Abstract
Objectives
Platelet activation and aggregation play an important role in the pathogenesis of cardiovascular disease. We examined the association of a single nucleotide polymorphism (SNP) in the GPIIIa platelet glycoprotein (Leu33Pro) with carotid artery plaque morphology and with expression of platelet markers using data from the Atherosclerosis Risk in Communities (ARIC) Carotid MRI study.
Methods
The study sample consisted of 1,202 Caucasian members of the ARIC study cohort recruited in 2004-2005 to participate in the Carotid MRI Substudy under stratified sampling based on maximum carotid artery wall thickness. The Leu33Pro polymorphism was identified as SNP rs5918 in the ITGB3 gene. Plaque visualization was accomplished with contrast enhanced MRI examination of the thickest segment of the carotid artery. Expression of platelet markers was measured using fasting whole blood flow cytometry.
Results
This cross-sectional analysis based on age and gender adjusted weighted linear regression models suggests that those homozygous for the Leu33Pro risk allele (C) have decreased mean and minimum fibrous cap thickness. We did not observe differences in plaque lipid volume or maximum carotid artery wall thickness across SNP rs5918 genotypes. Carriers of the Leu33Pro polymorphism, as compared to major allele homozygotes, had greater percent of platelets expressing P-selectin, a platelet glycoprotein indicating activation status. Prevalent coronary heart disease did not affect estimates of fibrous cap thickness or of platelet activation.
Conclusion
Our results suggest that individuals with Leu33Pro polymorphism of the GPIIIa glycoprotein may be predisposed to increased risk of atherosclerotic plaque rupture.
Keywords: plaque, platelets, atherosclerosis, coronary heart disease, Magnetic Resonance Imaging (MRI)
Introduction
The term ”vulnerable plaque” [1] refers to an atherosclerotic lesion in which a lipid-rich core, infiltrated by macrophages, is contained within a thin fibrous cap. Based on examination of autopsy specimens Kolodgie et al.[2] suggested that vulnerability of the plaque to rupture is defined primarily by the thinness of the fibrous cap and proposed a term “thin cap fibroatheroma” that identifies morphologically distinct lesions which vary in the volume and area of the lipid core, but which share the characteristically thin fibrous cap. The focus of this study is the association of a polymorphism in the platelet integrin GPIIb/IIIa with atherosclerotic plaque characteristics.
The GPIIb/IIIa integrin, the most abundant platelet–specific glycoprotein [3], exists as a heterodimer of two subunits: the αIIb chain and the β3 chain. This integrin functions as a receptor for ligands such as fibrinogen, von Willebrand factor and vitronectin [4-6] and is critical for the binding of platelets to the extracellular matrix of the blood vessel wall and to each other, thus facilitating platelet aggregation. Platelet aggregation is an essential step in formation of the thrombus [7] and in subsequent blood vessel wall remodeling [8]. In quiescent platelets, the αIIbβ3 glycoprotein is in a conformation which does not allow for ligand binding. Activation of platelets induces a conformational change in the cytoplasmic portion of the glycoprotein and subsequently, in the process of inside-out signaling [9], in the extracellular portion, exposing the extracellular Arg-Gly-Asp (RGD) ligand binding tripeptide [10]. In the GPIIIa protein (β3 chain), the RGD binding sequence is located in the same area as the most common platelet polymorphism, known as the Leu33Pro polymorphism or the PlA1/PlA2 polymorphism. This genetic variation has been examined for association with clinical coronary artery disease endpoints, but the results are not conclusive [11, 12].
Evaluation of the association of the Leu33Pro polymorphism with intermediate phenotypes characterizing atherosclerotic lesions is possible only in studies that are designed to examine atherosclerotic plaque morphology. The Carotid MRI component of the Atherosclerosis Risk in Communities (ARIC) Study is one of the few studies that provide that information. We therefore used data from the ARIC Carotid MRI study to examine the association of the Leu33Pro single nucleotide polymorphism (SNP) in the ITGB3 gene coding for the GPIIIa platelet glycoprotein with MRI measurements defining carotid artery plaque morphology and with expression of platelet markers.
