Abstract
A spotted fever rickettsia quantitative PCR assay (SQ-PCR) was developed for the detection and enumeration of Rickettsia rickettsii and other closely related spotted fever group rickettsiae. The assay is based on fluorescence detection of SYBR Green dye intercalation in a 154-bp fragment of the rOmpA gene during amplification by PCR. As few as 5 copies of the rOmpA gene of R. rickettsii can be detected. SQ-PCR is suitable for quantitation of R. rickettsii and 10 other genotypes of spotted fever group rickettsiae but not for R. akari, R. australis, R. bellii, or typhus group rickettsiae. The sensitivity of SQ-PCR was comparable to that of a plaque assay using centrifugation for inoculation. The SQ-PCR assay was applied successfully to the characterization of rickettsial stock cultures, the replication of rickettsiae in cell culture, the recovery of rickettsial DNA following different methods of extraction, and the quantitation of rickettsial loads in infected animal tissues, clinical samples, and ticks.
Rickettsia rickettsii is an obligately intracellular bacterium. In nature, R. rickettsii circulates in a zoonotic cycle that includes transovarial and transstadial maintenance by its arthropod hosts, ixodid ticks, and horizontal transmission to its vertebrate hosts, which include a wide range of small and medium-sized mammals. Following an inopportune tick bite, R. rickettsii is transmitted to humans and causes Rocky Mountain spotted fever, a severe febrile disease, which is associated with proinflammatory and procoagulant changes and development of a systemic vasculitis.
Because of the intracellular habitat of rickettsiae and their slow generation time, the use of traditional techniques to quantify the number of viable rickettsiae in a sample is laborious, inaccurate, and tedious. In principle, the absolute number of rickettsial particles can be determined by microscopic observation of smears prepared from cosuspension with a standardized bacterial suspension following staining with chemical or fluorescent dyes (3, 19) or fluorescein-labeled antibodies (20). Quantitation by this method may be inconsistent because of the variable distribution of microorganisms in the smear and because direct counts of bacterial particles by microscopic observation suffer from subjectivity and lack of reproducibility. The assay is also a time-intensive procedure that may not work well for infected animal tissues. Alternatively, the number of viable rickettsiae can be estimated by measurement of metabolically 35S-labeled rickettsiae or 32P-labeled rickettsiae (21, 22), by titration of infected samples in susceptible animals and embryonated chicken eggs (2, 5), and by tissue culture procedures, including plaque assays (23, 24). However, only infectious and/or metabolically active rickettsiae can be measured by using these biological approaches. These last techniques are expensive, require special facilities for work with radioactive materials or for housing of infected animals, and cannot be used with samples contaminated with other microorganisms or yeast. Except for the metabolic and tissue culture infectivity assays, they may require 5 to 10 days for completion.
Although quantitative PCR assays have been developed and applied to studies with rickettsial relatives in the family Anaplasmataceae (13, 14), a comparable procedure has not been developed and tested on diverse rickettsial samples. Rolain et al. (16) developed a quantitative PCR assay using the Roche LightCycler based on the conserved rickettsial gltA primers 877F and 1258R. The assay was used to monitor the growth kinetics and antibiotic susceptibility of R. conorii, R. typhi, and R. felis in Vero or XTC-2 cells. However, although both probe- and SYBR Green-based assays were mentioned, Rolain et al. did not specify the probe used or completely describe the methods for calibration and optimization of the assay. We describe here the detailed characterization of an alternative rOmpA-based spotted fever rickettsia quantitative PCR (SQ-PCR) assay that we found to be more suitable for quantifying R. rickettsii and other closely related spotted fever group (SFG) rickettsiae. The SQ-PCR assay was compared with the plaque assay for the ability to quantify R. rickettsii. Its utility for detection and quantitation of rickettsiae in experimental samples from cell cultures, animal tissues, and tick and clinical samples was determined.
MATERIALS AND METHODS
Rickettsiae.
Rickettsiae were grown in Vero cell monolayers, purified by centrifugation through a sucrose cushion, and stored in aliquots at −80°C in sucrose potassium glutamate buffer supplemented with 5 mM MgCl2 and 1% Renografin as described elsewhere (2, 5). The characteristics of R. rickettsii strains Bitterroot, Sheila Smith, Long Horse Canyon, 84JG, Morgan, Hlp#2, Brazil, Colombia, and Price T have been previously reported (6). The viable titer of purified R. rickettsii was determined by PFU assay according to the method of Wike and Burgdorfer (23). PFU titers were determined following overnight storage of stock cultures at −80°C and also after 1 to 4 years of storage of frozen purified rickettsiae at the same temperature. To increase the adherence of rickettsiae to Vero cells to more accurately determine total viable counts, rickettsiae were also centrifuged onto the monolayers at 1,000 rpm (200 × g) (table top centrifuge; Beckman GRP, Hamburg, Germany) for 20 min at room temperature, followed by an additional 30-min incubation at 37°C before the addition of the agarose overlay.
