Abstract
The peroxisome proliferator activated receptor (PPAR)-γ is a nuclear receptor that is activated by lipids to induce the expression of genes involved in lipid and glucose metabolism, thereby converting nutritional signals into metabolic consequences1. PPARγ is the target of the thiazolidinedione (TZD)-class of insulin-sensitizing drugs, which have been widely prescribed to treat Type 2 Diabetes Mellitus (T2DM). A common side effect of treatment with TZDs is weight gain2. Here we report a novel role for central nervous system (CNS) PPARγ in the regulation of energy balance. We found that both acute and chronic activation of CNS PPARγ, by TZDs or by hypothalamic over-expression of a VP16-PPARγ fusion protein, led to positive energy balance in rats. Blocking the endogenous activation of CNS PPARγ, with pharmacological antagonists or with shRNA, led to negative energy balance, restored leptin-sensitivity in high-fat diet (HFD)-fed rats, and blocked the hyperphagic response to oral TZD treatment. These findings have implications for the widespread clinical use of TZD drugs and for understanding the etiology of diet-induced obesity.
Despite intense investigation, PPARγ remains an orphan nuclear receptor; i.e., a high-affinity endogenous ligand has not yet been identified. Rather, several dietary fats and their metabolites bind to PPARγ, but with moderate affinity, leading to the suggestion that the physiological role of PPARγ is to act as a sensor for the integrated flux of multiple fatty acids1. Consistent with this possibility, PPARγ is highly expressed in white adipose tissue (WAT) where it is a key regulator of adipogenesis3,4 and where PPARγ activation promotes increased lipid storage5,6. Chronic peripheral administration of exogenous PPARγ agonists, including the TZD Rosiglitazone (RSG), improves glycemic control at the expense of increased caloric intake, body weight and body-fat gain2,7,8. Chronic peripheral administration of PPARγ antagonists also confers protection from diet-induced obesity9.
The traditional view has been that these changes in energy balance are mediated primarily by the actions of PPARγ to induce adipogenesis in WAT. However we would emphasize the point, made elsewhere by Rosen and Spiegelman10, that simply “having more fat cells does not make an animal fatter. In the absence of altered energy balance, an increase in adipogenesis will result in smaller fat cells with no change in total adiposity.” Pertinent to this, PPARγ is also expressed in regions of the hypothalamus important for the central regulation of energy balance11-13. We therefore hypothesized that: 1) activation of CNS PPARγ by RSG contributes to its effect on energy balance, and 2) activation of CNS PPARγ by its endogenous lipid agonists provides a direct mechanism underlying HFD-induced hyperphagia and leptin resistance.
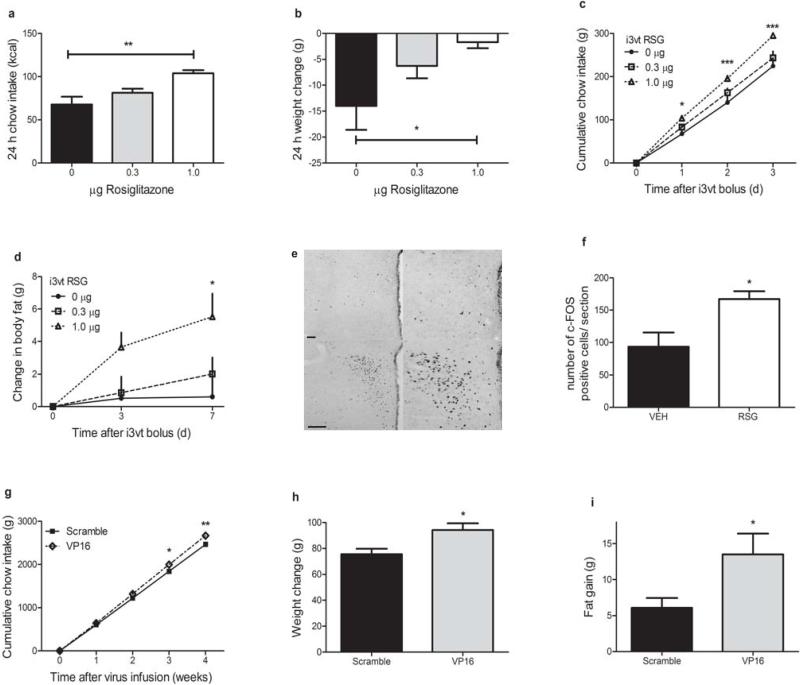