Methods
Study participants
The ARIC Study includes a population based cohort of men and women aged 45-64 at baseline (1987-1989) selected as a probability sample from four communities in the United States [13]. Participants of the ARIC Carotid MRI study [14] were selected from the population of ARIC Study members alive in 2004, such that 1200 were recruited based on their high (field center-specific cutpoints) carotid intima media thickness (IMT) levels determined at the last ARIC study visit (1993-1996) and 800 participants were recruited from the remaining cohort population through stratified random sampling. In total, 4306 ARIC cohort members were invited to participate in the ARIC Carotid MRI study. Of those, 1403 refused participation, 837 were ineligible for an MRI examination, leaving 2066 as the final number of Carotid MRI study participants. Individuals were ineligible for the study if they had any of the following: implanted metallic devices; carotid revascularization on either side for the low IMT group or on the side selected for imaging on the high IMT group; weight greater than 320 pounds; difficulties in understanding questions; or difficulties in completing the informed consent form. We excluded from the present study individuals with missing MRI scans (n=127), those for whom all attributes could not be ascertained (n=169), individuals who underwent chemotherapy or steroid treatment four weeks or less prior to the study visit (n= 75), those with missing information for all platelet variables (n=118), those missing information on plaque variables (n=360), and those missing genotype information (n=109). We further restricted the study to whites, as the total number of black study participants with the minor risk allele was too low for meaningful analyses (n=2). The final study sample consisted of 1,202 ARIC study participants. Institutional Review Boards of each study center approved the study protocols and all study participants provided informed consent.
Genotyping
TagSNPs within selected candidate genes were derived using the Haploview Program based on two sources of SNPs: the Caucasian (CEU) and Yoruban (YRI) population from the International HapMap project. The data were analyzed by the ARIC study DNA laboratory in a race specific manner using the gene definitions provided by the HapMap database. The dbSNP database was used as the primary source for the data on each gene. Genotyping was performed using a custom designed iSelect Infinium BeadChip with 7,600 bead types. Concordance of duplicated samples for all SNPs was 99.998% .
The Leu33Pro polymorphism was identified as SNP rs5918 in the ITGB3 gene located on chromosome 17. Minor allele frequency for this SNP in the ARIC Carotid MRI population was 0.15 among whites and 0.09 among blacks. The corresponding minor allele frequencies reported in the HapMap project are 0.14 for the CEU population and 0.13 for the YRI population.
MRI acquisition
A contrast-enhanced MRI exam was performed using a standard protocol as previously reported [15]. Exams were acquired on 1.5 T MRI scanners (Excite platform, GE Medical Systems; Symphony Maestro, Siemens Medical Solutions) using 4-element phased array carotid coils (Machnet, The Netherlands). A 3D time-of-flight MR angiogram (MRA) was acquired to include both extracranial carotid bifurcations. Detailed high-resolution black blood MRI (BBMRI) images were then acquired through the carotid with the higher maximum wall thickness based on a prior ultrasound, unless the contralateral carotid wall appeared thicker on the MRA to the technologist, in which case the contralateral carotid was chosen for detailed imaging. BBMRI imaging was performed using a 2D electrocardiogram (ECG)-gated double inversion recovery fast spin-echo sequence with the inversion time set to suppress the signal of luminal blood (repetition time/echo time, 1 RR/5 msec; acquired in-plane resolution, 0.51×0.58×2 mm3). Images were acquired before and 5 minutes after the injection of gadodiamide (Omniscan, GE Healthcare), 0.1mmol/kg. For this study, all MRI variables were derived from the postcontrast series that included 16 slices (3.2cm longitudinal coverage).
MRI Image Analysis
The postcontrast BBMRI images were analyzed by seven trained readers using semiautomated software (VesselMASS, Leiden University Medical Center). Details of the analysis were previously described [15]. Readers drew contours to delineate the outer wall, lumen, and lipid core. The fibrous cap contour was automatically generated based on the lumen and lipid core contours. Only 8 of the 16 slices were analyzed (1.6 cm segment), selected as those centered on the slice depicting the thickest wall. Each exam received an image quality and protocol adherence score and failed exams were not analyzed.