R. prowazekii Breinl, R. typhi Wilmington, R. canadensis McKiel and CA410, R. africae ETH SF2500, R. akari CW-PP Hartford, R. parkeri HmacA, R. sibirica 246, R. bellii 369C, R. australis Cutlack, R. montanensis OSU#85-930, R. rhipicephali 12T, human Astrakhan spotted fever rickettsia A-1, Israeli spotted fever rickettsia CDC, and Thai tick typhus rickettsia TT118 were cultivated in Vero cells as described elsewhere (8, 9).
Preparation of DNA template.
Several methods were employed for the preparation of rickettsial DNA templates. R. rickettsii strain Bitterroot DNA was used for the evaluation of standard PCR conditions and was prepared by standard lysozyme and protease K treatment and phenol-chloroform extraction of purified rickettsiae (18). To obtain crude DNA extracts from R. rickettsii isolates, sucrose-purified rickettsiae were suspended in 100 μl of distilled water and boiled for 10 min and cell debris was removed by centrifugation. The cleared supernatant was used as DNA template for the PCR assay.
To obtain purified DNA from other spotted fever group rickettsiae and typhus group rickettsiae, infected Vero cells were lysed and total host and rickettsial DNA was extracted by use of the DNeasy kit (Qiagen, Germantown, Md.) according to the manufacturer's protocol (kit N69506). Briefly, infected Vero cells grown in a T150 (150 cm2) flask were concentrated by centrifugation for 30 min at 10,000 × g (Sorvall centrifuge) and the pellet was resuspended in 0.5 ml of Tris-EDTA buffer. AL lysis buffer (200 μl; Qiagen) and protease K (20 μl) were added, mixed with a vortex machine, and incubated for 10 min at 70°C. Undigested proteins were precipitated after the addition of 200 μl of ethanol, and DNA was separated by centrifugation of samples in a Qiagen column for 1 min at 10,000 × g in a MiniCentrifuge (Sorvall Fresco Biofuge). Purified DNA was eluted with 400 μl of distilled water, and the eluate was supplemented with 100 μl of Tris-EDTA buffer and refrigerated for 1 to 7 days prior to quantitation.
DNA from uninfected Vero cells was prepared according to a standard phenol-chloroform procedure (18).
Primer design and selection.
Primer sequences were selected with the aid of the software program Oligo 6.4.4.0 (Oligo MFC; W. Rychlik) and were derived from the gene sequence of rOmpA of R. rickettsii (GenBank accession number M31227.1 [1]). Primers were selected to amplify DNA fragments of 100 to 150 bp (Table 1). Primer sequences were selected based on lack of predicted formation of primer dimers, a predicted Tm of ≥60°C, and an absence of stretches of identical nucleotides. In addition, the 532- and 632-bp fragments of the rOmpA gene, amplified with Rr190.70n-601p and Rr190.70n-701p primers, respectively, and a 381-bp fragment of gltA (877 to 1,258 nucleotides [nt]) (15, 17) were evaluated as potential amplicons in the quantitative PCR assay.
TABLE 1.
Oligonucleotide primer pairs used for development of SQ-PCR assay for R. rickettsii
| Target gene | Primer designation | Nucleotide sequence 5′→3′ | Amplified fragment size (nt) | Tm (°C) | Reference |
|---|---|---|---|---|---|
| gltA | RpCS877F | GGG GAC CTG CTC ACG GCG G | 381 | 68 | 15 |
| RpCS1258R | ATT GCA AAA AGT ACA GTG AAC A | 58 | |||
| rompA | RR190.70F | ATG GCG AAT ATT TCT CCA AAA A | 632 | 58 | 17 |
| RR190.701R | GTT CCG TTA ATG GCA GCA TCT | 62 | |||
| RR190.70F | ATG GCG AAT ATT TCT CCA AAA A | 532 | 58 | 15 | |
| RR190.602R | AGT GCA GCA TTC GCT CCC CCT | 68 | |||
| RR190.70F | ATG GCG AAT ATT TCT CCA AAA A | 77 | 58 | This study | |
| RR147.147R | CTA CTC AGC ATT ATC GCT GCG | 64 | |||
| RR190.70F | ATG GCG AAT ATT TCT CCA AAA A | 97 | 58 | This study | |
| RR190.167R | ACA CCT GTA GCA ACA CCG AGT | 64 | |||
| RR190.547F | CCT GCC GAT AAT TAT ACA GGT TTA | 154 | 66 | This study | |
| RR190.701R | GTT CCG TTA ATG GCA GCA T | 62 | |||
| RR190.588F | GGA GCG AAT GCT GCA CTA AT | 113 | 60 | This study | |
| RR190.701R | GTT CCG TTA ATG GCA GCA TCT | 62 |
SQ-PCR.
PCR amplification was conducted in a 25-μl volume, using SYBR Green PCR kit reagents according to the manufacturer's instructions (PE Applied Biosystems, Foster City, Calif.). Each assay contained 1.5 μl of diluted template or plasmid DNA; primers were used at a final concentration of 0.5 mM. After the template DNA was denatured for 3 min at 95°C, amplification conditions consisted of 40 cycles of 30 s at 95°C and 20 s at 57°C followed by extension at 65°C for 1 min. The reaction was concluded with a final extension at 72°C for 5 min. Amplification, data acquisition, and data analysis were carried out with an iCycler and iQ Real-Time PCR detection system software, versions 2.0 and 3.0A (Bio-Rad, Richmond, Calif.). To establish the optimal conditions for each primer pair tested, temperature gradient PCR was conducted within the range of 47 to 63°C and monitored by analysis of melting curves of the products amplified under these conditions, as recommended by the manufacturer.
