Abstract
Background
Factors contributing to chronic inflammation appear to be associated with increased risk of ovarian cancer. The purpose of this study was to assess the association between circulating levels of inflammation mediators and subsequent risk of ovarian cancer.
Methods
We conducted a case-control study of 230 cases and 432 individually-matched controls nested within three prospective cohorts to evaluate the association of pre-diagnostic circulating levels of inflammation-related biomarkers (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, TNFα, IL-1Ra, sIL-1RII, sIL-2Ra, sIL-4R, sIL-6R, sTNF-R1, and sTNF-R2) measured using Luminex xMap™ technology with risk of ovarian cancer.
Results
We observed a trend across quartiles for IL-2 (ORQ4 vs. Q1: 1.57, 95% CI: 0.98, 2.52, p= 0.07), IL-4 (ORQ4 vs. Q1: 1.50, 95% CI: 0.95, 2.38, p= 0.06), IL-6 (ORQ4 vs. Q1: 1.63, 95% CI: 1.03, 2.58, p= 0.03), IL-12p40 (ORQ4 vs. Q1: 1.60, 95% CI: 1.02, 2.51, p= 0.06), and IL-13 (ORQ4 vs. Q1: 1.42, 95% CI: 0.90, 2.26, p= 0.11). Trends were also observed when cytokines were modeled on the continuous scale for IL-4 (p-trend=0.01), IL-6 (p-trend=0.01), IL-12p40 (p-trend=0.01), and IL-13 (p-trend=0.04). Odds ratios were not materially different after excluding cases diagnosed less than five years after blood donation or when limited to serous tumors.
Conclusions and Impact
This study provides the first direct evidence that multiple inflammation markers, specifically IL-2, IL-4, IL-6, IL-12, and IL-13, may be associated with risk of epithelial ovarian cancer, and adds to the evidence that inflammation is involved in the development this disease.
Keywords: Inflammation, Ovarian Cancer, Cytokines, Serum, Plasma
Introduction
Epidemiological evidence suggests that inflammation may be an underlying mechanism in the development of ovarian cancer (1). The chronic inflammatory state is characterized by dysregulation of cytokine secretion, thereby increasing the likelihood of excessive cell growth, malignant transformation, and survival of transformed cells (2). Cytokines can act to promote the secretion of other cytokines and regulate the expression of their soluble receptors/modulators (3, 4), thus we hypothesized that both cytokines and cytokine modulators are associated with increased risk of ovarian cancer. To date, the only biomarker of inflammation that has been examined in relation to the development of ovarian cancer is C-reactive protein (CRP). One study found that women with CRP levels in the highest third of the distribution had a 70% increased risk of ovarian cancer versus women in the lowest third (5), though a second study conducted by our group only found the association among women with the highest CRP levels (>10 mg/L) (6).
The purpose of the present study was to assess the relationship between inflammatory cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, TNFα) and cytokine modulators (IL-1Ra, sIL-1RII, sIL-2Ra, sIL-4R, sIL-6R, IL-12p40 and sTNF-R1/R2) and subsequent risk of epithelial ovarian cancer. We conducted a case-control study nested within three prospective cohort studies: 1) the New York University Women’s Health Study (NYUWHS); 2) the Northern Sweden Health and Disease Study (NSHDS); and 3) the Italian Hormones and Diet in the Etiology of Cancer Study (ORDET). Markers were selected based on their biological relevance to normal and malignant ovarian processes and adequate temporal reproducibility over a 2–3 year period in a preliminary reliability study (7–9).
Methods
Parent Cohorts
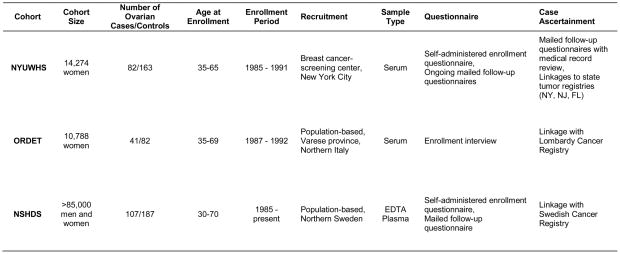
The parent prospective cohort studies have been described in detail previously (10–13). Figure 1 gives a brief description of each cohort.
Figure 1.
Description of the Parent Cohorts
Study Design and Subjects
All incident cases of invasive epithelial ovarian cancer confirmed through linkages with tumor registries or review of pathology reports throughout the most recent complete follow-up period for each cohort were included. Cases with any cancer diagnosed prior to ovarian cancer were ineligible.
For each case, two controls were selected at random from cohort members who fulfilled the risk set criteria. The risk set for a case consisted of all women in the same cohort who were alive and free of cancer at the time of diagnosis and who matched the case on age (± 6 months), date of blood donation (± 3 months), and menopausal status at the time of blood donation. Age was included as a matching factor because of its strong association with ovarian cancer, date of blood donation to control for length of sample storage which may affect biomarker levels, and menopausal status because the nested control-case study was designed to assess the association of ovarian cancer risk with a broad range of biomarkers, including endogenous sex hormones which vary strongly with menopausal status. Controls could not have had a bilateral oophorectomy prior to the diagnosis of the case. Participants using exogenous hormones (oral contraceptives or hormone replacement therapy) at the time of blood donation were not eligible for inclusion in the NYUWHS or ORDET cohorts. Although women using exogenous hormones were eligible to enter the NSHDS cohort, they were excluded from the nested case-control study, in order to increase comparability with women from the two other cohorts.
Laboratory Methods
Seventeen cytokines and inflammation-related markers were measured in serum and plasma samples using Luminex xMap technology (14) using assay kits and procedures described previously (7). In a preliminary reproducibility study, we observed that serum and EDTA plasma measurements were correlated for most inflammation markers (r > 0.6) (9).
Samples from matched case-control sets were assayed together in the same batch to reduce technical variability across batches. Laboratory personnel were blinded as to the case-control status of the samples. A minimum of 5 replicates from a large pool were included in each batch in a blinded fashion. The average intra-batch CVs were all below 10%, except for three markers: IL-1β (16%), IL-5 (15%), IL-12p70 (14%). The inter-batch coefficients of variation (CVs) were 10% or below except for 6 markers: IL-1β (32%), IL-2 (19%), IL-5 (19%), IL-10 (15%), IL-12p70 (36%), and TNFα (20%).
Statistical Methods
When 5% or more of the measurements for an inflammation marker were below the lower limit of detection (LLD) we imputed values below the LLD using a maximum likelihood estimation procedure developed by Lubin et al. for multiple imputation in the presence of detection limits (15). When less than 5% of measurements were below the LLD, we assigned a value equal to the midpoint between the LLD and zero. Cytokine measurements above the LLD, but below the lowest point on the standard curve, were extrapolated beyond the standard curve (less than 5% of values for all markers, except IL-5 and sIL-6R, which had up to 20% extrapolated values).
