Abstract
Purpose
Nucleotide oligomerization domain-2 (NOD2) plays an important role in innate immunity to sense muramyl dipeptide (MDP), a component of bacterial cell walls. Notably, NOD2 is linked to eye inflammation because mutations in NOD2 cause a granulomatous type of uveitis called Blau syndrome. A mouse model of NOD2-dependent ocular inflammation was employed to test the role of a cytokine strongly implicated in granuloma formation, IFN-γ, in order to gain insight into downstream functional consequences of NOD2 activation within the eye triggering uveitis.
Methods
Mice deficient in IFN-γ, NOD2, or CD11b and their wild-type controls were treated with intravitreal injection of MDP in the presence or absence of IFN-γ. IFN-γ production in the eye was measured by ELISA. The intravascular inflammatory response within the iris was quantified by intravital microscopy.
Results
NOD2 activation resulted in the production of IFN-γ within the eye. Deficiency in IFN-γ diminished the development of MDP-induced uveitis, indicating its crucial role in downstream inflammatory events triggered by NOD2. Moreover, exogenous IFN-γ markedly exacerbated MDP-induced ocular inflammation in a NOD2-dependent mechanism. The potential of IFN-γ to enhance inflammation required the adhesion molecule CD11b because CD11b-deficient mice failed to show the synergistic effects of IFN-γ and MDP cotreatment on adhering and infiltrating cells.
Conclusions
IFN-γ was identified as a downstream mediator of NOD2-driven inflammation and the capacity of IFN-γ in vivo to enhance the inflammatory potential of NOD2 was demonstrated. Extrapolation of these findings in mice suggests that the dysregulation of IFN-γ may occur in patients with Blau syndrome, thereby contributing to the granulomatous nature of the disease.
The innate immune system is the line of protection first against invading microbial pathogens. Immune detection of microbes is mediated by pathogen recognition receptors, including membrane-bound TLRs and the nucleotide oligomerization domain (NOD)-like receptors (NLRs) located within the cytoplasm. The NLR family member NOD2 plays an important role in host defense against intracellular bacteria through its ability to sense muramyl dipeptide (MDP),1,2 a naturally occurring degradation product of peptidoglycan (PGN) present in Gram-positive and Gram-negative bacterial cell walls. Activated NOD2 exerts its inflammatory effects through its interactions with the adaptor protein RIP2, which results in the stimulation of NF-κB.3,4 NOD2 has also been implicated in the induction of other signal transduction pathways involving p38, JNK,5,6 and caspase-1.7–9
The importance of NOD2 in inflammation is further underscored by the fact that rare mutations in NOD2 cause the autoinflammatory disease Blau syndrome.10–14 Blau syndrome is an autosomal dominant disease, wherein granulomatous inflammation develops predominantly within the eye, skin, and joints.15–17 In addition, polymorphic variations in NOD2 increase the risk of Crohn disease,18–20 another inflammatory syndrome that results in granulomatous inflammation sometimes associated with uveitis. Thus, investigation of the functions of NOD2 within the eye may provide important insight into mechanisms involved in uveitis as occurs in Blau syndrome or even mechanisms involved in more common forms of uveitis. Such has been the case for NALP3-associated auto-inflammatory diseases. Elucidating the role of NALP3 in rare diseases such as Muckle-Wells syndrome has provided valuable insight into the regulation of IL-1β in more common diseases, including gout and delayed-type hypersensitivity responses.21
In addition to its expression in immune cells, NOD2 is present in nonlymphoid tissue including the eye.22,23 We have shown that NOD2 is functionally active in the eye because local treatment with MDP in mice elicits an ocular inflammatory response characterized by increased leukocyte rolling and adherence within the iris vasculature and infiltration within iris tissue.24 Importantly, NOD2 knockout (KO) mice fail to show ocular inflammation in response to MDP, indicating the essential role for NOD2 in MDP-induced uveitis. We have demonstrated that in this model system, IL-1β is a downstream product of NOD2 activation, yet caspase-1 and IL-1β fail to contribute to the development of ocular inflammation. However, we did find that IL-1β contributes to systemic inflammation triggered by NOD2.9 Thus, NOD2-driven uveitis involves distinct mediators other than IL-1 signaling. Here, we further explored the role of NOD2 in regulating inflammatory responses within the eye and tested the hypothetical role of the cytokine IFN-γ as a modulator of MDP-induced uveitis.
