Abstract
Antibiotic-resistant international clones of Streptococcus pneumoniae are increasingly reported in different parts of the world. We investigated the spread of these clones through an active surveillance performed at the Israeli Streptococcal National Center during 1998 and 1999. Isolates were tested for antibiotic susceptibility, serotyped, and genotyped by random amplified polymorphic DNA analysis and pulsed-field gel electrophoresis. Of 437 isolates, 276 (63.4%) were antibiotic resistant and 156 (35%) were penicillin nonsusceptible (PNS). The PNS isolates were less frequently encountered in southern Israel (27 of 136 [20%]) than in other regions (127 of 301 [42%]). Among 276 antibiotic-resistant isolates, 43 fingerprint patterns were observed. The most common clones were 9V/14-a (19.2%), 5-a (17.8%), and 1-a (10%). The 9V/14-a clone was less common, while the 1-a clone was more frequent in the south than in other regions. The 5-a clone was more common in Jerusalem than in other regions. Among the Jewish and Arab populations the most frequent clones were 9V/14-a (20%) and 1-a (25%), respectively. Three international clones, 9V/14-a-Spain9V-3, 6B-a-Spain6B-2, and 5-a-Colombia5-19, comprised 40% of all antibiotic-resistant isolates and 56% of all PNS isolates. The seven-valent conjugate vaccine covers 58% of the most common clones, all highly PNS clones, and 94% of the multidrug-resistant clones in Israel, while the nine-valent vaccine covers all of them. The most common antibiotic-resistant invasive pediatric S. pneumoniae clones—mainly the three international ones—contribute significantly to increases in antibiotic resistance. Their geographic distribution varies within the country and between the different populations.
Streptococcus pneumoniae is the most common cause of acute otitis media, pneumonia, bacteremia, and bacterial meningitis in children (18). It is estimated that on an annual basis worldwide this organism causes at least 1 million deaths among children younger than 5 years of age (9, 10, 18). The only national survey of invasive S. pneumoniae infections in Israel was reported in 1992 (3). The annual reported incidence was 42 per 100,000 for children younger than 5 years of age and 104 per 100,000 for those <12 months old. The study also showed that different population groups showed various frequencies of infection with S. pneumoniae: the non-Jewish population was more susceptible to infections with S. pneumoniae than the Jewish population (3, 12).
Treatment of invasive disease caused by penicillin-nonsusceptible S. pneumoniae (PNSP) has become a challenge; and reports of treatment failures, especially of invasive multidrug-resistant (MDR) S. pneumoniae infections like meningitis, have increased in Israel (28). In southern Israel the rate of penicillin resistance among invasive pediatric S. pneumoniae isolates was recently reported to be 22%, 51% of which were resistant to one or more antibiotics and 7% were MDR (12).
Phenotypic and genotypic methods have been developed to assist in epidemiological investigations. These methods enable recognition of clusters of genetically identical or closely related isolates both within and between serotypes, facilitating more accurate epidemiological investigations (15). The use of molecular typing has enabled the identification of the international clonal spread of penicillin-resistant as well as multiantibiotic-resistant clones. The 16 most prevalent S. pneumoniae international clones were defined by an international committee and are available for epidemiological studies (17). The random amplified polymorphic DNA (RAPD) method has been used previously to investigate the epidemiology of S. pneumoniae in other countries (14). In addition, the pulsed-field gel electrophoresis (PFGE) method is commonly used for molecular typing (30). The use of more than one molecular typing method for molecular epidemiological studies is well accepted (17). Herein we present the findings from a study in which we used both methods for molecular typing of invasive S. pneumoniae isolates from children younger than 13 years of age in Israel between 1998 and 1999.
The aims of the present study were to determine (i) the antibiotic resistance rate among S. pneumoniae isolates causing invasive pediatric infections in Israel, (ii) the most prevalent antibiotic-resistant invasive pediatric S. pneumoniae clones and their distributions among different geographic and ethnic populations in Israel, and (iii) the prevalence of known international clones among invasive pediatric S. pneumoniae isolates in Israel.
MATERIALS AND METHODS
Study design.
