Summary
The pro-apoptotic lipid sphingosine is phosphorylated by sphingosine kinases 1 and 2 (SK1 and SK2) to generate the mitogenic lipid sphingosine-1-phosphate (S1P). We previously reported that inhibition of SK activity delays tumor growth in a mouse mammary adenocarcinoma model. Because SK inhibitors and the multikinase inhibitor sorafenib both suppress the MAP kinase pathway, we hypothesized that their combination may provide enhanced inhibition of tumor growth. Therefore, we evaluated the effects of two SK inhibitors, ABC294640 (a SK2-specific inhibitor) and ABC294735 (a dual SK1/SK2 inhibitor), alone and in combination with sorafenib on human pancreatic adenocarcinoma (Bxpc-3) and kidney carcinoma (A-498) cells in vitro and in vivo. Exposure of either Bxpc-3 or A-498 cells to combinations of ABC294640 and sorafenib or ABC294735 and sorafenib resulted in synergistic cytotoxicity, associated with activation of caspases 3/ 7 and DNA fragmentation. Additionally, strong decreases in ERK phosphorylation were observed in Bxpc-3 and A-498 cells exposed to either the sorafenib/ABC294640 or the sorafenib/ABC294735 combination. Oral administration of either ABC294640 or ABC294735 to mice led to a delay in tumor growth in both xenograft models without overt toxicity to the animals. Tumor growth delay was potentiated by co-administration of sorafenib. These studies show that combination of an SK inhibitor with sorafenib causes synergistic inhibition of cell growth in vitro, and potentiates antitumor activity in vivo. Thus, a foundation is established for clinical trials evaluating the efficacy of combining these signaling inhibitors.
Keywords: Targeted therapy, Sphingosine kinase, Sorafenib, Apoptosis, MAPK pathway
Introduction
There has been progressive improvement in the treatment of many types of cancer; however, severe side effects and the development of drug resistance in patients receiving anticancer therapies are continuing problems. These issues have prompted searches for new pharmacological approaches that target signaling pathways critical for cancer cell proliferation. A number of small molecules and antibodies that target such pathways have demonstrated activity in pre-clinical tumor models and in patients [1]. Development of these “targeted” therapies has been facilitated by new data revealing molecular pathways and mediators of cell survival and apoptosis. Importantly, a number of those pathways and mediators appear to be “druggable”. For example, sphingolipids have been extensively studied due to their involvement in apoptosis and cell survival [2]. In mammalian cells, sphingomyelin in the plasma membrane is enzymatically cleaved to yield ceramide, which is acted upon by ceramidase to produce sphingosine [3]. Sphingosine is then phosphorylated by either of two isozymes - sphingosine kinases 1 and 2 (SK1/ 2) to yield sphingosine 1-phosphate (S1P) [4]. This enzymatic processing of sphingolipids determines the balance between the pro-survival lipid S1P and pro-apoptotic species ceramide and sphingosine (frequently called the “ceramide/S1P rheostat”) [5]. In addition, several cellular processes such as proliferation, growth, migration, differentiation and senescence are regulated by either the addition of exogenous S1P or overexpression of SK enzymes [6]. Additionally, exposure of cancer cells to a variety of mitogens leads to increases in the intracellular levels of S1P as a result of increased enzymatic activity of SK [7]. In solid tumors, overexpression of SK1 is associated with an increase in cell survival and chemo-resistance. Conversely, down-regulation or pharmacological inhibition of SK activity reduces cell growth and enhances chemosensitivity [8, 9]. Taken together, it is clear that inhibition of SK activity provides an attractive, yet inadequately explored, target for cancer chemotherapy.
We have previously shown that pharmacological inhibition of SK activity by several structurally-unrelated non-lipid small molecules delays tumor growth in a mouse model of adenocarcinoma [9, 10]. Recently, we synthesized a series of novel small molecules based on a phenyladamantane core that inhibit SK activity at low micromolar concentrations [11]. The SK2-specific inhibitor 3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide (ABC294640) (Fig. 1a) inhibits mitogen-stimulated production of S1P, and the migration and proliferation of endothelial cells [12]. Furthermore, ABC294640 has antitumor activity, associated with decreased Akt and MAPK signaling in the mouse JC tumor model [11].
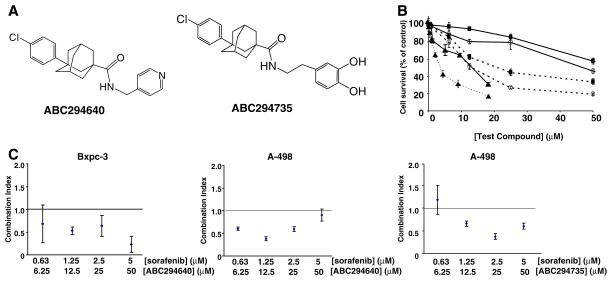
Fig. 1.
Cytotoxicities of ABC294640 and ABC294735 alone and in combination with sorafenib. a: Structures of ABC294640, 3-(4-chlorophenyl)adamantane-1-carboxylic acid (pyridin-4-ylmethyl)am-ide and ABC294735, 3-(4-chlorophenyl)adamantane-1-carboxylic acid 3,4-dihydroxybenzylamide. b: Bxpc-3 (solid lines) or A-498 cells (dashed lines) were exposed to the indicated concentrations of ABC294640 (black squares), ABC294735 (open circles) or sorafenib (solid triangles) for 48 hr. Standard SRB assays were performed to assess cytotoxicity. Data represent the mean±standard error for three independent experiments. c: Bxpc-3 or A-498 cells were exposed to the indicated concentrations of ABC294640+ sorafenib or ABC294735+ sorafenib for 72 hr, and cell survival was measured by the SRB assay. Combination indices were calculated as described in the Materials and Methods section. A Combination Index of: 1.0 indicates additive cytotoxicity; <1.0 indicates synergy; and >1.0 indicates antagonism. Data represent the mean±standard error for three independent experiments
The inhibitory affects of numerous small molecules on MAPK signaling have been explored in attempts to control tumor growth by pharmacological intervention on that pathway. For example, sorafenib is a potent inhibitor of Raf-1, a member of the RAF/MEK/ERK signaling pathway [13]. Sorafenib also has significant inhibitory activity against several receptor tyrosine kinases involved in neo-vascularization and tumor progression, including vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-3, platelet-derived growth factor receptor beta, Flt-3, and c-KIT [14]. In vivo, sorafenib has antitumor activity in colon, breast and non-small-cell lung cancer xenograft models [15]. This inhibition of tumor growth is associated with the inhibition of ERK1/2 phosphorylation, which is consistent with inhibition of the RAF/MEK/ERK pathway [15]. Therefore, agents that inhibit MEK pathway signaling have excellent potential for use against several types of cancer, and sorafenib (Nexavar) has been approved for the treatment of renal and hepatic cancers.