Plasmid standard for absolute quantitation of DNA copy number.
A plasmid standard for template quantitation was prepared as follows. The 732-bp fragment of the rOmpA gene of R. rickettsii strain Bitterroot was PCR amplified with the primers Rr190.70n and Rr190.701p and cloned into the pCR2.1-TOPO plasmid by use of the TA cloning kit (Invitrogen, NV Leek, The Netherlands), and the vector was transformed into Escherichia coli DH5α competent cells (GIBCO BRL). The plasmid insert was sequenced to confirm that it was that expected for rOmpA from R. rickettsii. To standardize the gltA amplicon, the 381-bp DNA fragment from R. rickettsii was similarly cloned and analyzed.
Specificity and sensitivity of SQ-PCR.
To determine the detection limits of the PCR assay, serial 10-fold dilutions of the plasmid standards were assayed. To determine the specificity of the PCR assay, its ability to amplify DNA obtained from a collection of spotted fever and typhus group rickettsiae was tested. The effect of heterologous DNA was determined by supplementing samples of R. rickettsii DNA with different concentrations of purified Vero cell DNA. The threshold was calculated using baseline cycles 2 to 10.
Cell culture infection.
Confluent monolayer cultures of Vero cells were prepared in 25-cm2 flasks and infected with purified R. rickettsii Bitterroot at a multiplicity of infection of 0.2 and 2.0 rickettsial uncentrifuged PFU per cell. After rickettsiae were allowed to attach for 1 h at room temperature, the inoculum was aspirated, the monolayers were rinsed with phosphate-buffered saline (pH 7.6), and 5 ml of fresh RPMI 1640, with 5% fetal bovine serum and 1 mM l-glutamine, was added. Infected cells and media were harvested with 3-mm-diameter glass beads at time zero (after aspiration of the inoculum) and 8, 24, 48, 72, and 96 h after infection. Harvested suspensions were pelleted by centrifugation at 13,000 rpm for 5 min in a Sorvall Biofuge, the supernatant was discarded, and the cell pellet was frozen. To prepare DNA templates, uninfected and infected Vero cell pellets were suspended in distilled water and boiled for 10 min, membrane fragments and cell debris were pelleted by centrifugation for 10 min at 14,000 rpm, and the supernatants were used for PCR.
Other samples.
DNA from brains and lungs of Microtus pinetorum infected with R. rickettsii strain Bitterroot and from uninfected control animals was prepared by use of a two-step Trizol RNA-DNA (Invitrogen, GIBCO BRL) extraction protocol and a DNeasy protocol (Qiagen) according to the manufacturers' instructions. Details on the animal infection protocol are described elsewhere (4). Qiagen kit-purified DNA from SFG rickettsiae-infected and uninfected Amblyomma americanum ticks and humans was a gift from John Sumner (Centers for Disease Control, Atlanta, Ga.).
Statistical analysis.
Where applicable, the experiments were conducted in three replicates for each experimental variable. The means, standard deviations, and standard errors of the means were calculated. Statistical significance was assessed by Student's t test (α = 0.05).
RESULTS
Development of optimal conditions for SQ-PCR.
Seven pairs of primers were tested for their suitability in the quantitative PCR assay. They originated from the 5′ end of the rOmpA gene of R. rickettsii, from nt 70 to 701, and from a gltA fragment, two target sequences that are commonly used for identification of SFG rickettsiae (Table 1) (7, 15, 17). Temperature gradient PCR and analysis of melting curves of the amplified products were conducted for each primer pair, using highly purified DNA extracted from sucrose-purified R. rickettsii Bitterroot. Primers amplifying fragments gltA, Rr190.70-601, and Rr190.70-701 were not considered further because of the large amplicons they produced and because additional secondary PCR products were formed after 40 cycles of amplification (data not shown). We were not able to replicate the melting curve obtained by Rolain et al. (16) for the gltA primer pair under a variety of conditions, possibly because they used a probe rather than SYBR Green dye for their melting curve analysis. Furthermore, this primer pair often gives rise to primer dimers, which contribute to high background levels when SYBR Green is used as the fluorophore, as it intercalates in both the dimers and the specific products. Amplification of the Rr190.70-147, Rr190.70-167, and Rr190.588-701 fragments was also unsatisfactory due to the formation of products with heterogeneous melting curves under different amplification conditions. In contrast, good results were obtained with the Rr190.547F and Rr190.701R primers, which generated a 154-bp fragment with a sharp melting curve profile.
To optimize amplification, the PCR cocktail mixture was supplemented with 1, 3, or 5 mM MgCl2, and the melting profile was determined for products that were amplified at temperatures ranging from 57 to 63°C. No amplification was obtained in the presence of 1 mM MgCl2, while amplification with 3 or 5 mM MgCl2 was equally efficient. An annealing temperature of 57°C and 3 mM MgCl2 were selected for further experiments to ensure high quality and stringency of amplification.