This prospective study was conducted between 1998 and 1999. Sixteen representative centers serving more than two-thirds of all pediatric patients in Israel routinely send all S. pneumoniae isolates to the Central Streptococcal Reference Laboratory of the Ministry of Health (CSRL-MOH), located in Jerusalem.
Case definition.
Blood or cerebrospinal fluid from patients younger than 13 years of age from whom S. pneumoniae was isolated was sent to CSRL-MOH.
Microbiology evaluation.
All organisms obtained from CSRL-MOH were reidentified at the Clinical Microbiology Laboratory of the Soroka University Medical Center and were serotyped at the Pediatric Infectious Disease Unit Research Laboratories of the Soroka University Medical Center.
S. pneumoniae isolates were grown on Trypticase soy agar plates with 5% sheep blood and incubated at 35°C in a 5% CO2-enriched atmosphere with tension. Isolates were identified as S. pneumoniae by their colonial morphology, inhibition by optochin, and a positive result by the slide agglutination test (Phadebact; Pharmacia Diagnostics, Uppsala, Sweden).
Testing of the susceptibilities of the isolates to oxacillin, erythromycin, clindamycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole (SXT) was performed by the disk diffusion method of Bauer and Kirby, following National Committee for Clinical Laboratory Standards recommendations (19). Isolates exhibiting a zone of inhibition with a radius ≤19 mm around a 1-μg oxacillin disk were further tested for susceptibility to penicillin by E-test (PDM, Epsilometer; AB Biodisk, Solna, Sweden) following the instructions of the manufacturer (16). MDR was defined as resistance to three or more antibiotic drug classes (SXT was considered one drug class).
Serogrouping and serotyping were performed on the basis of capsular swelling (Quellung reaction) (1) with antisera provided by Statens Seruminstitut, Copenhagen, Denmark.
Molecular typing methods.
All isolates were initially analyzed by RAPD analysis, and clones were determined. All unique strains and representative isolates of each clone were further analyzed by PFGE.
Primer 213 (5′ to 3′ sequence, CAGCGAACTA) was used for RAPD analysis. Each reaction mixture (25 μl) contained 15 ng of genomic DNA, and the PCR was performed as described previously (14). For PFGE analysis, genomic DNA was prepared in situ in agarose blocks and was digested with SmaI (30). PFGE was carried out with a contour-clamped homogeneous electric field apparatus (CHEF DR-III; Bio-Rad). The following parameters were used for electrophoresis: run time at 5.3 V/cm, 23 h; initial pulse time, 5 s; and final time, 35 s. All isolates with RAPD and PFGE banding patterns which appeared to be similar visually were run again, side by side on the same gel, to confirm their identities and were judged once more visually.
The clones were marked by the serotype and by the letters a, b, and c, for example, 23F-a and 23F-b. In the case of switch clones, the two serotypes were included; for example, clone 9V/14-a included serotypes 9V and 14 and the “a” represents the first clone. In addition, if a clone was identified as an international clone, the international nomenclature was used.
Statistical analysis.
The Epi-Info 2000 statistical package was used to test the differences in proportions (by the chi-square test or Fisher's exact test, as appropriate). A P value <0.05 was considered significant.
RESULTS
A total of 437 invasive pediatric S. pneumoniae isolates were sent to CSRL-MOH between 1 January 1998 and 31 December 1999. Resistance to one or more antibiotic classes was found in 276 (63.4%) of all isolates, and MDR was found in 66 (15%) of all isolates.
The isolates were mostly nonsusceptible to SXT (51%), penicillin (35%), and erythromycin (10%). Resistance to tetracycline, clindamycin, and chloramphenicol was found in 11, 5, and 2% of the isolates, respectively. Of the 154 isolates that were nonsusceptible to penicillin, 97 of 154 (63%) were intermediately susceptible (MICs, >0.1 but <2.0 μg/ml) and 37 of 154 (37%) were highly penicillin resistant (MICs, ≥2.0 μg/ml). These two groups comprised 22 and 13% of all S. pneumoniae isolates, respectively. Of all isolates, 390 (89.8%) were susceptible to ceftriaxone, the ceftriaxone MIC was 1.0 μg/ml for 46 (10%) of the isolates, and the ceftriaxone MIC was 2.0 μg/ml for only 1 (0.2%) isolate.
