Abstract
We propose genetic guidelines for the classification of rickettsial isolates at the genus, group, and species levels by using sequences of the 16S rRNA (rrs) gene and four protein-coding genes, the gltA, ompA, and ompB genes and gene D. To be classified as a member of the genus Rickettsia, an isolate should exhibit degrees of rrs and gltA homology with any of the 20 Rickettsia species studied of ≥98.1 and ≥86.5%, respectively. A member of the typhus group should fulfill at least two of the following four criteria: pairwise nucleotide sequence homologies with rrs, gltA, ompB, and gene D of either Rickettsia typhi or Rickettsia prowazekii of ≥99.4, ≥96.6, ≥92.4, and ≥91.6%, respectively. A member of the spotted fever group should either possess the ompA gene or fulfill at least two of the following four criteria: pairwise nucleotide sequence homologies with rrs, gltA, ompB, and gene D of any member of this group of ≥98.8, ≥92.7, ≥85.8, and ≥82.2%, respectively. The existence of a distinct “ancestral” group should be questioned. To be classified as a new Rickettsia species, an isolate should not exhibit more than one of the following degrees of nucleotide similarity with the most homologous validated species: ≥99.8 and ≥ 99.9% for the rrs and gltA genes, respectively, and, when amplifiable, ≥98.8, ≥99.2, and ≥99.3% for the ompA and ompB genes and gene D, respectively. By use of our classification scheme, “Rickettsia heilongjiangii” belongs to a new species for which we officially propose the name Rickettsia heilongjiangensis sp. nov.
The order Rickettsiales initially consisted of most of the bacteria associated with eukaryotic cells (63). On the basis of their 16S rRNA gene (rrs) sequences, Rickettsiella grylli, Coxiella burnetii, Wolbachia persica, Rochalimaea spp., Grahamella spp., Bartonella spp., Eperithrozoon ovis, and Hemobartonella spp. have been removed from the order Rickettsiales (5, 7, 41, 43, 62), which comprises only the genera Rickettsia, Orientia, Ehrlichia, Anaplasma, Neorickettsia, and Wolbachia. Historically, the genus Rickettsia was divided into three groups on the basis of phenotypic criteria: the typhus group, the spotted fever group, and the scrub typhus group (63). In 1995, Tamura et al. (53) proposed reclassification of Rickettsia tsutsugamushi (33) into a new genus, Orientia, using 16S rRNA gene sequence analysis. The same year, Stothard and Fuerst (52) suggested that Rickettsia bellii (36) and Rickettsia canadensis (27) represented a phylogenetic line that predated the typhus-spotted fever group split and, thus, were included into a fourth group named the “ancestral” group. At present, the genus Rickettsia contains 21 validated species classified into three groups: (i) the ancestral group described above; (ii) the typhus group, which includes Rickettsia prowazekii (12) and Rickettsia typhi (34); and (iii) the spotted fever group, which consists of Rickettsia rickettsii (9), Rickettsia conorii (10), Rickettsia africae (20), Rickettsia sibirica (67), Rickettsia slovaca (48), Rickettsia honei (51), Rickettsia japonica (56), Rickettsia australis (35), Rickettsia akari (19), Rickettsia felis (6), Rickettsia aeschlimannii (1), Rickettsia helvetica (2), Rickettsia massiliae (3), Rickettsia rhipicephali (11), Rickettsia montanensis (63), Rickettsia parkeri (22), and Rickettsia peacockii (31). In addition to the 21 recognized species, more than 20 rickettsial isolates which have not been fully characterized or which have not received a species designation have been described, and the classification of these isolates is confusing (42).
The most widely accepted description of bacterial species is based on the results of DNA-DNA hybridization and the description of phenotypic characteristics in a polyphasic classification strategy (18, 61). However, when the 70% DNA-DNA relatedness cutoff (61) is applied to rickettsiae, R. rickettsii, R. conorii, R. sibirica, and R. montanensis would belong to the same species (28, 58); thus, classification of members of the genus Rickettsia is still based on the mouse serotyping method developed in 1978 (37). This test detects specific epitopes of the high-molecular-mass, surface-exposed protein antigens (rOmpA, rOmpB, and 120-kDa proteins) of rickettsiae. However, mouse immunization is not highly reproducible and requires a large amount of work because each new isolate must be compared to all previously described species. Therefore, new taxonomic methods for the classification of rickettsiae should be developed.
In recent years, several genes have been sequenced for most of the known rickettsial isolates, including the panbacterial genes encoding 16S rRNA (rrs) (44) and citrate synthase (gltA) (46) and the Rickettsia-specific ompA (16) and ompB (45) genes and gene D (47), which encode the surface-exposed, high-molecular-weight proteins rOmpA, rOmpB, and PS120, respectively. The usefulness of DNA taxonomy has been recognized for living organisms (54), and Maiden et al. (26) have demonstrated the usefulness of sequencing multiple genes for taxonomic purposes. The Ad Hoc Committee for the Re-Evaluation of the Species Definition in Bacteriology (50) emphasized the need to sequence a minimum of five genes, including protein-coding genes. In an attempt to obtain gene sequence-based data for the classification of Rickettsia at various taxonomic levels, including the genus, group, and species levels, we compared the published sequences of the complete rrs, gltA, and ompB genes and gene D as well as the 5′ end of the ompA gene of all validated Rickettsia species except R. peacockii, for which the rrs, gltA, and ompA sequences available in GenBank are only partial and the ompB and gene D sequences are not available. Our aim was to set up objective guidelines that would allow any scientist to classify bacterial isolates as members or not members of the Rickettsia genus at various taxonomic levels.
MATERIALS AND METHODS
Selection, editing, and comparison of nucleotides sequences.
We used the nucleotide sequences available in GenBank of the rrs, gltA, ompA, and ompB genes and gene D of both validated and as yet unclassified Rickettsia species and of one member of each of the genera Orientia, Ehrlichia, Anaplasma, Neorickettsia, and Wolbachia, which, together with the genus Rickettsia, constitute the order Rickettsiales. Sequences were aligned by using the multiple-sequence alignment program CLUSTAL W (55). Sequences were edited by removal of fragments at the 5′ and 3′ ends so that their lengths matched that of the longest sequence common to all species compared. By comparison with R. rickettsii, we used the sequences of fragments between bases 17 and 1,424 for the rrs gene (GenBank accession number L36217), bases 87 and 1,134 for the gltA gene (GenBank accession number U59729), bases 1 and 590 for the 5′ end of the ompA gene (U43804), bases 296 and 5,141 for the ompB gene (GenBank accession number X16353), and bases 33 and 2,979 for gene D (GenBank accession number AF163000). In order to avoid misclassifications linked to deletions and/or insertions that were not inherited from common ancestors, as has been observed for the ompA gene among R. conorii strains (16), but that were caused by various events, including errors in DNA replication, only pairwise transitions and transversions between sequences, not deletions and insertions, were taken into account to calculate the degree of sequence homology (in percent).
In order to estimate the neutrality of the nucleotide sequence variations within the four protein-encoding genes used in this study, we used the Z test for large data sets (29) within the MEGA (version 2.1) software package (21). We used this test to estimate the neutral evolution of our sequences by calculation of the differences in synonymous (dS) and nonsynonymous (dN) substitutions among them. The variance of the differences between the average dS and the average dN was estimated by bootstrap analysis. A bootstrap value of <0.05 indicated that the gene did not undergo neutral evolution.
Calculation of cutoff values at genus, group, and species levels.