In the present studies, we compare the in vitro and in vivo anticancer activities of ABC294640, a selective SK2 inhibitor and (3-(4-chloro-phenyl)-adamantane-1-carboxylic acid 3,4-dihydroxy-benzylamide (ABC294735), a new, dual SK1/2 inhibitor (Fig. 1a). Additionally, the abilities of these compounds to synergize with sorafenib are examined. The data demonstrate that both ABC294640 and ABC294735 delay tumor growth as single agents, and this delay is potentiated by the co-administration of sorafenib.
Materials and methods
Cell lines and animals
Human pancreatic adenocarcinoma cells (Bxpc-3) and human kidney carcinoma cells (A-498) cells were purchased from American Type Culture Collection, and grown in RPMI 1640 (Bxpc-3) or EMEM (A-498) medium containing 10% Fetal Bovine Serum (Invitrogen; Carlsbad, CA) and 50 μg/ml gentamycin sulfate. SCID mice were purchased from the National Institutes of Health (Bethesda, MD).
Compounds
Sorafenib [16] and ABC294640 [12] were synthesized as described previously. ABC294735 (3-(4-chlorophenyl)ada-mantane-1-carboxylic acid [2-(3,4-dihydroxyphenyl)ethyl] amide) was synthesized by adding adamantane-1-carboxylic acid to a mixture of AlCl3 and Br2 at 0°C, and stirring at 0–10°C for 48 hr. The temperature of the mixture was then raised to 20°C for 5 hr, and the mixture was poured onto crushed ice, diluted with CHCl3 and decolorized with solid Na2S2O5. The aqueous phase was extracted twice with ether, and the combined organic phases were washed with water and extracted with 10 % NaOH. The alkaline extract was neutralized with H2SO4, yielding 3-bromoadamantane-1-carboxylic acid (~76 %). 3-bromoadamantane-1-carboxylic acid was dissolved in dry chlorobenzene and added to dry chlorobenzene containing AlCl3. The mixture was warmed to room temperature for 1 hr and then heated to 90°C for 10 hr. The mixture was then poured onto crushed ice, yielding 3-(4-chlorophenyl)adamantane-1-carboxylic acid (79 %). 3-(4-chlorophenyl)adamantane-1-carboxylic acid was dissolved in toluene, mixed with thionyl chloride under dry nitrogen, and refluxed for 1 hr. The resulting 3-(4-chlorophenyl)-adamantane-1-carbonyl chloride was added to 3-hydroxytyramine hydrochloride, NaOH and Na2CO3 in DMF under N2, stirred at 60°C for 24 hr and then cooled to room temperature. The reaction mixture was evaporated under vacuum, extracted with CHCl3, washed three times with water and dried with anhydrous Na2SO4, filtered and concentrated to produce ABC294735 as a white crystal with a yield of 83% and a melting point of 146–148°C; (C25H28ClNO3. 1/2 DMF). 1H NMR(500 MHz, DMSO) δ 1.63–1.68 (m, 2H, Admant-H), 1.71–1.83 (m, 10H, Admant-H), 2.14 (s, 2H, Admant-H), 2.50–2.53 (m, 2H, CH2), 3.15–3.18(t, J=7.5 Hz, 2H, NCH2), 3.40 (s, 1H, NH), 6.40–6.42 (d, J=10 Hz, 1H, Ar-H), 6.56 (s, 1H, Ar-H), 6.62–6.63 (d, J=5 Hz, 1H, Ar-H), 7.36–7.41 (m, 4H, Ar-H), 8.68 (s(br), 2H, OH); 13C NMR(500 MHz, DMSO) δ 28.8, 31.3, 35.0, 35.5, 36.3, 36.6, 38.3, 41.0, 41.3, 42.0, 44.3, 115.8, 116.4, 119.8, 127.4, 128.5, 130.7, 131.0, 149.6, 162.9, 176.8; MS m/z (rel intensity) 426.18 (MH+, 100), 427.18(68), 428.18(75).
Cell proliferation and apoptosis assays
Cell proliferation was measured using the standard sulforhodamine B assay [17]. Combined-effects analyses were conducted to establish whether combination of SK inhibitors with sorafenib results in synergism, additivity or antagonism for inhibition of cell proliferation using CalcuSyn software (Biosoft, Ferguson, MO), which is based on the method of Chou and Talalay [18]. For cell cycle analyses and quantification of genomic DNA fragmentation, cells were exposed to various concentrations of ABC294640, ABC294735 and/or sorafenib for 48 hr, washed twice with PBS and incubated in 0.5 ml of PI staining solution (50 μg/ml of propidium iodide, 40 μg/ml RNase A in PBS) for 30 min at 37°C. Cell cycle distributions were analyzed by the MUSC flow cytometry facility with a Becton Dickinson FACSCalibur Analytical Flow Cytometer. The activities of caspases 3 and 7 were measured by the Caspase-Glo 3/7 Assay (Promega, Madison, WI) according to manufacturer’s instructions. Briefly, A-498 or Bxpc-3 cells were grown in white 96-well plates at a density of 10,000 cells per well. After incubation with the test compound, 100 μl of the caspase reagent was added and plates were incubated at room temperature for 30 min. After incubation, luminescence was measured using a Molecular Devices SpectraMax M5 plate reader. Cells exposed to cisplatin were used as positive controls for apoptosis. For TUNEL analyses, cells were grown in Lab-Tek 8-well chamber slides (Rochester, NY), exposed to SK inhibitors alone or with sorafenib, fixed in 4 % paraformaldehyde and the TUNEL staining procedure was performed as described below.