Odds ratios (OR) and 95% confidence intervals (CI) for ovarian cancer risk were estimated for cytokines coded as continuous (log2 scale) and categorical (quartile) variables using the conditional logistic regression model, which takes into account the risk set sampling and the matching factors. Cytokine values were log2-transformed to reduce departures from the normal distribution and to yield the odds ratio associated with a doubling in cytokine level. Quartile divisions were based on the distribution in the controls and were cohort-specific. The distribution of IL-12p70 in controls was more reasonably divided using three categories, because 60–75% of subjects in each cohort had values in a very narrow range (0.1–1 pg/mL). To test for linear trend, inflammation marker quartiles were included as a single ordinal variable (1, 2, 3, 4) in the conditional logistic regression models. A likelihood ratio test was used to evaluate heterogeneity across cohorts by comparing models with and without cross-product terms for cohort. We used Rosner’s Generalized ESD Many-Outlier Procedure (16) to detect cohort-specific outlying cytokine values. Our preliminary reproducibility study (7, 9) estimates of within-individual variability were used to perform measurement error correction of our odds ratio and 95% confidence interval estimates using a regression calibration method (17).
Multivariate models are adjusted for parity (ever/never) and oral contraceptive (OC) use (ever/never) because they are well-established risk factors for ovarian cancer. Models are also adjusted for body mass index (BMI) at blood donation (weight in kg divided by height in m squared) because it was associated with several cytokines and is a potential risk factor for ovarian cancer. We considered the potential confounding effect of a number of other covariates: use of hormone replacement therapy (ever/never), age at menarche, age at menopause, and smoking status at blood donation (current vs. former/never). We also considered whether adjustment for first degree family history of breast or ovarian cancer (yes/no), use of NSAIDS at time of blood donation (yes/no), and use of vitamin supplements at time of blood donation (yes/no) influenced the ORs in models restricted to the NYUWHS and NSHDS cases and controls, since these variables were not available for the ORDET subjects. Inclusion of these variables did not alter the ORs by more than 10%, and thus were not included in the final models. Cytokine-associated ORs were not appreciably different in unconditional logistic regression models that were adjusted for the matching factors versus conditional logistic regression models. Thus, the relationship between inflammation mediators and ovarian cancer was evaluated in subgroups (according to BMI, menopausal status, never users of oral contraceptives, never users of NSAIDs, and never smokers) by breaking the matching and adjusting for the matching factors and BMI.
To avoid losing case-control sets because of missing data on potential confounders (21% of cases and 20% of controls had missing data on one or more potential confounders), we report results of analyses where we singly-imputed missing covariates using the cohort-specific mean (~25 kg/m2 in each cohort for BMI) or proportion (~ 0.36 for OC use and ~0.83 for parity) in the controls. Analyses excluding subjects with missing data (complete-case analysis) or using multiply-imputed covariate data (18) showed similar results (data not shown).
Inflammation mediators have naturally occurring circulating modulators, including receptor antagonists and soluble cytokine receptors. Because many soluble cytokine receptors have multiple functions (19, 20), their overall activity is difficult to characterize. However, all of the modulators can act as cytokine antagonists. Therefore, we hypothesized that having high levels of a cytokine and low levels of its respective modulator, as compared to having low levels of both, is associated with increased risk of ovarian cancer because the former group may have unchecked cytokine signaling. High versus low levels were defined as below or above the median of the distribution, and women were classified into 1 of 4 groups (low cytokine/low modulator, low/high, high/low, and high/high) for each cytokine-modulator pair.
In addition to regulation of cytokines by specific cytokine modulators, cytokines themselves can regulate the production/secretion of other cytokines. Because Th1, Th2, and Th17 cells secrete cytokines that can regulate each of the other Th-cell type cytokines, we also assessed whether an imbalance in archetypical cytokines for one Th-cell type vs. each of the other Th-cell types may be associated with increased risk of ovarian cancer, according to combinations of high versus low levels (defined as above/below the median of the distribution) of IL-2 (Th1) and IL-4 (Th2), IL-4 (Th2) and IL-6 (Th17), and IL-2 (Th1) and IL-6 (Th17) (21).
All tests for statistical significance were two-sided. Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
The Institutional Review Board of New York University School of Medicine, the Ethical Review Board of the National Cancer Institute of Milan (Italy) and the Regional Ethical Committee of the University of Umea, Sweden, and the Swedish Data Inspection Board reviewed and approved this study.
Results
Descriptive Characteristics
In total, 230 ovarian cancer cases and 432 matched controls (NYUWHS:82 cases and 163 controls; ORDET:41 cases and 82 controls; NSHDS:107 cases and 187 controls) were included. Table 1 gives descriptive characteristics of the cases and controls. The median age at enrollment was 54 for cases and 55 for controls and the median lag time between blood donation and diagnosis was 6.3 years. Over 90% of the subjects were Caucasian. As expected, ovarian cancer cases were generally more likely than controls to have a family history of breast or ovarian cancer (23% vs. 15%, p=0.07), to be nulliparous (23% vs. 18%, p=0.17), to have never used oral contraceptives (70% vs. 64%, p=0.26), and to have ever used HRT (35% vs. 25%, p=0.02).
Table 1.