To calculate cutoff values at the genus and group levels, we first calculated the mean sequence homology between species accepted as belonging to the genus Rickettsia or to each group within this genus on the basis of phenotypic criteria. For the genus and the spotted fever group means, the standard deviation (SD) was calculated. The cutoff was then defined as the mean less 3 SDs. Thus, a strain exhibiting a degree of homology at least 3 SDs lower than the mean sequence divergence between each pair of species belonging to a given group would be likely (with more than 99% probability) not to belong to that group.
To test the validity of the criteria established in this way, the entire similarity matrix that included each appropriate species for each gene was recalculated iteratively, with each accepted species omitted in succession. In this manner, 104 independent “omit” matrices were determined. The means and SDs of these new omit similarity matrices were calculated and used for comparison with the values for the sequences of the omitted species to determine whether it could be classified correctly. The sequences of several species had sufficient divergence that their inclusion skewed the validity of the comparisons with sequences and tests for several calculations, suggesting that their omission would result in more valid classification criteria. When the inclusive similarity matrices were then recalculated by omitting the values only for the outlying species that introduced bias for that specific gene alignment, the criteria were retested and found to more frequently predict the correct taxonomic assignment for accepted species, group, and genera. Thus, the data for the sequences of the outlying species were omitted from the final matrices and calculations used to determine inclusion or exclusion cutoff criteria.
The variance of the p distance was calculated for each of the five genes studied by using the MEGA (version 2.1) software package (21) and was used to estimate the normality of the distribution of sequence similarities among the Rickettsia species studied (30). A variance of <0.05 indicated that the similarity values were not normally distributed.
In order to validate each cutoff, we applied it to the pairwise sequence homology rates among all species used to establish the cutoff as well as the species used as outgroups. We determined the sensitivity of a cutoff for a given group by dividing the number of pairwise sequence homology rates that were above the cutoff among members of this group by the total number of pairwise comparisons within this group. Conversely, we calculated the specificity of this cutoff by dividing the number of pairwise sequence homology rates that were above the cutoff among the species used as outgroups by the total number of pairwise comparisons among these species. However, as each of the typhus and ancestral groups contained only two validated species, we used the homology rate between the two species as cutoffs and could not perform any omit test.
The inclusion of a bacterial isolate into the genus Rickettsia was based on the cutoffs for rrs and gltA, as only the sequences of these two genes were available for the outgroup species, i.e., Orientia, Ehrlichia, Anaplasma, Neorickettsia, and Wolbachia (Table 1). A bacterial isolate not fulfilling the genus criterion was not processed for further classification.
TABLE 1.
Accession numbers of gene sequences used in the present study and their original sources
| Species | Strain | GenBank accession no.
|
||||
|---|---|---|---|---|---|---|
| 16SrDNA | gltA | ompA | ompB | Gene D | ||
| Validated species | ||||||
| R. prowazekiia | Breinl, ATCC VR-142T | M21789 | M17149 | NAb | AF123718 | AF200340 |
| R. typhia | Wilmington, ATCC VR-144T | L36221 | U59714 | NA | L04661 | AF188482 |
| R. belliia | 369L42-1 | L36103 | U59716 | NA | NA | NA |
| R. canadensisa | 2678 | L36104 | U59713 | NA | NA | NA |
| R. helveticaa | C9P9 | L36212 | U59723 | NA | AF123725 | AF163009 |
| R. rickettsiia | R (Bitteroot), ATCC VR-891T | L36217 | U59729 | U43804 | X16353 | AF163000 |
| R. conoriia | Seven (Malish), ATCC VR-613T | AF541999 | U59730 | U43806 | AF123721 | AF163008 |
| R. africaea | ESF-5 | L36098 | U59733 | U43790 | AF123706 | AF151724 |
| R. sibiricaa | 246, ATCC VR-151T | L36218 | U59734 | U43807 | AF123722 | AF155057 |
| R. honeia | TT-118, ATCC VR-599T | L36220 | U59726 | U43809 | AF123724 | AF163004 |
| R. slovacaa | 13-B | L36224 | U59725 | U43808 | AF123723 | AF155054 |
| R. parkeria | Maculatum 20 | L36673 | U59732 | U43802 | AF123717 | AF155059 |
| R. japonicaa | YM | L36213 | U59724 | U43795 | AF123713 | AF155055 |
| R. akaria | MK (Kaplan), ATCC VR-148T | L36099 | U59717 | NA | AF123707 | AF213016 |
| R. australisa | Phillips | L36101 | U59718 | AF149108 | AF123709 | AF187982 |
| R. felisa | URRWXCal2, ATCC VR-1525 | L28944 | AF210692 | AF210694 | AF210695 | AF196973 |
| R. massiliaea | MtulT | L36214 | U59719 | U43799 | AF123714 | AF163003 |
| R. montanensisa | M/5-6 | L36215 | U74756 | U43801 | AF123716 | AF163002 |
| R. rhipicephalia | 3-7-6 | L36216 | U59721 | U43803 | AF123719 | AF155053 |
| R. aeschlimanniia | MC16T | U74757 | U59722 | U43800 | AF123705 | AF163005 |
| Species used as outgroup at genus level | ||||||
| Orientia tsutsugamushi | Gilliam | L36222 | NA | NA | NA | NA |
| Anaplasma phagocytophilum | Webster | AY055469 | AF304136 | NA | NA | NA |
| Ehclichia chaffeensis | Arkansas | AF147752 | AF304142 | NA | NA | NA |
| Neorickettsia sennetsu | Miyayama | M73225 | AF304148 | NA | NA | NA |
| Wohlbachia pipientis | Isolate from Folsomia candida | AF179630 | AF332584 | NA | NA | NA |
| Strains used to test criteria at species level | ||||||
| R. prowazekii | Madrid E | NA | AJ235273 | NA | NA | NA |
| R. prowazekii | Virginia | NA | NA | NA | AF211821 | NA |
| R. prowazekii | Florida | NA | NA | NA | AF211820 | NA |
| R. conorii | Moroccan, ATCC VR-141 | L36105 | Identical to U59730 | U45244 | AF123721 | AF163008 |
| R. conorii | Indian tick typhus rickettsia, ATCC VR-597 | L36107 | Identical to U59730 | U43794 | AF123726 | AF163005 |
| R. massiliae | GS | NA | NA | U43793 | NA | NA |
| R. honei | RB | NA | NA | NA | AF123711 | NA |
| R. australis | PHS | NA | NA | AF149108 | NA | NA |
| Unvalidated rickettsial strains | ||||||
| “R. mongolotimonae” | HA91, ATCC VR-1526 | L36219 | U59731 | U43796 | AF123715 | AF151725 |
| BJ-90 | AF178036 | AF178035 | AF179365 | AY331393 | AY331397 | |
| Strain S | U25042 | U59735 | U43805 | AF123720 | AF163001 | |
| Israeli spotted fever rickettsia | ISTT CDC1 | L36223 | U59727 | U43797 | AF123712 | AF155058 |
| Astrakhan fever rickettsia | A-167 | L36100 | U59728 | U43791 | AF123708 | AF163007 |
| Bar 29 | L36102 | U59720 | U43792 | AF123710 | AF155056 | |
| “R. heilongjiangii” | 054, ATCC VR-1524 | AF178037 | AF178034 | AF179362 | AY260451 | AY331396 |
Species used to establish the taxonomic criteria.
NA, sequences not amplifiable.
