Williams P. (2001) Mol. Microbiol. 41, 503–512 [DOI] [PubMed] [Google Scholar]
- 12. Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. (1986) Mol. Gen. Genet. 202, 58–61 [DOI] [PubMed] [Google Scholar]
- 13. Morfeldt E., Tegmark K., Arvidson S. (1996) Mol. Microbiol. 21, 1227–1237 [DOI] [PubMed] [Google Scholar]
- 14. Novick R. P., Projan S. J., Kornblum J., Ross H. F., Ji G., Kreiswirth B., Vandenesch F., Moghazeh S. (1995) Mol. Gen. Genet. 248, 446–458 [DOI] [PubMed] [Google Scholar]
- 15. Thoendel M., Horswill A. R. (2009) J. Biol. Chem. 284, 21828–21838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L., Gray L., Novick R. P., Ji G. (2002) J. Biol. Chem. 277, 34736–34742 [DOI] [PubMed] [Google Scholar]
- 17. Jensen R. O., Winzer K., Clarke S. R., Chan W. C., Williams P. (2008) J. Mol. Biol. 381, 300–309 [DOI] [PubMed] [Google Scholar]
- 18. Chen L. C., Tsou L. T., Chen F. J. (2009) J. Microbiol. 47, 572–581 [DOI] [PubMed] [Google Scholar]
- 19. Geisinger E., George E. A., Muir T. W., Novick R. P. (2008) J. Biol. Chem. 283, 8930–8938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koenig R. L., Ray J. L., Maleki S. J., Smeltzer M. S., Hurlburt B. K. (2004) J. Bacteriol. 186, 7549–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji G., Beavis R., Novick R. P. (1997) Science 276, 2027–2030 [DOI] [PubMed] [Google Scholar]
- 22. Lyon G. J., Mayville P., Muir T. W., Novick R. P. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 13330–13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakayama J., Uemura Y., Nishiguchi K., Yoshimura N., Igarashi Y., Sonomoto K. (2009) Antimicrob. Agents Chemother. 53, 580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park J., Jagasia R., Kaufmann G. F., Mathison J. C., Ruiz D. I., Moss J. A., Meijler M. M., Ulevitch R. J., Janda K. D. (2007) Chem. Biol. 14, 1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaufmann G. F., Park J., Mayorov A. V., Kubitz D. M., Janda K. D. (2011) Methods Mol. Biol. 692, 299–311 [DOI] [PubMed] [Google Scholar]
- 26. Marks J. D., Tristem M., Karpas A., Winter G. (1991) Eur. J. Immunol. 21, 985–991 [DOI] [PubMed] [Google Scholar]
- 27. Harlow E., Lane D. (1988) Antibodies: A Laboratory Manual, pp. 626–631, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York [Google Scholar]
- 28. Otwinoski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 29. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gruber K., Zhou B., Houk K. N., Lerner R. A., Shevlin C. G., Wilson I. A. (1999) Biochemistry 38, 7062–7074 [DOI] [PubMed] [Google Scholar]
- 31. Emsley P., Cowtan K. (2004) Acta. Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 32. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta. Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 33. Al-Lazikani B., Lesk A. M., Chothia C. (1997) J. Mol. Biol. 273, 927–948 [DOI] [PubMed] [Google Scholar]
- 34. Schüttelkopf A. W., van Aalten D. M. (2004) Acta. Crystallogr. D Biol. Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 35. Collaborative Computational Project (1994) Acta. Crystallogr. D Biol. Crystallogr. 50, 760–76315299374 [Google Scholar]
- 36. Blanc E., Roversi P., Vonrhein C., Flensburg C., Lea S. M., Bricogne G. (2004) Acta. Crystallogr. D Biol. Crystallogr. 60, 2210–2221 [DOI] [PubMed] [Google Scholar]
- 37. Abhinandan K. R., Martin A. C. (2008) Mol. Immunol. 45, 3832–3839 [DOI] [PubMed] [Google Scholar]
- 38. Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) Nucleic Acids Res. 35, W375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheriff S., Silverton E. W., Padlan E. A., Cohen G. H., Smith-Gill S. J., Finzel B. C., Davies D. R. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 8075–8079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Connolly M. L. (1993) J. Mol. Graph. 11, 139–141 [DOI] [PubMed] [Google Scholar]
- 41. McDonald I. K., Thornton J. M. (1994) J. Mol. Biol. 238, 777–793 [DOI] [PubMed] [Google Scholar]
- 42. Malone C. L., Boles B. R., Lauderdale K. J., Thoendel M., Kavanaugh J. S., Horswill A. R. (2009) J. Microbiol. Methods. 77, 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dougherty D. A. (1996) Science 271, 163–168 [DOI] [PubMed] [Google Scholar]
- 44. Gallivan J. P., Dougherty D. A. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 9459–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brandl M., Weiss M. S., Jabs A., Sühnel J., Hilgenfeld R. (2001) J. Mol. Biol. 307, 357–377 [DOI] [PubMed] [Google Scholar]
- 46. Burley S. K., Petsko G. A. (1985) Science 229, 23–28 [DOI] [PubMed] [Google Scholar]
- 47. Debler E. W., Kaufmann G. F., Kirchdoerfer R. N., Mee J. M., Janda K. D., Wilson I. A. (2007) J. Mol. Biol. 368, 1392–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wright J. S., 3rd, Lyon G. J., George E. A., Muir T. W., Novick R. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 16168–16173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wright J. S., 3rd, Traber K. E., Corrigan R., Benson S. A., Musser J. M., Novick R. P. (2005) J. Bacteriol. 187, 5585–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]