Methods
Reagents
Synthetic MDP (Bachem, Torrance, CA), lipopolysaccharide from Escherichia coli serotype 055:B5 (Sigma, St. Louis, MO), and mouse recombinant IFN-γ purchased from R&D Systems (Minneapolis, MN) served as the reagents. For in vivo injections, reagents were dissolved in sterile saline. For uveitis studies, mice were given intravitreal injection (2 μL) of each reagent using a Hamilton syringe with a 30-gauge half-inch needle. For peritonitis studies, mice were administered intraperitoneal injection of 100 μg MDP (in 0.5 mL), and the neutrophil influx in the peritoneal exudates was quantified by flow cytometry 5 hours later, as previously described.9
Mice
NOD2 KO mice (obtained from Richard Flavell of Yale University) were backcrossed onto a BALB/c background for 10 generations. IFN-γ KO mice and BALB/c control mice were purchased from Jackson Laboratories (Bar Harbor, ME). CD11b knockout mice on a C57Bl/6 background were obtained from C. Wayne Smith of Baylor University and then crossed with C57Bl/6 mice that lack the expression of tyrosinase (Jackson Laboratories) to generate tyrosinase mutant × CD11b KO mice. The tyrosinase mutant mice lacked pigment, a phenotype essential for performing intravital microscopy of iris. Mice were housed in a facility approved by the Association of Assessment and Accreditation of Laboratory Animal Care International. Procedures were carried out according to National Institutes of Health, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the Oregon Health & Science University Institutional Animal Care and Use policies. Age-matched (8- to 10-week-old) female mice were used in all experiments.
Intravital Microscopy
The leukocyte response within the vasculature and extravascular tissue of the iris was assessed by intravital microscopy, as previously described.24 Digital images of the iris vasculature were captured with a black-and-white video camera (Kappa Scientific, Gleichen, Germany) on an epifluorescence intravital microscope (modified Orthoplan; Leica, Wetzlar, Germany) in three independent regions. Diameter and length of each vessel segment or iris tissue and leukocyte phenotype (rolling, adhering, infiltrating) were quantified off-line with ImageJ analysis software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html), as previously described.24
ELISA
Protein was extracted from eye tissue, as previously described,9 in a buffer containing a protease inhibitor cocktail (Roche, Mannheim, Germany). Protein concentrations were determined (BCA kit; Pierce-Endogen, Rockford, IL), and equal amounts of protein for each sample were used for the measurement of cytokines by ELISA. IFN-γ, IL-12p40, IL-27, and IL-17 production were measured using commercially available ELISA kits (R&D Systems).
Statistical Analysis
Data are represented as mean ± SEM. Mean differences between treatment and genotype were analyzed using two-way and one-way analysis of variance with Bonferroni test or t-test post hoc analyses. Differences were considered statistically significant when P < 0.05.
Results
IFN-γ Production within the Eye as a Result of NOD2 Activation
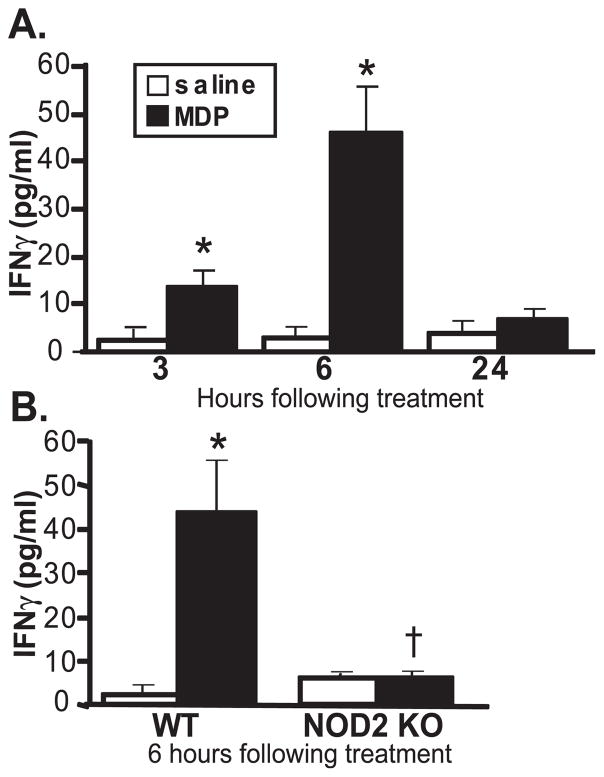
As mentioned earlier, several pathogenic mutations of NOD2 have been identified, yet the functions of NOD2 that trigger uveitis remain poorly understood. We used a previously characterized mouse model of NOD2-dependent ocular inflammation induced by local treatment with MDP,24 wherein the peak of the intravascular response occurred at 6 hours. We first tested the capacity of MDP to upregulate IFN-γ production within the eye. Within 3 hours of intraocular injection of MDP, IFN-γ levels were significantly increased in the eye (Fig. 1A). IFN-γ levels in the eye returned to baseline by 24 hours after MDP treatment, at which time the cellular inflammatory response in the eye resolved as previously reported.24 MDP-induced IFN-γ production was abolished as a consequence of NOD2 deficiency (Fig. 1B), indicating NOD2 as an essential mediator of MDP-induced IFN-γ production within the eye.