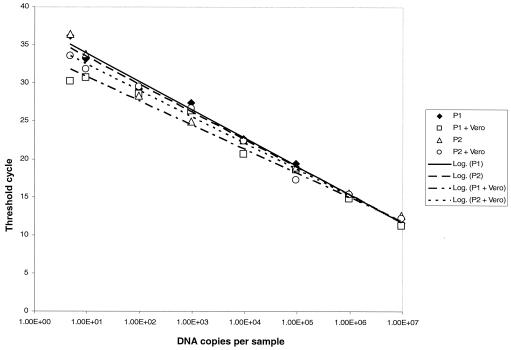
SQ-PCR using the Rr190.547F-701R primer pair could detect 5 copies of the cloned plasmid, while reagent controls containing no DNA were not amplified (Fig. 1). Amplification of 10-fold dilutions of standard plasmid exhibited linearity within the whole range of 9.5 × 107 to 4.75 copies per sample. Amplification efficiency averaged 0.99 ± 0.02 for 10 different experiments. Vero cell DNA at a final assay concentration of 0.4 μg/ml did not affect the efficiency of amplification of rickettsial DNA within the range of the calibration curve (Fig. 2). The correlation coefficient for the standard curves of two independently cloned plasmid preparations, with and without DNA, was 0.984 ± 0.004.
FIG. 1.
Standard calibration curve for SQ-PCR for Rickettsia with plasmid pCR2.1-TOPO containing the cloned Rr190.547F-Rr190.701R fragment of R. rickettsii Bitterroot. CF RFU, curve-fit relative fluorescence units.
FIG. 2.
Effect of added Vero cell DNA on detection and quantitation of the R. rickettsii rOmpA fragment, using the Rr190.547F and Rr190.701R primer pair. Serial dilutions of two independently isolated and cloned standard plasmid preparations (plasmid P1 and P2) were made in distilled water with or without Vero cell DNA to give a final assay concentration of 0.4 μg/ml. PCR conditions were described in Materials and Methods.
The effect of different DNA extraction methods on quantitation of R. rickettsii was compared (Table 2). The number of copies of rOmpA was determined in DNA samples prepared from equal aliquots of Vero cells infected with R. rickettsii and from R. rickettsii purified through Renografin density gradients by heat treatment alone, treatment with Chelex, or DNeasy extraction. The highest copy numbers were determined for both samples prepared with the DNeasy extraction protocol (P < 0.05). In contrast, the DNA yield was 10 times smaller for samples prepared by 10 min of heating at 95°C (P < 0.05), likely due to insufficient release of DNA and/or the presence of inhibitors that can be removed in part by Chelex treatment when low amounts of protein are present (Table 2).
TABLE 2.
Effect of DNA extraction methods on the quantitation of R. rickettsii by SQ-PCR
| Sample | No. of rOmpA copies per μl of DNA template by indicated extraction procedurea
|
||
|---|---|---|---|
| Boiling | Boiling plus Chelex | DNeasy kit | |
| Infected Vero cells | 1.81 × 104 ± 0.32 × 104b | 3.79 × 104 ± 1.75 × 104b | 6.11 × 105 ± 0.43 × 105 |
| Renografin gradient density-purified rickettsiae | 4.52 × 104 ± 0.84 × 104b | 2.96 × 105 ± 0.11 × 105b | 4.22 × 105 ± 0.35 × 105 |
The data are given as means±standard errors of the means for three independent preparations, each assayed in triplicate.
P < 0.05 compared to DNeasy extraction.
Specificity of SQ-PCR.
DNA samples from Vero cells infected with different typhus and spotted fever group rickettsia isolates were tested for the ability to amplify homologues of the Rr190.547-701 rOmpA gene fragment (Table 3). DNA samples from 11 spotted fever group rickettsiae were amplified, with threshold cycle (Ct) numbers ranging from 15 to 18, while similarly infected samples from R. akari, R. australis, and R. bellii had Ct values of ≥37. The latter Ct values were insignificant, as they were identical to those determined for a negative control containing DNA from uninfected Vero cells. Similarly, DNA from the typhus group rickettsiae, R. prowazekii, R. typhi, and R. canadensis was not amplified with these primers (Table 3).
TABLE 3.