Western blot analyses
Whole cell lysates were prepared and western blotting was carried out as previously reported [19]. Akt, phospho-Akt, pS259 Raf-1 and pan-Raf-1 antibodies were from Cell Signaling Technology (Beverly, MA); ERK and p-ERK antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); LC3 antibody was from Novus Biologicals (Littleton, CO). Beclin antibody was from Abcam (Cambridge, MA). Proteins were visualized by enhanced chemiluminescence using anti-rabbit or anti-mouse horseradish peroxidase-conjugated IgG (Pierce; Appleton, WI). Equal loading was confirmed by probing the blots with mouse anti-actin antibody (Abcam, Cam-bridge, MA).
Antitumor studies
SCID bearing xenografts of either kidney carcinoma A-498 or pancreatic adenocarcinoma Bxpc-3 cells were established as previously described [20, 21]. Animal care and procedures were in accordance with guidelines and regulations of the Medical University of South Carolina. Animals were housed under 12 hr light/dark cycles, with food and water provided ad libitum. Tumor cells (5×106 for A-498 or 3×106 for Bxpc-3 cells) were injected subcutaneously, and tumor volume was calculated using the equation: (length × width2)/2. Upon detection of tumors, mice were randomized into treatment groups (n=7–8). Mice were then treated daily with 50 mg/kg of ABC294640 or 50 mg/kg of ABC294735, dissolved in vehicle (50 % water and 50% PEG-200), and/or 10 mg/kg sorafenib every other day. Whole body weight and tumor volume measurements were performed twice a week. P-values were determined using two-way ANOVA using GraphPad InStat (San Diego, CA). After 4–5 weeks of treatment, 3 animals from each cohort were sacrificed and tumors were excised, fixed in paraformaldehyde and embedded in paraffin. Representative sections were deparaffinized and rehydrated in graded alcohols and xylene using standard procedures, and either stained with haematoxylin and eosin for histology, TUNEL using a kit (Roche, Mannheim, Germany) for apoptosis, or p-ERK for signaling. The percentages of TUNEL-positive cells within the tumor sections were determined by counting at least 100 cells each from at least three randomly selected fields. For p-ERK immunohistochemistry, after blocking in 10% normal goat serum in a humid chamber for 30 min, sections were incubated in primary antibodies overnight at 4°C followed by secondary antibody for 60 min at room temperature.
Results
In vitro anticancer effects of combination of SK inhibitors with sorafenib
To assess the anti-proliferative effects of the SK inhibitors, kidney carcinoma (A-498) or pancreatic adenocarcinoma (Bxpc-3) cells were plated in 96-well plates and exposed to various concentrations of an SK inhibitor. Following 48 hr of exposure, cell survival was measured by the standard sulforhodamine B assay. As shown in Fig. 1b, IC50 values for ABC294640 were approximately 50 and 60 μM for A-498 and Bxpc-3 cells, respectively; whereas the IC50 values for ABC294735 were approximately 20 and 40 μM for these cells. Sorafenib was more potent than these SK inhibitors, with IC50 values of 5 μM (A-498 cells) and 15 μM (Bxpc-3 cells). These data indicate that sorafenib is the most toxic and ABC294640 is the least toxic inhibitor of the two cancer cell lines in vitro.
Since both SK inhibitors [9–12] and sorafenib [22] decrease cancer cell survival by interfering with the pro-survival MAPK pathway signaling, we hypothesized that combining the two may result in synergistic cytotoxic effects in vitro. Therefore, A-498 or Bxpc-3 cell lines were treated with increasing concentrations of an SK inhibitor in the presence of a constant ratio of sorafenib. Following 48 h of treatment, cell viability was assessed and the Combination Index (CI) was calculated to determine whether the drug combination resulted in additive (CI=1.0), synergistic (CI<1.0) or antagonistic (CI > 1.0) toxicity. In Bxpc-3 cells, combinations of ABC294640 and sorafenib resulted in moderate to strong synergism (Fig. 1c). No synergism was observed in these cells when ABC204735 was combined with sorafenib (data not shown). Combination of either ABC294640 or ABC294735 with sorafenib in A-498 cells at constant ratios (10:1 respectively) resulted in moderate synergy (Fig. 1c), particularly at lower concentrations (<50 μM ABC294640 and <5 μM sorafenib). Therefore, ABC294640 or ABC294735 synergistically increased the cytotoxicity of sorafenib in kidney and pancreatic cancer cells.
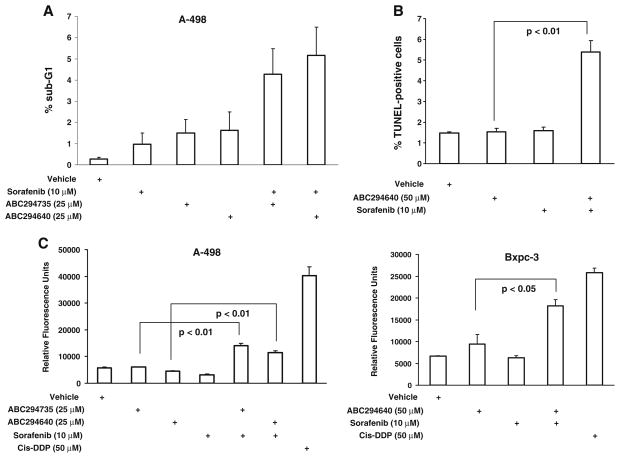
Because SK inhibitors and sorafenib cooperatively increased cell death, the underlying mechanism was examined. To establish if this cooperative effect is due to increases in apoptosis, we examined several apoptotic markers. First, genomic DNA fragmentation was measured by flow cytometry (Fig. 2a). We observed increased genomic DNA fragmentation in A-498 cells treated with combinations of either ABC294640 or ABC294735 plus sorafenib compared with cells exposed to single agents. Second, TUNEL assays were performed to quantify apoptotic DNA fragmentation in Bxpc-3 cells (Fig. 2b). These studies also demonstrated increases in apoptosis when the cells were treated with the ABC294640 plus sorafenib combination. Finally, the activities of caspases 3/ 7 in drug-treated cells were assessed, using cisplatin as a positive control. As shown in Fig. 2c, activation of caspases 3/7 was observed in A-498 cells exposed to combinations of either ABC294640 or ABC294735 with sorafenib. Similarly, activation of caspase activity was observed for the ABC294640 plus sorafenib combination in Bxpc-3 cells (Fig. 2c). Taken together, these data indicate that SK inhibitors cooperate with sorafenib to increase apoptosis in the two tumor cell lines.
