Abstract
Background
While gastric noncardia adenocarcinoma (GNCA) incidence rates in the US have decreased, the rates of gastric cardia adenocarcinoma (GCA) and esophageal adenocarcinoma (EADC) have increased. Obesity increases the risks of GCA and EADC, and the associations may be partially mediated by insulin resistance. A few case-control studies have shown an association between diabetes and an increased risk of EADC.
Methods
We prospectively examined the association between diabetes and upper gastrointestinal (UGI) cancers in a cohort of 469,448 people in the US, ages 50-71 at baseline. Cox proportional hazards regression was used to estimate the hazard ratios (HR) and 95% confidence intervals (CI) for diabetes and UGI cancers, controlling for multiple potential confounders, including body mass index (BMI).
Results
We observed no association of self-reported diabetes with risk of EADC, HR (95%CI) = 0.98 (0.73-1.31), esophageal squamous cell carcinoma (ESCC), HR (95%CI) = 1.02 (0.60-1.74), or GNCA, HR (95%CI) = 0.98 (0.70-1.37). However, diabetes was significantly associated with an increased risk of GCA, HR (95%CI) = 1.89 (1.43-2.50). The significant association between diabetes and risk of GCA remained after adjustment for BMI, HR (95%CI) = 1.70 (1.28-2.26) and did not differ by BMI strata (pinteraction =0.83). The significant association was unchanged when restricting to only overweight subjects (BMI 25 - ≤30), HR (95%CI) = 1.83 (1.18-2.85).
Conclusions
We found a significant association between self-reported diabetes and increased risk of GCA.
Impact
Our results suggest that the metabolic and hormonal changes related to diabetes may play a role in the etiology of GCA independently from BMI.
Keywords: Esophageal adenocarcinoma, gastric adenocarcinoma, diabetes, BMI
Introduction
Diabetes and cancer are prevalent diseases with increasing incidence. Recently, results from a series of studies and meta-analyses have indicated that some cancers develop more commonly in patients with diabetes (1). Type 2 diabetes is associated with increased risk of several cancer types, including non-Hodgkin lymphoma (2), postmenopausal breast (3), colorectal (4), endometrial (5), liver (6), bladder (7), and pancreatic (8) cancers, whereas prostate cancer (9) occurs less often in men with diabetes. Some of these associations appear to be independent of body mass index (BMI) (10). Few studies to date have examined links with Type 1 diabetes, usually an early-onset autoimmune disease that is relatively rare compared with Type 2 diabetes; due to physiologic differences, Type 1 and Type 2 diabetes may have different associations with cancer risk.
Type 2 diabetes and cancer share some similar risk factors, including obesity and a sedentary lifestyle. Characterized by hyperinsulinemia, Type 2 diabetes involves a loss of glucose homeostasis or the inability of tissues to respond to insulin. The chronic increase in circulating glucose and lipids may in turn further impair insulin secretion, and the aberrations in the insulin-like growth factor pathways and steroid hormone metabolism may play a role in predisposing diabetics to cancer. Hyperinsulinemia may promote cancer in diabetic patients because insulin is a growth factor with metabolic as well as mitogenic effects. Obesity, hyperglycemia, and increased oxidative stress may act together to contribute to the increase in cancer risk among diabetics.
In the US, the incidence of esophageal adenocarcinoma (EADC) has risen 350% since the mid-1970s (11-13), and to a lesser extent, the incidence of gastric cardia adenocarcinoma (GCA) has also increased (14), although improved cancer site classification may largely account for the increase (15). In contrast, the incidence rates of esophageal squamous cell carcinoma (ESCC) and gastric noncardia adenocarcinoma (GNCA) have decreased (13, 15-16). The increase of EADC is concordant with the increasing prevalence of obesity, and adiposity has been a consistent risk factor for EADC. Obesity may increase intra-abdominal pressure, with a mechanical effect on the lower esophageal sphincter that predisposes to gastroesophageal reflux disease (GERD); however, some studies have shown that obesity is associated with EADC even in the absence of reflux (17-18). Thus, insulin resistance, which results from the metabolic and hormonal changes of obesity, may be a risk factor for cancers of the gastroesophageal junction. Here, we prospectively examined the association between self-reported diabetes and UGI cancers using the NIH-AARP Diet and Health Study cohort.