Specificity of SQ-PCR assay for different genotypes of Rickettsia
| Isolate | Ct value (mean ± SE) | No. of DNA copies per T150 flask (mean ± SE) |
|---|---|---|
| R. rickettsii Bitterroot | 16.02 ± 0.006 | 6.07 × 108 ± 0.02 × 108 |
| R. montanensis OSU 85-930 | 17.30 ± 0.42 | 2.49 × 108 ± 0.71 × 108 |
| R. rhipicephali 12T | 17.17 ± 0.15 | 2.52 × 108 ± 0.29 × 108 |
| R. parkeri HmacA | 15.96 ± 0.05 | 6.39 × 108 ± 0.24 × 108 |
| SFG isolate 364-D | 16.40 ± 0.33 | 4.82 × 108 ± 1.23 × 108 |
| R. slovaca B | 17.95 ± 0.20 | 1.41 × 108 ± 0.21 × 108 |
| R. sibirica 246 | 16.01 ± 0.39 | 6.49 × 108 ± 2.12 × 108 |
| R. africae ETH SF-2500 | 17.32 ± 0.20 | 2.27 × 108 ± 0.32 × 108 |
| Israeli tick typhus CDC | 14.87 ± 0.08 | 1.48 × 109 ± 0.09 × 109 |
| Astrakhan spotted fever A-1 | 16.97 ± 0.23 | 3.00 × 108 ± 0.57 × 108 |
| R. honei TT118 | 18.88 ± 0.34 | 7.17 × 107 ± 2.03 × 107 |
| R. akari Hartforda | >37 | <1 |
| R. australis JCa | >37 | <1 |
| R. bellii 369C42a | >37 | <1 |
| R. canadensis McKiela | >37 | <1 |
| R. canadensis CA410a | >37 | <1 |
| R. prowazekii Breinla | >37 | <1 |
| R. typhi Wilmingtona | >37 | <1 |
rOmpA gene is absent from these rickettsiae or has low homology to the R. rickettsii rOmpA gene at the location of the primer sequences.
The Rr190.547-701 primers were designed based on the rOmpA gene sequence of the Bitterroot strain of R. rickettsii. To ensure that the primers selected did not exhibit strain specificity, DNA templates were also prepared from 10 strains of R. rickettsii that differ in virulence and geographic origin. Their Ct values varied from 20 to 25, which corresponded to 1.21 × 1010 to 4.97 × 108 DNA copies/ml (Table 4).
TABLE 4.
Quantitation of sucrose-purified seeds of different R. rickettsii isolates by plaque assay and SQ-PCR
| Isolate | No. of rickettsiae (PFU/ml [PFU/ml after centrifugation])a
|
Ct | No. of calculated DNA copies/ml after 1 to 4 yrs of seed storage | |
|---|---|---|---|---|
| After overnight freeze | After 1 to 4 yrs of storage | |||
| Brazil | 3.80 × 107 ± 0.92 × 107 | 2.30 × 107 ± 0.45 × 107 (1.30 × 109 ± 0.23 × 109) | 24.80 ± 0.23 | 4.97 × 108 ± 0.91 × 108 |
| 84JG | 1.92 × 107 ± 0.43 × 107 (1.5 × 109) | 1.45 × 107 ± 0.15 × 107 (7.05 × 109 ± 1.28 × 109) | 21.92 ± 0.25 | 4.74 × 108 ± 0.86 × 109 |
| Hlp#2 | 6.0 × 105 | 4.33 × 105 ± 0.58 × 105 (1.10 × 107 ± 0.26 × 107) | 24.47 ± 0.83 | 2.83 × 108 ± 1.72 × 108 |
| Bitterroot | 9.13 × 107 ± 2.02 × 107 | 2.90 × 106 ± 0.73 × 106 (6.48 × 107 ± 1.56 × 107) | 23.80 ± 0.24 | 1.38 × 109 ± 0.27 × 109 |
| Colombia | 2.55 × 107 ± 0.05 × 107 | 1.97 × 107 ± 0.15 × 107 (6.30 × 108 ± 0.89 × 108) | 23.27 ± 0.25 | 1.13 × 109 ± 0.20 × 109 |
| Lost Horse Canyon | 1.03 × 107 ± 0.25 × 107 | 2.15 × 107 ± 0.45 × 107 (4.58 × 108 ± 0.92 × 108) | 22.99 ± 0.27 | 3.11 × 109 ± 0.61 × 109 |
| Morgan | 2.10 × 106 ± 0.63 × 106 | 1.70 × 107 ± 0.60 × 107 (7.43 × 108 ± 0.57 × 108) | 25.18 ± 0.45 | 4.01 × 108 ± 2.05 × 108 |
| Price T | 4.0 × 107 ± 1.05 × 107 | 1.77 × 107 ± 0.23 × 107 (4.60 × 108 ± 0.46 × 108) | 20.13 ± 0.47 | 1.21 × 1010 ± 0.38 × 1010 |
| Sheila Smith | 3.90 × 107 ± 1.06 × 107 (9.0 × 108 ± 5.0 × 108) | Not done | 21.98 ± 0.59 | 3.16 × 108 ± 1.06 × 108b |
Numbers in parentheses are PFU titers determined after centrifugation of rickettsiae onto Vero cells. Data are means±standard errors of the means.
The DNA copy number for isolate Sheila Smith was determined only for freshly prepared frozen seed.
Comparison of the sensitivities of SQ-PCR and PFU assays.