Characteristics of ovarian cancer cases and matched controls; NYUWHS, ORDET, and NSHDS
| Characteristics | Cases (N=230) | Controls (N=432) |
|---|---|---|
| Age at blood sampling, y, median (10th, 90th) | 54 (40, 64) | 55 (40, 64) |
| Time to diagnosis, y, median (10th, 90th) | 6.3 (1.3, 13.9) | |
| Age at menarche, y, median (10th, 90th) | 13 (11, 15) | 13 (11, 15) |
| Unknown, n | 24 | 29 |
| Body Mass Index, kg/m2, median (10th, 90th) | 24.6 (20.4, 30.1) | 25.0 (21.0, 31.1) |
| Unknown, n | 15 | 22 |
| Menopausal status at baseline, n(%) | ||
| Premenopausal | 83 (36.2) | 157 (36.4) |
| Postmenopausal | 146 (63.8) | 274 (63.6) |
| Unknown | 1 | 1 |
| Family history of breast or ovarian cancer, n(%) | ||
| No | 125 (77.6) | 270 (84.9) |
| Yes | 36 (23.4) | 48 (15.1) |
| Unknown† | 69 | 114 |
| Parity, n(%) | ||
| Nulliparous | 45 (22.5) | 70 (17.5) |
| Parous | 155 (77.5) | 331 (82.5) |
| Unknown | 30 | 31 |
| Age at first pregnancy, y, median (10th, 90th)* | 25 (21, 31) | 25 (20, 33) |
| Unknown, n | 56 | 121 |
| Use of oral contraceptives, n(%) | ||
| Never | 139 (69.8) | 237 (64.4) |
| Ever | 60 (30.2) | 131 (35.6) |
| Unknown | 31 | 64 |
| Use of hormone replacement therapy, n(%)‡ | ||
| Never | 123 (65.4) | 269 (75.4) |
| Ever | 65 (34.6) | 88 (24.6) |
| Unknown | 42 | 75 |
| Smoking status at baseline, n(%) | ||
| Former or never | 163 (74.4) | 303 (78.2) |
| Current | 56 (25.6) | 84 (21.8) |
| Unknown | 11 | 45 |
| Use of NSAIDs at baseline, n(%) | ||
| No | 167 (88.8) | 312 (89.1) |
| Yes | 21 (11.2) | 38 (10.9) |
| Unknown† | 42 | 82 |
| Use of vitamins at baseline, n(%) | ||
| No | 126 (67.0) | 228 (65.3) |
| Yes | 62 (32.0) | 121 (34.7) |
| Unknown† | 42 | 83 |
| Histology, n(%) | ||
| Serous | 120 (52.2) | |
| Endometriod | 30 (13.0) | |
| Clear cell | 16 (7.0) | |
| Mucinous | 22 (10.0) | |
| Undifferentiated | 8 (3.5) | |
| Not Otherwise Specified | 21 (9.1) | |
| Unknown | 13 (5.7) | |
Among everparous women
High frequency of missing because variable is not available from the ORDET cohort (41 cases and 82 controls)
Cases and controls were significantly different with regard to ever use of hormone replacement therapy (p=0.02)
As shown in Table 2, cases generally had higher levels of cytokines than controls, but patterns in the levels of cytokine modulators were not apparent. We observed a smaller proportion of samples with cytokine values below the LLD in the plasma samples (NSHDS cohort) vs. the serum samples (NYUWHS and ORDET cohorts) for IL-1β (0% for NSHDS controls vs. 9/11% below LLD for NYUWHS/ORDET) and IL-2 (1% for NSHDS controls vs. 23/28% for NYUWHS/ORDET), which was likely due to the differences in the biological matrix of the samples.
Table 2.
Inflammation marker levels in cases and controls
| Inflammation Markers | Intra-batch CV (%) | Below LLD* | Cases (n=230) | Controls (n=432) | |
|---|---|---|---|---|---|
| Cases n (%) | Controls n (%) | Median (10%, 90%), pg/mL | Median (10%, 90%), pg/mL | ||
| Cytokines | |||||
| IL-1β | 15.9 | 13 (5.7) | 24 (5.6) | 0.8 (0.1, 4.8) | 0.7 (0.1, 3.2) |
| IL-2 | 7.8 | 32 (13.9) | 64 (14.8) | 14.6 (0.1, 61.1) | 11.4 (0.1, 47.6) |
| IL-4 | 5.0 | 9 (3.9) | 27 (6.3) | 47.6 (1.5, 611) | 21.6 (1.4, 414) |
| IL-5 | 14.5 | 56 (24.3) | 100 (23.1) | 0.1 (0.0, 1.1) | 0.1 (0.0, 1.0) |
| IL-6 | 6.2 | 7 (3.0) | 15 (3.5) | 7.0 (0.9, 52.9) | 4.8 (0.7, 42.8) |
| IL-10 | 5.1 | 1 (0.4) | 1 (0.2) | 5.6 (2.1, 37.8) | 5.4 (2.1, 21.2) |
| IL-12p40 | 2.4 | 0 (0) | 1 (0.2) | 163 (81.9, 418) | 152 (73.0, 335) |
| IL-12p70 | 13.8 | 29 (12.6) | 75 (17.4) | 0.8 (0.1, 13.1) | 0.6 (0.1, 6.9) |
| IL-13 | 4.0 | 29 (12.6) | 61 (14.1) | 22.8 (0.1, 160) | 15.3 (0.1, 132) |
| TNFα | 6.4 | 1 (0.4) | 0 (0) | 3.2 (1.7, 6.4) | 3.0 (1.7, 5.8) |
| Cytokine Modulators | |||||
| IL-1Ra | 4.1 | 56 (24.3) | 103 (23.8) | 517 (83.9, 5595) | 547 (83.9, 3660) |
| sIL-1Rll | 0.8 | 0 (0) | 0 (0) | 5255 (3245, 8321) | 5536 (3061, 8832) |
| sIL-2Ra | 1.8 | 0 (0) | 2 (0.5) | 556 (331, 982) | 539 (311, 952) |
| sIL-4R | 1.3 | 0 (0) | 1 (0.2) | 631 (420, 1211) | 616 (400, 1126) |
| sIL-6R | 2.3 | 0 (0) | 0 (0) | 44896 (28730, 139267) | 51354 (27632, 160798) |
| sTNF-R1 | 1.1 | 0 (0) | 0 (0) | 1244 (815, 2126) | 1215 (813, 2024) |
| sTNF-R2 | 1.0 | 0 (0) | 1 (0.2) | 765 (428, 1097) | 759 (411, 1166) |
Note: LLD=Lower Limit of Detection. Measurements below the lower limit of detection were assigned the value at the midpoint between the lower limit of detection and zero unless more than 5% of samples were below the LLD, upon which values were multiply imputed using maximum likelihood estimation (15).
The proportion of values below the lower limit of detection did not differ substantially across cohorts for any of the markers except IL-1β (below LLD for 9–11% of NYUWHS and ORDET controls and 0% of NSHDS controls) and IL-2 (below LLD for 23–28% of NYUWHS and ORDET controls and only 1% of NSHDS controls).
Inflammation Markers and Risk of Ovarian Cancer
Table 3 shows ORs and 95% confidence intervals for the association between each cytokine and risk of ovarian cancer. We observed evidence of an increasing trend in risk across quartiles of IL- 2 (ORQ4vs.Q1: 1.60, 95% CI: 1.01, 2.55, p-trend =0.05), IL-4 (ORQ4vs.Q1: 1.57, 95% CI: 0.99, 2.47, p- trend=0.04), IL-6 (ORQ4vs.Q1: 1.64, 1.04, 2.58, p-trend=0.03), and IL-13 (ORQ4vs.Q1: 1.50, 95% CI: 0.95, 2.26, p-trend=0.07). Trends remained significant or borderline significant after adjustment for potential confounders (parity, OC use, and BMI). Associations for IL-4 (OR: 1.08, 95% CI: 1.02, 1.13, p=0.01), IL-6 (OR: 1.10, 95% CI: 1.02, 1.19, p=0.01), IL-12p40 (OR: 1.19, 95% CI: 1.05, 1.48, p=0.01), IL-12p70 (OR: 1.08, 95% CI: 1.02, 1.13, p=0.01), and IL-13 (OR: 1.06, 95% CI: 1.01, 1.13, p=0.01) were significant when cytokines were modeled on the continuous scale in both unadjusted and adjusted models. We detected significant heterogeneity between cohorts for IL-4 (p-interaction= 0.01) and IL-13 (p-interaction= 0.02). Cohort-specific ORs for a doubling in these markers were positively associated or null for NYUWHS and NSHDS, respectively, and inversely associated with risk for ORDET: IL-4 [NYUWHS OR: 1.15, (95% CI: 1.05, 1.25); ORDET OR: 0.89, (0.77, 1.02); NSHDS OR: 1.04, (0.97, 1.12)] and IL-13 [NYUWHS OR:1.14, (1.04, 1.26); ORDET OR: 0.94, (0.85, 1.05); NSHDS OR: 1.05, (0.95, 1.15)]. Associations were not significant for these two markers (IL-4 and IL-13) in the ORDET and NSHDS cohorts, but cohort-specific tests were limited in power due to the small sample size (particularly for the ORDET cohort with n=41 cases and 82 controls). Removal of potential outlying values did not change the ORs substantially (data not shown).