Materials and Methods
Study population
The details of the NIH-AARP Diet and Health study have been described elsewhere (19). Briefly, between 1995 and 1996, a questionnaire was mailed to 3.5 million AARP members who were between 50 and 71 years of age and residing in six US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan). The questionnaire elicited information on demographic characteristics, dietary intake, and health-related behaviors. Out of the 617,119 people who completed and returned the questionnaire, 566,402 completed the questionnaire in satisfactory detail and consented to be in the study. We excluded proxy-responders (15,760), subjects with a cancer prior to baseline (51,234), those with calorie intake of more than two interquartile ranges from the median (4,417), those with unknown BMI or diabetes status (12,523), and those who died or were diagnosed with cancer on the first day of follow-up (12). Due to concerns about reverse causation, those subjects with less than two years of follow-up were also excluded (13,020). The resulting cohort included 469,448 participants: 280,883 men and 188,565 women.
Follow-up and case ascertainment
We calculated follow-up time from two years after the day of study entry until diagnosis of an upper gastrointestinal cancer, death, or the current end of follow-up (31 December 2006). The cohort database was matched annually to that of the National Change of Address (NCOA) maintained by the US Postal Service (USPS) so that the address of each cohort member could be updated. Annual linkage of the cohort to the Social Security Administration Death Master File (SSA DMF) on deaths in the US, searches of the National Death Index (NDI), and linkage to cancer registries were performed to ascertain vital status.
Cancer cases were identified by cancer registries in the original eight states or metropolitan areas and two additional states (Arizona and Texas); cancers were identified by anatomic site and histologic code of the International Classification of Disease for Oncology, 3rd edition (ICD-O). Tumors with site codes C15.0-15.9 were esophageal cancer, and ESCC and EADC were identified by histology; tumors with site code C16.0 and adenocarcinoma histology were classified as GCA; and tumors with site codes C16.1-16.9 and adenocarcinoma histology or those without a specified location were categorized as GNCA.
Exposure assessment
All exposure variables were derived from the information provided in the baseline questionnaire, which inquired about demographics, tobacco and alcohol use, physical activity, and diet using a 124-item food frequency questionnaire (19). History of diabetes was assessed with the question, “Have you ever been told by a doctor that you had diabetes?” BMI was calculated from self-reported baseline height and weight and was categorized as five quantiles based on WHO standard definitions: <18.5, 18.5-<25, 25-<30, 30-<35, and ≥35 kg/m2. Calories were assessed as kcal per day. Fruit and vegetable intake were measured separately as non-alcohol energy-adjusted pyramid equivalents per day. Alcohol consumption was measured as drinks per day, and tobacco smoking was categorized as: never smokers, former smokers of ≤20 cigarettes/day, former smokers of >20 cigarettes/day, current smokers of ≤20 cigarettes/day, and current smokers of >20 cigarettes/day. Participants were grouped into four race and ethnicity categories: white, not Hispanic; black, not Hispanic; Hispanic; and Asian, Pacific Islander, and American Indian/Alaska Native. Education was categorized into four ordinal categories: high school graduate or less, technical school or some college, college graduate, and post-graduate education. We included two physical activity variables: usual physical routine throughout the day and vigorous physical activity at baseline. Activity throughout the day included: sit all day, sit much of the day/walk some times, stand/walk often/no lifting, lift/carry light loads, and carry heavy loads. Vigorous physical activity included: never, rarely, 1-3 times/month, 1-2 times/week, 3-4 times/week, 5 or more times per week. Missing indicator variables were used for those who had missing values.
Statistical analysis
All analyses were conducted using SAS (SAS Institute, Cary, NC). We interpreted p < 0.05 and 95% confidence intervals (CIs) excluding 1 as statistically significant. We used multivariate Cox proportional hazards models to estimate hazard ratios (HRs) and two-sided 95% CIs for self-reported history of diabetes. Variables considered a priori as important potential confounders were included in the models, and we show both age-adjusted and multivariate-adjusted effect estimates. To detect deviations from the proportional hazards assumption, we examined potential differential effects by follow-up time by fitting the models using cases from each individual year of follow-up.
We examined the association between self-reported history of diabetes and UGI cancers separately in men and women. We examined the association with and without adjusting for continuous BMI and the WHO categories of BMI. We also fit separate models in BMI strata that contained ≥10 cancer cases each. In addition, to increase the comparability across studies, we examined the association when the obese and extremely obese categories (BMI 30− <35 and BMI 35+, respectively) were combined as BMI ≥30. We tested for effect modification (interaction) using cross-product terms and modeled BMI as a continuous variable for this purpose.