Fig. 2.
Effects of SK inhibitors alone or in combination with sorafenib on apoptosis. a: A-498 cells were treated with the indicated concentrations of ABC294640, ABC294735 and/or sorafenib for 48 hr. Nuclei were harvested and stained with propidium iodide and the DNA content was analyzed by flow cytometry as described in the Materials and Methods section. b: Bxpc-3 cells were treated with the indicated concentrations of ABC294640 and/or sorafenib for 48 hr, fixed with paraformaldehyde and TUNEL-positive cells were quantified as described in the Materials and Methods section. c: A-498 or Bxpc-3 cells were exposed to the indicated concentrations of ABC294640, ABC294735 and/or sorafenib for 48 hr. Caspases 3/7 activity was measured by luminescence as described in the Materials and Methods section. Data represent mean±standard error for three independent experiments. Cisplatin (Cis-DDP) was used as a positive control
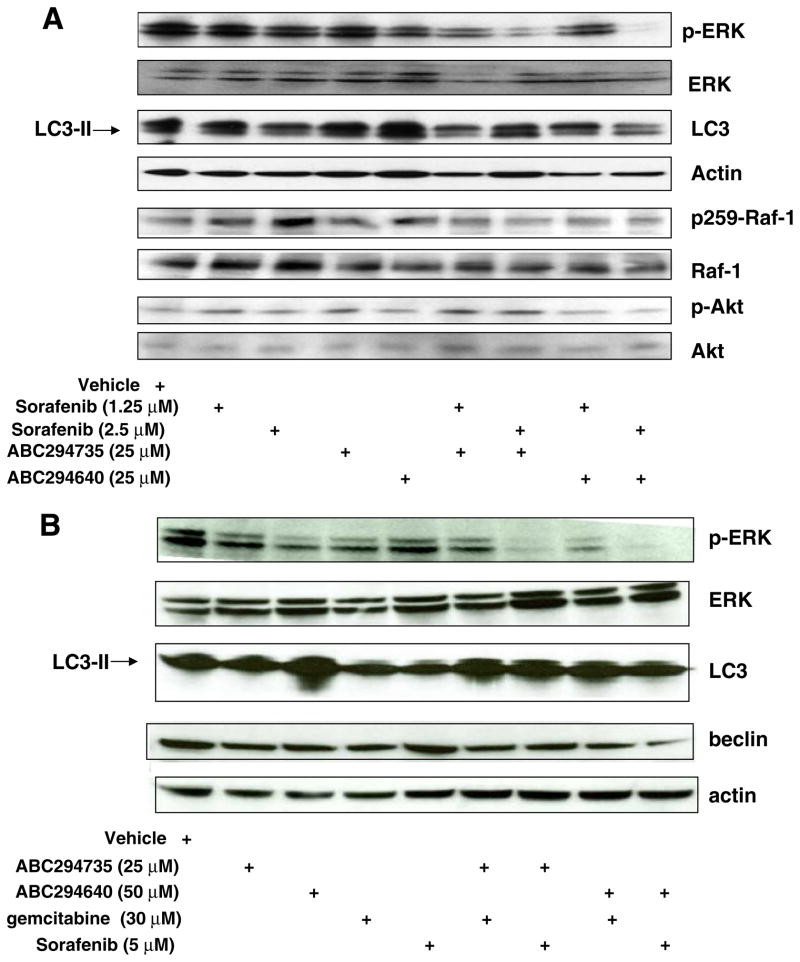
To gain insight into the signaling mechanisms underlying the combined cytotoxicity, the affects of the SK inhibitor / sorafenib combinations on MAPK pathway signaling was examined by western blotting. At 48 hrs of drug exposure, low doses of sorafenib and either ABC294640 or ABC294735, but not the individual agents, decreased the levels of phospho-ERK1/2 (pERK) in both A-498 (Fig. 3a) and Bxpc-3 (Fig. 3b) cells. We included Bxpc-3 cells treated with gemcitabine (a standard drug for pancreatic cancer) alone or in combination with ABC294640 for comparison. A decrease of p-ERK was also observed in cells exposed to gemcitabine and either ABC294640 or ABC294735, although this decrease was smaller than the response to the SK inhibitor plus sorafenib combinations. No changes were observed for total ERK1/2 protein by any of the drug treatments (Fig. 3a, b). Therefore, combined exposure of kidney carcinoma or pancreatic adenocarcinoma cells to an SK inhibitor and sorafenib leads to down-regulation of pro-survival MAPK signaling. In contrast, no differences in the levels of p-Akt in A-498 cells were observed between the treatments (Fig. 3a). In addition, we probed the levels of phosphorylation of the serine-259 residue (p-S259) of Raf-1 (Fig. 3a). Phosphorylation of this site has been linked to the inhibition of Raf-1 signaling due to the binding of 14-3-3ζ; whereas, dephosphorylation of S259 has been associated with activation of Raf-1 [23]. The ratio of p-S259 / Raf-1 protein did not change substantially after treatment with any of the drug treatments.
Fig. 3.