The sensitivity of the quantitative PCR assay was compared with results obtained by classical PFU assay (Table 4). PFU assays were conducted on freshly frozen stock cultures immediately after purification of rickettsiae by use of a sucrose gradient and were also conducted after these rickettsial stock cultures had been frozen for 1 to 4 years. Low-speed centrifugation was employed to increase the efficiency of rickettsiae-Vero cell attachment to more accurately assess total viable counts. The titer of freshly purified uncentrifuged rickettsiae was typically 105 to 107 PFU/ml. Titers of six stored stock cultures were typically within a twofold difference of the fresh stock cultures, a difference that is within the range expected for interexperiment variability. However, strain Bitterroot inexplicably declined 33-fold, while strain Morgan increased 8-fold, suggesting that those stock cultures may not have been uniformly suspended. These PFU numbers were increased 23- to 75-fold following centrifugation, a result consistent with inefficient plaque formation due to poor interaction with the monolayer at room temperature. Surprisingly, four stock cultures (Brazil, 84JG, Morgan, and Sheila Smith) had postcentrifugation PFU titers that were two- to threefold higher than the calculated DNA copy numbers determined by quantitative PCR (Table 4). This suggests that DNA extraction by boiling of these stock cultures was insufficient in releasing all of the DNA present or that some inhibitors of the PCR were present (Table 2). For the other four stock cultures (Hlp#2, Colombia, Lost Horse Canyon, and Price T), the centrifuged PFU titers were significantly less than the number of DNA copies, which suggests that many dead rickettsiae were present in these stock cultures.
Quantitation of R. rickettsii growth in monolayers of Vero cells by SQ-PCR.
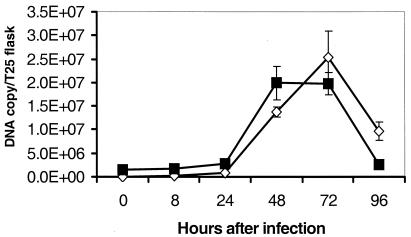
The SQ-PCR assay was used to quantify the growth of R. rickettsii Bitterroot isolate in monolayers of Vero cells (Fig. 3). The Ct values for samples prepared from cells infected at 0.2 rickettsia per cell steadily decreased for the first 72 h after inoculation (P < 0.05), and this correlated with a logarithmic increase of DNA copies (from 1.3 × 103 per flask to 5.5 × 106 per flask) recovered from a T25 cell culture flask (P < 0.05). The number of rickettsiae declined to 1.9 × 106 per flask 96 h after infection (P < 0.05).
FIG. 3.
Detection of R. rickettsii growth in Vero cells by SQ-PCR. Vero cell monolayers were infected with 2.0 rickettsial PFU per cell (squares) or 0.2 rickettsial PFU per cell (diamonds). Each value is the average ± standard error of three replicates. Some error bars are too small to be seen.
Similarly, threshold values for rickettsial DNA in samples of Vero cells infected with 2 rickettsiae per cell decreased from days 0 to 3 after infection (P < 0.05), stayed at the maximum level from 48 to 72 h (P = 0.99), and quickly decreased by 96 h (P < 0.05). That the low and high inoculation doses reached the same peak number of DNA copies per flask was probably due to the fixed number of host cells available for rickettsial replication. The relative yield of DNA per inoculum was approximately 140 times higher for the low than for the high infectious dose used (P < 0.05), as calculated at the highest point of the growth curve 72 h after infection.
Detection and quantitation of rickettsiae in experimental, clinical, and field samples.
The SQ-PCR assay was used to quantify DNA from R. rickettsii present in a blood sample obtained from a patient with Rocky Mountain spotted fever: 2.39 × 105 ± 0.02 × 105 DNA copies were detected per ml, while no background signal was detected with a healthy donor blood sample. A series of DNAs from blood samples that were negative for rickettsial DNA by conventional PCR amplification of endogenous rickettsial DNA were spiked with purified R. rickettsii DNA. Since similar Ct values (not shown) were found for the rickettsial DNA alone and in the spiked blood samples, SQ-PCR does not appear to be sensitive to potential inhibitors.
Rickettsemia was detected in the blood of experimentally infected M. pinetorum at 8.51 × 104 ± 2.74 × 104 DNA copies/ml, a value that is within the range of that detected in the clinical sample. The quantities of rickettsiae detected in the brains and lungs of acutely infected voles were 8.7 × 104 and 3.9 × 104 DNA copies/mg, respectively, although they were two to five times higher in tissues of animals that died from experimental infection (Table 5). With the exception of one animal, in which R. rickettsii persisted in the brain at day 23 of infection, other tissues were cleared of rickettsiae, so no signal was obtained with the quantitative PCR assay.
TABLE 5.