Figure 1.
Activation of NOD2 results in IFN-γ production within the eye. Mice were treated with intravitreal injection of 100 μg MDP or saline. IFN-γ production in whole eye tissue homogenates was measured by ELISA as a function of time after treatment (A) or in NOD2 knockout (NOD2 KO) compared with their control (wild-type [WT]) mice (B) 6 hours after treatment. Data are mean ± SEM. *P < 0.05 comparison between MDP and saline within a genotype. †P < 0.05 comparison between MDP-treated NOD2 KO and WT mice (n = 6–8 mice/treatment/time).
IFN-γ, a Downstream Mediator of NOD2-Dependent Ocular Inflammation
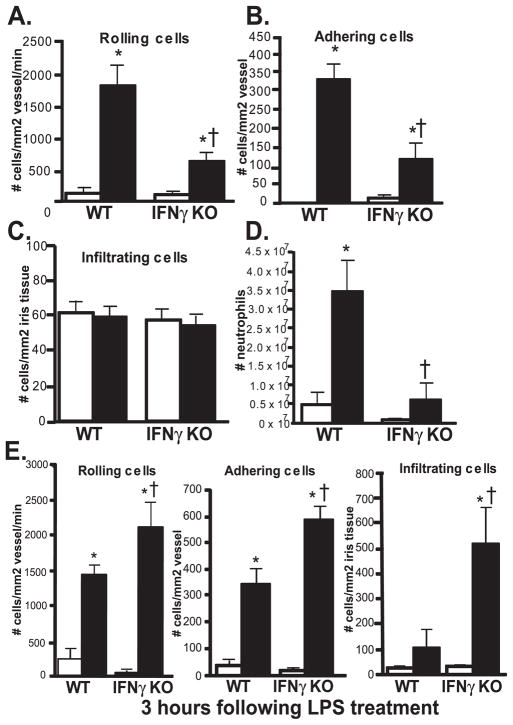
A role for IFN-γ as a downstream mediator of NOD2 responses in vivo has yet to be established. IFN-γ KO mice were used to elucidate the functional involvement of IFN-γ in MDP-triggered ocular inflammation. We found that a deficiency in IFN-γ significantly reduced two main hallmarks of the cellular inflammatory response: leukocyte rolling and adherence induced by MDP injection (Figs. 2A, 2B). The number of infiltrators was not altered by IFN-γ deficiency (Fig. 2C), but this was not surprising given our earlier characterization of the kinetics of MDP-induced uveitis,24 wherein infiltration occurred at later time points. We did, however, assess inflammation at 24 hours, when MDP-induced inflammation was completely resolved, and we did not observe any difference between WT and IFN-γ KO mice, indicating that IFN-γ deficiency did not alter the kinetics or delay the inflammatory response elicited by MDP.
Figure 2.
IFN-γ is a downstream mediator of NOD2-dependent inflammation. (A) IFN-γ knockout mice (IFN-γ KO) and their controls (WT) were treated with intravitreal injection of 100 μg MDP or saline. (A–C) The ocular inflammatory response within the iris vasculature was assessed by intravital microscopy 6 hours after treatment (peak of intravascular inflammatory response to MDP), and the numbers of rolling, adhering, and infiltrating leukocytes were quantified. (D) IFN-γ KO or WT mice were treated with intraperitoneal injection of 100 μg MDP, and the neutrophil influx in peritoneal exudates was quantified by flow cytometry 6 hours later. (E) IFN-γ KO or WT mice were given intravitreal injection of 250 ng LPS, and ocular inflammation was assessed by intravital microscopy 3 hours later for quantification of leukocyte rolling, adhering, and infiltration. Data are mean ± SEM. *P < 0.05 comparison between saline and treatment within a genotype. †P < 0.05 comparison between treated-IFN-γ KO and WT mice (n = 6–8 mice/genotype/treatment).
Our earlier studies demonstrated differential requirements for IL-1 signaling in NOD2-driven inflammation in the eye compared with peritoneal inflammation.9 Understanding the unique mechanisms within the eye compared with systemic responses may be relevant to the development of therapeutic approaches for uveitis and Blau syndrome, which involves a systemic component of disease in addition to uveitis. Therefore, we tested the role of IFN-γ as a downstream mediator of NOD2-triggered systemic inflammation in a model of MDP-induced peritonitis. We previously demonstrated that intraperitoneal injection of MDP results in a significant neutrophil influx in peritoneal exudates in mice that is completely abolished in the absence of NOD2.9 We show here that IFN-γ deficiency abrogates neutrophil influx in response to MDP (Fig. 2D). These findings indicate that in contrast to IL-1β, IFN-γ is a pivotal mediator of NOD2-inflammation in more than one organ system because ocular and systemic forms of inflammation were reduced by the absence of IFN-γ. Thus, IFN-γ may be an especially relevant regulator of NOD2 and Blau syndrome. Interestingly, we found that in contrast to MDP-induced uveitis, IFN-γ deficiency resulted in exacerbated ocular inflammation in a different and well-established model of endotoxin-induced uveitis (EIU) (Fig. 2E). We also observed that IFN-γ deficiency exacerbated endotoxin-induced uveitis 6 hours after treatment in mice treated with suboptimal doses of 20 ng LPS (data not shown), further supporting the protective role for IFN-γ in EIU. Thus, IFN-γ appeared to play opposing roles in two different models of uveitis, indicating that it may have a positive or a negative influence, depending on the initiating agent.