We hypothesized that direct activation of CNS PPARγ would result in positive energy balance. To test this, we administered small doses of RSG or its vehicle directly into the 3rd-cerebral ventricle (i3vt) of male Long-Evans rats in the area of the ventral hypothalamus. Acute i3vt RSG resulted in a 50% higher caloric intake over 24 h, with a corresponding higher body weight change (Fig. 1a,b) compared to i3vt vehicle alone. Furthermore, a single bolus of i3vt RSG led to significantly greater food intake for as many as 3 d (Fig. 1c) and body fat gain was still higher 7 d following the single injection (Fig. 1d), compared to i3vt vehicle alone. We found no differences in chow intake following an oral dose of RSG (0 vs. 0.1 mg kg·bw–1) roughly 30 times greater than our central dose (VEH: 26.34 g ± 0.58, RSG: 27.64 g ± 0.71), ruling out that our i3vt treatments have peripheral orexigenic effects. To determine whether RSG could activate neuronal populations involved in the regulation of energy balance, we measured c-Fos immunoreactivity in rat hypothalamus 1 h following an acute i3vt injection of RSG. There was a significantly greater induction of c-Fos in the paraventricular (PVH, Fig. 1e,f) but not in the arcuate (ARH) or dorsomedial nucleus of the hypothalamus (DMH) (Supplementary Fig. 1a,b) among rats injected with RSG compared to those injected with vehicle alone.
Figure 1. Activation of hypothalamic PPARγ leads to positive energy balance.
a,b) 24 h caloric intake (a) and weight change (b) following i3vt RSG or vehicle (Kruskal-Wallis, Dunn's posthoc) c,d) Cumulative food intake (c) and body fat gain (d) following the bolus infusion of RSG or vehicle on day 0 (RM ANOVA with Tukey posthoc) e) Representative sections (top = vehicle, bottom = RSG; left = 10X, right = 20X) showing c-Fos immunoreactivity in the PVH at 1 h following i3vt RSG or vehicle. Scale bar = 100 μm f) Quantification of c-Fos response to 1 μg RSG or its vehicle i3vt (Mann-Whitney test) g,h,i) Caloric intake (g), body weight change (h), and body fat gain (i) 4 weeks following over-expression of a constitutively active form of PPARγ (VP16-PPARγ) or its scrambled control in the medial hypothalamus (RM ANOVA with Tukey posthoc, T-tests) * = p < 0.05, ** = p < 0.01, *** = p < 0.001. The mean for each group is represented ± S.E., n = 3–6 animals per group for the c-Fos experiment, for all other experiments n = 7–12 animals per group.
To investigate the effects of chronic activation of CNS PPARγ, we used a lentiviral vector to over-express VP16-PPARγ fusion protein locally in the hypothalamus of male rats (Supplementary Fig. 2a,b). Fusion of the viral transcriptional activator, VP16, to PPARγ potently and constitutively activates PPARγ in the absence of ligand14, and has been used to explore the effects of chronic tissue-specific PPARγ activation15,16. Over the course of 4 weeks following the lentivirus infusions, rats that had received the VP16-PPARγ virus consumed more calories, gained more weight, and gained twice as much body fat as control-treated rats (Fig. 1g–i). Collectively these data suggest a potentially important role for hypothalamic PPARγ in both the acute and chronic regulation of food intake and adiposity.