Effects of SK inhibitors and sorafenib on signaling pathways in A-498 and Bxpc-3 cells. A-498 cells (a) or Bxpc-3 cells (b) were exposed to the indicated concentrations of ABC294640, ABC294735 and/ or sorafenib for 48 hr. Cell lysates were then fractionated by SDS-PAGE, and probed with antibodies to detect p-ERK, pan-ERK, LC3, actin, p-Raf-1, pan-Raf-1, p-Akt, pan-Akt or beclin-1 as described in the Materials and Methods section
Because we previously found that SK inhibitors induce autophagy which leads to the death of A-498 cells [19], we also assessed levels of LC3-II (an indicator of autophagy) by immunoblotting [24] in cells that were exposed to various treatments. No significant differences in the levels of LC3-II were observed in cells treated with an SK inhibitor plus sorafenib or gemcitabine compared with cells treated with the individual compounds, indicating that enhanced autophagy is not responsible for the combined cytotoxicities. We also assessed levels of beclin 1 (another indicator of autophagy) in Bxpc-3 cells and observed no differences in levels of this protein among the treatments (Fig. 3b).
In vivo antitumor effects of combination of SK inhibitors with sorafenib
Because the combination of an SK inhibitor with sorafenib is synergistically cytotoxic toward cancer cells in vitro, their combined effects on tumor growth were also examined in two xenograft models. A-498 kidney carcinoma or Bxpc-3 pancreatic adenocarcinoma cells were implanted subcutaneously into SCID mice, and upon the development of measurable tumors, typically 3–4 weeks later, mice were randomized into groups (n=7–8) and treated with: vehicle (PEG-300/DMSO=50/50); ABC294640; ABC294735; sorafenib; ABC294640 plus sorafenib; or ABC294735 plus sorafenib. SK inhibitors were administered orally every day (Monday to Friday) at 50 mg/kg body weight, and sorafenib was administered intraperitoneally at 10 mg/kg body weight on alternate days (Monday, Wednesday and Friday). Tumors were measured with calipers, and animals were weighed twice a week to assess systemic toxicity.
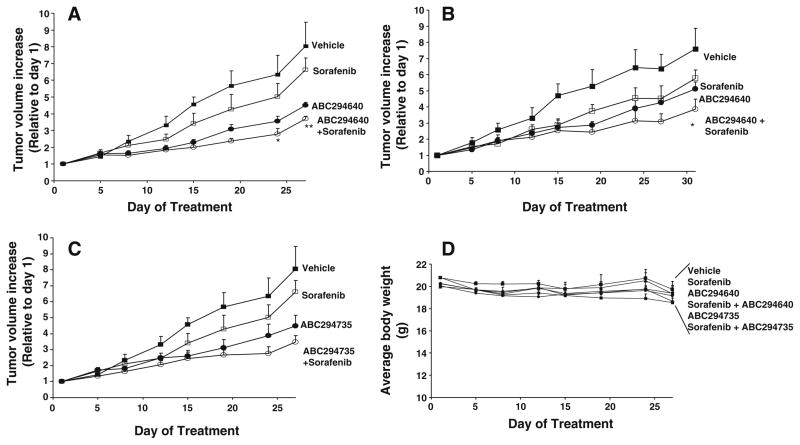
Single-agent administration of ABC294640, ABC294735 or sorafenib to mice reduced tumor growth in both xenograft models (Fig. 4). Similar to the in vitro results, combination of sorafenib with ABC294640 resulted in statistically-significant reductions of tumor growth compared with either single agent in both models (Fig. 4a, b). Combining ABC294735 with sorafenib was also more effective than the single agent treatments; however, this decrease in tumor growth did not reach statistical significance (Fig. 4c). To assess the systemic toxicity of the treatments, we measured mouse total body weight, and found no significant weight loss for any treatment group (Fig. 5d). To assess the potential toxicity of the drug treatments on normal tissues, sections prepared from the small intestine and liver of the test mice were stained for TUNEL-positive cells. Treatment with an SK inhibitor alone or combined with sorafenib did not result in increased numbers of apoptotic cells in the normal tissues compared with samples from the vehicle-treated control mice (data not shown).
Fig. 4.
Effects of SK inhibitors and sorafenib on the growth of kidney carcinoma or pancreatic adenocarcinoma cells in xenograft models. Female SCID mice (n=8 per group) were injected subcutaneously with A-498 cells (a and c) or Bxpc-3 cells (b) suspended in PBS. After palpable tumors were formed, the animals were treated 5 days per week with vehicle, 50 mg/kg of ABC294640 (aand b), 50 mg/kg ABC294735 (c), 10 mg/kg sorafenib (three times per week) or combinations as indicated in the Figure. Values represent the mean±standard error tumor volume normalized to treatment Day 1 for each mouse. *, p< 0.05; **, p<0.01. d: The average mouse weights (mean±standard error) for the treatment groups are shown
Fig. 5.