Greater Ocular Inflammation in Mice in a NOD2-Dependent Fashion due to Synergy of IFN-γ with MDP
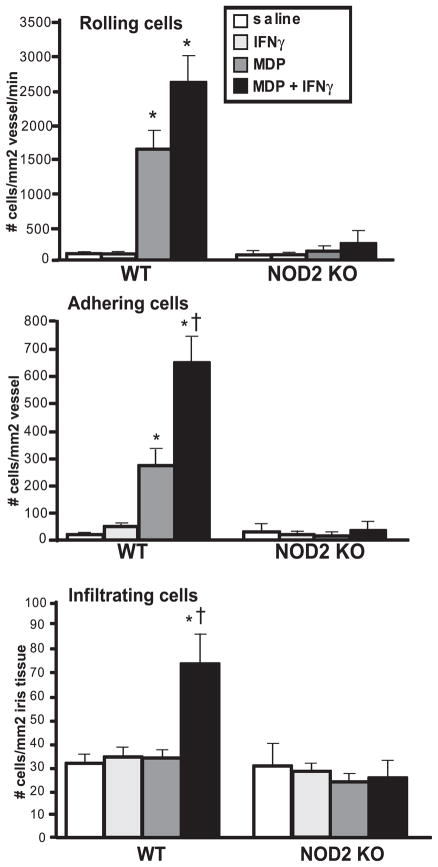
Given our data here demonstrating that IFN-γ is a downstream mediator of NOD2-induced ocular inflammation, we further hypothesized that IFN-γ could, in turn, amplify the inflammatory potential of NOD2 in vivo. IFN-γ has previously been implicated in regulating the amplitude of TLR responses in macrophages,25,26 wherein IFN-γ cotreatment exacerbated TLR-triggered cytokine production. Whether IFN-γ regulates NOD2 function within the eye in a similar capacity, as in TLRs, is unknown. To address this question, mice were treated locally with MDP in the presence or absence of IFN-γ, and ocular inflammation was assessed at 6 hours by intravital microscopy (Fig. 3). Interestingly, we found that the administration of IFN-γ alone failed to cause ocular inflammation. Indeed, treatment with IFN-γ up to a dose of 2 μg did not cause any ocular inflammation (data not shown), indicating that it was not an especially inflammatory stimulant by itself. However, when coinjected with MDP, IFN-γ markedly exacerbated ocular inflammation because the number of adhering and infiltrating leukocytes was significantly increased compared with MDP treatment alone. We did not observe a significant effect of IFN-γ cotreatment on the number of rolling leukocytes, though a trend for increased rolling was observed. These data indicate that IFN-γ preferentially enhances leukocyte-endothelial interactions involved in cell adherence and subsequent infiltration when NOD2 is activated. We also demonstrate that the synergistic effect of MDP and IFN-γ on the ocular inflammatory response was abolished in NOD2 KO mice (Fig. 3), indicating the essential role for NOD2 in this phenomenon.
Figure 3.
IFN-γ exacerbates ocular inflammation in vivo in a NOD2-dependent fashion. Mice were treated with intravitreal injection of 100 μg MDP or saline in the presence or absence of 500 ng mouse recombinant IFN-γ. The ocular inflammatory response within the iris vasculature was assessed by intravital microscopy 6 hours after treatment (peak of inflammatory response to MDP), and the numbers of rolling, adhering, and infiltrating leukocytes were quantified. Data are mean ± SEM. *P < 0.05 comparison between saline and treatment within a genotype. †P < 0.05 comparison between MDP and MDP + IFN-γ within a genotype (n = 6–10 mice/treatment).