The analysis software divided vessel walls into 12 radial segments and fibrous caps into radial segments at 15° increments for each slice. Mean thickness values were generated for the vessel wall and area measurements were generated for the lipid core. Volumetric data were computed by integrating area measurements over the eight contiguous analyzed slices. Mean cap thickness was defined as the mean thickness of all caps segments at two adjacent slices with the largest core, and minimum cap thickness was defined as the mean of the two minimum cap segment thicknesses at these two slices with the largest core. Reliability estimates for these derived MRI variables were previously reported [15].
Flow cytometry
Flow cytometry was performed using fasting whole blood samples which were stored in Cyto-Chex® BCT vacutainer tubes (Streck, Omaha, NE) containing EDTA and a blood cell stabilizer. The tubes were shipped at room temperature in temperature-stabilizing packages overnight to a central flow-cytometry laboratory. Details of the flow cytometry protocols and reproducibility of used methods have been described elsewhere [16]. We examined percent cells positive for selected platelet surface glycoproteins and for median fluorescence intensity associated with those markers. Antigen-negative controls were used to set the threshold between positive and negative cell populations. The coefficient of variation was less than 10% for most analytes. A validation study conducted among 55 study participants, indicated high repeatability (R>0.60) for the measurement of the CD62P antigen.
Statistical analysis
We used weighted linear regression analysis to determine age-, gender- and study center-adjusted means of selected characteristics of the study participants as well as plaque and platelet characteristics. Analyses were weighted by the inverse of the sampling fractions used in the design of the ARIC Carotid MRI study. All analyses were cross-sectional and were based on a recessive model. Analyses of atherosclerotic plaque characteristics were limited to individuals with maximum segmental wall thickness equal to or greater than 1.5 mm in order to exlude those cases with cores too small to be detected by the resolution constraints of the MRI scanner, and analyses of the fibrous cap thickness were limited to participants with maximum segmental wall thickness equal to or greater than 1.5 mm and with a present lipid core. Additionally, we explored a vector of plaque vulnerability using the method of principal components analysis [17] based on the following variables associated with plaque morphology: mean cap thickness, minimum cap thickness, maximum lipid core volume, and maximum segmental wall thickness. Weighted linear regression analysis was performed on the relevant principal components to examine their relationship to the genotypes as described above.
Analyses were performed using STATA 10.0 (STATACORP LP, College Station, Texas) and SUDAAN (RTI International, Research Triangle Park, Durham, NC)
Results
The minor allele (C) frequency of SNP rs5918 of the ITGB3 gene was 15% among ARIC whites, similar to the 14% reported among the HapMap CEU population. The genotype distribution conformed to Hardy-Weinberg equilibrium.
We did not observe many differences in the characteristics of study participants according to the SNP rs5918 genotype (Table 1). The percent of individuals with self-reported use of anti-hyperlipidemic medication was higher among individuals homozygous for the major allele as compared to heterozygotes and those homozygous for the minor allele. Levels of glucose, mean total cholesterol, mean HDL-cholesterol, and mean LDL cholesterol were similar among major allele homozygotes and heterozygotes, but differed significantly for minor allele homozygotes.
Table 1.
