Abstract
Borna disease virus (BDV) is a neurotropic RNA virus with a wide host range. Human infections, although controversial, have been described in Europe, Asia, and the United States. The present study investigated the existence of BDV infections in immunocompromised human beings, namely, 82 human immunodeficiency virus (HIV)-infected and 80 therapeutically immunosuppressed patients. BDV p40 RNAs were detected in peripheral white blood cells with reverse transcription-nested PCR and hybridization in, respectively, 11 (13.41%) and 1 (1.25%) of the two groups of patients. BDV p24 RNAs were identified in only one of those. BDV RNA was detected in the absence of any neuropsychiatrical illness, suggesting that BDV infections may occur in asymptomatic carriers. The severity and particularity of cellular immunosuppression could explain the significantly increased detection of BDV RNA in HIV-infected patients.
Borna disease virus (BDV) represents the prototype of the Bornaviridae, genus Bornavirus, among the Mononegavirales (13, 18, 26). Its genome, a negative-strand nonsegmented RNA molecule, 8.9 kb in size, is characterized by a surprising stability (15, 35, 36), in spite of its wide host range (22, 31, 38). BDV is mainly neurotropic, with a preferential localization to the limbic system (19), which could explain the various behavior abnormalities observed in infected animals. Borna disease was first described in Germany in horses. Clinical symptoms could depend upon the animal species (horse, donkey, cattle, sheep, goat, nonhuman primate, rabbit, cat, lynx, or birds), the age of primary infection, the host immune status, the site of virus penetration into the organism, and strain characteristics. Asymptomatic carriage may be common. The natural viral reservoir is currently unknown but could include rodents and wild birds (5, 38).
Several seroepidemiological and molecular studies performed in Europe, Asia, and the United States demonstrated that BDV could also infect humans (25, 31, 38). An association between BDV infection and psychiatric illnesses, especially bipolar disease and schizophrenia, was repeatedly suggested (2, 6-9, 20, 21, 23, 32-35, 42, 43). However, the alleged prevalence of human BDV infection varied considerably from one study to another, partly because of the absence of a consensus virological technique (30), the apparent low avidity of human immunoglobulins G for BDV antigens (1), and the brief and low viral load in circulating blood (10, 25). Therefore, there is some controversy regarding the validity of positive results. Such results could even arise from possible contamination of samples with laboratory strains because of sequence similarities between human BDV isolates and BDV laboratory strains (29, 37, 38).
Little is known about human BDV infections in France, where symptomatic epidemic animal infections do not seem to occur, although antibodies to BDV and BDV RNA have been detected in horses, domestic animals, and foxes (14, 16). The aim of the present study was to investigate the existence of BDV infections in two groups of immunocompromised patients treated in Bordeaux University Hospital. Its goal was not to establish prevalence. Such patients were selected because rare viral infections are known to occur more frequently, although sometimes asymptomatically, in immunocompromised hosts.
MATERIALS AND METHODS
Patients and blood samples.
Informed consent was obtained from the patients, and human experimentation guidelines of Bordeaux University Hospital (CCCPRB Bordeaux 1) were followed in this clinical research. One hundred sixty-two patients were randomly tested. The first group included 82 human immunodeficiency virus (HIV)-infected patients with leukocyte counts over 1.5 g/liter. The second group comprised 80 patients treated with immunosuppressive drugs, including cyclosporine or tacrolimus and corticosteroids after liver transplantation (n = 40) and cyclophosphamide or azathioprine and corticosteroids for severe lupus erythematosus (n = 40). None of the patients was recorded as presenting psychiatric symptoms. The patients were tested in a double-blind manner, independently of their belonging to one group or the other, in chronological order.
Ten milliliters of peripheral blood were collected on EDTA. Blood cells were separated by 1,200 × g (2,500 rpm) centrifugation for 5 min. Red blood cells were subsequently lysed on ice for 30 min with 8 ml of blood lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA; pH 7.4). After centrifugation at 1,200 × g for 10 min, the pellet was resuspended with 0.2 ml of sterile water and separated into two tubes. Leukocyte integrity was examined by Trypan blue exclusion. Total leukocyte aliquots were stored at −80°C before use.
Molecular procedures.