Results
Among the eligible cohort of 469,448 participants, 41,388 (8.82%) reported a diabetes diagnosis. Table 1 presents the cohort characteristics by diabetes status. More men than women reported having a history of diabetes. Overall, diabetics were older, had higher BMI and higher caloric intake, drank less alcohol, were more likely to have smoked, had less education, and were less physically active than non-diabetics. The diabetics group had higher percentages of non-whites than the non-diabetics group. Among the cohort, we identified 534 EADC, 172 ESCC, 352 GCA, and 379 GNCA incident cases. The average length of follow-up was 7.96 years. Table 2 presents the case counts and age-adjusted and multivariate-adjusted HRs and 95% CIs for the associations between self-reported diabetes and risk of EADC, ESCC, GCA, and GNCA for the whole cohort and for men and women separately. While no significant associations were observed between self-reported diabetes and EADC, ESCC, and GNCA, there was significantly increased risk for GCA. The risk estimates for ESCC, EADC, and GNCA were null among both men and women. We had low power to assess the risk estimates for each cancer site among women because fewer cases occurred among women, so the risk estimates for women had larger confidence intervals and may be considered exploratory. We found no evidence for effect modification by sex: for ESCC, pinteraction = 0.60; EADC, pinteraction =0.68; GCA, pinteraction =0.95; and GNCA, pinteraction =0.30.
Table 1.
NIH-AARP Study of Diet and Health cohort subject characteristics overall and by diabetes status
| Characteristic | Whole cohort | Non-diabetic | Diabetic |
|---|---|---|---|
| n (%) | 469,448 | 428,060 (91.18) | 41,388 (8.82) |
|
| |||
| Female sex, n (%) | 188,565 (40.17) | 175,060 (40.90) | 13,505 (32.63) |
|
| |||
| Age at entry (years), mean (SD) | 61.98 (5.37) | 61.90 (5.39) | 62.81 (5.14) |
|
| |||
| BMI (kg/m^2), mean (SD) | 27.10 (4.81) | 26.83 (4.63) | 29.83 (5.72) |
| <18.5 (underweight), n (%) | 4,417 (0.94) | 4,206 (0.98) | 211 (0.51) |
| 18.5-<25 (normal weight), n (%) | 161,582 (34.42) | 154,381 (36.07) | 7,201 (17.40)) |
| 25-<30 (overweight), n (%) | 200,267 (42.66) | 183,671 (42.91) | 16,596 (40.10) |
| 30-<35 (obese), n (%) | 73,974 (15.76) | 63,216 (14.77) | 10,758 (25.99) |
| ≥35 (obese), n (%) | 29,208 (6.22) | 22,586 (5.28) | 6,622 (16.00) |
|
| |||
| Calories intake (kcal/day), mean (SD) | 1835.79 (803.92) | 1835.05 (801.38) | 1843.40 (829.68) |
|
| |||
| Fruit (servings/day), mean (SD) | 1.19 (0.81) | 1.19 (0.81) | 1.18 (0.79) |
|
| |||
| Vegetables (servings/day), mean (SD) | 1.13 (0.59) | 1.13 (0.59) | 1.15 (0.59) |
|
| |||
| Alcohol (drinks/day), mean (SD) | 0.91 (2.37) | 0.94 (2.40) | 0.56 (2.01) |
|
| |||
| Smoking* | |||
| Never, n (%) | 165,659 (36.64) | 152,910 (37.09) | 12,749 (32.01) |
| Former, ≤20cigs/d, n (%) | 125,566 (27.77) | 114,882 (27,86) | 10,684 (26.83) |
| Former, >20cigs/d, n (%) | 97,636 (21.59) | 86,051 (20.87) | 11,585 (29.09) |
| Current, ≤20cigs/d, n (%) | 40,958 (9.06) | 38,109 (9.24) | 2,849 (7.15) |
| Current, >20cigs/d, n (%) | 22,309 (4.93) | 20,352 (4.94) | 1,957 (4.91) |