Weighted means (SE) or percent of study participant characteristics according to SNP rs5918 genotype: The ARIC Carotid MRI Study
| SNP rs5918 genotype |
|||
|---|---|---|---|
| Characteristic | TT (n=1,165) |
TC (n=335) |
CC (n=38) |
| Age (years) | 70.7 (0.2) | 70.6 (0.2) | 70.7 (0.4) |
| Gender (% male) | 44.5 | 46.7 | 49.4 |
| BMI (kg/m2) | 28.4 (0.2) | 27.9 (0.4) | 28.2 (0.9) |
| Glucose (mg/dL) | 106.5 (1.0) | 107.9 (1.6) | 104.7 (3.0) |
| Total cholesterol (mg/dl) | 190.7 (1.6) | 201.1 (3.5) | 179.4 (7.7)* |
| HDL-cholesterol (mg/dL) | 49.6 (0.6) | 50.6 (1.2) | 47.2 (2.4) |
| LDL-cholesterol (mg/DL) | 110.1 (1.5) | 118.2 (3.0) | 101.3 (7.5)* |
| Triglycerides (mg/dL) | 190.7 (1.6) | 201.1 (3.5) | 179.4 (7.7) |
| Diabetes (%) | 19.8 | 22.1 | 20.2 |
| Hypertension (%) | 59.0 | 64.0 | 56.8 |
| Prevalent CHD (%) | 11.6 | 6.8 | 11.1 |
| Use of anticoagulants (%) | 4.6 | 2.1 | 0* |
| Use of anti-hyperlipidemic medication (%) | 47.8 | 37.6 | 35.6 |
| Use of antiplatelet medication (%) | 3.2 | 1.4 | 2.4 |
| Use of aspirin (%) | 54.5 | 47.5 | 55.9 |
| Current smokers (%) | 7.6 | 6.8 | 8.9 |
Levels of total cholesterol and LDL-cholesterol were significantly different (p<0.05) between individuals homozygous for the minor allele (CC) and heterozygotes. Use of anticoagulants among those homozygous for the minor allele was significantly different (p<0.05) from anticoagulant use in both the heterozygotes and in the major allele homozygotes. No other statistically significant differences in the distribution of population characteristics were observed.
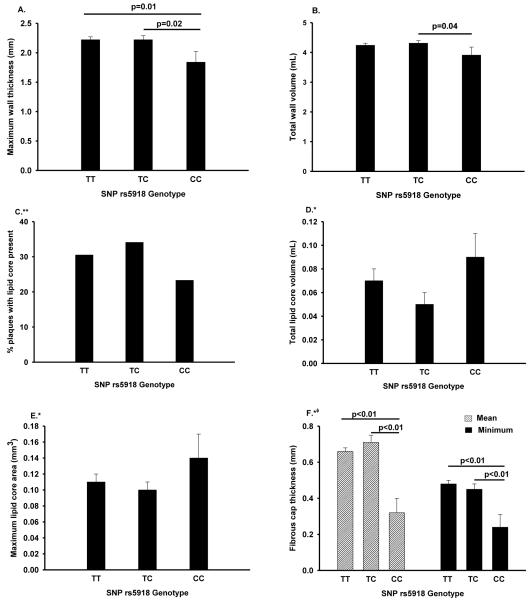
Figure 1 shows atherosclerotic plaque characteristics stratified by ITGB3 rs5918 genotype. In comparison with heterozygotes and with individuals homozygous for the major allele, individuals homozygous for the minor allele had a smaller total wall volume (p=0.04) and smaller maximum segmental wall thickness (p=0.01). The total lipid core volume and the maximum lipid core area was the highest among those homozygous for the minor allele, although the differences were not statistically significant. The percent of individuals with visible lipid core presence in any of the eight MRI slices was lower among individuals homozygous for the minor allele in comparison with heterozygotes and with those homozygous for the major allele. For both measures of the fibrous cap thickness (mean and minimum), individuals homozygous for SNP rs5918 minor allele exhibited values that were significantly lower than those observed for either heterozygotes or for major allele homozygotes. Initial analysis was performed with adjustment for age, gender, maximum segmental wall thickness, and ARIC study center. No significant attenuation of estimates was observed in adjustment for smoking (current, former, or never), prevalent coronary heart disease, diabetes, levels of total and non-HDL cholesterol and use of antihyperlipidemic medication and aspirin.
Figure 1.
Association of the SNP rs5918 polymorphism of the platelet GPIIIa glycoprotein with atherosclerotic plaque characteristics. Adjustment for age, gender, study center, and smoking.
The ARIC Carotid MRI Study.