Table 3.
Odds ratios and 95% confidence intervals for the association between circulating levels of inflammation markers and ovarian cancer risk
| Cytokine | Continuous | Cohort-specific Quartiles* | |||||
|---|---|---|---|---|---|---|---|
| OR (95%CI) for a doubling in marker level | p for trend | 1 | 2 | 3 | 4 | p for trend | |
| IL-1β | |||||||
| Unadjusted† | 1.08 (1.00, 1.17) | 0.06 | 1.0 (ref) | 1.67 (1.06, 2.65) | 1.32 (0.83, 2.10) | 1.45 (0.92, 2.28) | 0.21 |
| Multivariate‡ | 1.07 (0.99, 1.17) | 0.09 | 1.0 (ref) | 1.72 (1.08, 2.74) | 1.29 (0.80, 2.06) | 1.49 (0.94, 2.35) | 0.20 |
|
| |||||||
| IL-2 | |||||||
| Unadjusted† | 1.03 (0.98, 1.10) | 0.25 | 1.0 (ref) | 1.29 (0.82, 2.05) | 1.33 (0.84, 2.12) | 1.60 (1.01, 2.55) | 0.05 |
| Multivariate‡ | 1.03 (0.97, 1.09) | 0.32 | 1.0 (ref) | 1.30 (0.82, 2.06) | 1.32 (0.82, 2.11) | 1.57 (0.98, 2.52) | 0.07 |
|
| |||||||
| IL-4 | |||||||
| Unadjusted† | 1.06 (1.01, 1.11) | 0.02 | 1.0 (ref) | 1.21 (0.76, 1.92) | 1.38 (0.87, 2.20) | 1.57 (0.99, 2.47) | 0.04 |
| Multivariate‡ | 1.08 (1.02, 1.13) | 0.01 | 1.0 (ref) | 1.17 (0.73, 1.88) | 1.40 (0.88, 2.24) | 1.50 (0.95, 2.38) | 0.06 |
|
| |||||||
| IL-5 | |||||||
| Unadjusted† | 1.02 (0.97, 1.07) | 0.49 | 1.0 (ref) | 1.02 (0.65, 1.60) | 1.54 (0.98, 2.41) | 1.24 (0.79, 1.93) | 0.16 |
| Multivariate‡ | 1.03 (0.98, 1.08) | 0.30 | 1.0 (ref) | 1.00 (0.63, 1.57) | 1.55 (0.99, 2.43) | 1.23 (0.79, 1.92) | 0.16 |
|
| |||||||
| IL-6 | |||||||
| Unadjusted† | 1.09 (1.02, 1.17) | 0.02 | 1.0 (ref) | 1.22 (0.77, 1.94) | 1.33 (0.84, 2.09) | 1.64 (1.04, 2.58) | 0.03 |
| Multivariate‡ | 1.10 (1.02, 1.19) | 0.01 | 1.0 (ref) | 1.22 (0.77, 1.95) | 1.37 (0.86, 2.17) | 1.63 (1.03, 2.58) | 0.03 |
|
| |||||||
| IL-10 | |||||||
| Unadjusted† | 1.07 (0.97, 1.17) | 0.18 | 1.0 (ref) | 1.10 (0.70, 1.73) | 0.96 (0.60, 1.53) | 1.54 (0.96, 2.47) | 0.14 |
| Multivariate‡ | 1.08 (0.98, 1.19) | 0.14 | 1.0 (ref) | 1.09 (0.69, 1.72) | 0.96 (0.60, 1.54) | 1.54 (0.96, 2.49) | 0.14 |
|
| |||||||
| IL-12p40 | |||||||
| Unadjusted† | 1.19 (1.01, 1.39) | 0.03 | 1.0 (ref) | 1.21 (0.78, 1.89) | 1.12 (0.71, 1.78) | 1.46 (0.95, 2.26) | 0.12 |
| Multivariate‡ | 1.24 (1.05, 1.48) | 0.01 | 1.0 (ref) | 1.30 (0.82, 2.03) | 1.18 (0.74, 1.87) | 1.60 (1.02, 2.51) | 0.06 |
|
| |||||||
| IL-12p70 | |||||||
| Unadjusted† | 1.07 (1.02, 1.12) | 0.01 | 1.0 (ref) | 1.59 (1.06, 2.39) | 1.27 (0.86, 1.88) | N/A | 0.12 |
| Multivariate‡ | 1.08 (1.02, 1.13) | 0.01 | 1.0 (ref) | 1.53 (1.02, 2.32) | 1.28 (0.86, 1.90) | N/A | 0.12 |
|
| |||||||
| IL-13 | |||||||
| Unadjusted† | 1.05 (1.01, 1.11) | 0.06 | 1.0 (ref) | 1.14 (0.72, 1.80) | 1.27 (0.80, 2.01) | 1.50 (0.95, 2.37) | 0.07 |
| Multivariate‡ | 1.06 (1.01, 1.13) | 0.04 | 1.0 (ref) | 1.07 (0.68, 1.70) | 1.23 (0.77, 1.95) | 1.42 (0.90, 2.26) | 0.11 |
|
| |||||||
| TNFα | |||||||
| Unadjusted† | 1.11 (0.92, 1.35) | 0.28 | 1.0 (ref) | 0.75 (0.48, 1.18) | 1.05 (0.67, 1.64) | 1.11 (0.70, 1.75) | 0.54 |
| Multivariate‡ | 1.19 (0.96, 1.47) | 0.11 | 1.0 (ref) | 0.77 (0.49, 1.21) | 1.12 (0.70, 1.78) | 1.23 (0.77, 1.97) | 0.31 |
|
| |||||||
| IL-1Ra | |||||||
| Unadjusted† | 1.02 (0.96, 1.09) | 0.56 | 1.0 (ref) | 0.80 (0.49, 1.32) | 0.71 (0.44, 1.14) | 0.95 (0.61, 1.49) | 0.76 |
| Multivariate‡ | 1.03 (0.97, 1.11) | 0.31 | 1.0 (ref) | 0.82 (0.50, 1.36) | 0.73 (0.45, 1.18) | 1.00 (0.64, 1.57) | 0.91 |
|
| |||||||
| sIL-1Rll | |||||||
| Unadjusted† | 0.90 (0.70, 1.15) | 0.39 | 1.0 (ref) | 0.98 (0.62, 1.56) | 1.16 (0.75, 1.80) | 0.79 (0.49, 1.28) | 0.55 |
| Multivariate‡ | 0.91 (0.70, 1.19) | 0.49 | 1.0 (ref) | 1.02 (0.64, 1.63) | 1.24 (0.79, 1.94) | 0.81 (0.50, 1.32) | 0.66 |