The inclusion of a rickettsial isolate within the spotted fever group was prioritized on the basis of the presence of the ompA gene, because this gene has been demonstrated to be specific for the spotted fever group rickettsiae (16). In cases in which ompA was not detected, a rickettsial isolate was classified within the spotted fever group on the basis of similarities in rrs, gltA, ompB, and gene D. The cutoffs defining inclusion in the typhus group were calculated by using the rrs, gltA, ompB, and gene D sequences. For the ancestral group, only the rrs and gltA gene sequences were used, as the ompA, ompB, and gene D sequences are not available for these species (16, 45, 47).
As too few sequences from a given gene were available for various strains of a given species to establish any reliable variability measure, we could not calculate cutoffs at the species level. Instead, we selected as a cutoff value for each gene the highest degree of pairwise nucleotide sequence similarity observed among the 20 recognized species. In order to estimate the validity of these criteria, we applied them to sequences available in GenBank from strains of validated species not used to establish the criteria.
Finally, in order to estimate the applicability of our genetic criteria, we applied them to seven rickettsiae previously classified as members of the spotted fever group, i.e., “Rickettsia mongolotimonae” (66), which is phylogenetically closely related to R. sibirica on the basis of genotypic and phenotypic criteria (66); BJ-90 (70), which is considered an R. sibirica strain or subspecies on the basis of genotypic data; strain S (14), which has not been found to be phenotypically and genotypically different enough from R. africae to be classified as a new species; two members of the R. conorii complex, Israeli spotted fever rickettsia (17) and Astrakhan fever rickettsia (15), both of which are considered at present to belong to the species R. conorii (59); Bar 29 (4), which is considered to belong to R. massiliae; and “R. heilongjiangii” (69), a Chinese strain most closely related to R. japonica but considered a separate species on the basis of epidemiological characteristics and the mouse serotyping assay.
PCR amplification and DNA sequencing of missing gene sequences.
As the ompB sequence from BJ-90 and the gene D sequence from “R. heilongjiangii” were missing, we amplified and sequenced them using the primers and methods described previously (45, 47). As no DNA was available from “Rickettsia hulinii” (69), another Chinese strain for which the gene D sequence was also missing, we did not include it in our study.
Phylogenetic analysis.
In order to compare the taxonomic classification obtained from our genetic criteria to that deduced from phylogenetic analysis, we inferred from the same sequence alignments the phylogenetic relationships among the rickettsiae studied using the maximum-parsimony and neighbor-joining methods within the MEGA (version 2.1) software package (21) and the maximum-likelihood method within the PHYLIP software package (40). Bootstrap replicates obtained from 100 trees were performed to estimate the node reliabilities of the phylogenetic trees obtained by the three methods (8).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA, gltA, ompA, ompB, and gene D genes of R. heilongjiangensis sp. nov. have been deposited in the GenBank database under accession numbers AF178037, AF178034, AF179362 (69), AY260451, and AY331396, respectively.
RESULTS
Nucleotide sequences.
R. peacockii, the 21st validated Rickettsia species, was excluded from our analysis, as only incomplete sequences of rrs, gltA, and ompA and no ompB and gene D sequences were available in GenBank; and we did not have this species to determine the missing sequences. Following removal of deletions and insertions, nucleotide sequence fragments of 1,400 and 1,047 bp of the 16S rRNA and gltA genes, respectively, of the 20 valid Rickettsia species; one species of each of the genera Orientia, Ehrlichia, Anaplasma, Neorickettsia, and Wolbachia; and several unclassified Rickettsia strains were studied (Table 1). Nucleotide sequence fragments of 4,682 and 2,725 bp of the ompB gene and gene D, respectively, of 16 valid Rickettsia species of the spotted fever group, both valid species of the typhus group, and several unclassified Rickettsia strains were studied (Table 1). ompB and gene D sequences were not available for members of the ancestral group (45, 47). For ompA, we studied a fragment of 565 bp that was available for 14 valid Rickettsia species of the spotted fever group (Table 1). Although R. akari and R. helvetica are members of the spotted fever group, as well as members of the ancestral group, they were not included in the ompA sequence analysis, as this gene could not be identified in either species (16).
The variances of the differences between the average dS and the average dN values were 0.8, 0.2, 0.2, and 0.3 for the gltA, ompA, and ompB genes and gene D, respectively. The variances of the p distances were 0.048, 0.09, 0.08, 0.08, and 0.07 for the rrs, gltA, ompA, and ompB genes and gene D, respectively.
Determination of cutoff values at the genus level (Fig. 1). (i) rrs.
FIG. 1.
Genotypic scheme for classification of the rickettsiae at the genus and group levels.
Within the genus Rickettsia, the pairwise nucleotide sequence similarities ranged from 97.7% between R. prowazekii and R. akari to 99.8% between R. sibirica and R. rickettsii (Table 2). The mean level of nucleotide sequence similarity less 3 SDs among the 20 species was 97.9%. However, by using the omit test, R. akari was sufficiently different from the other species to be excluded from the complete analysis. Therefore, we recalculated the cutoff value as 98.1%. When this value was applied to the 20 Rickettsia species, it was validated for 168 of 180 similarity rates (sensitivity, 93.3%). None of the five outgroup species fulfilled this criterion with any of the 20 Rickettsia species (specificity, 100%) (Table 3).
TABLE 2.
Pairwise nucleotide sequence similarities for each of the five genes studied among the three Rickettsia groups
| Group and gene | % Similarity
|
||
|---|---|---|---|
| Ancestral group | Typhus group | Spotted fever group | |
| Ancestral | |||
| rrs | 98.9 | ||
| gltA | 85.5 | ||
| Typhus | |||
| rrs | 97.8-98.4% | 99.4 | |
| gltA | 85.7-89.9% | 96.6 | |
| ompB | NAa | 92.5 | |
| Gene D | NA | 91.6 | |
| Spotted fever | |||
| rrs | 97.6-99.3 | 97.7-98.8 | 97.9-99.8 |
| gltA | 85.9-92.2 | 90.3-93.2 | 93.3-99.9 |
| ompA | NA | NA | 55.4-98.8 |
| ompB | NA | 68.9-83.0 | 72.4-99.2 |
| Gene D | NA | 77.0-81.2 | 85.4-99.3 |
NA, not applicable.
TABLE 3.
Pairwise nucleotide sequence similarities of species of the genera Orientia, Anaplasma, Ehrlichia, Wolbachia, Neorickettsia, and strains of validated species not used to calculate the taxonomic criteria by comparison with validated Rickettsia species
| Species | Strain | Gene | Range of % Pairwise nucleotide sequence similarity |
|---|---|---|---|
| O. tsutsugamushi | Gilliam | rrs | 90.2-91.0 |
| A. phagocytophilum | Webster | rrs | 83.8-83.9 |
| gltA | 56.6-58.1 | ||
| E. chaffeensis | Arkansas | rrs | 82.5-83.3 |
| gltA | 60.6-63.1 | ||
| W. pipientis | Isolate from F. candida | rrs | 83.1-84.0 |
| gltA | 62.1-63.4 | ||
| N. sennetsu | Miyayama | rrs | 82.5-83.1 |
| gltA | 54.9-56.9 | ||
| R. prowazekii | Madrid E | gltA | 85.6-99.9 |
| Virginia | ompB | 69.0-99.8 | |
| Florida | ompB | 69.1-99.9 | |
| R. conorii | Moroccan | rrs | 98.3-100 |
| gltA | 86.9-100 | ||
| ompA | 57.0-100 | ||
| ompB | 75.4-100 | ||
| Gene D | 80.0-100 | ||
| Indian tick typhus rickettsia | rrs | 98.3-100 | |
| gltA | 87.0-100 | ||
| ompA | 57.0-99.8 | ||
| ompB | 75.4-99.8 | ||
| Gene D | 79.9-99.9 | ||
| R. massiliae | GS | ompA | 57.3-100 |
| R. honei | RB | ompB | 75.5-99.8 |
| R. australis | PHS | ompA | 55.4-99.9 |
(ii) gltA.