* Analysis limited to whites with maximum wall thickness ≥1.5mm
**Analysis limited to whites with maximum wall thickness ≥1.5mm with additional adjustment for maximum wall thickness
*φAnalysis limited to whites with maximum wall thickness ≥1.5mm and with presence of lipid core.
A. maximum wall thickness; B. total wall volume; C. percent of plaques with lipid core; D. total lipid core volume; E. maximum lipid core area; F. fibrous cap thickness
Principal components analysis of mean cap thickness, minimum cap thickness, maximum lipid core volume, and maximum segmental wall thickness identified two principal components, one associated with a thick fibrous cap, thick wall, and large volume of the lipid core; the other was positively associated with fibrous cap thickness and to a lesser degree negatively associated with lipid core volume and maximum segmental wall thickness (data not shown). In regression analysis adjusted for age and gender, we found the second principal component to be inversely associated with the presence of both minor alleles of SNP rs5918 (mean difference: −1.3737, 95% CI: −1.9223, −0.8248), while regression analyses performed with the first principal component were null (data not shown).
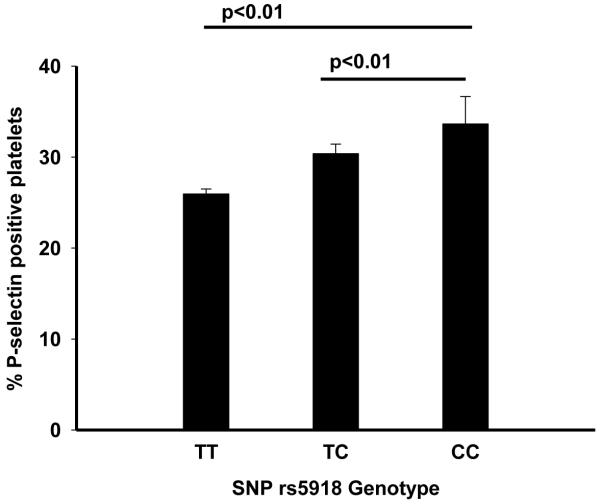
We observed a gradient in levels of platelet activation marker P-selectin across the SNP rs5918 genotype (Figure 2). Individuals homozygous for the major allele (T) had 25.9 (SE 0.5) percent of platelets expressing P-selectin. That percent increased among heterozygotes to 30.7 (SE 1.0) and it was 38.1 (SE 3.2) among minor allele (C) homozygotes. The differences in levels of P-selectin between the genotype groups were statistically significant (p<0.05).
Figure 2.
Association of SNP rs5918 of the GPIIIa platelet glycoprotein with platelet activation. Age and gender adjusted percent (SE). Whites only. p-for trend <0.01. The ARIC Carotid MRI study
Discussion
This cross-sectional analysis based on age- and gender-adjusted weighted linear regression models suggests that the Leu33Pro polymorphism may be associated with changes in the structure of atherosclerotic plaques. Specifically, individuals homozygous for the risk allele (C) of SNP rs5918 in the ITGB3 gene may have decreased mean and minimum thickness of the atherosclerotic plaque fibrous cap and increased total lipid core volume, as compared to heterozygotes and major allele homozygotes. We did not observe significant differences in maximum carotid artery wall thickness across SNP rs5918 genotypes. Carriers of the Leu33Pro polymorphism, as compared to major allele homozygotes, had greater percent of platelets expressing P-selectin, a platelet glycoprotein that indicates platelet activation status. Prevalent coronary heart disease did not affect estimates of fibrous cap thickness or levels of platelet markers.
The GPIIb/IIIa glycoprotein plays an essential role in the final common pathway leading to platelet aggregation and formation of the thrombus plug [18]. Receptors found at the extracellular portion of this transmembrane integrin protein serve to bind activated platelets to ligands such as fibrinogen and von Willebrand factor [19] thus facilitating platelet-platelet binding and adhesion of platelets to the exposed extracellular matrix of the vascular wall. Glycoprotein IIb/IIIa antagonists have been used clinically in prevention of subsequent thrombotic events among patients with acute coronary syndromes and those undergoing percutaneous intervention [20, 21].