|
| |||||||
| sIL-2Ra | |||||||
| Unadjusted† | 1.24 (0.94, 1.64) | 0.13 | 1.0 (ref) | 1.19 (0.73, 1.94) | 1.23 (0.76, 1.99) | 1.30 (0.79, 2.15) | 0.31 |
| Multivariate‡ | 1.24 (0.93, 1.67) | 0.15 | 1.0 (ref) | 1.19 (0.73, 1.94) | 1.30 (0.80, 2.12) | 1.35 (0.81, 2.23) | 0.22 |
|
| |||||||
| sIL-4R | |||||||
| Unadjusted† | 1.26 (0.93, 1.70) | 0.14 | 1.0 (ref) | 0.94 (0.60, 1.48) | 0.83 (0.52, 1.32) | 1.24 (0.78, 1.97) | 0.49 |
| Multivariate‡ | 1.29 (0.93, 1.78) | 0.12 | 1.0 (ref) | 0.94 (0.60, 1.48) | 0.84 (0.53, 1.33) | 1.19 (0.74, 1.89) | 0.60 |
|
| |||||||
| sIL-6R | |||||||
| Unadjusted† | 0.86 (0.70, 1.05) | 0.14 | 1.0 (ref) | 0.83 (0.53, 1.30) | 0.98 (0.62, 1.54) | 0.63 (0.39, 1.01) | 0.12 |
| Multivariate‡ | 0.86 (0.69, 1.06) | 0.16 | 1.0 (ref) | 0.81 (0.51, 1.27) | 0.97 (0.61, 1.52) | 0.62 (0.38, 1.01) | 0.12 |
|
| |||||||
| sTNF-R1 | |||||||
| Unadjusted† | 1.11 (0.85, 1.44) | 0.45 | 1.0 (ref) | 0.95 (0.60, 1.51) | 1.14 (0.72, 1.81) | 1.17 (0.73, 1.87) | 0.40 |
| Multivariate‡ | 1.23 (0.92, 1.63) | 0.17 | 1.0 (ref) | 1.00 (0.63, 1.58) | 1.21 (0.76, 1.94) | 1.26 (0.78, 2.02) | 0.26 |
|
| |||||||
| sTNF-R2 | |||||||
| Unadjusted† | 0.97 (0.77, 1.23) | 0.83 | 1.0 (ref) | 1.25 (0.80, 1.94) | 1.24 (0.78, 1.94) | 0.92 (0.57, 1.48) | 0.81 |
| Multivariate‡ | 1.02 (0.80, 1.31) | 0.85 | 1.0 (ref) | 1.28 (0.82, 2.01) | 1.28 (0.81, 2.03) | 0.94 (0.58, 1.51) | 0.87 |
Quantile cut points were selected based on the distribution of values in the controls, independently for each cohort; p for trend estimated by entering the cytokine quantiles as an ordinal variable (1, 2, 3, 4) into the conditional logistic regression models
Odds ratios from conditional logistic regression models, which controls for matching factors (age, cohort, and menopausal status) only
Odds ratios from conditional logistic regression models, which controls for matching factors, but also adjusted for ever pregnant, ever use of oral contraceptives, and BMI. Missing values for ever pregnant, ever use of oral contraceptives, and BMI were imputed using the proportion/distribution of the controls separately for each cohort.
Stratified/subgroup Analyses
ORs did not differ appreciably in analyses stratified by BMI (< 25/≥25 kg/m2) or time to diagnosis (< 5/≥5 years after blood donation). ORs were also not appreciably different after excluding individuals diagnosed less than 2 years after blood donation. We did not detect significant statistical interaction with BMI or menopausal status at the time of blood donation. ORs were similar to those from the overall analysis in the subgroups of never smokers, ever-users of oral contraceptives (no participants were current users), and non-users of NSAIDs at blood donation, though results in subgroups were generally no longer statistically significant (data not shown). Results were also similar in analyses limited to the serous histological subtype (supplementary Table 1).
We considered the influence of cytokines in conjunction with their naturally occurring modulators (agonists and/or antagonists). ORs in the high cytokine/low modulator combination (our a priori definition of an unbalanced cytokine vs. modulator response) versus the low cytokine/low modulator combination was significant for IL-6 vs. sIL-6R (OR: 1.65, 95% CI: 1.05, 2.61), IL-12p70 vs. IL-12p40 (OR: 1.62, 95% CI: 1.01, 2.58), and TNFα vs. sTNF-R1 (OR: 2.13, 95% CI: 1.33, 3.41) (Table 4). The TNFα vs. sTNF-R1 and IL-12p70 vs. IL-12p40 combinations were also associated with risk when either one or both of the markers were high.
Table 4.