|
| |||
| Race* | |||
| White, not Hispanic, n (%) | 429,375 (92.63) | 393,673 (93.10) | 35,702 (87.69) |
| Black, not Hispanic, n (%) | 17,641 (3.81) | 14,709 (3.48) | 2,932 (7.20) |
| Hispanic, n (%) | 8,878 (1.92) | 7,686 (1.82) | 1,192 (2.93) |
| Asian, Pac Isl, Am Indian/Alaska Native n (%) | 7,659 (1.65) | 6,769 (1.60) | 890 (2.19) |
|
| |||
| Education* | |||
| High school graduate or less, n (%) | 118,035 (25.87) | 105,347 (25.31) | 12,688 (31.71) |
| Technical/some college, n (%) | 155,472 (34.08) | 141,137 (33.91) | 14,335 (35.83) |
| College graduate, n (%) | 89,083 (19.52) | 82,245 (19.76) | 6,838 (17.09) |
| Postgraduate, n (%) | 93,668 (20.53) | 87,526 (21.03) | 6,142 (15.35) |
|
| |||
| Activity throughout the day* | |||
| Sit during day, not much walking, n (%) | 36,489 (7.95) | 31,678 (7.56) | 4,811 (11.96) |
| Sit much of the day, walk a fair amount, n (%) | 151,173 (32.92) | 136,653 (32.62) | 14,520 (36.09) |
| Stand/walk a lot, no lifting, n (%) | 177,038 (38.55) | 162,459 (38.78) | 14,579 (36.24) |
| Lift carry light loads, n (%) | 80,932 (17.62) | 75,502 (18.02) | 5,430 (13.50) |
| Heavy work, n (%) | 13,560 (2.95) | 12,669 (3.02) | 891 (2.21) |
|
| |||
| Vigorous physical activity* | |||
| never, n (%) | 19,895 (4.28) | 16,803 (3.96) | 3,092 (7.57) |
| rarely, n (%) | 62,823 (13.52) | 55,233 (13.03) | 7,590 (18.58) |
| 1-3/mo, n (%) | 63,664 (13.70) | 58,069 (13.70) | 5,595 (13.70) |
| 1-2/wk, n (%) | 101,366 (2.18) | 93,220 (21.99) | 8,146 (19.95) |
| 3-4/wk, n (%) | 126,406 (27.20) | 116,723 (27.54) | 9,683 (23.71) |
| 5+/wk, n (%) | 90,567 (19.49) | 83,833 (19.78) | 6,734 (16.49) |
Participants with missing values for these variables were excluded from this table. Smoking: 17,320 excluded; Race: 5,895 excluded; Education: 13,190 excluded; Activity throughout the day: 10,256 excluded; Vigorous physical activity: 4,727 excluded.
Table 2.
Association between self-reported diabetes and UGI cancers
| Cancer | Non-diabetics, n | Diabetics, n | Age-adjusted HR (95% CI) |
Multivariate-adjusted* HR (95% CI) |
|
|---|---|---|---|---|---|
| Entire cohort | ESCC | 157 | 15 | 1.03 (0.60-1.74) | 1.02 (0.60-1.74) |
| EADC | 482 | 52 | 1.16 (0.87-1.54) | 0.98 (0.73-1.31) | |
| GCA | 291 | 61 | 2.23 (1.69-2.94) | 1.89 (1.43-2.50) | |
| GNCA | 340 | 39 | 1.20 (0.86-1.68) | 0.98 (0.70-1.37) | |
|
| |||||
| Males only | ESCC | 103 | 12 | 1.11 (0.61-2.01) | 1.08 (0.59-1.98) |
| EADC | 446 | 50 | 1.06 (0.79-1.42) | 1.00 (0.74-1.34) | |
| GCA | 256 | 56 | 2.07 (1.55-2.76) | 1.89 (1.41-2.54) | |
| GNCA | 239 | 27 | 1.05 (0.71-1.57) | 0.90 (0.60-1.34) | |
|
| |||||
| Females only | ESCC | 54 | 3 | 0.74 (0.23-2.35) | 0.87 (0.27-2.82) |
| EADC | 36 | 2 | 0.75 (0.18-3.13) | 0.68 (0.16-2.85) | |
| GCA | 35 | 5 | 1.83 (0.72-4.67) | 1.77 (0.68-4.58) | |
| GNCA | 101 | 12 | 1.53 (0.84-2.78) | 1.24 (0.67-2.28) | |
Potential confounders for the entire cohort models included age, sex, calories, alcohol consumption, smoking, fruit consumption, vegetable consumption, ethnicity, education, and physical activity; confounders for the males only or females only models included all these variables except sex.