No Synergistic Effect of MDP and IFN-γ in CD11b-Deficient Mice
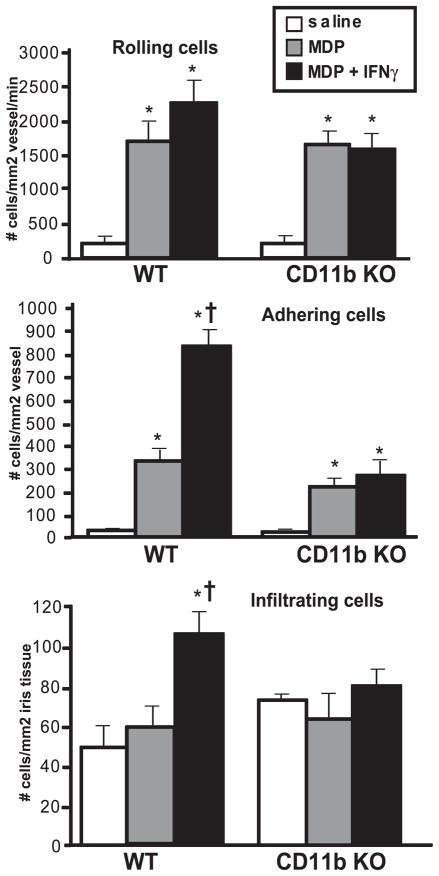
The inflammatory cascades triggered by NOD2 involving leukocyte recruitment and endothelial interactions have not been described. Because our findings indicated that IFN-γ potentiated MDP-induced leukocyte adherence and infiltration, we further explored the mechanism by which cellular adherence and infiltration could be enhanced. A particular advantage of intravital microscopy is that it allows visualization of the cellular inflammatory responses occurring within the eye in real-time and dissection of distinct leukocyte functions such as leukocyte rolling, adherence, or tissue infiltration. All three of these processes occur in a stepwise continuum mediated by different cell adhesion molecules.27 Our findings indicated that IFN-γ potentiated leukocyte adherence and infiltration; therefore, we hypothesized that IFN-γ enhances ocular inflammation by promoting leukocyte-endothelial interactions in a CD11b-dependent fashion. CD11b is an adhesion molecule expressed on myeloid cells and is responsible for leukocyte-endothelial adherence through its interaction with ICAM-1 expressed on endothelial cells. The interaction between these two adhesion molecules is pivotal for cell adherence and, thus, was likely to play a role here in MDP-IFN-γ synergy. To test the functional involvement of CD11b in the exacerbated cellular response in the eye induced by IFN-γ, we used CD11b KO mice and assessed their ocular inflammation by intravital microscopy (Fig. 4). In contrast to congenic control mice that demonstrate exacerbated adherence and infiltration in response to MDP and IFN-γ cotreatment, CD11b KO mice failed to exhibit worsened inflammation in the presence of MDP and IFN-γ. This finding indicates the essential role for CD11b in the exacerbated leukocyte adherence and most likely subsequent infiltration in the presence of MDP and IFN-γ. Interestingly, CD11b deficiency did not appear to affect MDP-induced adherence, suggesting that combination NOD2 and IFN-γ signaling results in unique inflammatory responses possibly involving different adhesion molecules.
Figure 4.
Exacerbation of cellular responses in the eye by IFN-γ is blocked by CD11b deficiency. CD11b knockout mice (CD11b KO) and their controls (WT) were treated with intravitreal injection of 100 μg MDP or saline in the presence or absence of 500 ng mouse recombinant IFN-γ, and the ocular inflammatory response was assessed by intravital microscopy at 6 hours. Data are mean ± SEM. *P < 0.05 comparison between saline and treatment within a genotype. †P < 0.05 comparison between MDP and MDP + IFN-γ within a genotype (n = 6–8 mice/treatment).
Discussion
In addition to skin and joints, the eye is one of the three organs most consistently affected in Blau syndrome, which results in granuloma formation in affected tissues. Thus, understanding the function of NOD2 within this specific tissue is critical to clarifying this disease. Investigation of a gene that so clearly links innate immunity to disease could also further our understanding of more common forms of uveitis. To gain insight into functional consequences of NOD2 activation on eye inflammation, we investigated the role of IFN-γ, a cytokine involved in granuloma formation, in a previously established mouse model of MDP-induced uveitis. Taken together, our findings reveal a novel role for IFN-γ as a downstream mediator of NOD2 in ocular inflammation and demonstrate that IFN-γ has the capacity to act as a regulator of NOD2 function in vivo, thereby synergizing with MDP-induced ocular inflammation.
The role of IFN-γ as a participant in NOD2 inflammation has not been previously studied in vivo. Our findings identify IFN-γ as a downstream modulator of NOD2 inflammatory functions in the eye and systemically. Thus, in contrast to our earlier findings with IL-1β, IFN-γ appears to be a regulator of NOD2-driven inflammation in the eye and peritoneum. MDP activated IFN-γ production within the eye in a NOD2-dependent fashion, consistent with an earlier report indicating that MDP stimulation results in IFN-γ production in freshly isolated hepatocytes.28 Moreover, our findings demonstrate that in the absence of IFN-γ, ocular inflammation is diminished. IFN-γ is a cytokine regulated transcriptionally by NF-κB. Because most of the inflammatory effects of NOD2 are believed to be mediated by NF-κB activation, it seems likely that this could be the mechanism by which IFN-γ could be produced in response to MDP. NOD2 is expressed in immune cells, including neutrophils and macrophages, and in nonlymphoid tissue including endothelial cells within the choroid and retina,23 thereby suggesting that activation of NOD2 within the vascular endothelium—or perhaps activation in neutrophils, macrophages, NK cells or possibly even T cells— could result in IFN-γ production, though this remains to be tested. Indeed, in the case of Salmonella or Listeria infection wherein innate immunity and NOD2 play pivotal roles in host defense, IFN-γ –producing phagocytes predominate in the early course of infection.29,30 Future investigation should elucidate the mechanisms by which IFN-γ is regulated by NOD2 within the eye.