Odds ratios and 95% confidence intervals by inflammatory profile
| Cytokine vs. Modulator | Low/Low | Low/High | High/Low | High/High |
|---|---|---|---|---|
| IL-1β vs. IL-1Ra | ||||
| # cases/# controls | 72/139 | 39/85 | 42/71 | 77/137 |
| OR (95% CI) | 1.0 (ref) | 0.91 (0.57, 1.47) | 1.15 (0.71, 1.85) | 1.09 (0.73, 1.64) |
| p-value | 0.71 | 0.57 | 0.67 | |
| IL-1β vs. sIL-1Rll | ||||
| # cases/# controls | 46/105 | 65/119 | 70/109 | 49/99 |
| OR (95% CI) | 1.0 (ref) | 1.20 (0.76, 1.92) | 1.43 (0.90, 2.27) | 1.11 (0.68, 1.81) |
| p-value | 0.43 | 0.13 | 0.69 | |
| IL-2 vs. sIL-2Ra | ||||
| # cases/# controls | 48/118 | 59/107 | 63/99 | 60/108 |
| OR (95% CI) | 1.0 (ref) | 1.36 (0.85, 2.19) | 1.56 (0.98, 2.47) | 1.36 (0.85, 2.16) |
| p-value | 0.20 | 0.06 | 0.20 | |
| IL-4 vs. sIL-4R | ||||
| # cases/# controls | 61/121 | 44/103 | 53/95 | 72/113 |
| OR (95% CI) | 1.0 (ref) | 0.85 (0.53, 1.36) | 1.11 (0.70, 1.76) | 1.28 (0.84, 1.97) |
| p-value | 0.50 | 0.65 | 0.25 | |
| IL-6 vs. sIL-6R | ||||
| # cases/# controls | 52/116 | 51/110 | 68/94 | 59/112 |
| OR (95% CI) | 1.0 (ref) | 1.05 (0.66, 1.68) | 1.65 (1.05, 2.61) | 1.19 (0.76, 1.89) |
| p-value | 0.84 | 0.03 | 0.45 | |
| IL-12p70 vs. IL-12p40 | ||||
| # cases/# controls | 49/129 | 54/100 | 58/96 | 69/107 |
| OR (95% CI) | 1.0 (ref) | 1.46 (0.92, 2.34) | 1.62 (1.01, 2.58) | 1.74 (1.11, 2.74) |
| p-value | 0.11 | 0.04 | 0.02 | |
| TNFα vs. sTNF-R1 | ||||
| # cases/# controls | 48/137 | 67/83 | 62/85 | 63/127 |
| OR (95% CI) | 1.0 (ref) | 2.12 (1.30, 3.45) | 2.13 (1.33, 3.41) | 1.51 (0.95, 2.41) |
| p-value | 0.002 | 0.002 | 0.08 | |
| TNFα vs. sTNF-R2 | ||||
| # cases/# controls | 62/129 | 43/91 | 53/86 | 72/126 |
| OR (95% CI) | 1.0 (ref) | 0.98 (0.61, 1.58) | 1.28 (0.81, 2.03) | 1.18 (0.76, 1.82) |
| p-value | 0.94 | 0.29 | 0.46 | |
Note: Each marker was classified as high or low based on the median value and then four groups were created for each pair. Models are unconditional logistic regression models, adjusted for the matching factors (age, cohort, menopausal status)
We observed that any combination of high levels of the Th1, Th2, and Th17 archetypical cytokines was associated with increased risk of ovarian cancer: The OR associated with having high levels of both IL-2 and IL-4 versus low levels of both cytokines was 1.55 (95% CI: 1.01, 2.39); the OR associated with having high levels of both IL-6 and IL-4 was 1.43 (95% CI: 1.00, 2.03), and the OR associated with having high levels of both IL-6 and IL-2 was 1.58 (95% CI: 1.04, 2.42) (Table 5).
Table 5.
Odds ratios and 95% confidence intervals by T-helper cytokine marker profile
| Low/Low | Low/High | High/Low | High/High | |
|---|---|---|---|---|
| IL-2/IL-4 (Th1/Th2) | ||||
| # cases/# controls | 56/131 | 51/94 | 49/95 | 74/112 |
| OR (95% CI) | 1.0 (ref) | 1.29 (0.81, 2.06) | 1.21 (0.76, 1.93) | 1.55 (1.01, 2.39) |
| p-value | 0.28 | 0.42 | 0.05 | |
| IL-6/IL-4 (Th17/Th2) | ||||
| # cases/# controls | 86/188 | 17/38 | 19/38 | 108/168 |
| OR (95% CI) | 1.0 (ref) | 1.00 (0.53, 1.88) | 1.13 (0.61, 2.09) | 1.43 (1.00, 2.03) |
| p-value | 0.99 | 0.70 | 0.05 | |
| IL-6/IL-2 (Th17/Th1) | ||||
| # cases/# controls | 57/136 | 46/90 | 50/89 | 77/117 |
| OR (95% CI) | 1.0 (ref) | 1.22 (0.76, 1.95) | 1.37 (0.85, 2.18) | 1.58 (1.04, 2.42) |
| p-value | 0.42 | 0.19 | 0.03 | |
Note: Each marker was classified as high or low based on the median value and then four groups were created for each pair. Models are unconditional logistic regression models, adjusted for the matching factors (age, cohort, menopausal status)
Discussion
We found an increasing risk of ovarian cancer with a doubling in the levels of IL-4, IL-6, IL-12p40, IL-12p70, and IL-13 and observed a trend across quartiles of IL-2, IL-4, IL-6, IL-12p40 and IL-13. These trends were statistically significant or of borderline significance after adjustment for parity, oral contraceptive use, and BMI.
Factors that inhibit ovulation, such as parity and oral contraceptive use, have consistently been shown to be inversely associated with ovarian cancer risk. The physiological process of ovulation has many characteristics of an inflammatory reaction, including the generation of an abundance of inflammatory cytokines to facilitate growth, development, and remodeling of the follicle and repair of the ovulatory wound (22, 23). The repeated wounding and healing process is thought to contribute to DNA and cellular damage at the ovarian surface epithelium (OSE) and/or in ovarian inclusion cysts (OIC), the putative site of origin for several ovarian tumor types (24–28). The fallopian tube fimbria, which has recently been identified as another site of origin for ovarian tumors, is exposed to inflammatory cytokines due to retrograde transport of menstrual fluid and infections from the lower genital tract (23, 28, 29). Local inflammatory conditions such as endometriosis, exposure to irritants such as talc, and low grade systemic inflammation due to factors such as obesity are also associated with increased ovarian cancer risk. Both ovulation-specific and ovulation-independent low-grade inflammatory processes are associated with increased ovarian cancer risk, which may be one reason why we did not observe evidence of effect modification by menopausal status. Inflammation mediators can act as tumor promoters by providing a proliferative, anti-apoptotic, and angiogenic environment for transformed cells in the fallopian tubes and ovaries (30, 31). The involvement of the global immune network in the regulation of physiologically normal ovarian processes (32–41), suggests that these organs may be particularly exposed to systemic as well as local inflammation.
IL-2, IL-4, and IL-6 are archetypical cytokines expressed in Th1, Th2, and Th17 inflammatory responses, respectively (21). These cytokines’ pivotal role in inflammation as well as their association with normal (41–45) and malignant (44, 46–52) ovarian processes offer biologic plausibility to our findings. We observed that IL-2, IL-4, and IL-6 were associated with significantly increased risk. For IL-2 and IL-6, this association was particularly apparent when their modulators (sIL-2Ra and sIL-6R, respectively) were expressed at low levels, suggesting that an imbalance in cytokines and their modulators, which can act as antagonists of cytokine signaling, may be associated with increased risk. The T-helper (Th) immune cell paradigm suggests that Th1 cell populations secrete cytokines which can inhibit the Th2 cell type (and vice versa) to regulate cell-mediated as well as antibody-mediated immunity (53). Furthermore, depending on their relative concentrations, Th1 and Th2 cytokines can also promote or inhibit Th17 cells, which are associated with immune-mediated tissue damage (53). Although this classification system is thought to be an oversimplification of cytokine production and function, improved cytokine classification systems have not yet been developed. Our hypothesis was that having high Th1- or Th17-related cytokines and low Th2-related cytokines would be associated with increased risk of ovarian cancer due to the pro-inflammatory nature of Th1 and Th17 versus Th2 subtypes. However, we found that for the pairs IL-2 (Th1) and IL-4 (Th2), IL-6 (Th17) and IL-4 (Th2), and IL-2 (Th1) and IL-6 (Th17), having high levels of both markers was associated with a significant increase in risk, and having high levels of only one was not. This finding suggests that a persistent inflammatory state, involving elevations in all Th-cell type cytokine responses, may be associated with increased risk, though we had somewhat limited power to detect associations within subgroups.