Detection and quantitation of R. rickettsii DNA in clinical and experimental samples
| Sample no. | Origin | Type of sample | Ct value (mean ± SE) | No. of calculated DNA copies (mean ± SE)a | Additional information |
|---|---|---|---|---|---|
| 1 | Human patient | Whole blood | 26.19 ± 0.10 | 2.39 × 104 ± 0.20 × 105 | Acute RMSFc |
| 2 | Human donor | Whole blood | >36 | ≤1b | |
| 3 | Human donor | Skin biopsy | >36 | ≤1b | |
| 4 | M. pinetorum | Whole blood | 27.69 ± 0.51 | 8.51 × 104 ± 2.74 × 104 | Acute experimental RMSF |
| 5 | M. pinetorum | Brain | 29.41 ± 0.29 | 4.56 × 104 ± 1.03 × 104 | Acute experimental RMSF |
| 6 | M. pinetorum | Brain | 29.08 ± 0.48 | 8.70 × 104 ± 2.42 × 104 | Fatal experimental RMSF |
| 7 | M. pinetorum | Brain | >37 | ≤1 | Convalescent from experimental RMSF |
| 8 | M. pinetorum | Lung | 30.57 ± 0.33 | 3.91 × 104 ± 2.07 × 104 | Acute experimental RMSF |
| 9 | M. pinetorum | Lung | 28.26 ± 0.73 | 2.45 × 104 ± 0.81 × 105 | Fatal experimental RMSF |
| 10 | M. pinetorum | Lung | >37 | ≤1 | Convalescent from experimental RMSF |
| 11 | Amblyomma tick | Whole tick | 23.20 ± 0.58 | 3.45 × 106 ± 0.10 × 106 | Questing tick |
| 12 | Amblyomma tick | Whole tick | 21.38 ± 0.27 | 1.17 × 107 ± 0.10 × 107 | Questing tick |
| 13 | Amblyomma tick | Whole tick | >37 | ≤1b | Questing tick |
DNA copy numbers were calculated per milliliter of blood, milligram of tissue, or whole tick.
The absence of amplification of rickettsial DNA was confirmed by an independent PCR assay.
RMSF, Rocky Mountain Spotted fever.
Amblyomma ticks were found to have 106 to 107 DNA copies of R. rickettsii per tick. Samples of ticks that were negative for the presence of rickettsiae by an alternative PCR assay used to amplify the gene for the 17-kDa protein of Rickettsia were also negative by the quantitative PCR assay (data not shown).
DISCUSSION
We describe here a new PCR-based assay for quantitation of spotted fever group rickettsiae and demonstrate its utility for use with a variety of samples. The SQ-PCR measures the fluorescence of SYBR Green, a dye that binds to double-stranded amplified DNA, and it can detect 5 copies of a standard plasmid DNA per sample. The SQ-PCR assay has significant advantages over classical methods used for quantitation of rickettsiae and also over nonquantitative PCR methods. These include a shorter assay time, no requirement for gel electrophoresis, and the capacity for analyzing large numbers of samples with fully automated equipment. In contrast to a TaqMan-based quantitative PCR assay, SQ-PCR does not require synthesis of an expensive specific probe. This reduces the cost of each analysis, a significant advantage when a large number of similar samples need to be compared.
The SQ-PCR assay is compatible with different procedures for DNA template preparation and also can detect rickettsial DNA in the presence of heterologous DNA of different origins. However, because different sample handling procedures can result in significantly different efficiencies of DNA extraction, adherence to a single extraction technique within an experiment is recommended to ensure reproducibility and comparability of quantitative data. Rickettsial DNAs from purified organisms, Vero cell culture samples, human and animal blood, animal tissues, and ticks were tested and found in the amounts expected.
SQ-PCR can be used for detection of R. rickettsii isolates and any other spotted fever group rickettsiae that share the Rr190.547F and Rr190.701R conserved rOmpA primer sequences (17). Since divergent homologous sequences are present in the rOmpA genes of R. akari, R. australis, R. bellii, and R. canadensis (10, 17) and since an intact rOmpA gene is not present in R. typhi and R. prowazekii (5), DNA from these species was not detected by SQ-PCR.
On a geometric scale, the number of DNA copies per sample determined by SQ-PCR matched very well the number of PFU determined by a classical centrifuged PFU titration method applied to samples of purified viable rickettsiae. Some discrepancy was expected since plaque assays are only accurate to a half-log (12) and the recovery of DNA from samples may vary. A boiling procedure was chosen to avoid losses from multiple-step processing, but it may not fully release all copies of DNA to hybridize with primers (Table 2). Also, the precise number of DNA copies per rickettsial cell that are present in stock cultures harvested under different growth conditions and passage levels is unknown and requires further examination.
The SQ-PCR assay was used to measure the growth of the R. rickettsii Bitterroot isolate in cell culture. Similar growth curves were obtained for two inoculation doses, 0.2 and 2 rickettsiae per cell, but less total replication was observed at the higher dose and the DNA degraded more rapidly from its maximum level. These results resembled the growth curve of R. rickettsii that was determined by counting of rickettsiae in Gimenez-stained slides (11, 24).
In conclusion, we have validated a new quantitative PCR assay that is highly versatile and sensitive and that is a valuable addition to current methods for quantifying rickettsiae.
Acknowledgments
This study was supported by a Genetic Working Group Grant from the National Center for Infectious Diseases (M.E.E.), a New Faculty Intramural Grant of the University of Maryland School of Medicine (M.E.E.), and NIAID grant AI-17416 (D.J.S. and M.E.E.).
We thank Robert Massung and Racheal Priestley for helpful discussions, John Sumner for the clinical and tick DNA samples, and Zhongxing Liang and Qiang Yu for their technical assistance with sample preparation.