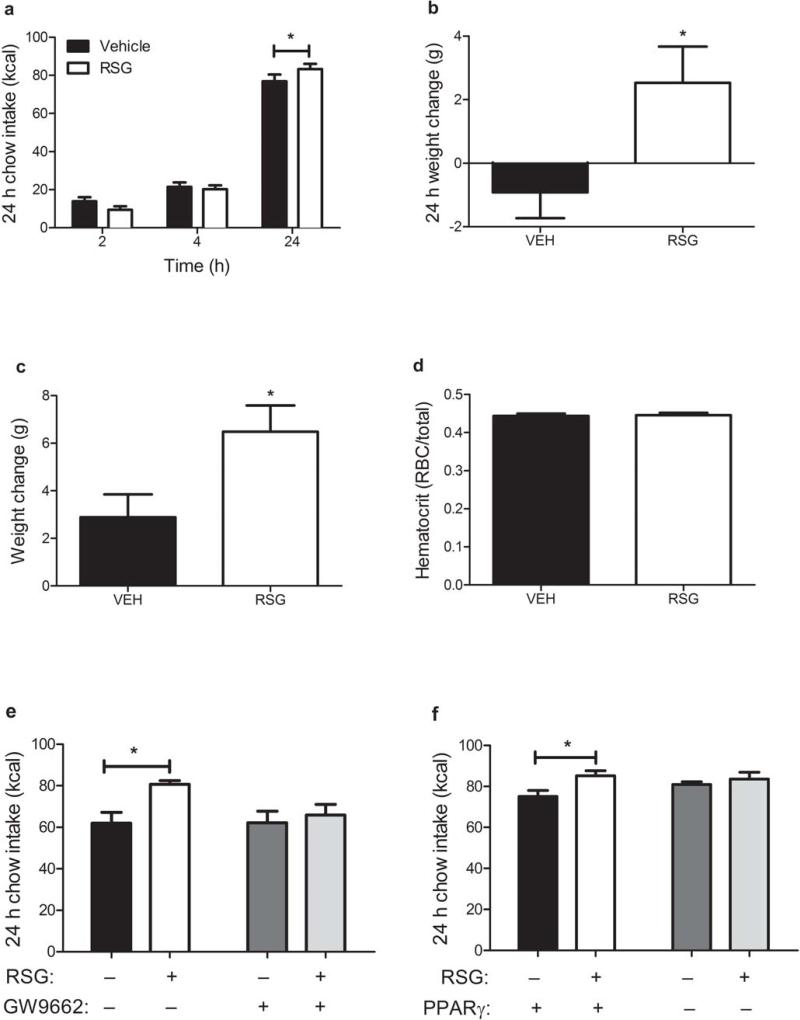
These findings additionally suggest that peripheral administration of TZD drugs might increase weight gain by activating CNS PPARγ. To test this hypothesis explicitly, we administered RSG or its vehicle via oral gavage and simultaneously infused the PPARγ antagonist GW9662 or its vehicle i3vt. It is well-established that treatment with oral TZDs over the course of weeks or months induces weight gain in humans7,8,17 and in rodents2,18. We observed that a single oral dose of RSG induced hyperphagia and greater body weight gain within 24 h (Fig. 2a,b) compared to vehicle alone. To rule out that this weight gain was associated with greater plasma volume19, we measured hematocrits from rats following oral RSG or its vehicle (Fig. 2c,d). Further, when CNS PPARγ receptors were blocked by administering 1 μg of GW9662 i3vt, the acute orexigenic effect of oral RSG was completely attenuated (Fig. 2e). To test the hypothesis in a second way, we administered an shRNA against PPARγ locally into the hypothalamus using a lentiviral vector (Supplementary Fig. 3). Acute oral RSG again elicited greater food intake, compared to its vehicle, among rats that had received the scrambled control shRNA, but this effect was blunted in rats receiving the shRNA against PPARγ (Fig. 2f). These data suggest that peripheral administration of TZDs results in activation of hypothalamic PPARγ that can drive higher food intake.
Figure 2. Activation of CNS PPARγ is required for the hyperphagic effect of oral RSG.
a,b) Caloric intake (a) and body weight change (b) 24 h following 30 mg kg–1 RSG or its vehicle by oral gavage (RM ANOVA with Tukey posthoc) c,d) Body weight gain (c) and hematocrits (d) 24 h following 10 mg kg–1 RSG or its vehicle by oral gavage (T-tests) e) Caloric intake 24 h following 10 mg kg–1 RSG or its vehicle by oral gavage and 1 μg GW9662 or its vehicle i3vt (ANOVA with Tukey posthoc) f) Caloric intake 24 h following 10 mg kg–1 RSG or its vehicle by oral gavage in rats previously infected with a lentivirus expressing an shRNA targeted against PPARγ in the medial hypothalamus (RM ANOVA with Tukey posthoc). * = p < 0.05. The mean for each group is represented ± S.E., n = 5–12 animals per group.