Within the genus Rickettsia, the pairwise nucleotide sequence similarities ranged from 85.5% between R. canadensis and R. bellii to 99.9% between R. parkeri and R. sibirica (Table 2). The mean level of nucleotide sequence similarity less 3 SDs among the 20 species was 86.5%. The omit test showed a high degree of homogeneity of the gltA sequences among the 20 species. When this cutoff was applied to the 20 Rickettsia species and the 4 outgroup species, it had a sensitivity of 173 of 180 (96.1%) and a specificity of 84 of 84 (100%) (Table 3).
Determination of cutoff values at the group level (Fig. 1). (i) Ancestral group.
Members of the ancestral group exhibited degrees of pairwise nucleotide sequence similarity of 98.9 and 85.5% for the rrs and gltA genes, respectively (Table 2). These cutoffs had specificities of 25 of 36 (69.4%) and 0 of 36 (0%), respectively.
(ii) Typhus group.
The typhus group was characterized by degrees of pairwise nucleotide sequence similarity of 99.4, 96.6, 92.4, and 91.6% for the rrs, gltA, and ompB genes and gene D, respectively. These cutoff values had specificities of 36 of 36 (100%), 36 of 36 (100%), 32 of 32 (100%), and 32 of 32 (100%) for the rrs, gltA, and ompB genes and gene D, respectively.
(iii) Spotted fever group.
Within the spotted fever group, the degrees of pairwise nucleotide sequence similarity ranged from 97.9% between R. akari and R. parkeri to 99.8% between R. conorii and R. sibirica for the rrs gene; from 93.3% between R. akari and R. honei to 99.9% between R. parkeri and R. sibirica for the gltA gene; from 72.4% between R. akari and R. helvetica to 99.2% between R. africae and R. sibirica, R. parkeri, or R. slovaca for the ompB gene; and from 84.5% between R. rickettsii and R. akari to 99.3% between R. conorii and R. slovaca for gene D (Table 2). The mean level of rrs (without R. akari) nucleotide sequence similarity less 3 SDs among the 16 spotted fever group species was 98.8%. This cutoff had a sensitivity of 95 of 112 (84.8%) and a specificity of 50 of 64 (78.1%). The mean level of gltA nucleotide sequence similarity less 3 SDs among the 16 spotted fever group species was 92.7%. This cutoff had a sensitivity of 112 of 112 (100%) and a specificity of 40 of 64 (68.7%). The mean level of ompB nucleotide sequence similarity less 3 SDs among the 16 spotted fever group species was 76.7%. However, by the omit test, R. helvetica was different enough from the other species to be excluded from the analysis. Therefore, we recalculated the cutoff value as 85.8%. All spotted fever group species except R. helvetica fulfilled this criterion (sensitivity, 97 of 112 [86.6%]), and the specificity was 32 of 32 (100%). The mean level of gene D nucleotide sequence similarity less 3 SDs among the 16 spotted fever group species was 82.2%. The omit test showed a high level of homogeneity of the gene D sequences among the 16 species. All spotted fever group species fulfilled this criterion (sensitivity, 112 of 112 [100%]), and the specificity was 32 of 32 (100%).
Application of genus and group criteria to valid species.
When the combination of the genus and group criteria (Fig. 1) was applied to the 20 valid Rickettsia species and 5 outgroup species, all of them were correctly classified (sensitivity and specificity, 100%).
Determination of cutoff values at the species level.
The highest pairwise nucleotide sequence similarity rates among the 20 validated species were 99.8, 99.9, 98.8, 99.2, and 99.3% for the rrs, gltA, ompA, and ompB genes and gene D, respectively (Table 2). All strains of validated species that were not used in the analysis exhibited levels of nucleotide sequence similarity to other strains of their respective species equal to or higher than these cutoffs (Table 3).
Application of the criteria to R. akari.
As R. akari was excluded from the calculation of the rrs cutoff and was not included in the determination of the ompA cutoff, we applied our criteria to this species to estimate their validity for this species. The nucleotide sequences of the rrs and gltA genes of R. akari exhibited similarity rates ranging from 97.7 to 98.6% and 85.9 to 97.0%, respectively, to those of the other 19 valid Rickettsia species. These values classified R. akari within the genus Rickettsia. At the group level, this species was classified within the spotted fever group (Table 4), and at the species level it was considered a separate species.
TABLE 4.
Pairwise nucleotide sequence similarities for various rickettsial isolates with each of the three Rickettsia groups and with the 20 validated species
| Group and gene | % Similarity
|
% Pairwise similarity with phylogenetically closest validated speciesa | ||
|---|---|---|---|---|
| Ancestral group | Typhus group | Spotted fever group | ||
| R. akari | ||||
| rrs | 97.6-98.3 | 97.7-97.9 | 98.1-98.6 | 98.6 |
| gltA | 85.9-89.3 | 90.9-91.2 | 93.3-97.0 | 97.0 |
| ompB | NAb | 80.0-80.3 | 86.1-94.2 | 94.2 |
| Gene D | NA | 77.6-77.8 | 84.5-94.9 | 94.9 |
| “R. mongolotimonae” | ||||
| rrs | 98.3-99.0 | 98.2-98.3 | 98.0-100 | 100 |
| gltA | 87.0-91.5 | 92.8-93.0 | 94.0-99.8 | 99.8 |
| ompA | NA | NA | 59.1-99.8 | 99.0 |
| ompB | NA | 82.1-82.4 | 76.9-99.6 | 99.6 |
| Gene D | NA | 80.5-80.6 | 84.0-99.4 | 99.4 |
| BJ-90 | ||||
| rrs | 98.1-98.9 | 98.1-98.2 | 97.8-99.8 | 99.8 |
| gltA | 87.2-91.7 | 93.0-93.1 | 94.2-100 | 100 |
| ompA | NA | NA | 58.8-99.8 | 99.8 |
| ompB | NA | 82.1-82.4 | 86.1-99.7 | 99.7 |
| Gene D | NA | 80.8-81.6 | 84.9-99.9 | 99.9 |
| Strain S | ||||
| rrs | 98.2-99.0 | 98.2-98.3 | 98.1-99.8 | 99.8 |
| gltA | 87.1-91.6 | 92.7-93.0 | 94.0-99.8 | 99.4 |
| ompA | NA | NA | 59.1-99.5 | 99.5 |
| ompB | NA | 82.0-82.5 | 77.0-99.0 | 99.0 |
| Gene D | NA | 80.0-80.5 | 83.6-99.2 | 99.2 |
| Israeli spotted fever rickettsia | ||||
| rrs | 98.3-99.0 | 98.3-98.4 | 98.0-99.8 | 99.8 |
| gltA | 86.7-91.5 | 92.7-93.0 | 94.0-99.8 | 99.3 |
| ompA | NA | NA | 58.6-99.3 | 98.3 |
| ompB | NA | 81.8-82.1 | 76.7-98.6 | 98.6 |
| Gene D | NA | 80.2-80.6 | 84.0-99.3 | 99.3 |
| Astrakhan fever rickettsia | ||||
| rrs | 98.3-99.0 | 98.3-98.5 | 98.3-99.9 | 99.9 |
| gltA | 86.9-91.6 | 92.9-93.0 | 94.1-99.5 | 99.5 |
| ompA | NA | NA | 58.6-98.4 | 98.3 |
| ompB | NA | 81.9-82.3 | 76.8-98.7 | 98.7 |
| Gene D | NA | 80.3-80.8 | 84.0-99.4 | 99.4 |
| Bar 29 | ||||
| rrs | 98.3-99.0 | 98.2-98.3 | 98.3-99.9 | 99.9 |
| gltA | 87.0-91.5 | 92.8-93.0 | 94.0-99.8 | 99.8 |
| ompA | NA | NA | 58.1-99.5 | 99.5 |
| ompB | NA | 82.5-82.8 | 77.3-99.4 | 99.4 |
| Gene D | NA | 79.8-80.3 | 84.0-98.7 | 98.7 |
| “R. heilongjiangii” | ||||
| rrs | 96.5-97.0 | 96.7 | 96.2-98.0 | 98.0 |
| gltA | 86.9-91.6 | 93.0 | 93.9-99.6 | 99.6 |
| ompA | NA | NA | 58.8-97.2 | 97.2 |
| ompB | NA | 82.4-82.7 | 87.2-98.8 | 98.8 |
| Gene D | NA | 81.0-81.3 | 85.1-99.4 | 99.4 |
The species to which each of the seven unvalidated species studied belonged were R. australis for R. akari, R. sibirica for “R. mongolotimonae” and BJ-90, R. africae for strain S, R. conorii seven for Israeli spotted fever and Astrakhan fever rickettsiae, R. massiliae for Bar 29, and R. japonica for “R. Leilongjiangii.”