A significant difference (P < 0.001) in the proportion of penicillin-nonsusceptible isolates was found between southern Israel (27 of 136 [20%] isolates) and the rest of the country (18 of 37 [49%], 60 of 131 [46%], and 49 of 133 [37%] isolates in northern Israel, the Tel Aviv area, and the Jerusalem area, respectively).
Nineteen different serotypes were identified among the antibiotic-resistant isolates. The most common serotypes were 14 (18%), 5 (18%), 1 (11%), and 23F (11%). One hundred thirty-nine (50%) of all antibiotic-resistant isolates are covered by the recently licensed seven-valent conjugated vaccine, and 218 (79%) are covered by the candidate nine-valent vaccine. Serotypes 3 and 7F (to be included in the future 11-valent pneumococcal vaccine) were not found among the antibiotic-resistant isolates. One hundred eighteen (77%) and 121 (79%) of the 154 penicillin-nonsusceptible serotypes are covered by the seven- and nine-valent vaccines, respectively; 62 (94%) of the 66 MDR isolates are covered by both vaccines; and 28 (18%) isolates are of serotypes immunologically related to the serotypes covered by the vaccine (i.e., serotypes 6A and 19A).
Among the 276 antibiotic-resistant S. pneumoniae isolates, 43 fingerprint patterns were observed. The most common clones were 9V/14-a (19.2%), 5-a (17.8%), 1-a (10.9%), 19A-a, 19F-a, 6B-a, and two clones of serotype 23F, clones a and b (Table 1).
TABLE 1.
The more prevalent antibiotic-resistant invasive pediatric S. pneumoniae clones in Israel, 1998 and 1999
| Serotype | Clone | No. of isolates | % of all antibiotic- resistant isolates (n = 276) | Antibiotic to which resistance was detecteda |
|---|---|---|---|---|
| 9V/14 | 9V/14-a | 53 | 19.2 | PEN, SXT |
| 5 | 5-a | 49 | 17.8 | SXT |
| 1 | 1-a | 30 | 10.9 | SXT |
| 23F | 23F-a | 16 | 5.8 | PEN, SXT |
| 23F | 23F-b | 14 | 5 | PEN, SXT |
| 19A | 19A-a | 9 | 3.3 | PEN, SXT |
| 19F | 19F-a | 9 | 3.3 | PEN, ERY, SXT, TET |
| 6B | 6B-a | 9 | 3.3 | PEN, CLI, ERY, TET, SXT |
| Total | 189 | 68.5 |
PEN, penicillin; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; ERY, erythromycin; CLI, clindamycin.
The eight most common clones each represented more than 3% of all antibiotic-resistant isolates and together constituted 68.5% of all isolates. All these clones were SXT resistant, and two clones (1-a and 5-a) were resistant to this drug only (Table 1). Six clones were mostly penicillin resistant (25% of all 437 isolates). The most common penicillin-resistant clone was 9V/14-a, which accounted for 12% of all isolates. Two clones, 19F-a and 6B-a, were MDR and accounted for 6.5% of all antibiotic-resistant isolates.
One hundred ten isolates belonging to the eight most common clones are covered by the seven-valent conjugated vaccine and constituted 25% of all isolates, 40% of all antibiotic-resistant isolates, and 71% of all PNSP isolates. Ten clones are covered by the nine-valent conjugated vaccine and represented 43% of all isolates and 68% of all antibiotic-resistant isolates (Table 1).
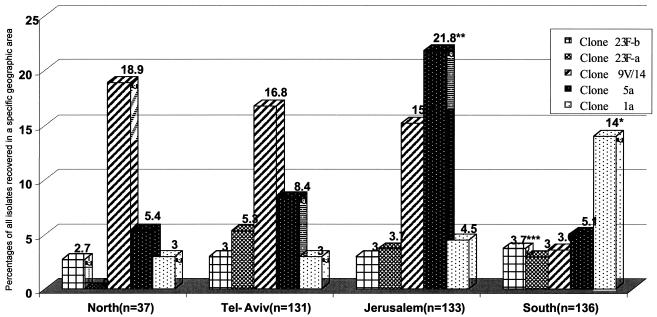
The geographic distributions of the various clones showed geographic clustering. The 9V/14-a clone was more prevalent in the northern part of the country and in the Tel Aviv and Jerusalem areas, but it was significantly less common in the southern part of the country. In contrast, the 1-a clone was significantly more prevalent in the south than in the other areas. The 5-a clone was the most prevalent in the Jerusalem area and occurred significantly more frequently there than in any other region. The geographic distributions of both serotype 23F-a and 23F-b clones were similar throughout the country (Fig. 1).