Platelet glycoproteins are highly polymorphic, with the Leu33Pro polymorphism of the GPIIIa glycoprotein ITGB3 gene as the most common platelet polymorphism. The association of the Leu33Pro polymorphism with the incidence of coronary heart disease first reported by Marian et al. [22] has been examined in numerous other studies with mixed results [12]. Meta-analysis results suggest a slight increase in coronary heart disease risk among individuals with the Leu33Pro polymorphism; however, the range of reported effect estimates is very wide. We sought to examine the association of this polymorphism with intermediate phenotypes which ultimately may lead to clinically manifest coronary heart disease, namely plaque morphology and platelet activation. Our observation that the Leu33Pro polymorphism is associated with decreased fibrous cap thickness of the atherosclerotic plaque confirms reports of a positive association of this genetic variant with increased risk of thrombosis and increased extent of coronary artery disease [23, 24].
We observed a gradient in levels of platelet surface glycoprotein P-selectin across genotypes of the rs5918 SNP. P-selectin, stored in the cytosolic Wabe-Pallade bodies of the platelet, is released upon platelet activation and expressed on the platelet surface. Thus, an increase in percent of platelets expressing P-selectin is considered a marker of increased platelet activation. While in vitro studies of the association of the Leu33Pro polymorphism with platelet activation are not conclusive [25-27], it is possible that the conflicting reports may reflect modification of the effect of the genotype on platelet aggregability by fibrinogen and perhaps other ligands.
The majority of the studies examining the association of the Leu33Pro variant with clinical endpoints have combined the risk allele homozygotes with heterozygotes in the exposure category. However, our results suggest that the effect of the Leu33Pro polymorphism on plaque characteristics is observed only for risk allele homozygotes. Inclusion of heterozygotes in the exposure category would dilute the effect estimates for intermediate phenotypes and, by extension, for clinical endpoints. This dilution may be potentiated by the significantly greater heterozygote genotype frequency, as compared to that of risk allele homozygotes. Several studies [28-30] have reported wide ethnic variation in the distribution of the Leu33Pro polymorphism, with whites exhibiting the largest mean minor allele frequency. Analyses of the association of this polymorphism with clinical outcomes in populations of mixed race and ethnicity may dilute the effect estimate and bias results towards the null. Analyses presented here were performed only among whites.
Our study had several limitations. Most importantly, the number of individuals with the minor allele of SNP rs5918 of the ITGB3 gene was very small. Our small sample size was partly dictated by the fact that the MRI resolution limited our analysis to the largest observed plaques. We further constrained the analysis of plaque morphology to include only individuals with thick arterial walls. It is therefore possible that our observations were due to chance. In a sensitivity analysis we examined the association of two distinct chromosome 17 SNPs (rs8069732 and rs7214096) which are in linkage disequilibrium with SNP rs5918 with the selected plaque and platelet outcomes. They both showed associations similar to those observed with SNP rs 5918; however, the minor allele frequency for those SNPs was similarly less than 15%. Low minor allele frequency and small sample size disallowed analyses in the black participants of the ARIC Carotid MRI study. Despite the resulting small sample size, our effect estimates were stable and consistent with clinical observations. We were not able to perform a replication study for this analysis because no other study exists that provides similar data. To our knowledge, the Multi-Ethnic Study of Atherosclerosis (MESA) is to date the only other population-based study of carotid artery plaque morphology by magnetic resonance imaging with extant data. However, the number of completed MRIs in MESA in individuals with plaques thick enough to analyze is only 214, and thus would provide very limited power for replication that is already compromised by the low minor allele frequency of the rs5918 SNP.
The major strength of this study is that it was performed using validated data from an established cohort study. Our data show internal consistency, such that low thickness of the fibrous cap among the minor allele homozygotes corresponded with a relatively greater lipid core area and volume in that group, as compared to the heterozygotes and the major allele homozygotes, suggesting the presence of lipid-rich thin cap fibroatheromas.