Consistent with these findings, two groups have reported that a modest amount of systemically-delivered RSG crosses the blood-brain-barrier18,20. However, those groups draw opposing conclusions regarding the implications of these findings. One study18 concluded that the modest levels of RSG found in whole mouse brain following its intravenous administration were insufficient to account for blunted thyroid status and sympathetic tone observed following peripheral RSG. This conclusion was based primarily on the failure to observe any changes in energy balance following a 14 d continuous i.c.v. infusion of RSG via osmotic pump in a small number of rats (n = 4 group–1). The discrepancy between these findings and ours might be explained if RSG was unstable in an aqueous solution at body temperature over this time frame. Alternatively, they may result from other differences in experimental design. Conversely, another study20 concluded that the modest levels of RSG present in mouse cortex following oral delivery of a therapeutic dose (a comparable dose to ours, 10 mg kg·bw–1 d–1) exceeded the EC50 for RSG at PPARγ and could account directly for changes they observed in the CNS following an oral dose.
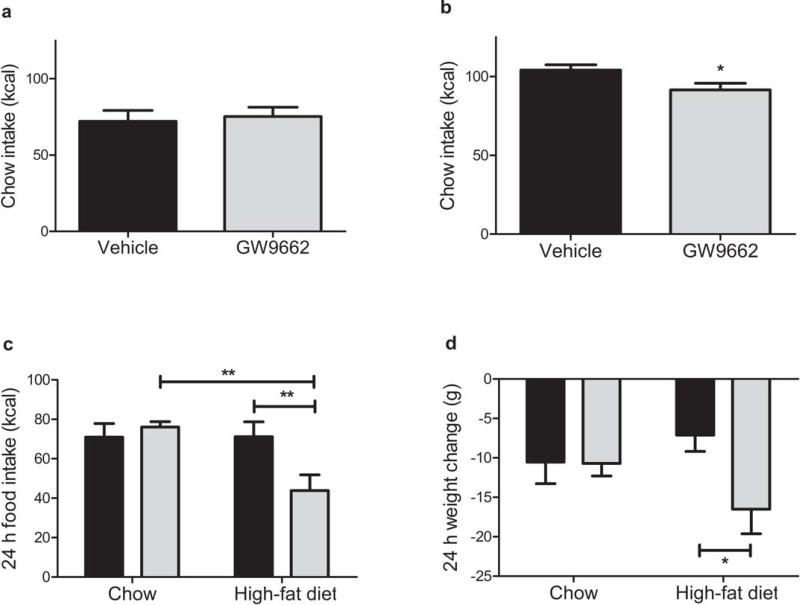
Next we investigated the physiological role of CNS PPARγ in the regulation of energy balance. If hypothalamic PPARγ has a physiologically-important role, blocking its endogenous activation locally within the CNS should lead to negative energy balance. We found that fasted, but not ad-libitum fed, rats maintained on a low-fat chow diet and treated with acute i3vt GW9662 exhibited lower 24 h food intake compared to those treated with vehicle alone (Fig. 3a,b). When GW9662 or its vehicle was administered i3vt to HFD-induced obese rats and their age-matched chow-fed controls, the antagonist-treated HFD-fed rats consumed 40% fewer calories compared to vehicle controls, but the same dose had no effect in the chow-fed animals (Fig. 3c). This difference in caloric intake was reflected by a lower body weight change in HFD-fed, but not chowfed, rats (Fig. 3d). Consistent with this, a previous study12 reported that neuron-specific PPARγ knockout mice exhibit no metabolic phenotype when maintained on a chow diet; no data were presented from HFD-fed mice. Together these data suggest that the physiological role of CNS PPARγ is greater during fasting or in rats fed a high-fat diet (HFD), both physiological states characterized by an increased flux of fatty acids.
Figure 3. Blocking the activation of CNS PPARγ with GW9662 leads to negative energy balance.
a,b) 24 h chow intake following 1 μg GW9662 or vehicle i3vt to ad lib-fed (a), or 24 h fasted (b) rats (T-tests) c) Caloric intake 24 h following 1μg GW9662 (grey bars) or vehicle (black bars) i3vt, to ad lib-fed rats maintained on either chow or HFD (RM ANOVA with Tukey posthoc). d) Weight change 24 h following 1μg GW9662 (grey bars) or vehicle (black bars) i3vt, to ad lib-fed rats maintained on either chow or HFD (T-tests). * = p < 0.05, ** = p < 0.01. Means are depicted ± S.E., n = 9–12 rats per group.