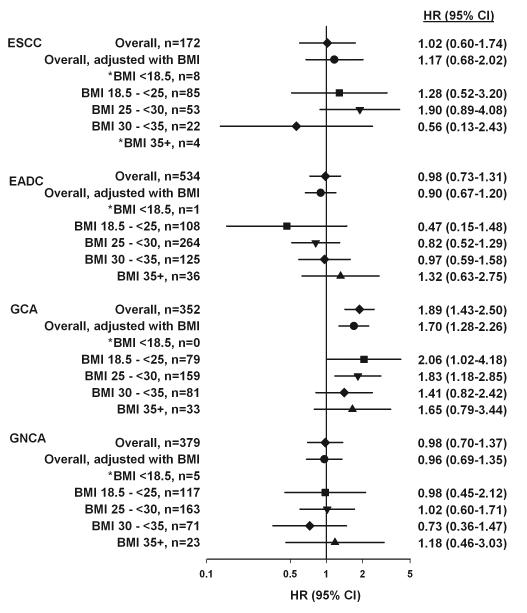
Figure 1 presents the overall risk estimates from the cohort before and after adjustment for BMI and by strata of WHO-defined BMI categories. Additional adjustment for BMI only marginally changed the risk estimates. Also, the risk estimates for all the strata of BMI remained null for ESCC, EADC, and GNCA. The risk estimates for GCA remained similarly elevated among participants who were in each of the WHO-defined BMI categories. When we considered participants with BMI ≥30 as a single category, we observed the following results: ESCC, HR 0.41, 95% CI 0.10-1.74; EADC, HR 1.08, 95% CI 0.72-1.62; GCA, HR 1.51, 95% CI 0.98-2.33; and GNCA, HR 0.84, 95% CI 0.48-1.47. We found no evidence for effect modification by BMI: for ESCC, pinteraction =0.56; EADC, pinteraction = 0.89; GCA, pinteraction = 0.83; GNCA, pinteraction = 0.53.
Figure 1.
Association between self-reported diabetes and the risk of UGI cancers upon adjustment for and stratification by BMI. The overall risk estimates from the cohort in comparison to risk estimates calculated upon adjustment with BMI and stratification by WHO-defined BMI categories. Potential confounders included in all models were age, sex, caloric intake, alcohol consumption, smoking status, fruit and vegetable intake, ethnicity, education, and two physical activity variables. Models that adjusted for BMI included the categorical BMI variable. We found no evidence for effect modification by BMI: for ESCC, pinteraction =0.56; EADC, pinteraction = 0.89; GCA, pinteraction = 0.83; GNCA, pinteraction = 0.53.*Due to instability, we do not provide estimates for BMI categories with <10 UGI cases per category.
Discussion
We found a significant association between self-reported diabetes and an increased risk of GCA, which was independent of BMI. We saw no clear association between self-reported diabetes and risk of EADC, ESCC, or GNCA.
Few studies have examined the association between diabetes and UGI cancers, and inconsistent associations have been observed. A case-control study of US veterans with GERD found no evidence of an association between diabetes and EADC or GCA (20). Another case-control study of British women found that diabetes was non-significantly associated with an increased risk of EADC (21). However, a case-control study from Australia found a significantly increased risk of EADC associated with diabetes (22). In contrast to the risk estimates in our study that were independent of BMI, the risk estimates observed in both the Australian and British studies were attenuated upon adjusting for BMI (21-22).
While our large prospective study examined the association between diabetes and the histologic subsites of esophageal and stomach cancers, some other studies did not differentiate the subsites. For example, a study that used the Swedish national registries found that patients discharged from the hospital for Type 2 diabetes had increased risks of esophageal and stomach cancers (23) but did not specify the risks for the histologic subsites. In Japan, a case control study found increased risks of esophageal and stomach cancers among diabetic men and women, respectively (24), and a large prospective cohort study found a significantly increased risk of stomach cancer associated with diabetes in women (25), but neither study specified the subsites.