NA, not applicable.
Application of the taxonomic criteria to unvalidated species.
By using the taxonomic criteria, all seven unvalidated species studied belonged to the genus Rickettsia and to a single group, the spotted fever group (Table 4). “R. mongolotimonae” and BJ-90 belonged to the species R. sibirica on the basis of four and five validated cutoffs, respectively. Strain S belonged to the species R. africae on the basis of two validated cutoffs. Israeli tick typhus rickettsia and Astrakhan fever rickettsia belonged to the species R. conorii each on the basis of two validated cutoffs. Strain Bar 29 belonged to the species R. massiliae on the basis of three validated cutoffs. “R. heilongjiangii” fulfilled only one cutoff and, thus, was classified as a separate species.
Comparison of sequence-based criteria and phylogeny.
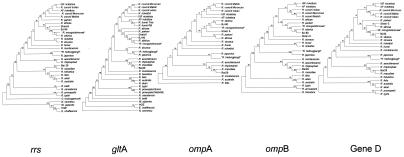
The phylogenetic classification at the genus level matched our findings perfectly (Fig. 2), as none of the rickettsiae studied clustered with any of the bacteria used as outgroups for the rrs and gltA genes. At the group level, we observed a discrepancy in the position of the ancestral group between the analyses of these two genes, since, by using rrs, the typhus group was the ancestor of the ancestral group, whereas the opposite result was observed by using gltA sequences. At the species level, all rickettsiae closely related to R. conorii formed a homogeneous cluster together in analyses with all genes except rrs, with which R. parkeri grouped with R. conorii, and gltA, with which the Astrakhan fever rickettsia was grouped with R. honei. This group was supported by high bootstrap values for all genes except the rrs and gltA genes. “R. mongolotimonae” and BJ-90 formed a reliable cluster with R. sibirica in analyses with all genes. Likewise, Bar 29 was reliably associated with R. massiliae in analyses with all five genes. “R. heilongjiangii” was grouped with R. japonica with high bootstrap values in analyses with all five genes. However, it was impossible to deduce from the phylogenetic analysis that “R. heilongjiangii” was a strain of a species distinct from R. japonica (Fig. 2). In addition, it was not clear from the phylogenetic analysis to which species strain S belonged, as it was grouped with R. africae, as expected from our results, in analyses with the rrs gene and gene D and with the R. sibirica group in analyses with the gltA and ompA genes.
FIG. 2.
Unrooted trees showing the phylogenetic relationships among the rickettsiae studied, as inferred from sequence analysis of each of the five genes by the maximum-parsimony method. Bootstrap values are indicated at the nodes. ISF rickettsia, Israeli spotted fever rickettsia; AF rickettsia, Astrakhan fever rickettsia; HGE, the former human granulocytic ehrlichiosis agent (Anaplasma phagocytophilum).
DISCUSSION
We propose gene sequence-based criteria for the taxonomic classification of rickettsial isolates at the genus, group, and species levels. To date, attempts to define a species among strictly intracellular bacteria have been very difficult. The application of the phenotypic characteristics used for extracellular bacteria to the order Rickettsiales is limited since few are expressed by these bacteria. Rickettsiologists have demonstrated that the 70% DNA-DNA homology criterion (61) does not adequately differentiate rickettsiae. Among the classical laboratory methods for the identification rickettsiae, cross immunity and vaccine protection tests with guinea pigs (13), complement fixation tests with washed specific particulate antigens (38), and mouse toxin neutralization tests (22) have largely been superceded by mouse serotyping assays for the spotted fever group rickettsiae (37). Although monoclonal antibodies are helpful (60, 65), they are limited by the lack of an accepted standardized panel directed to all known isolates. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and pulsed-field gel electrophoresis (PFGE) have proven to be useful for differentiating rickettsiae, but their values are limited because of the variations in the molecular weights of rOmpA and rOmpB within species for SDS-PAGE and the absence of any database allowing the comparison of profiles for PFGE.
Recently, a number of genes, including those encoding 16S rRNA (rrs), citrate synthase (gltA), the 17-kDa common antigen, and surface-exposed, high-molecular-weight antigenic proteins of the sca family (ompA, ompB, gene D) (32), have been used to rapidly differentiate spotted fever group rickettsiae either by analysis of restriction fragment length polymorphisms of PCR products or by direct sequence determination (16, 45-47). The usefulness of these genes for taxonomic purposes was demonstrated by the valid description of two new, at that time, uncultured Rickettsia species, R. felis (6) and R. peacockii (31), mainly on the basis of genotypic criteria. The classification of these two rickettsiae was later confirmed by the study of isolates in vitro (23, 39, 49). Despite these examples and the demonstration that nucleotide sequence-based taxonomy was more discriminative than taxonomy based on phenotypic characteristics, no formal guidelines have been accepted among rickettsiologists for the use of this tool in classifying rickettsial isolates. Polyphasic taxonomy, which integrates phenotypic, genotypic, and phylogenetic data, has proven to be very useful for the taxonomic classification of several bacterial genera (57). For that objective, multilocus sequencing, which reduces the risk of error caused by lateral gene transfer, has been demonstrated to be suitable for the taxonomic classification of bacteria (26). Recently, La Scola et al. (24) have demonstrated that gene sequence-based criteria are useful for species definition within the genus Bartonella. However, multilocus sequencing must use genes that are undergoing neutral selection (50). In our study, the Z test demonstrated that the four protein-coding genes were undergoing stabilizing selection, thus confirming their suitability for our study. In addition, we estimated the normality of the distribution of similarity values used to infer our criteria for each gene. With the exception of the rrs gene, which is highly conserved among spotted fever group rickettsiae, the test showed that our data set was normally distributed and thus constituted a reliable basis on which to establish our criteria.