Notably, we also found that plasma thyroid-stimulating hormone (TSH) was more than 5-fold higher among DIO rats acutely-treated with i3vt GW9662 compared to those treated with vehicle alone (Supplementary Fig. 4a). These data agree with previous reports of cross-talk between PPARγ and the HPT axis 21,22. Moreover, they are consistent with reduced thyroid status previously observed among adult rats following oral RSG18, and they fit with an overall catabolic effect of the antagonist. In contrast, a recent study reported that RSG increases thyroid status in newborn mice22, suggesting that development may be a critical factor modulating this interaction. We did not observe differences in thyrotropin-releasing hormone (Trh) mRNA (Supplementary Fig. 4), and there was a trend for higher plasma tri-iodothyronine (T3) (Supplementary Fig. 4b). Given the relative sluggishness of the HPT axis, we suspect that T3 had not yet reached its peak response to increased TSH at this time point. Thus the role of the HPT axis to mediate effects of CNS PPARγ signaling must therefore remain speculative, and is a priority for future research.
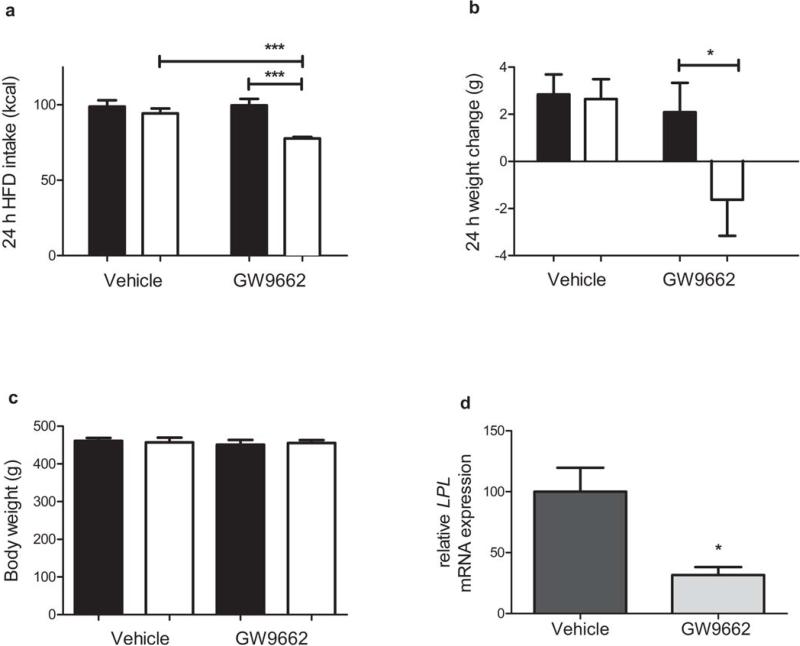
A hallmark of HFD-induced obesity is leptin resistance. Leptin is a peptide hormone secreted by WAT in proportion to total adiposity, thereby providing an indicator of the levels of stored energy in adipose tissue23. Leptin activates its receptors in various hypothalamic nuclei; its downstream effectors are integrated in the PVH, where Trh is also expressed24. Leptin's actions in the hypothalamus and in other areas of the CNS reduce food intake and deplete energy storage in WAT25. Leptin signaling in the hypothalamus is blunted in animals fed a HFD26, and this leptin resistance is thought to contribute to the continued accumulation of body fat. Because the endogenous ligands of PPARγ are thought to be fatty acids, and given the results of our previous experiments, we hypothesized that hypothalamic PPARγ may contribute to the development of HFD-induced leptin resistance. We predicted that chronic antagonism of CNS PPARγ with GW9662 would restore leptin sensitivity in HFD-induced leptin-resistant rats. To test this, we chronically delivered GW9662 or its vehicle into the lateral ventricle of HFD rats, using a sub-threshold dose that has no effect on body weight but that results in significantly lower hypothalamic expression of the PPARγ target gene lipoprotein lipase (Lpl) (Fig. 4). After 14 d, we challenged these rats with exogenous i.p. leptin at a dose that reduces caloric intake and body weight in lean animals27. Among rats concurrently receiving i.c.v. GW9662, those also receiving i.p. leptin exhibited lower 24 h food intake (Fig. 4c) and body weight change (Fig. 4d) compared to those also receiving i.p. vehicle, whereas the weight-matched vehicle-treated rats remained leptin-resistant. These findings implicate PPARγ as a potential molecular link between fatty acids and leptin action to regulate food intake.