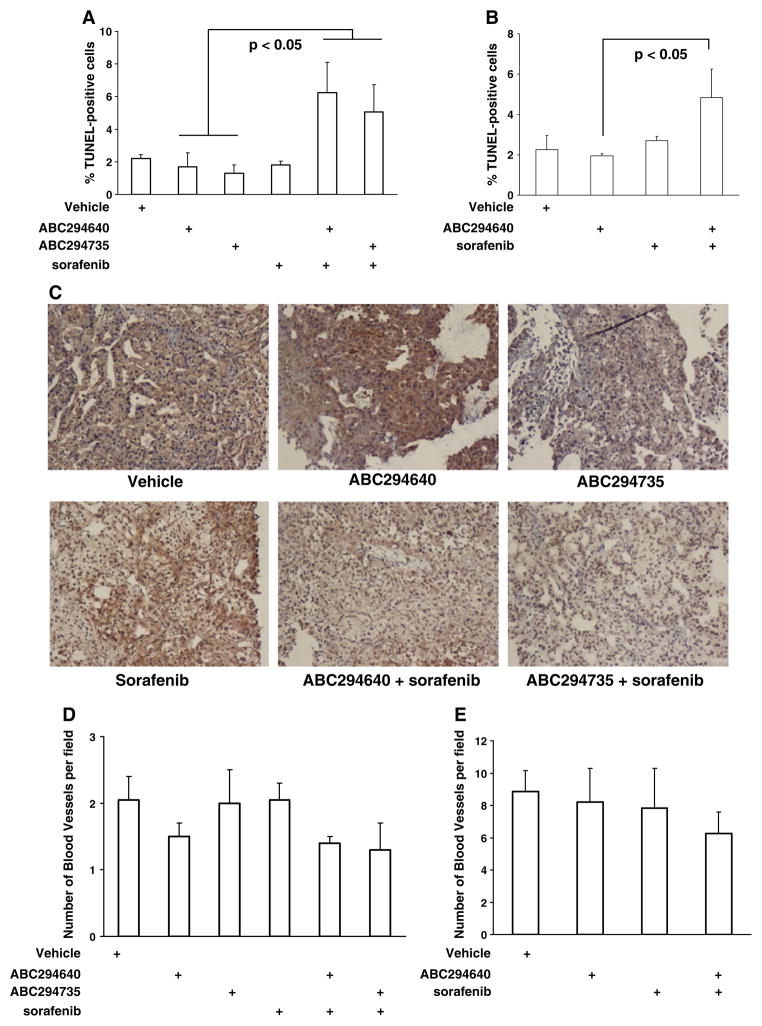
Histological characterization of SK inhibitor- and/or sorafenib-treated tumors. Mice described in Fig. 4 were sacrificed after 27 (RCC model) or 31 (PA model) days of treatment, tumors were fixed and sections were stained with hematoxylin, and then counterstained for TUNEL-positive cells, Von Willebrandt factor or p-ERK as described in the Materials and Methods section. a and b: Quantification of TUNEL-positive cells in A-498 (a) and Bxpc-3 (b) tumors. The bars represent the average±standard error number of TUNEL-positive counted in 45 microscopic fields (n=3). c: Immunostaining of A-498 tumor tissues for p-ERK. d and e: Quantification of blood vessels in A-498 (d) and Bxpc-3 (e) tumor tissues by staining for Van Willebrandt protein. The bars represent average±standard error number of blood vessels counted in 30 microscope fields (n=3)
To examine the underlying mechanism(s) leading to the reduction in tumor growth for the SK inhibitor plus sorafenib combinations, the effects of these agents on apoptosis, vascular development and ERK signaling in the tumor tissues were investigated. Consistent with the in vitro studies described above, the numbers of TUNEL-positive cells in A-498 tumors from mice treated with sorafenib and either ABC294640 or ABC294735 were substantially higher than those observed in tumors from animals treated with individual agents (Fig. 5a). Similar enhancements of tumor cells apoptosis were observed in Bxpc-3 tumors in mice treated with the ABC294640 plus sorafenib combination (Fig. 5b). Tumor tissues were immunostained for pERK to determine if the increases in apoptosis were associated with down-regulation of MAPK signaling. As shown in Fig. 5c, decreases in p-ERK levels were observed in A-498 tumors from mice treated with sorafenib and either ABC294640 or ABC294735.
To assess the effects of the SK inhibitors and sorafenib on the development of the vasculature in A-498 and Bxpc-3 tumors, we immunostained the tumor sections for von Willebrand factor and counted vessel numbers. As indicated in the Fig. 5d, e, we did not observe statistically-significant decreases in the number of blood vessels in either of the tumor models in animals treated with an SK inhibitor and/ or sorafenib.
Together, the data described in this section indicate that SK inhibitor plus sorafenib combinations significantly elevate apoptosis in tumor tissues (but not normal tissues), and lead to decreases in the ERK pro-survival signaling pathway compared to treatment with the single agents. In contrast, no effects on the angiogenesis were observed.
Discussion
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults, responsible for approximately 80% of cases, and it is also the most lethal of all genitourinary tumors [25]. For non-metastatic tumors, the 5-year survival rate is 60–70%, but this is lowered considerably when metastases have spread [26]. This metastatic form of the disease is resistant to radiation therapy and standard chemotherapy, although targeted cancer therapies such as sunitinib, temsirolimus, bevacizumab, interferon-alpha, and sorafenib have improved progression-free survival [27]. The most common type of pancreatic cancer is adenocarcinoma, which accounts for 85% of all newly diagnosed cases. Pancreatic adenocarcinoma is the 4th-leading cause of cancer deaths in the United States [28]. Disease progression in pancreatic cancer involves multiple pathways and mutations, and this molecular heterogeneity is a major reason for failure of targeted therapies. Hepatocellular carcinoma has an annual worldwide incidence of more than 600,000 cases and a mortality rate greater than 95%, making it the third most common cause of death from cancer worldwide. The multikinase inhibitor sorafenib is the only FDA-approved drug for advanced hepatocellular carcinoma, but increases the median duration of survival by only 3 months (from 7.9 month on placebo to 10.7 months on sorafenib). Clearly, new and more effective drugs and/or combinations are needed for the treatment of these highly fatal cancers.
Sorafenib is a small molecule inhibitor of Raf kinases and several tyrosine protein kinases that are overactive in many of the molecular pathways that facilitate cancer cell proliferation and survival (Fig. 6). These pathways include Raf-1 kinase, Platelet-Derived Growth Factor, VEGF Receptor 2 and 3, and c-Kit [13, 14]. Once activated, Raf-1 can phosphorylate and thereby activate the dual specificity protein kinases MEK1 and MEK2, which in turn phosphorylate and activate the serine/threonine protein kinases ERK1 and ERK2 [14]. Sorafenib is currently approved for the treatment of renal cell carcinoma and hepatocellular carcinoma, and is also being tested for the treatment of pancreatic adenocarcinoma, lung and thyroid cancers, alone or in combination with other therapeutics [15].
Fig. 6.