The mechanistic link between diabetes and gastric cancer has been explored in diabetic mice, which have been shown to be more susceptible to chemical induction of gastric carcinogenesis (26). Inflammation has been implicated as an important etiologic factor in both insulin resistance and Type 2 diabetes (27), and chronic inflammation also plays a role in the development of gastric atrophy and cancer (28). Interestingly, over the past two decades, bariatric surgeons have recognized that gastric bypass surgery, particularly total gastrectomy and to a lesser extent gastric binding, causes a durable improvement in Type 2 diabetes; remarkably, the normalization of glucose metabolism occurs within several weeks of surgery, long before any substantial weight loss occurs (29). This amelioration of diabetes has been shown consistently in obese patients (29-31) undergoing gastric bypass surgery and somewhat consistently in patients undergoing surgery for other indications, such as gastric cancer or gastric ulcer disease (32-34) In particular, bypass surgery alters the dietary exposure of the gastric cardia tissue. Therefore, the hormonal changes as a result of gastrectomy in bypass surgery may be implicated in changes in glucose metabolism (35-36).
Diabetes may be in the causal pathway between higher BMI and cancer risk. Therefore, we modeled the association between diabetes and cancer risk with and without adjusting for BMI and also across BMI strata. We found no significant association between diabetes and risk for ESCC, EADC, and GNCA, but a significantly increased risk for GCA. The minimal change in the estimate of the effect of diabetes on GCA risk after adjustment for BMI and the similarity of effect of diabetes in different BMI strata suggest that the effect of diabetes is independent of BMI.
The significant association between diabetes and GCA could reflect reverse causality because undiagnosed cancer may have induced diabetes. To limit this potential problem, we excluded the first two years of follow-up after the completion of the baseline questionnaire. In fact, we performed multiple sub-analyses to examine a potential latency effect, but we found no effect between early and late follow-up (beyond six years of follow-up). In addition, a diagnosis of diabetes may increase vigilance and medical care and subsequently, the diagnosis of cancer. Yet, if this was the issue, we would expect to find an increased risk for the other cancer sites as well.
The significant association found between diabetes and GCA, but not EADC, GNCA, or ESCC, could also reflect a chance finding. However, the etiologies of the UGI cancer histologic subsites have been shown to be different. For example, BMI ≥35 was shown to be significantly associated with increased risks for EADC and GCA, but not for GNCA (37). In addition, infection with Helicobacter pylori (H pylori), but not gastric atrophy, is associated with a reduced risk of EADC, whereas the opposite seems to be true for GCA, and both infection and gastric atrophy may be associated with increased risk of ESCC (38-40).
This study has many strengths. The prospective design avoided exposure recall bias and included a very large sample size, over 450,000 participants, almost 9% of whom had self-reported diabetes. The baseline questionnaire assessed many potential confounding factors; however, unmeasured residual confounding may still be a possibility. An additional strength of this study was the ability to examine site-specific UGI cancers separately.
This study also had some limitations. Self-reported diabetes may be biased because participants may not be aware of their disease status. Nonetheless, self-reported diabetes is a robust exposure measure; recent studies in the US on the concordance between patient self-report of diabetes and medical record audits have found excellent agreement of greater than 92% and kappa scores of greater than 0.80 (41-42). While the questionnaire used in this study did not specify Type 1 or Type 2 diabetes, we expected that the majority of self-reported diabetes were Type 2; according to the National Institute of Diabetes and Digestive and Kidney Diseases, 90-95% of diabetes in the US are Type 2 (43). Nonetheless, a previous cohort study of patients hospitalized for Type 1 diabetes found an elevated risk of stomach cancer (44). Our study used self-reported diabetes assessed at baseline and did not have follow-up information regarding the diabetes status of those who were non-diabetic at baseline. If non-diabetics developed diabetes after the baseline assessment, the potential misclassification may bias the results toward the null.
We were limited by the information provided by the NIH-AARP Diet and Health Study baseline questionnaire. Therefore, we could not consider alternative measurements of obesity such as waist-to-hip ratio. We did not have information on H pylori infection status, which may have been important because H pylori gastritis has been suggested to enhance glucose-stimulated insulin release by increasing gastrin secretion (45). Furthermore, we did not have information on the use of antidiabetic drugs. Unfortunately, we did not have information on the duration of diabetes, which may be important as a previous Australian case-control study found an increased risk of esophageal adenocarcinoma among those who had a history of diabetes for ≥10 years (22).
We examined the effect of some potential confounders for which we had information for only a subset of the cohort. For example, GERD may play a role in the development of cancers of the gastroesophageal junction, but we did not have information on GERD or the use of anti reflux therapies in these subjects. We did, however, have information on the use of antacids for a subset of the cohort, and in a sub-analysis, we observed similar results upon adding antacid use as a potential confounder. Similarly, the addition of aspirin and non-steroidal anti-inflammatory drug (NSAID) use did not alter the results. We also explored the effect of smoking duration on the risk estimates and found that its addition in the models did not alter our results.