Figure 4. Blocking the activation of CNS PPARγ with GW9662 leads to improved leptin sensitivity.
a,b) 24 h food intake (a) and body weight change (b), among ad lib HFD-fed rats receiving a chronic, sub-threshold i.c.v. dose of GW9662 (3 μg kg·bw–1) or its vehicle, 24 h following a 1 mg kg·bw–1 acute i.p. dose of leptin (white bars) or its vehicle (black bars) (ANOVA with Tukey posthoc) c) Body weights of rats in panels (a) and (b) (ANOVA) d) Hypothalamic expression of lipoprotein lipase (Lpl) relative to the housekeeping gene L32 (T-test), among ad lib HFD-fed rats receiving chronic sub-threshold i.c.v. dose of GW9662 (3 μg kg·bw–1) or its vehicle. * = p < 0.05, ** = p < 0.01, *** = p < 0.001. The mean for each group is depicted ± S.E., n = 4–7 rats per group.
The present data collectively reveal a novel role of hypothalamic PPARγ in the central regulation of energy balance, and they imply that CNS mechanisms may underlie at least some of the weight gain observed with PPARγ-modulating drugs. In further support of this possibility, Sugii et al.15 recently reported that constitutive PPARγ activation specifically in adipocytes improves the glucose tolerance of DIO mice but does not lead to increased weight gain. An additional implication, therefore, is that differential access to the CNS by different PPARγ ligands and their formulations may result in different therapeutic outcomes. Notably, these data also identify a physiological pathway by which increased consumption of lipids can promote leptin resistance and obesity via hypothalamic PPARγ.
Supplementary Material
Acknowledgements
This work was funded by the National Institutes of Diabetes and Digestive and Kidney Diseases (DK082173 to K.K.R., DK17844 to S.C.W. and DK056863, DK073505 to R.J.S.). The VP16-PPARγ construct was generously provided by M. Lazar (University of Pennsylvania).
Online methods
Animals
The Institutional Animal Care and Use Committee at the University of Cincinnati approved all animal protocols. We housed adult male Long-Evans rats (Harlan) individually in plastic tub cages and maintained them on a 12:12 h light : dark cycle with ad libitum access to water and pelleted rodent chow (Harlan Teklad) or pelleted 40% butter-fat diet (Research Diets) unless otherwise noted.
Intracerebroventricular cannulations
For i3vt studies, we outfitted rats with a cannula (Plastics One) directed toward the 3rd-cerebral ventricle and confirmed correct placement as previously described28. For chronic i.c.v. studies, we outfitted rats with brain infusion kits (Alzet) into the lateral cerebral ventricle and connected to subcutaneously-implanted osmotic pumps (model 2006, Alzet) with polyethylene tubing, according to the manufacturers instructions as previously described29.
Drugs
For central studies, we dissolved Rosiglitazone (Cayman Chemicals) in 25% dimethyl sulfoxide (DMSO) and injected it i3vt in a volume of 2 μl. For peripheral studies we suspended RSG in 0.5% methyl cellulose and administered it by oral gavage in a volume of about 4 ml. We dissolved GW9662 (Cayman Chemicals) in 33% DMSO and injected it i3vt in a volume of 2 μl. We dissolved leptin (PepProTech) in water and injected it i.p. in a volume of about 0.5 ml.
Food intake and body composition studies
We removed food 6 h prior to the onset of dark and assigned rats to weight-matched groups. We administered RSG, GW9662 or leptin beginning 3 h before the onset of dark. We returned food at lights out and recorded food and body weight at selected time points. We measured body composition in triplicate by Echo MRI.
c-Fos immunohistochemistry
We pre-treated weight-matched rats with i3vt RSG (1 μg) or vehicle. 1 h later, they were deeply anesthetized with sodium pentobarbital and perfused transcardially with 0.9% saline followed by 4.0% paraformaldehyde in sodium phosphate. We postfixed brains at 4°C for 24 h in 4.0% paraformaldehyde in sodium phosphate and stored them at 4°C in 30.0% sucrose/PBS.