Our findings identify IFN-γ as a promoter of NOD2 ocular inflammation, which would be contradictory to the role of IFN-γ in other models of uveitis in which T cells are critical regulators. Although autoimmune uveitis can occur with IFN-γ and IL-17 responses,31 IFN-γ has been considered protective.32,33 It is thought that the protective effects of IFN-γ result in part from its suppressive actions on IL-17–driven ocular inflammation. Notably, we have not been able to detect IL-17 protein production in the eye in response to MDP at 2 or 6 hours after treatment (data not shown). However, consistent with the role for IFN-γ, we have observed a significant increase in protein production of other Th1-associated cytokines, IL-12p40 and IL-27 (data not shown). The role for NOD2 in modulating the IL-17 axis has not been completely established. Activation of NOD2 expressed within dendritic cells promotes IL-17 production in human memory T cells in vitro.34 Our own studies in a mouse model of proteoglycan-induced arthritis support a role for MDP in promoting a Th1 response known to contribute to disease in this model.35–38 We have observed that MDP treatment exacerbates disease along with the antigen-mediated production of Th1 cytokines, IFN-γ and IL-12p40, but not Th17 cytokines such as IL-17, IL-6, TGF-β.39
It is also possible that immune status influences the nature of inflammatory responses. Contrary to earlier reports examining the role of IL-17, MDP-induced uveitis is likely to occur independently of T cells because it is an acute inflammatory response characterized primarily by innate immune cells such as neutrophils and macrophages. Interestingly, we demonstrated that IFN-γ deficiency exacerbated EIU in mice, which is consistent with an earlier report wherein IFN-γ played a role in the development of ocular inflammation.40 Moreover, administration of exogenous IFN-γ suppressed EIU,40 whereas our findings demonstrated a role for IFN-γ in exacerbating MDP-induced uveitis. This suggested that even innate-driven ocular inflammatory responses can differ with respect to the role of IFN-γ and that TLR4 and NOD2 may result in opposing mechanisms of uveitis.
The capacity of IFN-γ to regulate the amplitude of TLR responses in macrophages has been described; however, whether IFN-γ regulates NLR responses in a similar fashion has not been examined. A single report indicated that IFN-γ -and MDP-stimulated macrophages produce more NO in vitro,41,42 yet there has been no evidence to demonstrate that IFN-γ can enhance NOD2-induced cellular inflammatory responses in vivo. Our studies here indicate that IFN-γ and MDP synergize in vivo in the exacerbation of ocular inflammation. The mechanisms by which IFN-γ synergizes with NOD2 are not known. Others and we have shown that NOD2 expression in a variety of cell types can be enhanced by IFN-γ,28,42,43 including human ocular endothelial cells,23 suggesting that the increased expression of NOD2 could promote inflammation. Our findings here also indicate that IFN-γ appears to preferentially enhance leukocyte-endothelial interactions involving adherence and cellular infiltration, a process abolished in the absence of CD11b expression. We have found that the expression of CD11b and ICAM-1, the endothelial ligand for CD11b, in eye tissue is increased slightly less than twofold in response to cotreatment with MDP and IFN-γ (data not shown), indicating that changes in expression of these two adhesion molecules are not limiting factors in MDP-induced uveitis or MDP-IFN-γ synergy. IFN-γ by itself is a relatively weak inducer of NF-κB or MAPK. Rather, most of the more well-described biological activities of IFN-γ are mediated by the JAK-STAT signaling pathway. However, it is possible that in NOD2 activation, the NF-κB or MAPK pathways may be preferentially enhanced by IFN-γ. Or perhaps the expression of factors regulated by IFN-γ, such as IP-10, MIP-1α and MIP-1β, RANTES, and MCP-1, could provide unique signals for cellular recruitment that are not otherwise induced by NOD2. Given that NOD2 and TLR pathways share some common signaling mediators, it is intriguing to speculate whether an analogous mechanism by which IFN-γ amplifies TLR responses could occur for NOD2 responses. In enhanced TLRs, one theory is that IFN-γ synergizes with TLRs in part by suppressing a TLR-induced feedback inhibition, which is a response that involves IL-10 and STAT3.25 Further investigation of how inflammatory mediators are differentially regulated in the setting of IFN-γ and NOD2 signaling will be informative.