To our knowledge, this is the largest prospective study of the association between diabetes and UGI cancers with information on important confounders, such as smoking and physical activity. We found no association between self-reported diabetes and risk of EADC, ESCC, or GNCA. However, we did find a significant association between self-reported diabetes and an increased risk of GCA. Our results suggest that the metabolic and hormonal changes related to diabetes may play a role in the etiology of GCA independently from BMI.
Acknowledgments
Funding
This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Footnotes
Disclosures
No conflicts of interest exist.
References
- 1.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 2.Mitri J, Castillo J, Pittas AG. Diabetes and risk of Non-Hodgkin's lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008;31:2391–7. doi: 10.2337/dc08-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 5.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–74. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Larsson SC, Andersson SO, Johansson JE, Wolk A. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur J Cancer. 2008;44:2655–60. doi: 10.1016/j.ejca.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasper JS, Giovannucci E. A Meta-analysis of Diabetes Mellitus and the Risk of Prostate Cancer. Cancer Epidemiology Biomarkers & Prevention. 2006;15:2056–62. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 10.Renehan A, Smith U, Kirkman MS. Linking diabetes and cancer: a consensus on complexity. Lancet. 2010;375:2201–2. doi: 10.1016/S0140-6736(10)60706-4. [DOI] [PubMed] [Google Scholar]
- 11.Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998–2003. International Journal of Cancer. 2008;123:1422–8. doi: 10.1002/ijc.23691. [DOI] [PubMed] [Google Scholar]
- 12.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–9. [PubMed] [Google Scholar]
- 13.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–53. [PubMed] [Google Scholar]
- 14.Devesa SS, Fraumeni JF., Jr The rising incidence of gastric cardia cancer. J Natl Cancer Inst. 1999;91:747–9. doi: 10.1093/jnci/91.9.747. [DOI] [PubMed] [Google Scholar]
- 15.Corley DA, Kubo A. Influence of Site Classification on Cancer Incidence Rates: An Analysis of Gastric Cardia Carcinomas. J Natl Cancer Inst. 2004;96:1383–7. doi: 10.1093/jnci/djh265. [DOI] [PubMed] [Google Scholar]
- 16.Anderson WF, Camargo MC, Fraumeni JF, Jr., Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–8. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 18.Chow W-H, Fraumeni JF, Jr., Blot WJ, Vaughan TL, Stanford JL, Farrow DC, et al. Body Mass Index and Risk of Adenocarcinomas of the Esophagus and Gastric Cardia. J Natl Cancer Inst. 1998;90:150–5. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 19.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and Serendipity in Establishing a Large Cohort with Wide Dietary Intake Distributions : The National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 20.Rubenstein JH, Davis J, Marrero JA, Inadomi JM. Relationship between diabetes mellitus and adenocarcinoma of the oesophagus and gastric cardia. Aliment Pharmacol Ther. 2005;22:267–71. doi: 10.1111/j.1365-2036.2005.02544.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheng KK, Sharp L, McKinney PA, Logan RF, Chilvers CE, Cook-Mozaffari P, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000;83:127–32. doi: 10.1054/bjoc.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neale RE, Doecke JD, Pandeya N, Sadeghi S, Green AC, Webb PM, et al. Does type 2 diabetes influence the risk of oesophageal adenocarcinoma? Br J Cancer. 2009;100:795–8. doi: 10.1038/sj.bjc.6604908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist. 2010;15:548–55. doi: 10.1634/theoncologist.2009-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. European Journal of Cancer Prevention. 2007;16:83–9. doi: 10.1097/01.cej.0000228404.37858.40. 10.1097/01.cej.0000228404.37858.40. [DOI] [PubMed] [Google Scholar]