FIG. 1.
Distributions of the most common invasive clones of S. pneumoniae by geographic region in Israel. Percentages were calculated from the total number of S. pneumoniae strains isolated in the region. *, P < 0.01 between the south and the other regions; **, P < 0.01 between Jerusalem and the other regions; ***, P < 0.01 between the south and the other regions.
The ethnic origins of the patients from whom the isolates were obtained were known for 251 (91%) antibiotic-resistant isolates. In the Jewish population (171 [68%] isolates) the most common clones were 9V/14-a (20%) and 5-a (10%), while in the Arab population (80 [32%] isolates) the most common clones were 1-a (25%) and 5-a (13%).
Three international antibiotic-resistant clones were identified among the isolates: (i) the 9V/14-a clone, known as the Spain9V-3 international clone; (ii) the 6B-a clone, which was found to be identical to the Spain6B-2 international clone; and (iii) the 5-a clone, which was identified as the Colombia5-19 clone. The 9V/14-a (Spain9V-3) clone comprised 12% of all isolates, 19% of the antibiotic-resistant isolates, and 25% of all penicillin-nonsusceptible isolates. In our study, the 6B-a (Spain6B-2) clone, which was an MDR clone, constituted 2% of all isolates and 3% of all resistant isolates. The 5-a (Colombia5-19) clone comprised 17.8% of all antibiotic-resistant isolates, but this clone was penicillin susceptible. The three clones together comprised 40.3% of all antibiotic-resistant isolates and 56% of all penicillin-resistant isolates. None of the other invasive clones resembled any of the antibiotic-resistant international clones listed above.
DISCUSSION
Antibiotic-resistant S. pneumoniae is common worldwide (20, 23, 26, 31, 32) and is related to a relatively limited number of serotypes (6, 7, 32). This study documents the prevalence of antibiotic resistance among invasive S. pneumoniae isolates from children younger than 13 years of age in Israel. Resistance to at least one antibiotic class was seen in 63% of the isolates tested; 35% were PNSP, and 15% were MDR.
Previous studies from southern Israel have reported penicillin nonsusceptibility rates of between 22 and 36% and MDR rates up to 7% among invasive pediatric isolates (12). These rates are similar to those found in the present study among isolates from southern Israel, which were significantly lower than those among isolates from the rest of the country. The rates of PNSP in Israel may be considered intermediate compared with those in other regions in the world (11), since worldwide rates range from a low of 3% in Sweden (8) to a high of 76% in Taiwan (25). However, the rates in Israel are comparable to those in the United States, where the average proportion of PNSP isolates among invasive pediatric S. pneumoniae isolates is 20 to 44% (32). Marked differences in antibiotic resistance patterns were found among the regions, with an especially low prevalence of PNSP in the southern part of the country. However, this difference was due to the high prevalence of serotypes 1 and 5 in southern Israel, which were susceptible to penicillin.
In the present study the most common invasive PNSP serotypes were 9V, 14, 23F, 19F, 19A, and 6B. These serotypes have previously been reported to be the most prevalent antibiotic-resistant serotypes causing invasive infections in children in southern Israel (4, 12). Five of the six serotypes are covered by the seven-valent pneumococcal conjugated vaccine, and one (serotype 19A) is antigenically related to serotype 19F, which is covered by the seven-valent vaccine (24).
Molecular typing methods have demonstrated that a limited number of international clones constitute a large proportion of antibiotic-resistant S. pneumoniae isolates, but local clones are also prevalent (14, 17, 21, 23, 32). Three major clones, clones 9V/14-a, 1-a, and 5-a, accounted for 47% of all isolates. The 9V/14-a clone expressed resistance to penicillin and SXT. The Israeli 9V/14-a clone was found by PFGE analysis to be identical to the Spanish-French 9V/14 clone known as the Spain9V-3 international clone (17). This clone is the most common antibiotic-resistant clone among clones of invasive pediatric S. pneumoniae isolates. It constituted 34.4% of all PNSP isolates in our study and was the most predominant antibiotic-resistant clone mainly in the north of the country.