REFERENCES
- 1.Anderson, B. E., G. A. McDonald, D. C. Jones, and R. L. Regnery. 1990. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect. Immun. 58:2760-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasch, G. A., and E. Weiss. 1992. The genera Rickettsia, Rochalimaea, Ehrlichia, Cowdria, and Neorickettsia, p. 2407-2470. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, 2nd ed., vol. III. Springer-Verlag, New York, N.Y.
- 3.Emelyanov, V. V. 1990. A simple method for counting Rickettsia cells. Mikrobiol. Z. 52:91-95. [PubMed] [Google Scholar]
- 4.Eremeeva, M. E., Z. Liang, C. Paddock, S. Zaki, J. G. Vandenbergh, G. A. Dasch, and D. J. Silverman. 2003. Rickettsia rickettsii infection in the pine vole, Microtus pinetorum: kinetics of infection and quantitation of antioxidant enzyme gene expression by RT-PCR. Ann. N. Y. Acad. Sci. 990:468-473. [DOI] [PubMed] [Google Scholar]
- 5.Eremeeva, M. E., and G. A. Dasch. 2001. Rickettsia and Orientia, p. 2175-2216. In M. Sussman (ed.), Molecular medical microbiology, vol. 3. Academic Press, London, United Kingdom.
- 6.Eremeeva, M. E., G. A. Dasch, and D. J. Silverman. 2001. Quantitative analyses of variations in the injury of endothelial cells elicited by 11 isolates of Rickettsia rickettsii. Clin. Diagn. Lab. Immunol. 8:788-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eremeeva, M., X.-Y. Yu, and D. Raoult. 1994. Differentiation among spotted fever group rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J. Clin. Microbiol. 32:803-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eremeeva, M. E., N. M. Balayeva, V. F. Ignatovich, and D. Raoult. 1993. Proteinic and genomic identification of spotted fever group rickettsiae isolated in the former USSR. J. Clin. Microbiol. 31:2625-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eremeeva, M. E., V. Roux, and D. Raoult. 1993. Determination of genome size and restriction pattern polymorphism of Rickettsia prowazekii and Rickettsia typhi by pulsed field gel electrophoresis. FEMS Microbiol. Lett. 112:105-112. [DOI] [PubMed] [Google Scholar]
- 10.Fournier, P.-E., V. Roux, and D. Raoult. 1998. Phylogenetic analysis of spotted fever group rickettsiae by study of outer surface protein rOmpA. Int. J. Syst. Bacteriol. 48:839-849. [DOI] [PubMed] [Google Scholar]
- 11.Gimenez, D. F. 1964. Staining rickettsiae in yolk sac culture. Stain Technol. 39:135-140. [DOI] [PubMed] [Google Scholar]
- 12.Ormsbee, R., M. Peacock, R. Gerloff, G. Tallent, and D. Wike. 1978. Limits of rickettsial infectivity. Infect. Immun. 19:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pusterla, N., J. B. Huder, C. M. Leutenegger, U. Braun, J. E. Madigan, and H. Lutz. 1999. Quantitative real-time PCR for detection of members of the Ehrlichia phagocytophila genogroup in host animals and Ixodes ricinus ticks. J. Clin. Microbiol. 37:1329-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pusterla, N., C. M. Leutenegger, J.-S. Chae, H. Lutz, R. B. Kimsey, J. S. Dumler, and J. E. Madigan. 1999. Quantitative evaluation of ehrlichial burden in horses after experimental transmission of human granulocytic ehrlichia agent by intravenous inoculation with infected leukocytes and by infected ticks. J. Clin. Microbiol. 37:4042-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regnery, R. L., C. L. Spruill, and B. D. Plikaytis. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolain, J.-M., L. Stuhl, M. Maurin, and D. Raoult. 2002. Evaluation of antibiotic susceptibilities of three rickettsial species including Rickettsia felis by a quantitative PCR DNA assay. Antimicrob. Agents Chemother. 46:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux, V., P-E. Fournier, and D. Raoult. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 34:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning. A laboratory manual, vol. 1, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Silverman, D. J., P. Fiset, and C. L. Wisseman, Jr. 1979. Simple, differential staining technique for enumerating rickettsiae in yolk sac, tissue culture extracts, or purified suspensions. J. Clin. Microbiol. 9:437-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turco, J., and H. H. Winkler. 1993. Role of the nitric oxide synthase pathway in inhibition of growth of interferon-sensitive and interferon-resistant Rickettsia prowazekii strains in L929 cells treated with tumor necrosis factor alpha and gamma interferon. Infect. Immun. 61:4317-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker, T. S. 1984. Rickettsial interactions with human endothelial cells in vitro: adherence and entry. Infect. Immun. 44:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker, T. S., and H. H. Winkler. 1978. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazekii. Infect. Immun. 22:200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wike, D. A., and W. Burgdorfer. 1972. Plaque formation in tissue cultures by Rickettsia rickettsii isolated directly from whole blood and tick hemolymph. Infect. Immun. 6:736-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisseman, C. L., Jr., E. A. Edlinger, A. D. Waddell, and M. R. Jones. 1976. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect. Immun. 14:1052-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]