Our study has several important strengths. A major strength of our study is its prospective design, which ensured that samples were collected before diagnosis, which is required to infer the proper temporal sequence from cytokine elevations to cancer, and also minimizes selection bias. An additional strength was that we evaluated the temporal reliability of all biomarkers in preliminary studies. Measurement error adjusted estimates for the cytokines that were associated with ovarian cancer risk did not differ appreciably from unadjusted estimates (absolute increase of ≤0.01 in the adjusted vs. unadjusted ORs) (data not shown). These results were not surprising since these markers had intra-class correlation coefficient (ICC, fraction of total variation due to between-subject variability) above 0.5, with most ranging from 0.7–0.9, indicating that a single measurement of these markers is representative of an individual’s average marker level and can therefore be used to rank individuals (7–9).
A limitation of our study is that we did not have information on several factors that influence cytokine levels, such as the presence of autoimmune or infectious diseases. However, women were generally healthy at blood donation, and the proportion of women with serious infections and undiagnosed chronic diseases is likely to be very low. The main limitation of our study was its relatively small sample size resulting in sufficient power to detect moderate to strong ORs only, especially within subgroups. Another limitation is that multiple comparisons may have led to some spurious associations. However, associations were apparent for IL-4, IL-6, and IL-13 when modeled as continuous as well as categorical variables and after controlling for confounders, although less so for IL-2 and IL-12p40. Replication of our results in independent prospective studies, though, is needed, in particular for IL-4 and IL-13 for which there was some evidence of interaction by cohort and also for IL-12p70 which was associated with risk when modeled as a continuous variable, but did not show a trend across ordered categories. Finally, ovarian cancer is a heterogeneous disease, with multiple histological subtypes and diverse etiological pathways. Overall case-control comparisons may have prevented us from observing a cytokine effect that is limited to a specific subtype. However, our study is limited to ovarian cancers that originate from epithelial cells and each of the epithelial histological subtypes is associated with risk factors consistent with the inflammation hypothesis. When we restricted the analyses to the most common serous histological subtype, we found that odds ratios were not substantially different from overall analyses.
In summary, we found a positive association between IL-2, IL-4, IL-6, IL-12, and IL-13 and ovarian cancer risk. These findings provide support for the role of inflammation in the etiology of ovarian cancer.
Supplementary Material
Acknowledgments
We thank Jay Lubin for generously providing his SAS program for multiple imputation. We thank Yelena Afanasyeva for database management, and Lynne Quinones and Noriko Shimizu for administrative support.
Financial Support
This work was supported by research grants from the National Cancer Institute (R21 CA116585, R01 CA098661, and P30CA016087) and the National Institute of Environmental Health Sciences Center Grant (ES000260) at the National Institutes of Health.
References
- 1.Ness RB, Grisso JA, Cottreau C, Klapper J, Vergona R, Wheeler JE, et al. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000;11(2):111–7. doi: 10.1097/00001648-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Botran R. Soluble cytokine receptors: basic immunology and clinical applications. Crit Rev Clin Lab Sci. 1999;36(3):165–224. doi: 10.1080/10408369991239196. [DOI] [PubMed] [Google Scholar]
- 4.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64(2):135–46. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- 5.McSorley MA, Alberg AJ, Allen DS, Allen NE, Brinton LA, Dorgan JF, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gynecol. 2007;109(4):933–41. doi: 10.1097/01.AOG.0000257126.68803.03. [DOI] [PubMed] [Google Scholar]
- 6.Lundin E, Dossus L, Clendenen T, Krogh V, Grankvist K, Wulff M, et al. C-reactive protein and ovarian cancer: a prospective study nested in three cohorts (Sweden, USA, Italy) Cancer Causes Control. 2009;20(7):1151–9. doi: 10.1007/s10552-009-9330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Y, Zeleniuch-Jacquotte A, Linkov F, Koenig KL, Liu M, Velikokhatnaya L, et al. Reproducibility of serum cytokines and growth factors. Cytokine. 2009;45(1):44–9. doi: 10.1016/j.cyto.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- 9.Clendenen TV, Arslan AA, Lokshin AE, Idahl A, Hallmans G, Koenig KL, et al. Temporal reliability of cytokines and growth factors in EDTA plasma. BMC Res Notes. 2010;3:302–10. doi: 10.1186/1756-0500-3-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87(3):190–7. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 11.Toniolo PG, Pasternack BS, Shore RE, Sonnenschein E, Koenig KL, Rosenberg C, et al. Endogenous hormones and breast cancer: a prospective cohort study. Breast Cancer Res Treat. 1991;18 (Suppl 1):S23–6. doi: 10.1007/BF02633522. [DOI] [PubMed] [Google Scholar]
- 12.Hallmans G, Agren A, Johansson G, Johansson A, Stegmayr B, Jansson JH, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort - evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24. doi: 10.1080/14034950310001432. [DOI] [PubMed] [Google Scholar]
- 13.Berrino F, Muti P, Micheli A, Bolelli G, Krogh V, Sciajno R, et al. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 1996;88(5):291–6. doi: 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- 14.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243(1–2):243–55. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 15.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosner B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;25(2):165–72. [Google Scholar]
- 17.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol. 1992;136(11):1400–13. doi: 10.1093/oxfordjournals.aje.a116453. [DOI] [PubMed] [Google Scholar]
- 18.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 19.Heaney ML, Golde DW. Soluble cytokine receptors. Blood. 1996;87(3):847–57. [PubMed] [Google Scholar]
- 20.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 21.Peck A, Mellins ED. Plasticity of T-cell phenotype and function: the T helper type 17 example. Immunology. 2010;129(2):147–53. doi: 10.1111/j.1365-2567.2009.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50(2):233–8. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 23.Shan W, Liu J. Inflammation: a hidden path to breaking the spell of ovarian cancer. Cell Cycle. 2009;8(19):3107–11. doi: 10.4161/cc.8.19.9590. [DOI] [PubMed] [Google Scholar]