Serotypes 1 and 5 were previously reported (3, 12, 22) to be the most common invasive pediatric serotypes in Israel, especially in the southern part of the country, and were reported to be related to unique clones. The 5-a clone was the most predominant clone in the Jerusalem region and the second most common clone in other parts of the country. The 5-a clone was found to be identical to the Columbia5-19 clone (13, 22, 29). This clone is not included among the 16 most common international clones of S. pneumoniae (17). In contrast to the Israeli isolates of the 5-a clone, the Columbia5-19 clone expresses resistance to chloramphenicol and tetracycline, but like its Israeli counterpart, it is also susceptible to penicillin (13, 22, 29).
The 1-a clone is significantly more common in the southern part of Israel than in all other regions. It had previously been shown (12) that serotype 1 is significantly more common in the Bedouin population in that region and is associated with low socioeconomic levels and overcrowding. This clone was associated with an outbreak in a close community in the south of the country (5). The 1-a clone is unique and endemic in our country; the different rates of this clone in different regions of the country might be related to the different characteristics of the populations in the various regions.
The most common clone among the Jewish population was 9V/14-a. This clone was common in the Tel Aviv and Jerusalem areas. The proportion of children <14 years old varies throughout the country. The proportion of Jewish children is significantly higher in Tel Aviv (92%) and Jerusalem (75%) than in the rest of the country (27). In the Arab population the most common clone was 1-a, which was mainly found in the southern part of the country (14% of all isolates). Different minorities live in the northern part of the country, such as Druses and Christian Arabs, while the Bedouins are the predominant minority in the south (27). These demographic differences might explain the different distributions of the drug-resistant clones in the country. The number of isolates from the northern part of the country was relatively small (n = 37), and thus, comparisons between the different populations within the region are difficult.
In this study, we were able to identify three major antibiotic-resistant clones with different epidemiological backgrounds that have spread throughout Israel. Of the most common PNSP clones, two, clones Spain9V-3 and the Spain6B-2, are recognized as international clones. Of the two main penicillin-susceptible antibiotic-resistant clones, the 5-a (Colombia5-19) clone is also an international clone, while the 1-a clone is endemic in Israel, mainly in the southern part of the country, although it also spreads to other parts of the country. The different distributions of the international clones might be related to socioeconomic differences between different parts of the country and different populations; for example, the Spain9V-3 clone was found to be more common in the center of the country, where most of the population is Jewish, while the Colombia5-19 clone was distributed equally among Jews and Arabs throughout the country. Further epidemiological studies should investigate the spread of these clones within the country and, potentially, to other parts of the world.
The seven-valent pneumococcal conjugate vaccine used at present covers only 58% of the most common antibiotic-resistant invasive clones in Israel, while the nine-valent vaccine covers all of them. However, the seven-valent vaccine covers all of the highly penicillin-resistant S. pneumoniae isolates and 94% of all MDR isolates. These data should influence future decision making on vaccine usage in Israel and can be used to monitor the epidemiological changes that may occur after implementation of a vaccination program.
The strength of this study lies in the fact that all available cases were encountered in 16 medical centers (which serve more than two-thirds of all pediatric patients in Israel). The limitations are that these findings were collected only over a period of 2 years; thus, changes in antibiotic resistance patterns and clonal distributions over time could not be detected.
Knowledge of the clonal distributions of PNSP and MDR S. pneumoniae isolates within Israel will contribute to understanding the risk factors associated with their occurrence and spread. Identification of factors such as geographic location and the subpopulations at increased risk will help in the formulation of strategies aimed at preventing the spread of these organisms. Moreover, this database will contribute to the detection of changes following the future use of conjugate vaccines in Israel, such as genetic changes (i.e., capsular switches) among invasive S. pneumoniae isolates or the emergence of new local or international clones (2). In addition, these data will be used to develop a national database of PNSP and MDR S. pneumoniae isolates and will encourage continued collaboration with international groups with the goal of setting up an international database to detect the spread of these clones. They will also help decision makers implement newly developed vaccination strategies aimed at preventing invasive S. pneumoniae infections in children in the future.