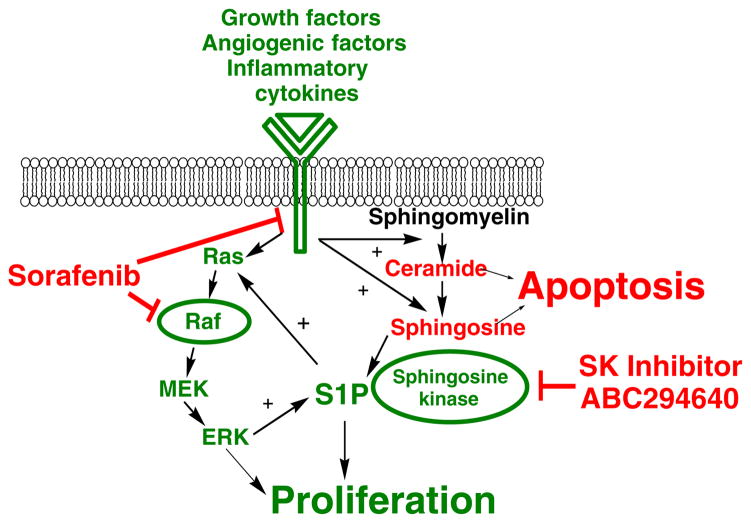
Rationale for simultaneous targeting of SK and Raf for cancer chemotherapy. Features in green represent pro-proliferative components, while features in red represent pro-apoptotic components. A variety of growth-stimulatory and angiogenic factors promote signaling through the Ras-Raf-MEK-ERK pathway that drives proliferation. Concurrently, these stimuli activate the sphingolipid metabolism pathway resulting in sequential the conversion of sphingomyelin to ceramide, sphingosine and S1P, which also drive proliferation. Crosstalk between the two pathways occurs at the level of SK, since S1P enhances the activation of Ras, and ERK phosphorylates and thereby stimulates SK activity. Treatment with sorafenib acts on components in the Ras-Raf-MEK-ERK pathway, while the SK inhibitors attenuate S1P-mediated proliferation. Importantly, dual inhibition of the interacting pathways prevents the amplification of signaling cycle, providing synergistic reduction in tumor cell proliferation
The involvement of sphingolipids in cancer biology has been the subject of a growing body of scientific investigation (reviewed in [29]). As indicated in Fig. 6, ceramide is produced by the hydrolysis of sphingomyelin in response to several growth stimulatory and/or inflammatory signals. Ceramide induces apoptosis in tumor cells without disrupting quiescent normal cells [30]. Additionally, ceramide can be further hydrolyzed by the action of ceramidase to produce sphingosine, which is phosphorylated by SK to produce S1P. Studies in various cell lines consistently indicate that S1P induces proliferation and protects cells from ceramide-induced apoptosis. Therefore, a critical balance between ceramide and S1P has been hypothesized to determine the fate of tumor cells [31]. In this model, the balance between ceramide and S1P determines whether a tumor cell proliferates or undergoes apoptosis.
There is substantial evidence that suggests that combination of sorafenib with inhibitors of sphingolipid metabolism may be therapeutically effective. For example, the activity profile of sorafenib involves signaling pathways also regulated by S1P, particularly the Ras-Raf-Mek-Erk pathway [13]. Interestingly, S1P promotes proliferation by a Ras-mediated pathway and Ras-mediated transformation is dependent on SK activity. Furthermore, SK activity is increased upon phosphorylation by ERK [32], setting up an amplification cascade for tumor cell proliferation (Fig. 6). Signaling through VEGF receptors is another point of convergence for the effects of sorafenib and an SK inhibitor, and so the combination could have anti-angiogenic activity. Additionally, the ability of ceramide to reduce drug resistance predicts that the combination of an SK inhibitor plus sorafenib will provide sensitization and consequently increased tumor cell killing. Therefore, there is an excellent chance that cancer patients will benefit from a sorafenib plus SK inhibitor chemotherapy regimen.
In the present work, we have examined the hypothesis that the antitumor effects of SK inhibitors can be potentiated by the simultaneous administration of sorafenib. Renal cell carcinoma and pancreatic adenocarcinoma cancer models were chosen because the progression of both tumor types depends, at least in part, on enhanced RAS/ERK signaling [33]. Point mutations of the K-ras gene occur in up to 90% of pancreatic adenocarcinomas, and represent one of the key and early events in carcinogenesis in this tissue. Downstream mediators are clearly activated, for example levels of p-ERK in pancreatectomy specimens were found to be inversely correlate with disease-free survival [34]. In kidney carcinoma, an underlying defect is chromosomal instability, and this likely results in sequence infidelity in the Ras pathway. In both renal and pancreatic cancers, targeting multiple oncogenic pathways using novel therapies could improve patient survival.
Herein, we show that the dual SK1/SK2 inhibitor ABC294375 and the SK2-selective inhibitor ABC294640 kill tumor cells at low μM concentrations in vitro, and delay growth of kidney and pancreatic cells in xenograft models. These activities are enhanced by combining the SK inhibitors with sorafenib. Specifically, exposure of A-498 cells or Bxpc-3 cells to an SK inhibitor plus sorafenib resulted in synergistic cytotoxicity in vitro. These cooperative effects in cell killing were associated with increases in apoptosis and caspase 3/7 activation. In addition, down-regulation of proliferative MAPK signaling in A-498 and Bxpc-3 tumor cells was observed for the combination of these agents. These data indicate that combining an SK inhibitor with sorafenib results in more efficient induction of apoptosis and decreased proliferative signaling in cancer cells.
Administration of these compounds to mice bearing either A-498 or Bxpc-3 tumor xenografts resulted in enhanced tumor growth inhibition without overt toxicity to mice. Similar to what was observed in cell culture systems, TUNEL staining in tumors from mice treated with the combinations indicated increases in tumor cell apoptosis compared with tumors from mice treated with the individual agents. The increases in apoptosis were most prominent in the A-498 tumor model treated with the ABC294640 plus sorafenib. Importantly, no indications of elevated apoptosis in normal tissues were observed in mice treated with an SK inhibitor plus sorafenib combination. Also, decreases in the levels of p-ERK were observed in the tumor tissues harvested from mice treated with the combination of agents. However, we did not find differences in the tumor vasculature between the various treatment groups. These finding indicate that SK inhibitors “cooperate” with sorafenib to decrease pro-survival signaling and increase apoptosis with minimal effect on angio-genesis in tumors.