- 25.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S, et al. Diabetes Mellitus and the Risk of Cancer: Results From a Large-Scale Population-Based Cohort Study in Japan. Arch Intern Med. 2006;166:1871–7. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa N, Yamaguchi H, Yamamoto M, Shimizu N, Furihata C, Tatematsu M, et al. Gastric carcinogenesis by N-Methyl-N-Nitrosourea is enhanced in db/db diabetic mice. Cancer Science. 2009;100:1180–5. doi: 10.1111/j.1349-7006.2009.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: ‘cause or effect’? Curr Diabetes Rev. 2006;2:195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- 28.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and Type 2 Diabetes after Bariatric Surgery: Systematic Review and Meta-analysis. The American Journal of Medicine. 2009;122:248–56. doi: 10.1016/j.amjmed.2008.09.041. e5. [DOI] [PubMed] [Google Scholar]
- 30.Herron DM, Tong W. Role of surgery in management of type 2 diabetes mellitus. Mt Sinai J Med. 2009;76:281–93. doi: 10.1002/msj.20114. [DOI] [PubMed] [Google Scholar]
- 31.Sultan S, Gupta D, Parikh M, Youn H, Kurian M, Fielding G, et al. Five-year outcomes of patients with type 2 diabetes who underwent laparoscopic adjustable gastric banding. Surgery for Obesity and Related Diseases. 2010;6:373–6. doi: 10.1016/j.soard.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 32.Herbella FA, Tineli AC, Wilson JL, Jr, Del Grande JC. Duodenal bypass does not decrease glucose levels of lean individuals with gastric cancer submitted to partial or total gastrectomy. Arquivos de Gastroenterologia. 2009;46:230–2. doi: 10.1590/s0004-28032009000300017. [DOI] [PubMed] [Google Scholar]
- 33.Lanzarini E, Csendes A, Lembach H, Molina J, Gutiérrez L, Silva J. Evolution of Type 2 Diabetes Mellitus in Non Morbid Obese Gastrectomized Patients with Roux en-Y Reconstruction: Retrospective Study. World Journal of Surgery. 2010 doi: 10.1007/s00268-010-0640-z. [DOI] [PubMed] [Google Scholar]
- 34.Zervos EE, Agle SC, Warren AJ, Lang CG, Fitzgerald TL, Dar M, et al. Amelioration of Insulin Requirement in Patients Undergoing Duodenal Bypass for Reasons Other than Obesity Implicates Foregut Factors in the Pathophysiology of Type II Diabetes. Journal of the American College of Surgeons. 2010;210:564–72. doi: 10.1016/j.jamcollsurg.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. discussion -5. [PubMed] [Google Scholar]
- 36.Saliba J, Wattacheril J, Abumrad NN. Endocrine and metabolic response to gastric bypass. Curr Opin Clin Nutr Metab Care. 2009;12:515–21. doi: 10.1097/MCO.0b013e32832e1b14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abnet CC, Freedman ND, Hollenbeck AR, Fraumeni JF, Jr., Leitzmann M, Schatzkin A. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465–71. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiteman DC, Parmar P, Fahey P, Moore SP, Stark M, Zhao ZZ, et al. Association of Helicobacter pylori Infection With Reduced Risk for Esophageal Cancer Is Independent of Environmental and Genetic Modifiers. Gastroenterology. 2010;139:73–83. doi: 10.1053/j.gastro.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, et al. Opposing Risks of Gastric Cardia and Noncardia Gastric Adenocarcinomas Associated With Helicobacter pylori Seropositivity. J Natl Cancer Inst. 2006;98:1445–52. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 40.Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, et al. Helicobacter pylori Infection and Gastric Atrophy: Risk of Adenocarcinoma and Squamous-Cell Carcinoma of the Esophagus and Adenocarcinoma of the Gastric Cardia. J Natl Cancer Inst. 2004;96:388–96. doi: 10.1093/jnci/djh057. [DOI] [PubMed] [Google Scholar]
- 41.Tisnado DM, Adams JL, Liu H, Damberg CL, Chen WP, Hu FA, et al. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44:132–40. doi: 10.1097/01.mlr.0000196952.15921.bf. [DOI] [PubMed] [Google Scholar]
- 42.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: experience from the Veterans Health Study. J Ambul Care Manage. 2005;28:102–10. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 43.National Diabetes Statistics, 2007 fact sheet. National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 2008. [Google Scholar]
- 44.Zendehdel K, Nyrén O, Östenson C-G, Adami H-O, Ekbom A, Ye W. Cancer Incidence in Patients With Type 1 Diabetes Mellitus: A Population-Based Cohort Study in Sweden. Journal of the National Cancer Institute. 2003;95:1797–800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 45.Acbay O, Celik AF, Gundogdu S. Does Helicobacter pylori-induced gastritis enhance food-stimulated insulin release? Dig Dis Sci. 1996;41:1327–31. doi: 10.1007/BF02088555. [DOI] [PubMed] [Google Scholar]