Acknowledgments
This study was funded by the Office of Israel's Chief Scientist (grant 80563201).
REFERENCES
- 1.Austrian, R. 1976. The Quellung reaction, a neglected microbiologic technique. Mt. Sinai J. Med. 43:699-709. [PubMed] [Google Scholar]
- 2.Beall, B., M. C. McEllistrem, R. E. Gertz, Jr., D. J. Boxrud, J. M. Besser, L. H. Harrison, J. H. Jorgensen, C. G. Whitney, and Active Bacterial Core Surveillance/Emerging Infections Program Network. 2002. Emergence of a novel penicillin-nonsusceptible, invasive serotype 35B clone of Streptococcus pneumoniae within the United States. J. Infect. Dis. 186:118-122. [DOI] [PubMed] [Google Scholar]
- 3.Dagan, R., D. Engelhard, E. Piccard, et al. 1992. Epidemiology of invasive childhood pneumococcal infections in Israel. JAMA 268:3328-3332. [PubMed] [Google Scholar]
- 4.Dagan, R., P. Yagupsky, A. Goldbart, A. Wasas, and K. Klugman. 1994. Increasing prevalence of penicillin-resistant pneumococcal infections in children in southern Israel: implications for future immunization policies. Pediatr. Infect. Dis. J. 13:782-786. [DOI] [PubMed] [Google Scholar]
- 5.Dagan, R., S. Gradstein, I. Belmaker, N. Porat, Y. Siton, G. Weber, J. Janco, and P. Yagupsky. 2000. An outbreak of Streptococcus pneumoniae serotype 1 in a closed community in southern Israel. Clin. Infect. Dis. 30:319-321. [DOI] [PubMed] [Google Scholar]
- 6.Di Fabio, J. L., E. Castaneda, C. I. Agudelo, F. De La Hoz, M. Hortal, T. Camou, G. Echaniz-Aviles, M. Noemi, C. Barajas, I. Heitmann, J. C. Hormazabal, M. C. Brandileone, V. S. Dias Vieiria, M. Regueira, R. Ruvinski, A. Corso, M. Lovgren, J. A. Talbot, C. De Quadros, et al. 2001. Evolution of Streptococcus pneumoniae serotypes and penicillin susceptibility in Latin America, Sireva-Vigia Group, 1933 to 1999. Pediatr. Infect. Dis. J. 120:959-967. [DOI] [PubMed] [Google Scholar]
- 7.Doit, C., C. Loukil, P. Geslin, and E. Bingen. 2002. Phenotypic and genetic diversity of invasive pneumococcal isolates recovered from French children. J. Clin. Microbiol. 8:2994-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson, M., B. Henriques, and K. Ekdahl. 2000. Epidemiology of pneumococcal infections in Swedish children. Acta Paediatr. Suppl. 89:35-39. [DOI] [PubMed] [Google Scholar]
- 9.Eskola, J., and M. Anttila. 1999. Pneumococcal conjugate vaccines. Pediatr. Infect. Dis. J. 18:543-551. [DOI] [PubMed] [Google Scholar]
- 10.Facklam, R. R., and R. F. Breiman. 1991. Current trends in bacterial respiratory pathogens. Am. J. Med. 91(Suppl. 6A):3S-11S. [DOI] [PubMed] [Google Scholar]
- 11.Fenoll, A., I. Jado, D. Vicioso, A. Perez, and J. Casal. 1998. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996). J. Clin. Microbiol. 36:3447-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, D., N. Givon-Lavi, N. Bilenko, and R. Dagan. 2001. A decade (1989-1998) of pediatric invasive pneumococcal disease in two populations residing in one geographic location: implications for vaccine choice. Clin. Infect. Dis. 33:421-427. [DOI] [PubMed] [Google Scholar]
- 13.Gamboa, L., T. Camou, M. Hortal, E. Castañeda, and the Sireva-Vigía Working Group. 2002. Dissemination of Streptococcus pneumoniae clone Columbia5-19 in Latin America. J. Clin. Microbiol. 40:3942-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg, D., D. P. Speert, E. Mahenthiralingam, D. A. Henry, M. E. Campbell, D. W. Scheifele, and The CPS/LCDC IMPACT Monitoring Network. 2002. Emergence of penicillin-nonsusceptible Streptococcus pneumoniae invasive clones in Canada. J. Clin. Microbiol. 40:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, L. M. C. 1998. Application of molecular typing to the epidemiology of Streptococcus pneumoniae. J. Clin. Pathol. 51:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs, M. R., S. Bajaksouzian, P. C. Appelbaum, and A. Bolstrom. 1992. Evaluation of the E-test for susceptibility testing of pneumococci. Diagn. Microbiol. Infect. Dis. 15:473-478. [DOI] [PubMed] [Google Scholar]