- 24.Hutson R, Ramsdale J, Wells M. p53 protein expression in putative precursor lesions of epithelial ovarian cancer. Histopathology. 1995;27(4):367–71. doi: 10.1111/j.1365-2559.1995.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 25.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 26.Murdoch WJ, Martinchick JF. Oxidative damage to DNA of ovarian surface epithelial cells affected by ovulation: carcinogenic implication and chemoprevention. Exp Biol Med (Maywood) 2004;229(6):546–52. doi: 10.1177/153537020422900613. [DOI] [PubMed] [Google Scholar]
- 27.Pothuri B, Leitao MM, Levine DA, Viale A, Olshen AB, Arroyo C, et al. Genetic Analysis of the Early Natural History of Epithelial Ovarian Carcinoma. PLoS ONE. 2010;5(4):e10358. doi: 10.1371/journal.pone.0010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karst AM, Drapkin R. Ovarian cancer pathogenesis: a model in evolution. J Oncol. 2010;2010:932371. doi: 10.1155/2010/932371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvador S, Gilks B, Kobel M, Huntsman D, Rosen B, Miller D. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer. 2009;19(1):58–64. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 30.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85(4):473–83. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431(7007):405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 32.Willis C, Morris JM, Danis V, Gallery ED. Cytokine production by peripheral blood monocytes during the normal human ovulatory menstrual cycle. Hum Reprod. 2003;18(6):1173–8. doi: 10.1093/humrep/deg231. [DOI] [PubMed] [Google Scholar]
- 33.Omu AE, Al-Azemi MK, Makhseed M, Al-Oattan F, Ismail AA, Al-Tahir S, et al. Differential expression of T-helper cytokines in the peritoneal fluid of women with normal ovarian cycle compared with women with chronic anovulation. Acta Obstet Gynecol Scand. 2003;82(7):603–9. doi: 10.1034/j.1600-0412.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 34.Abrahamsen B, Stilgren LS, Rettmer E, Bonnevie-Nielsen V, Beck-Nielsen H. Effects of the natural and artificial menstrual cycle on the production of osteoprotegerin and the bone resorptive cytokines IL-1beta and IL-6. Calcif Tissue Int. 2003;72(1):18–23. doi: 10.1007/s00223-002-2037-y. [DOI] [PubMed] [Google Scholar]
- 35.Verthelyi D, Klinman DM. Sex hormone levels correlate with the activity of cytokine- secreting cells in vivo. Immunology. 2000;100(3):384–90. doi: 10.1046/j.1365-2567.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu KM, Arnaud CD, Ju J, Mayes D, Bacchetti P, Weitz S, et al. Correlation of estradiol, parathyroid hormone, interleukin-6, and soluble interleukin-6 receptor during the normal menstrual cycle. Bone. 2000;26(1):79–85. doi: 10.1016/s8756-3282(99)00243-4. [DOI] [PubMed] [Google Scholar]
- 37.Steer CV, Campbell S, Pampiglione JS, Kingsland CR, Mason BA, Collins WP. Transvaginal colour flow imaging of the uterine arteries during the ovarian and menstrual cycles. Hum Reprod. 1990;5(4):391–5. doi: 10.1093/oxfordjournals.humrep.a137109. [DOI] [PubMed] [Google Scholar]
- 38.Scholtes MC, Wladimiroff JW, van Rijen HJ, Hop WC. Uterine and ovarian flow velocity waveforms in the normal menstrual cycle: a transvaginal Doppler study. Fertil Steril. 1989;52(6):981–5. doi: 10.1016/s0015-0282(16)53162-8. [DOI] [PubMed] [Google Scholar]
- 39.Collins W, Jurkovic D, Bourne T, Kurjak A, Campbell S. Ovarian morphology, endocrine function and intra-follicular blood flow during the peri-ovulatory period. Hum Reprod. 1991;6(3):319–24. doi: 10.1093/oxfordjournals.humrep.a137332. [DOI] [PubMed] [Google Scholar]
- 40.Tan SL, Zaidi J, Campbell S, Doyle P, Collins W. Blood flow changes in the ovarian and uterine arteries during the normal menstrual cycle. Am J Obstet Gynecol. 1996;175(3 Pt 1):625–31. doi: 10.1053/ob.1996.v175.a73865. [DOI] [PubMed] [Google Scholar]
- 41.Nash MA, Ferrandina G, Gordinier M, Loercher A, Freedman RS. The role of cytokines in both the normal and malignant ovary. Endocr Relat Cancer. 1999;6(1):93–107. doi: 10.1677/erc.0.0060093. [DOI] [PubMed] [Google Scholar]
- 42.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990;87(8):3092–6. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barak V, Mordel N, Zajicek G, Kalichman I, Treves AJ, Laufer N. The correlation between interleukin 2 and soluble interleukin 2 receptors to oestradiol, progesterone and testosterone levels in periovulatory follicles of in-vitro fertilization patients. Hum Reprod. 1992;7(7):926–9. doi: 10.1093/oxfordjournals.humrep.a137772. [DOI] [PubMed] [Google Scholar]
- 44.Ripley D, Shoup B, Majewski A, Chegini N. Differential expression of interleukins IL-13 and IL-15 in normal ovarian tissue and ovarian carcinomas. Gynecol Oncol. 2004;92(3):761–8. doi: 10.1016/j.ygyno.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215(1–2):135–41. doi: 10.1016/j.mce.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martinez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991;164(4):1038–42. doi: 10.1016/0002-9378(91)90582-c. discussion 42–3. [DOI] [PubMed] [Google Scholar]
- 47.Gastl G, Plante M, Finstad CL, Wong GY, Federici MG, Bander NH, et al. High IL-6 levels in ascitic fluid correlate with reactive thrombocytosis in patients with epithelial ovarian cancer. Br J Haematol. 1993;83(3):433–41. doi: 10.1111/j.1365-2141.1993.tb04668.x. [DOI] [PubMed] [Google Scholar]
- 48.Moradi MM, Carson LF, Weinberg B, Haney AF, Twiggs LB, Ramakrishnan S. Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer. 1993;72(8):2433–40. doi: 10.1002/1097-0142(19931015)72:8<2433::aid-cncr2820720822>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 49.Scambia G, Testa U, Panici PB, Martucci R, Foti E, Petrini M, et al. Interleukin-6 serum levels in patients with gynecological tumors. Int J Cancer. 1994;57(3):318–23. doi: 10.1002/ijc.2910570305. [DOI] [PubMed] [Google Scholar]
- 50.Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol. 1997;66(1):27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- 51.Lambeck AJ, Crijns AP, Leffers N, Sluiter WJ, ten Hoor KA, Braid M, et al. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res. 2007;13(8):2385–91. doi: 10.1158/1078-0432.CCR-06-1828. [DOI] [PubMed] [Google Scholar]
- 52.Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(4):981–7. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 53.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13(2):139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.