In conclusion, our observations suggest that the Leu33Pro polymorphism is associated with increased level of platelet activation as well as decreased fibrous cap thickness. Although those two manifestations of a prothrombotic state may be causally related, it is not possible to assess causality in this cross-sectional study. The results however, suggest that individuals with the Leu33Pro polymorphism of the GPIIIa glycoprotein may be predisposed to increased risk of atherosclerotic plaque rupture due to both a thinning fibrous cap and as a result of a sustained pro-inflammatory state.
Acknowledgements
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, and 5U01-HL-075572. The authors thank the staff and participants of the ARIC study for their important contributions.
The authors would also like to thank Dr. Richey Sharrett for providing helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004;13:125–38. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- [2].Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16:285–92. doi: 10.1097/00001573-200109000-00006. [DOI] [PubMed] [Google Scholar]
- [3].Bennett JS. Structure and function of the platelet integrin alphaIIbbeta3. J Clin Invest. 2005;115:3363–9. doi: 10.1172/JCI26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Andrieux A, Hudry-Clergeon G, Ryckewaert JJ, Chapel A, Ginsberg MH, Plow EF, et al. Amino acid sequences in fibrinogen mediating its interaction with its platelet receptor, GPIIbIIIa. Journal of Biological Chemistry. 1989;264:9258–65. [PubMed] [Google Scholar]
- [5].Farrell DH, Thiagarajan P, Chung DW, Davie EW. Role of fibrinogen alpha and gamma chain sites in platelet aggregation. Proc Natl Acad Sci U S A. 1992;89:10729–32. doi: 10.1073/pnas.89.22.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmugge M, Rand ML, Freedman J. Platelets and von Willebrand factor. Transfusion and Apheresis Science. 2003;28:269–77. doi: 10.1016/S1473-0502(03)00046-6. [DOI] [PubMed] [Google Scholar]
- [7].Furie B, Furie BC. Mechanisms of Thrombus Formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- [8].Ruggeri ZM, Mendolicchio GL. Adhesion Mechanisms in Platelet Function. Circ Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- [9].Rivera J, Lozano ML, Navarro-Nunez L, Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94:700–11. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992;71:671–8. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- [11].Di Castelnuovo A, de Gaetano G, Donati MB, Iacoviello L. Platelet glycoprotein receptor IIIa polymorphism PLA1/PLA2 and coronary risk: a meta-analysis. Thromb Haemost. 2001;85:626–33. [PubMed] [Google Scholar]
- [12].Zhu MM, Weedon J, Clark LT. Meta-analysis of the association of platelet glycoprotein IIIa PlA1/A2 polymorphism with myocardial infarction. Am J Cardiol. 2000;86:1000–5. A8. doi: 10.1016/s0002-9149(00)01136-x. [DOI] [PubMed] [Google Scholar]
- [13].The Aric I The Atherosclerosis Risk IN Communities (ARIC) Study:Design and Objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- [14].Wagenknecht L, Wasserman B, Chambless L, Coresh J, Folsom A, Mosley T, et al. Correlates of carotid plaque presence and composition as measured by MRI: the Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging. 2009;2:314–22. doi: 10.1161/CIRCIMAGING.108.823922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wasserman BA, Astor BC, Sharrett AR, Swingen C, Catellier D. MRI measurements of carotid plaque in the atherosclerosis risk in communities (ARIC) study: methods, reliability and descriptive statistics. J Magn Reson Imaging. 31:406–15. doi: 10.1002/jmri.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Catellier DJ, Aleksic N, Folsom AR, Boerwinkle E. Atherosclerosis Risk in Communities (ARIC) Carotid MRI flow cytometry study of monocyte and platelet markers: intraindividual variability and reliability. Clin Chem. 2008;54:1363–71. doi: 10.1373/clinchem.2007.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jolliffe IT. Principal components analysis. Springer-Verlag; 2002. [Google Scholar]