Our findings here along with the studies described support a role for NOD2 in promoting ocular inflammatory responses, yet they would be contradictory to the role of NOD2 in animal models of inflammatory bowel disease. In this case, NOD2 is considered to function as a negative regulator of PGN-induced responses such that in its absence, Th1 responses and colitis are exacerbated.44 The authors’ interpretation is that loss of function as a consequence of Crohn-associated mutations in NOD2 results in inappropriate intestinal inflammatory responses to TLRs over time and predisposes to disease. This differs from mutations in NOD2 causing Blau syndrome, which is an autosomal dominant disease in which infection does not appear to play a role. In contrast to polymorphisms associated with Crohn disease, the mutations causing Blau syndrome have been suggested to result in constitutive NOD2-driven NF-κB activity.45 IFN-γ is a cytokine regulated by NF-κB, and our data here indicate it is a downstream mediator of NOD2 inflammation, making it intriguing to speculate that in Blau syndrome, dysregulation of NOD2 activation could result in excessive IFN-γ production. Excessive IFN-γ production could in turn further enhance NOD2-induced inflammation. This hypothesis would be consistent with the known role of IFN-γ as a regulator of granuloma formation, a hallmark feature of Blau syndrome and Crohn disease. Although our model of MDP-induced eye inflammation is a valuable tool for exploring the inflammatory effects of NOD2 activation in vivo, we recognize that the ocular inflammation in our mouse model is transient and differs from sustained, granulomatous inflammation seen in Blau syndrome. Intriguingly, we have also found a role for NOD2 in promoting joint disease in a chronic model of immune-mediated arthritis.39 Clearly, how different mutations in NOD2 alter its function and cause different diseases is not entirely understood. Future investigation of NOD2 functions in human ocular tissue would be informative.
In conclusion, inflammatory responses must be modulated appropriately for the specific tissue environment, especially within the eye, where it is vital to avoid inflammation that can compromise vision. Thus, the investigation of NOD2 inflammatory functions within the eye, which is a tissue clearly affected in Blau syndrome, should provide important insight into underlying mechanisms of this disease. Our studies here contribute to the understanding of inflammatory functions of NOD2 involving IFN-γ.
Acknowledgments
Supported by National Eye Institute Grants F32-EY017254 and EY013093; Research to Prevent Blindness (JTR, TMM, Casey Eye Institute); and the Stan and Madelle Rosenfeld Family Trust.
The authors thank C. Wayne Smith of Baylor University and Richard Flavell of Yale Medical School for their donations of CD11b KO and NOD2 mice, respectively. They also thank Monica Jann, AiLien Truong, and Hari Sawkar for their technical contributions.
Footnotes
Disclosure: H.L. Rosenzweig, None; T. Kawaguchi, None; T.M. Martin, None; S.R. Planck, None; M.P. Davey, None; J.T. Rosenbaum, None
References
- 1.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 2.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K, Inohara N, Hernandez LD, et al. RICK/Rip2/CAR-DIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Kim YG, McDonald C, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 5.Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–190. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu YM, Zhang Y, You Y, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 7.Pan Q, Mathison J, Fearns C, et al. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82:177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 8.Ferwerda G, Kramer M, de Jong D, et al. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur J Immunol. 2008;38:184–191. doi: 10.1002/eji.200737103. [DOI] [PubMed] [Google Scholar]
- 9.Rosenzweig HL, Martin TM, Planck SR, et al. Activation of NOD2 in vivo induces IL-1 beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol. 2008;84:529–536. doi: 10.1189/jlb.0108015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Kuivaniemi H, Bonavita G, et al. CARD15 mutations in familial granulomatosis syndromes: a study of the original Blau syndrome kindred and other families with large-vessel arteritis and cranial neuropathy. Arthritis Rheum. 2002;46:3041–3045. doi: 10.1002/art.10618. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa T, Kikuchi T, Ohta K, Imai H, Yoshimura N. Ocular manifestations in Blau syndrome associated with a CARD15/Nod2 mutation. Ophthalmology. 2003;110:2040–2044. doi: 10.1016/S0161-6420(03)00717-6. [DOI] [PubMed] [Google Scholar]
- 13.Rose CD, Doyle TM, McIlvain-Simpson G, et al. Blau syndrome mutation of CARD15/NOD2 in sporadic early onset granulomatous arthritis. J Rheumatol. 2005;32:373–375. [PubMed] [Google Scholar]
- 14.Rose CD, Wouters CH, Meiorin S, et al. Pediatric granulomatous arthritis: an international registry. Arthritis Rheum. 2006;54:3337–3344. doi: 10.1002/art.22122. [DOI] [PubMed] [Google Scholar]