We propose sequence-based guidelines for the classification of new rickettsial isolates (Fig. 1). These criteria can be applied to a bacterium only if the sequences of the gene fragments used to establish the guidelines have been determined for this isolate. We are aware that these guidelines may later be updated by the introduction of additional genetic and/or phenotypic characteristics and of new Rickettsia species. Using the rrs gene, we determined that a rickettsia-like organism belongs to the Rickettsia genus if it exhibits a nucleotide sequence homology of ≥98.1% with any of the recognized species. The rrs gene has been proposed to be the best tool for the classification of prokaryotic taxa at the genus level (50). In addition, we also determined a cutoff value of ≥86.5% for the gltA gene, which is widely present among bacteria. Both the rrs and the gltA cutoffs were validated by using both reiterative omission tests and comparisons with five genera most closely related to Rickettsia. Both values were supported by elevated sensitivities and specificities. To belong to the genus Rickettsia a bacterium should exhibit degrees of rrs and gltA homology equal to or higher than our cutoffs with at least 1 of the 20 validated Rickettsia species.
Within the genus Rickettsia, we determined cutoff values to allow the classification of rickettsial isolates in the different groups. These taxonomic criteria for the classification at the group level were based on five genes for 14 of the 20 (70%) species, four genes for an additional 4 species (20%), and two genes for only 2 species (10%). To belong to the typhus group, an isolate should exhibit pairwise nucleotide sequence similarity with the sequence of either R. prowazekii or R. typhi with at least two of the following four cutoffs: ≥99.4, 96.6, 92.5, and 91.6% for rrs, gltA, ompB, and gene D, respectively. The presence of an ompA gene warrants classification of a rickettsial isolate into the spotted fever group (16). However, lack of detection of this gene does not exclude an isolate from the spotted fever group. Therefore, for ompA-negative isolates, a minimum of two of the following four cutoffs for homology with any member of the spotted fever group warrants inclusion in the spotted fever group: ≥98.8, 92.7, 85.8, and 82.2% for rrs, gltA, ompB, and gene D, respectively. The specificities obtained for the rrs and gltA genes were low, which may be because both genes are highly conserved (44, 46). In contrast, the sensitivities and specificities for the ompB and gene D cutoffs were elevated. For the ancestral group, both cutoff values exhibited low specificities, which may be due to either the high conservation of these genes or the heterogeneity of this group, which initially comprised two rickettsial species which could not be classified into either the typhus or the spotted fever group but which are also clearly different from each other (52). Therefore, we believe that R. bellii and R. canadensis do not belong to a single group but may be representatives of two distinct groups.
In order to classify a rickettsial isolate as a new species, we propose that candidates possess no more than one value above the following nucleotide sequence similarities by comparison with any validated Rickettsia species: ≥99.8 and 99.9% for the 16S rDNA and gltA genes, respectively, and, when available ≥98.8, 99.2, and 99.3% for the ompA and ompB genes and gene D, respectively. Our criteria should not be used for the official description of a rickettsia as a new species if no established isolate is available. By use of these criteria, eight strains of validated Rickettsia species were accurately classified.
When our taxonomic scheme was applied to R. akari, this species was correctly classified, despite its removal from a part of the analysis, thus strengthening our guidelines. In addition, the application of our criteria to R. peacockii by use of the similarity rates described by Niebylski et al. (31), i.e., 99.7 and 93.2% for rrs and ompA, respectively, with R. rickettsii would classify this rickettsia into the spotted fever group as a separate species.
When our taxonomic scheme was applied to seven rickettsial strains not previously officially classified, all of them were correctly classified into the spotted fever group within the genus Rickettsia. “R. mongolotimonae” and BJ-90 belonged to the species R. sibirica, strain S belonged to the species R. africae, the Israeli tick typhus and Astrakhan fever rickettsiae belonged to the species R. conorii, and Bar 29 belonged to the species R. massiliae. Our results for all strains except strain S were consistent with those obtained from the phylogenetic analysis. The phylogenetic position of strain S varied depending on the gene. Moreover, although the phylogenetic approach was valuable in grouping closely related rickettsiae, often there was no possibility to determine the species status of some rickettsiae, as was shown for “R. heilongjiangii.”
Our results confirm the previously established taxonomic classification of seven strains whose taxonomic status is not yet established. “R. heilongjiangii” exhibited low rates of similarity to R. japonica, which prevented its classification in this species. In 1982, “R. heilongjiangii” was first isolated as the Heilongjiang isolate or strain 054 from Dermacentor silvarum ticks collected in the city of Suifenhe in Heilongjiang Province of northeastern China (25). It was later isolated from patients with symptoms consistent with a spotted fever rickettsiosis in Suifenhe (64). On the basis of phenotypic and genotypic analyses, this strain was previously proposed to be a new species (64, 68). Our data demonstrate that this rickettsia belongs to a new species. Thus, we formally propose the creation of Rickettsia heilongjiangensis sp. nov., which contains strain heilongjiangii or strain 054 (type strain).
In conclusion, we propose objective, gene sequence-based guidelines for the taxonomic classification of new rickettsial isolates. These guidelines are designed mostly for use with sequenced amplified genes or gene fragments. Thus, absence of detection is not equivalent to absence of the gene, a concept accommodated by the criteria. Moreover, they are time-saving, do not require the availability of all known rickettsial strains for the classification of a new isolate, and are not subject to reproducibility problems such as those encountered with other methods, in particular, mouse serotyping assays. In addition, we propose the creation of one new species: R. heilongjiangensis sp. nov.
Description of Rickettsia heilongjiangensis sp. nov.
Rickettsia heilongjiangensis (hei.long.iang.en′sis. N.L. gen. n. heilongjiangensis, from Heilongjiang, the Chinese province where the D. silvarum tick providing the first isolate was collected [25]), is an obligate gram-negative intracellular bacterium. It grows on Vero cells at 32°C in minimal essential medium supplemented with 2% fetal calf serum and 2 mg of l-glutamine per ml. It is nonmotile. Pathogenicity for humans has been demonstrated. It is transmitted to humans through the bite of D. silvarum ticks.
The type strain is strain 054, which was isolated from D. silvarum ticks in 1982 in the city of Suifenhe in the Chinese province of Heilongjiang (25). Type strain 054 has been deposited in the American Type Culture Collection under the reference designation ATCC VR-1524 and in the Collection of the World Health Organization Collaborative Center for Rickettsioses, Borrelioses and Tick-Borne Infections, Marseille, France.
REFERENCES
- 1.Beati, L., M. Meskini, B. Thiers, and D. Raoult. 1997. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 47:548-554. [DOI] [PubMed] [Google Scholar]
- 2.Beati, L., O. Peter, W. Burgdorfer, A. Aeschlimann, and D. Raoult. 1993. Confirmation that Rickettsia helvetica sp. nov. is a distinct species of the spotted fever group of rickettsiae. Int. J. Syst. Bacteriol. 43:521-526. [DOI] [PubMed] [Google Scholar]
- 3.Beati, L., and L. Raoult. 1993. Rickettsia massiliae sp. nov., a new spotted fever group rickettsia. Int. J. Syst. Bacteriol. 43:839-840. [DOI] [PubMed] [Google Scholar]
- 4.Beati, L., V. Roux, A. Ortuno, J. Castella, F. Segura Porta, and D. Raoult. 1996. Phenotypic and genotypic characterization of spotted fever group rickettsiae isolated from Catalan Rhipicephalus sanguineus ticks. J. Clin. Microbiol. 34:2688-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birtles, R. J., T. G. Harrison, N. A. Saunders, and D. H. Molyneux. 1995. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int. J. Syst. Bacteriol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 6.Bouyer, D. H., J. Stenos, P. Crocquet-Valdes, C. Moron, P. Vsevolod, J. E. Zavala-Velasquez, L. Foil, D. Stothard, A. Azad, and D. Walker. 2001. Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int. J. Syst. Evol. Microbiol. 51:339-347. [DOI] [PubMed] [Google Scholar]
- 7.Brenner, D. J., S. O'Connor, H. H. Winkler, and A. G. Steigerwalt. 1993. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int. J. Syst. Bacteriol. 43:777-786. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. K. 1994. Bootstrap hypothesis tests for evolutionary trees and other dendrograms. Proc. Natl. Acad. Sci. USA 91:12293-12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brumpt, E. 1922. Les spirochétoses, p. 491-531. In H. Roger, G. W. Vidal, and P. Teissier (ed.), Nouveau traité de médecine. Masson, Paris, France.