- [18].Weiss EJ, Bray PF, Tayback M, Schulman SP, Kickler TS, Becker LC, et al. A Polymorphism of a Platelet Glycoprotein Receptor as an Inherited Risk Factor for Coronary Thrombosis. N Engl J Med. 1996;334:1090–4. doi: 10.1056/NEJM199604253341703. [DOI] [PubMed] [Google Scholar]
- [19].Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- [20].Coller BS. Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. The Journal of Clinical Investigation. 1997;99:1467–71. doi: 10.1172/JCI119307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zeymer U, Wienbergen H. A Review of Clinical Trials with Eptifibatide in Cardiology. Cardiovascular Drug Reviews. 2007;25:301–15. doi: 10.1111/j.1527-3466.2007.00022.x. [DOI] [PubMed] [Google Scholar]
- [22].Marian AJ, Brugada R, Kleiman NS, Carter AM, Ossei-Gerning N, Grant PJ, et al. Platelet Glycoprotein IIIa PlA Polymorphism and Myocardial Infarction. N Engl J Med. 1996;335:1071–4. doi: 10.1056/NEJM199610033351418. [DOI] [PubMed] [Google Scholar]
- [23].Mikkelsson J, Perola M, Laippala P, Savolainen V, Pajarinen J, Lalu K, et al. Glycoprotein IIIa PlA Polymorphism Associates With Progression of Coronary Artery Disease and With Myocardial Infarction in an Autopsy Series of Middle-Aged Men Who Died Suddenly. Arterioscler Thromb Vasc Biol. 1999;19:2573–8. doi: 10.1161/01.atv.19.10.2573. [DOI] [PubMed] [Google Scholar]
- [24].Mikkelsson J, Perola M, Laippala P, Penttila A, Karhunen PJ. Glycoprotein IIIa Pl(A1/A2) polymorphism and sudden cardiac death. J Am Coll Cardiol. 2000;36:1317–23. doi: 10.1016/s0735-1097(00)00871-8. [DOI] [PubMed] [Google Scholar]
- [25].Meiklejohn DJ, Urbaniak SJ, Greaves M. Platelet glycoprotein IIIa polymorphism HPA 1b (PlA2): no association with platelet fibrinogen binding. Br J Haematol. 1999;105:664–6. doi: 10.1046/j.1365-2141.1999.01413.x. [DOI] [PubMed] [Google Scholar]
- [26].Goodall AH, Curzen N, Panesar M, Hurd C, Knight CJ, Ouwehand WH, et al. Increased binding of fibrinogen to glycoprotein IIIa-proline33 (HPA-1b, PlA2, Zwb) positive platelets in patients with cardiovascular disease. Eur Heart J. 1999;20:742–7. doi: 10.1053/euhj.1998.1203. [DOI] [PubMed] [Google Scholar]
- [27].Michelson AD, Furman MI, Goldschmidt-Clermont P, Mascelli MA, Hendrix C, Coleman L, et al. Platelet GP IIIa Pl(A) polymorphisms display different sensitivities to agonists. Circulation. 2000;101:1013–8. doi: 10.1161/01.cir.101.9.1013. [DOI] [PubMed] [Google Scholar]
- [28].Kim HO, Jin Y, Kickler TS, Blakemore K, Kwon OH, Bray PF. Gene frequencies of the five major human platelet antigens in African American, white, and Korean populations. Transfusion. 1995;35:863–7. doi: 10.1046/j.1537-2995.1995.351096026369.x. [DOI] [PubMed] [Google Scholar]
- [29].Lim J, Lal S, Ng KC, Ng K-S, Saha N, Heng C-K. Variation of the platelet glycoprotein IIIa PIA1/A2 allele frequencies in the three ethnic groups of Singapore. International Journal of Cardiology. 2003;90:269–73. doi: 10.1016/s0167-5273(02)00567-3. [DOI] [PubMed] [Google Scholar]
- [30].Kekomaki S, Partanen J, Kekomaki R. Platelet alloantigens HPA-1, -2, -3, -5 and -6b in Finns. Transfus Med. 1995;5:193–8. doi: 10.1111/j.1365-3148.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]