We identified c-Fos positive cells using chromagen immunhistochemistry. We collected coronal hypothalamic sections (35 μm) in 1:3 series using a freezing microtome. We stored sections in ethylene glycol cryoprotectant at –20°C until time of use. Briefly, we removed tissue sections from cryoprotectant and washed them in 0.1 M phosphate-buffered saline (PBS). To block endogenous peroxidases, tissue was soaked in 9:1, methanol: 3% hydrogen peroxide solution for 15 min and subsequently rinsed in PBS. We then pre-incubated tissue in blocking buffer (PBS + 0.4 % triton X-100 + 2% normal donkey serum) for 30 min at room temperature, followed by incubation with cFos-specific antibody (1:2500, Santa Cruz Biotechnology, #sc–52) in blocking buffer for 48 h at 4°C. Following washes in PBS, we incubated tissue for 1 h in biotinylated donkey anti-rabbit antibody (1:300, Vector Laboratories, #BA–1000), and subsequently washed and incubated tissue in avidin-biotin solution (1:600, Vector Laboratories, #PK–6100) for 30 min. We visualized c-Fos immunoreactivity with 3,3’-diaminobenzidine-enhanced with nickel chloride.
We used The Rat Brain Atlas by Paxinos to match hypothalamic levels -1.3 to -3.30 mm caudal to bregma. Using a Zeiss Axioplan 2 Microscope coupled with a Zeiss Axiocam camera, we obtained adjacent images of c-Fos at 5X using Axiovision 4.8 Software. Using Scion Image 4.0.3.2., we uploaded images and subtracted the background. We optimized each density slice for positive staining and counted positive cells in the PVH, ARH and DMH. We averaged the number of c-Fos positive cells across each nucleus. An individual blinded to the experimental treatment groups scored the sections.
In vivo VP16-PPARγ studies
The Institutional Biosafety Committee at the University of Cincinnati approved all lentivirus protocols.
America Pharma Source concentrated the viruses to 108 viral units μl–1. Briefly, under ketamine / xylazine anesthesia, we used a Hamilton syringe mounted to the stereotaxic apparatus (KOPF) to directly infuse 2 μl of concentrated virus bilaterally into the medial hypothalamus (-2.2 mm from bregma, -8.5 mm from dura, ± 0.6 mm lateral of midline) of chow-fed rats. We infused virus over 20 min; the Hamilton syringe was allowed to rest for an additional 10 min and then we slowly withdrew it. A single dose of buprenorphine (Reckitt Benckiser Healthcare), 0.3 mg kg·bw–1, was given for analgesia at the time of the surgery.
In vivo shRNA studies
America Pharma Source concentrated the viruses to 109 viral units μl–1. Briefly, under isoflurane anesthesia, we outfitted rats with bilateral cannulas (Plastics One) directed into the medial hypothalamus (-2.2 mm from bregma, -7.5 mm from dura, ± 0.6 mm lateral of midline). We lowered an injector projecting 1 mm below each cannula and infused 2 μl of concentrated virus bilaterally over 20 min. We allowed the injector to rest for an additional 10 min, and then slowly withdrew the cannula and injector. A single dose of buprenorphine (Reckitt Benckiser Healthcare), 0.3 mg kg·bw–1, was given for analgesia at the time of the surgery. Rats recovered for 2 weeks prior to challenge with oral RSG.
Statistical Analyses
All values are reported as means ± S.E. We analyzed the data using student's t-test, Mann-Whitney, Kruskal-Wallis, or ANOVA. We made post-hoc multiple comparisons using Tukey's or Dunn's post-hoc tests. Significance was set at P < 0.05 for all analyses.
Footnotes
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at (http://www.nature.com/nm).