- 17.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-809. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial susceptibility test: 9th informational supplement. NCCLS document M100-S9, vol. 19, no. 1 National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Overweg, K., P. W. Hermans, K. Trzcinski, M. Sluijter, R. de Groot, and W. Hryniewicz. 1999. Multidrug-resistant Streptococcus pneumoniae in Poland: identification of emerging clones. J. Clin. Microbiol. 37:1739-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parry, C. M., N. M. Duong, J. Zhou, N. T. Mai, T. S. Diep, Q. Thinh Le, J. Wain, N. Van Vinh Chau, D. Griffiths, N. P. Day, N. J. White, T. T. Hien, B. G. Spratt, and J. J. Farrar. 2002. Emergence in Vietnam of Streptococcus pneumoniae resistant to multiple antimicrobial agents as a result of dissemination of the multiresistant Spain (23F)-1 clone. Antimicrob. Agents Chemother. 46:3512-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porat, N., R. Trefler, and R. Dagan. 2001. Persistence of two invasive Streptococcus pneumoniae clones of serotypes 1 and 5 in comparison to that of multiple clones of serotypes 6B and 23F among children in southern Israel. J. Clin. Microbiol. 39:1827-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setchanova, L., and A. Tomasz. 1999. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates from Bulgaria. J. Clin. Microbiol. 37:638-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinefield, H. R., S. Black, P. Ray, I. Chang, N. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. Siber, R. Kohberger, D. V. Madore, F. J. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757-763. [DOI] [PubMed] [Google Scholar]
- 25.Siu, L. K., M. L. Chu, M. Ho, Y. S. Lee, and C. C. Wang. 2002. Epidemiology of invasive pneumococcal infection in Taiwan: antibiotic resistant, serogroup distribution, and ribotypes analyses. Microb. Drug Resist. 8:201-208. [DOI] [PubMed] [Google Scholar]
- 26.Song, J. H., J. W. Yang, J. H. Jin, S. W. Kim, C. K. Kim, H. Lee, K. R. Peck, S. Kim, N. Y. Lee, M. R. Jacobs, P. C. Appelbaum, and The Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. 2000. Molecular characterization of multidrug-resistant Streptococcus pneumoniae isolates in Korea. J. Clin. Microbiol. 38:1641-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The State of Israel. 2002. Statistical abstract of Israel, no. 53, p. 2-28-2-32. Central Bureau of Statistics, Jerusalem, Israel.
- 28.Steinberg, T., A. Rachmel, Z. Samra, and S. Ashkenazi. 1997. Penicillin-resistant pneumococcal meningitis in Israel. Isr. J. Med. Sci. 33:757-759. [PubMed] [Google Scholar]
- 29.Tamayo, M., R. Sa-Leao, I. Santos Sanches, E. Castaneda, and H. de Lencastre. 1999. Dissemination of a chloramphenicol- and tetracycline-resistant but penicillin-susceptible invasive clone of serotype 5 Streptococcus pneumoniae in Colombia. J. Clin. Microbiol. 37:2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasz, A., A. Coraso, Members of the PAHO/Rockefeller University Workshop, G. Echaniz-Aviles, M. C. D. C. Brandileone, T. Cmou, E. Castaneda, O. Figueroa, A. Rossi, and E. P. Severina. 1998. Molecular epidemiological characterization of penicillin-resistant Streptococcus pneumoniae invasive pediatric isolates recovered in six South American countries. Microb. Drug Resist. 4:195-207. [DOI] [PubMed] [Google Scholar]
- 32.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, A. Schuchat, and Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]