- 15.Blau EB. Familial granulomatous arthritis, iritis, and rash. J Pediatr. 1985;107:689–693. doi: 10.1016/s0022-3476(85)80394-2. [DOI] [PubMed] [Google Scholar]
- 16.Pastores GM, Michels VV, Stickler GB, Su WP, Nelson AM, Bovenmyer DA. Autosomal dominant granulomatous arthritis, uveitis, skin rash, and synovial cysts. J Pediatr. 1990;117:403–408. doi: 10.1016/s0022-3476(05)81080-7. [DOI] [PubMed] [Google Scholar]
- 17.Jabs DA, Houk JL, Bias WB, Arnett FC. Familial granulomatous synovitis, uveitis, and cranial neuropathies. Am J Med. 1985;78:801–804. doi: 10.1016/0002-9343(85)90286-4. [DOI] [PubMed] [Google Scholar]
- 18.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 19.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 20.Hampe J, Frenzel H, Mirza MM, et al. Evidence for a NOD2-independent susceptibility locus for inflammatory bowel disease on chromosome 16p. Proc Natl Acad Sci U S A. 2002;99:321–326. doi: 10.1073/pnas.261567999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Martinez S, Cancino-Diaz ME, Jimenez-Zamudio L, Garcia-Latorre E, Cancino-Diaz JC. TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol. 2005;89:904–910. doi: 10.1136/bjo.2004.056218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT. Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvasc Res. 2006;71:103–107. doi: 10.1016/j.mvr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig HL, Martin TM, Jann MM, et al. NOD2, the gene responsible for familial granulomatous uveitis, is essential in a mouse model of muramyl dipeptide-induced uveitis. Invest Ophthalmol Vis Sci. 2008;49:1518–1524. doi: 10.1167/iovs.07-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 26.Bosisio D, Polentarutti N, Sironi M, et al. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–3431. doi: 10.1182/blood.v99.9.3427. [DOI] [PubMed] [Google Scholar]
- 27.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 28.Body-Malapel M, Dharancy S, Berrebi D, et al. NOD2: a potential target for regulating liver injury. Lab Invest. 2008;88:318–327. doi: 10.1038/labinvest.3700716. [DOI] [PubMed] [Google Scholar]
- 29.Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002;169:4450–4459. doi: 10.4049/jimmunol.169.8.4450. [DOI] [PubMed] [Google Scholar]
- 30.Czuprynski CJ, Haak-Frendscho M. Non-specific resistance mechanisms to listeriosis: implications for experimental and naturally occurring infection. Immunol Rev. 1997;158:47–56. doi: 10.1111/j.1600-065x.1997.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 31.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su SB, Grajewski RS, Luger D, et al. Altered chemokine profile associated with exacerbated autoimmune pathology under conditions of genetic interferon-gamma deficiency. Invest Ophthalmol Vis Sci. 2007;48:4616–4625. doi: 10.1167/iovs.07-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshimura T, Sonoda KH, Miyazaki Y, et al. Differential roles for IFN-gamma and IL-17 in experimental autoimmune uveoretinitis. Int Immunol. 2008;20:209–214. doi: 10.1093/intimm/dxm135. [DOI] [PubMed] [Google Scholar]
- 34.Van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Finnegan A, Grusby MJ, Kaplan CD, et al. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J Immunol. 2002;169:3345–3352. doi: 10.4049/jimmunol.169.6.3345. [DOI] [PubMed] [Google Scholar]
- 36.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 37.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–5390. [PubMed] [Google Scholar]
- 38.Doodes PD, Cao Y, Hamel KM, et al. Development of proteoglycan-induced arthritis is independent of IL-17. J Immunol. 2008;181:329–337. doi: 10.4049/jimmunol.181.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenzweig HL, Jann MM, Glant TT, et al. Activation of nucleotide oligomerization domain 2 exacerbates a murine model of proteoglycan-induced arthritis. J Leukoc Biol. doi: 10.1189/jlb.0808478. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukushima A, Ozaki A, Ishida W, Fukata K, Ueno H. Systemic interferon-gamma suppresses the development of endotoxin-induced uveitis in mice. Curr Eye Res. 2005;30:7–12. doi: 10.1080/02713680490522461. [DOI] [PubMed] [Google Scholar]
- 41.Kengatharan KM, De Kimpe S, Robson C, Foster SJ, Thiemermann C. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J Exp Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Totemeyer S, Sheppard M, Lloyd A, et al. IFN-gamma enhances production of nitric oxide from macrophages via a mechanism that depends on nucleotide oligomerization domain-2. J Immunol. 2006;176:4804–4810. doi: 10.4049/jimmunol.176.8.4804. [DOI] [PubMed] [Google Scholar]
- 43.Rosenstiel P, Fantini M, Brautigam K, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 45.Kanazawa N, Okafuji I, Kambe N, et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-κB activation: common genetic etiology with Blau syndrome. Blood. 2005;105:1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]