- 10.Brumpt, E. 1932. Longévité du virus de la fièvre boutonneuse (Rickettsia conorii n. sp.) chez la tique Rhipicephalus sanguineus. C. R. Soc. Biol. Filiales 110:1199-1209. [Google Scholar]
- 11.Burgdorfer, W., L. P. Brinton, W. L. Krinsky, and R. N. Philip. 1978. Rickettsia rhipicephali: a new spotted fever group rickettsia from the brown dog tick Rhipicephalus sanguineus, p. 307-316. In J. Kazar, R. A. Ormsbee, and I. V. Tarasevich (ed.), Rickettsiae and rickettsial diseases. House of the Slovak Academy of Sciences, Bratislava, Czechoslovakia.
- 12.da Rocha-Lima, H. 1916. Zur aetiologie des fleckenfiebers. Berl. Klin. Wochenschr. 53:567-569. [Google Scholar]
- 13.Davis, G. E. 1934. Comparative experiments on spotted fever and boutonneuse fever. Public Health Rep. 49:423. [Google Scholar]
- 14.Eremeeva, M., N. M. Balayeva, V. Roux, V. Ignatovich, M. Kotsinjan, and D. Raoult. 1995. Genomic and proteinic characterization of strain S, a rickettsia isolated from Rhipicephalus sanguineus ticks in Armenia. J. Clin. Microbiol. 33:2738-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eremeeva, M. E., L. Beati, V. A. Makarova, N. F. Fetisova, I. V. Tarasevich, N. M. Balayeva, and D. Raoult. 1994. Astrakhan fever rickettsiae: antigenic and genotypic of isolates obtained from human and Rhipicephalus pumilio ticks. Am. J. Trop. Med. Hyg. 51:697-706. [DOI] [PubMed] [Google Scholar]
- 16.Fournier, P. E., V. Roux, and D. Raoult. 1998. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Evol. Microbiol. 48:839-849. [DOI] [PubMed] [Google Scholar]
- 17.Goldwasser, R. A., Y. Steiman, W. Klingberg, T. A. Swartz, and M. A. Klingberg. 1974. The isolation of strains of rickettsiae of the spotted fever group in Israel and their differentiation from other members of the group by immunofluorescence methods. Scand. J. Infect. Dis. 6:53-62. [DOI] [PubMed] [Google Scholar]
- 18.Grimont, P. A. 1988. Use of DNA reassociation in bacterial classification. Can. J. Microbiol. 34:541-546. [DOI] [PubMed] [Google Scholar]
- 19.Huebner, R. J., W. L. Jellison, and C. Pomerantz. 1946. Rickettsialpox—a newly recognized rickettsial disease. IV. Isolation of a rickettsia apparently identical with the causative agent of rickettsialpox from Allodermanyssus sanguineus, a rodent mite. Public Health Rep. 61:1677-1682. [PubMed] [Google Scholar]
- 20.Kelly, P. J., L. Beati, P. R. Mason, L. A. Matthewman, V. Roux, and D. Raoult. 1996. Rickettsia africae sp. nov., the etiological agent of African tick bite fever. Int. J. Syst. Bacteriol. 46:611-614. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 22.Lackman, D. B., E. J. Bell, H. G. Stoenner, and E. G. Pickens. 1965. The Rocky mountain spotted fever group of rickettsiae. Health Lab. Sci. 2:135. [PubMed] [Google Scholar]
- 23.La Scola, B., S. Meconi, F. Fenollar, J. M. Rolain, V. Roux, and D. Raoult. 2002. Emended description of Rickettsia felis (Bouyer et al. 2001) a temperature-dependant cultured bacterium. Int. J. Syst. Evol. Microbiol. 52:2035-2041. [DOI] [PubMed] [Google Scholar]
- 24.La Scola, B., Z. Zeaiter, A. Khamis, and D. Raoult. 2003. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol. 11:318-321. [DOI] [PubMed] [Google Scholar]
- 25.Lou, D., Y. M. Wu, B. Wang, G. D. Lui, J. Z. Li, W. Wang, and Y. F. Han. 1985. A new member of the spotted fever group of rickettsiae—rickettsia. Chin. J. Microbiol. Immunol. 5:250-253. [Google Scholar]
- 26.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russel, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKiel, Y. A., E. J. Bell, and D. B. Lackman. 1967. Rickettsia canada: a new member of the typhus group of rickettsiae isolated from Haemaphylasis leporispalustris ticks in Canada. Can. J. Microbiol. 13:503-510. [DOI] [PubMed] [Google Scholar]
- 28.Myers, W. F., and C. L. Wisseman, Jr. 1981. The taxonomic relationship of Rickettsia canada to the typhus and spotted fever groups of the genus Rickettsia, p. 313-325. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, Inc., New York, N.Y.
- 29.Nei, M., and L. Jin. 1989. Variances of the average numbers of nucleotide substitutions within and between populations. Mol. Biol. Evol. 6:290-300. [DOI] [PubMed] [Google Scholar]
- 30.Nei, M., and S. Kumar. 2000. Evolutionary change of DNA sequences, p. 33-50. In M. Nei and S. Kumar (ed.), Molecular evolution and phylogenetics. Oxford University Press, New York, N.Y.
- 31.Niebylski, M. L., M. E. Schrumpf, W. Burgdorfer, E. R. Fischer, K. L. Gage, and T. G. Schwan. 1997. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int. J. Syst. Bacteriol. 47:446-452. [DOI] [PubMed] [Google Scholar]
- 32.Ogata, H., S. Audic, P. Renesto-Audiffren, P. E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J. M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 33.Ogata, N. 1931. Aetiologie der Tsutsugamushi-Krankheit: Rickettsia tsutsugamushi. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 122:249-253. [Google Scholar]
- 34.Philip, C. B. 1943. Nomenclature of the pathogenic rickettsiae. Am. J. Hyg. 37:301-309. [Google Scholar]
- 35.Philip, C. B. 1950. Miscellaneous human rickettsioses, p. 781-788. In R. L. Pullen (ed.), Communicable diseases. Lea and Febiger Co., Philadelphia, Pa.