References
- 1.Semple RK, Chatterjee VKK, O'Rahilly S. PPARγ and human metabolic disease. J. Clin. Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen PJ, et al. Differential Influences of Peroxisome Proliferator Activated Receptors– α and γ on Food Intake and Energy Homeostasis. Diabetes. 2003;52:2249–2259. doi: 10.2337/diabetes.52.9.2249. [DOI] [PubMed] [Google Scholar]
- 3.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 4.Rosen ED, et al. PPARγ Is Required for the Differentiation of Adipose Tissue In Vivo and In Vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 5.Patel NG, Holder JC, Smith SA, Kumar S, Eggo MC. Differential Regulation of Lipogenesis and Leptin Production by Independent Signaling Pathways and Rosiglitazone During Human Adipocyte Differentiation. Diabetes. 2003;52:43–50. doi: 10.2337/diabetes.52.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Lehrke M, Lazar MA. The Many Faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Annemiek MCPJ, Arjen HFB, Maarten JAG, Klaas RW. The effect of the PPARγ ligand rosiglitazone on energy balance regulation. Diabetes Metab. Res. Rev. 2006;22:204–210. doi: 10.1002/dmrr.592. [DOI] [PubMed] [Google Scholar]
- 8.Home PD, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 9.Nakano R, et al. Antagonism of peroxisome proliferator-activated receptor-γ prevents high-fat diet-induced obesity in vivo. Biochem. Pharmacol. 2006;72:42–52. doi: 10.1016/j.bcp.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid x receptors in the adult rat CNS. J. Neurosci. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 12.Sarruf DA, et al. Expression of PPARγ in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2008;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouihate A, Boisse L, Pittman QJ. A Novel Antipyretic Action of 15-Deoxy-Δ12,14-Prostaglandin J2 in the Rat Brain. J. Neurosci. 2004;24:1312–1318. doi: 10.1523/JNEUROSCI.3145-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Lazar MA. Differential Gene Regulation by PPARγ Agonist and Constitutively Active PPARγ2. Mol. Endocrinol. 2002;16:1040–1048. doi: 10.1210/mend.16.5.0825. [DOI] [PubMed] [Google Scholar]
- 15.Sugii S, et al. PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. 2009;106:22504–22509. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saez E, et al. PPARγ signaling exacerbates mammary gland tumor development. Genes Dev. 2004;18:528–40. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn SE, et al. Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. N. Engl. J. Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 18.Festuccia WT, et al. Peroxisome Proliferator-Activated Receptor-γ-Mediated Positive Energy Balance in the Rat Is Associated with Reduced Sympathetic Drive to Adipose Tissues and Thyroid Status. Endocrinology. 2008;149:2121–2130. doi: 10.1210/en.2007-1553. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, et al. Collecting duct-specific deletion of peroxisome proliferator-activated receptor-γ blocks thiazolidinedione-induced fluid retention. Proc. Natl. Acad. Sci. 2005;102:9406–11. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strum JC, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J. Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 21.Juge-Aubry CE, et al. Peroxisome proliferator-activated receptor mediates cross-talk with thyroid hormone receptor by competition for retinoid X receptor. Possible role of a leucine zipper-like heptad repeat. J. Biol. Chem. 1995;270:18117–22. doi: 10.1074/jbc.270.30.18117. [DOI] [PubMed] [Google Scholar]
- 22.Kouidhi S, et al. Peroxisome proliferator-activated receptor-γ (PPARγ) modulates hypothalamic Trh regulation in vivo. Mol. Cell. Endocrinol. 2010;317:44–52. doi: 10.1016/j.mce.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 24.Harris M, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J. Clin. Invest. 2001;107:111. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 26.Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. J. Neurosci. 2001;104:1111–1117. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 27.Kelly JF, et al. Ciliary Neurotrophic Factor and Leptin Induce Distinct Patterns of Immediate Early Gene Expression in the Brain. Diabetes. 2004;53:911–920. doi: 10.2337/diabetes.53.4.911. [DOI] [PubMed] [Google Scholar]
- 28.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav. Neurosci. 1995;109:528–531. doi: 10.1037//0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- 29.Naert G, Ixart G, Tapia-Arancibia L, Givalois L. Continuous i.c.v. infusion of brain-derived neurotrophic factor modifies hypothalamic-pituitary-adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. J. Neurosci. 2006;139:779–789. doi: 10.1016/j.neuroscience.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Reed JA, et al. GM-CSF action in the CNS decreases food intake and body weight. J. Clin. Invest. 2005;115:3035–44. doi: 10.1172/JCI25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.