In summary, we demonstrate that simultaneous targeting of the sphingolipid and MAPK pathways using SK inhibitors and sorafenib leads to more effective inhibition of cancer cell growth than do the single agents alone. Growth suppression is associated with increases in tumor cell apoptosis and down-regulation of MAPK signaling in cultured cells and intact tumors. Importantly, the combination does not induce apoptosis in normal tissues. Consequently, we believe that this combination of agents has the potential to be more effective for the treatment of kidney, liver and pancreatic cancers in patients. Thus, these studies establish the foundation for clinical trials evaluating the efficacy of combining ABC294640 and sorafenib for the treatment of patients with cancer.
Acknowledgments
Funding was provided by National Institutes of Health grant R01 CA122226.
Contributor Information
Vladimir Beljanski, Drug Discovery Core, Hollings Cancer Center and Department of Pharmaceutical and Biomedical Sciences, Medical University of South Carolina, Charleston, SC, USA.
Christian Knaak, Drug Discovery Core, Hollings Cancer Center and Department of Pharmaceutical and Biomedical Sciences, Medical University of South Carolina, Charleston, SC, USA.
Yan Zhuang, Apogee Biotechnology Corporation, Hummelstown, PA, USA.
Charles D. Smith, Email: smithchd@musc.edu, Drug Discovery Core, Hollings Cancer Center and Department of Pharmaceutical and Biomedical Sciences, Medical University of South Carolina, Charleston, SC, USA. Apogee Biotechnology Corporation, Hummelstown, PA, USA
References
- 1.Kessler T, Bayer M, Schwoppe C, Liersch R, Mesters RM, Berdel WE. Compounds in clinical Phase III and beyond. Recent Results Cancer Res. 2010;180:137–163. doi: 10.1007/978-3-540-78281-0_9. [DOI] [PubMed] [Google Scholar]
- 2.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 3.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn-Schmiedeberg’s Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 5.Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- 6.Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W, Levade T, Moreau-Gachelin F. Over-expression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106:1808–1816. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- 7.Safadi-Chamberlain F, Wang LP, Payne SG, Lim CU, Stratford S, Chavez JA, Fox MH, Spiegel S, Summers SA. Effect of a membrane-targeted sphingosine kinase 1 on cell proliferation and survival. Biochem J. 2005;388:827–834. doi: 10.1042/BJ20041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 9.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 10.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 11.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maines LW, French KJ, Wolpert EB, Antonetti DA, Smith CD. Pharmacologic manipulation of sphingosine kinase in retinal endothelial cells: implications for angiogenic ocular diseases. Invest Ophthalmol Vis Sci. 2006;47:5022–5031. doi: 10.1167/iovs.05-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young A, Lyons J, Miller AL, Phan VT, Alarcon IR, McCormick F. Ras signaling and therapies. Adv Cancer Res. 2009;102:1–17. doi: 10.1016/S0065-230X(09)02001-6. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–2421. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 17.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 19.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 333:454–464. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juang SH, Lung CC, Hsu PC, Hsu KS, Li YC, Hong PC, Shiah HS, Kuo CC, Huang CW, Wang YC, Huang L, Chen TS, Chen SF, Fu KC, Hsu CL, Lin MJ, Chang CJ, Ashendel CL, Chan TC, Chou KM, Chang JY. D-501036, a novel selenophene-based triheterocycle derivative, exhibits potent in vitro and in vivo antitumoral activity which involves DNA damage and ataxia telangiectasia-mutated nuclear protein kinase activation. Mol Cancer Ther. 2007;6:193–202. doi: 10.1158/1535-7163.MCT-06-0482. [DOI] [PubMed] [Google Scholar]
- 21.Van Quaquebeke E, Mahieu T, Dumont P, Dewelle J, Ribaucour F, Simon G, Sauvage S, Gaussin JF, Tuti J, El Yazidi M, Van Vynckt F, Mijatovic T, Lefranc F, Darro F, Kiss R. 2, 2, 2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1, 3-dioxo-2, 3-dihydro-1H-be nzo[de]isoquinolin- 5-yl}carbamoyl)acetamide (UNBS3157), a novel nonhematotoxic naphthalimide derivative with potent antitumor activity. J Med Chem. 2007;50:4122–4134. doi: 10.1021/jm070315q. [DOI] [PubMed] [Google Scholar]
- 22.Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4:677–685. doi: 10.1158/1535-7163.MCT-04-0297. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Viciana P, Oses-Prieto J, Burlingame A, Fried M, McCormick F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol Cell. 2006;22:217–230. doi: 10.1016/j.molcel.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facchini G, Perri F, Caraglia M, Pisano C, Striano S, Marra L, Fiore F, Aprea P, Pignata S, Iaffaioli RV. New treatment approaches in renal cell carcinoma. Anticancer Drugs. 2009;20:893–900. doi: 10.1097/CAD.0b013e32833123d4. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 27.Gkialas IK, Papadopoulos G. New therapeutic approaches in the management of metastatic renal cell carcinoma. J BUON. 2009;14:399–404. [PubMed] [Google Scholar]
- 28.Lee T, Kim J, Sohn U. Sphingosylphosphorylcholine-induced contraction of feline ileal smooth muscle cells is mediated by Galphai3 protein and MAPK. Cell Signal. 2002;14:989–997. doi: 10.1016/s0898-6568(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 29.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413–440. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 31.Spiegel S, Kolesnick R. Sphingosine 1-phosphate as a therapeutic agent. Leukemia. 2002;16:1596–1602. doi: 10.1038/sj.leu.2402611. [DOI] [PubMed] [Google Scholar]
- 32.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine kinase modulates micro-vascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 33.Stadler WM. Targeted agents for the treatment of advanced renal cell carcinoma. Cancer. 2005;104:2323–2333. doi: 10.1002/cncr.21453. [DOI] [PubMed] [Google Scholar]
- 34.Chadha KS, Khoury T, Yu J, Black JD, Gibbs JF, Kuvshinoff BW, Tan D, Brattain MG, Javle MM. Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann Surg Oncol. 2006;13:933–939. doi: 10.1245/ASO.2006.07.011. [DOI] [PubMed] [Google Scholar]