- 36.Philip, R. N., E. A. Casper, R. L. Anacker, J. Cory, S. F. Hayes, W. Burgdorfer, and E. Yunker. 1983. Rickettsia bellii sp. nov.: a tick-borne rickettsia, widely distributed in the United States, that is distinct from the spotted fever and typhus biogroups. Int. J. Syst. Bacteriol. 33:94-106. [Google Scholar]
- 37.Philip, R. N., E. A. Casper, W. Burgdorfer, R. K. Gerloff, L. E. Hugues, and E. J. Bell. 1978. Serologic typing of rickettsiae of the spotted fever group by micro-immunofluorescence. J. Immunol. 121:1961-1968. [PubMed] [Google Scholar]
- 38.Pickens, E. G., E. J. Bell, D. B. Lackman, and W. Burgdorfer. 1965. Use of mouse serum in identification and serologic classification of Rickettsia akari and Rickettsia australis. J. Immunol. 94:883-889. [PubMed] [Google Scholar]
- 39.Raoult, D., B. La Scola, M. Enea, P. E. Fournier, V. Roux, F. Fenollar, M. A. M. Galvao, and X. De Lamballerie. 2001. A flea-associated Rickettsia pathogenic for humans. Emerg. Infect. Dis. 7:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Retief, J. D. 2000. Phylogenetic analysis using PHYLIP. Methods Mol. Biol. 132:243-258. [DOI] [PubMed] [Google Scholar]
- 41.Rikihisa, Y., M. Kawahara, B. Wen, G. Kociba, P. Fuerst, F. Kawamori, C. Suto, S. Shibata, and M. Futohashi. 1997. Western immunoblot analysis of Haemobartonella muris and comparison of 16S rRNA gene sequences of H. muris, H. felis, and Eperythrozoon suis. J. Clin. Microbiol. 35:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roux, V. 1999. Phylogenetic analysis and taxonomic relationships among the genus Rickettsia, p. 52-66. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millennium. Elsevier, Marseille, France.
- 43.Roux, V., M. Bergoin, N. Lamaze, and D. Raoult. 1997. Reassessment of the taxonomic position of Rickettsiella grylli. Int. J. Syst. Bacteriol. 47:1255-1257. [DOI] [PubMed] [Google Scholar]
- 44.Roux, V., and D. Raoult. 1995. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146:385-396. [DOI] [PubMed] [Google Scholar]
- 45.Roux, V., and D. Raoult. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 50:1449-1455. [DOI] [PubMed] [Google Scholar]
- 46.Roux, V., E. Rydkina, M. Eremeeva, and D. Raoult. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 47:252-261. [DOI] [PubMed] [Google Scholar]
- 47.Sekeyova, Z., V. Roux, and D. Raoult. 2001. Phylogeny of Rickettsia spp. inferred by comparing sequences of ′gene D,' which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 51:1353-1360. [DOI] [PubMed] [Google Scholar]
- 48.Sekeyova, Z., V. Roux, W. B. Xu, J. Rehacek, and D. Raoult. 1998. Rickettsia slovaca sp. nov., a member of the spotted fever group rickettsiae. Int. J. Syst. Bacteriol. 48:1455-1462. [DOI] [PubMed] [Google Scholar]
- 49.Simser, J., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl. Environ. Microbiol. 67:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stackebrandt, E., W. Frederiksen, G. M. Garrity, P. A. D. Grimont, P. Kampfer, M. C. J. Maiden, X. Nesme, R. Rossello-Mora, J. Swings, H. G. Truper, L. Vauterin, A. C. Ward, and W. B. Whitman. 2002. Report of the Ad Hoc Committee for the Re-Evaluation of the Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047. [DOI] [PubMed] [Google Scholar]
- 51.Stenos, J., V. Roux, D. Walker, and D. Raoult. 1998. Rickettsia honei sp. nov., the aetiological agent of Flinders Island spotted fever in Australia. Int. J. Syst. Evol. Microbiol. 48:1399-1404. [DOI] [PubMed] [Google Scholar]
- 52.Stothard, D. R., and P. A. Fuerst. 1995. Evolutionary analysis of the spotted fever and typhus groups of Rickettsia using 16S rRNA gene sequences. Syst. Appl. Microbiol. 18:52-61. [Google Scholar]
- 53.Tamura, A., N. Ohashi, H. Urakami, and S. Miyamura. 1995. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int. J. Syst. Bacteriol. 45:589-591. [DOI] [PubMed] [Google Scholar]
- 54.Tautz, D., P. Arctander, A. Minelli, R. H. Thomas, and A. P. Vogler. 2003. A plea for DNA taxonomy. Trends Ecol. Evol. 18:70-74. [Google Scholar]
- 55.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchida, T., T. Uchiyama, K. Kumano, and D. H. Walker. 1992. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int. J. Syst. Bacteriol. 42:303-305. [DOI] [PubMed] [Google Scholar]
- 57.Vandamme, P., B. Pot, M. Gillis, P. De Vos, K. Kersters, and J. Swings. 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60:407-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker, D. H. 1989. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin. Microbiol. Rev. 2:227-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker, D. H., H. M. Feng, J. I. Saada, P. Croquet-Valdes, S. Radulovic, V. L. Popov, and E. Manor. 1995. Comparative antigenic analysis of spotted fever group rickettsiae from Israel and other closely related organisms. Am. J. Trop. Med. Hyg. 52:569-576. [DOI] [PubMed] [Google Scholar]
- 60.Walker, D. H., Q. H. Liu, X. J. Yu, H. Li, C. Taylor, and H. M. Feng. 1992. Antigenic diversity of Rickettsia conorii. Am. J. Trop. Med. Hyg. 47:78-86. [DOI] [PubMed] [Google Scholar]
- 61.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Truper. 1987. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]
- 62.Weisburg, W. G., M. E. Dobson, J. E. Samuel, G. A. Dasch, L. P. Mallavia, O. Baca, L. Mandelco, J. E. Sechrest, E. Weiss, and C. R. Woese. 1989. Phylogenetic diversity of the rickettsiae. J. Bacteriol. 171:4202-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss, E., and J. W. Moulder. 1984. Order I. Rickettsiales Gieszczkiewicz 1939, p. 687-703. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology. The Williams & Wilkins Co., Baltimore, Md.
- 64.Wu, Y. M., S. R. Yu, and D. Lou. 1994. Western-blot analysis of Rickettsia heilongjiangi. J. Prev. Med. P. L. A. 12:28-30. [Google Scholar]
- 65.Xu, W. B., and D. Raoult. 1998. Taxonomic relationships among spotted fever group rickettsiae as revealed by antigenic analysis with monoclonal antibodies. J. Clin. Microbiol. 36:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu, X., Y. Jin, M. Fan, G. Xu, Q. Liu, and D. Raoult. 1993. Genotypic and antigenic identification of two new strains of spotted fever group rickettsiae isolated from China. J. Clin. Microbiol. 31:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zdrodovskii, P. F. 1949. Systematics and comparative characterization of endemic rickettsioses. Z. Mikrobiol. Epidemiol. 10:19-28. [Google Scholar]
- 68.Zhang, J. Z., M. Y. Fan, and D. Z. Bi. 1996. Isolation and identification of a new species of spotted fever group rickettsiae. Chin. J. Zoonoses 5:2-8. [Google Scholar]
- 69.Zhang, J. Z., M. Y. Fan, Y. M. Wu, P. E. Fournier, V. Roux, and D. Raoult. 2000. Genetic classification of Rickettsia heilongjiangii and Rickettsia hulinii, two Chinese spotted fever group rickettsiae. J. Clin. Microbiol. 38:3498-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, J. Z., M. Y. Fan, X. J. Yu, and D. Raoult. 2000. Phylogenetic analysis of the chinese isolate BJ-90. Emerg. Infect. Dis. 6:432-433. [DOI] [PMC free article] [PubMed] [Google Scholar]