Total RNA was extracted from the 162 blood cell samples according to Chomczynski's technique, based on guanidinium thiocyanate and phenol treatment, isopropanol precipitation, and ethanol washes (12). At the end of the procedure, after pooling of each patient's extracts, the total volume of an RNA extract was 60 μl. Reverse transcription (RT) was performed with RT-murine leukemia virus and the antisense primer, using 5 μl of the extract, allowing transcription of viral messenger RNAs; cDNA amplification (PCR) was obtained by a nested procedure (Genset [Paris, France] for the primers, Roche Molecular Diagnostics [Meylan, France] for all other reagents). All primers and RT-PCR techniques were as previously published (23, 35). The samples were first subjected to RT-nested PCR in the BDV p40 gene, the most abundantly transcribed in infected cells (35). Whenever the result was positive, RT-nested PCR in the BDV p24 gene was attempted (23). In 10 BDV p40-positive samples, RT-PCR for measles virus (MV) was performed (27). RT-PCR for α-actin mRNAs was applied successfully to BDV p40-negative RNA extracts.
Detection of amplified DNA was obtained by 1.5% agarose gel electrophoresis and ethidium bromide staining. Next, we carried out an enzyme-linked immunosorbent assay-format molecular hybridization (Hybridowell kit; Argene Biosoft, Varhiles, France). After chemical denaturation of amplified products and their adsorption onto a microtitration plate, hybridization with an in-house-designed p40- or p24-specific biotinylated probe was carried out. Detection was performed with a streptavidin-peroxidase conjugate associated with tetramethylbenzidine substrate. Optical density was measured on a spectrophotometer at 450 nm. The cutoff value was arbitrarily determined according to the manufacturer's recommendations, by adding 0.150 to the mean optical density of negative controls. This method was validated by using a panel of dilutions of positive controls. Primer and probe sequences are listed in Table 1.
TABLE 1.
Primers and probes used in this study
| Primer or probe | Sequence | Use | Nucleotide positionsa | Gene |
|---|---|---|---|---|
| Primer A | 5′-TTCATACAGTAACGCCCAGC-3′ | First-round PCR | 259-278 (S) | p40 |
| Primer B | 5′-GCAACTACAGGGATTGTAAGGG-3′ | First-round PCR | 829-808 (AS) | p40 |
| Primer C | 5′-GCCTTGTGTTTCTATGTTTGC-3′ | Second-round PCR | 277-297 (S) | p40 |
| Primer D | 5′-GCATCCATACATTCTGCGAG-3′ | Second-round PCR | 805-766 (AS) | p40 |
| Probe 1 | 5′-CCGGCCATCCCATGGTGAGAC-3′ | Hybridization | 575-595 (S) | p40 |
| Primer D2 | 5′-TGACCCAACCAGTAGACCA-3′ | First-round PCR | 1387-1405 (S) | p24 |
| Primer A1 | 5′-GTCCCATTCATCCGTTGTC-3′ | First-round PCR | 1865-1847 (AS) | p24 |
| Primer D3 | 5′-TCAGACCCAGACCAGCGAA-3′ | Second-round PCR | 1443-1461 (S) | p24 |
| Primer A2 | 5′-AGCTGGGGATAAATGCGCG-3′ | Second-round PCR | 1834-1816 (AS) | p24 |
| Probe 2 | 5′-TCCAGACAGCTCAGCGGTGCG-3′ | Hybridization | 1627-1647 (S) | p24 |
S, sense; AS, antisense.
Negative controls included RNA-free samples, cultured C6 rat glioma cells, patients' RNA samples subjected to RT in the absence of reverse transcriptase and RNA extracted from MV-infected and vesicular stomatitis virus (VSV; strain Indiana)-infected cell cultures.
At the beginning of the study, RNA extracts from BDV-infected C6 rat glioma cells, cultivated in Dulbecco's modified Eagle medium and 10% fetal calf serum (33), were used to determine the RT-nested PCR threshold of detection. BDV-infected C6 cells were cultured in a security laboratory (containment level 3), located in a first building, and the amplifications assays were performed with these samples in the same building. As soon as it was available, RNA transcribed from plasmid p40 INS, which contains a 56-nucleotide insert that allows its distinction from the wild-type BDV genome, was used to assess the sensitivity of the method and then as a positive control for RT-nested PCR assays on the patient samples (35). Extractions and amplifications of clinical samples were performed in a second, distant building. Strict measures to prevent laboratory contamination, including separate rooms dedicated to sample preparation, extraction, mix preparation, amplification, and detection, were observed in the two buildings.
The 528-bp BDV p40 PCR products were sequenced after purification on S400 spin columns (Amersham Pharmacia Biotech, Orsay, France). Both strands were used as templates in a single-cycle reaction by the dideoxy-chain termination method (D-rhodamine terminator cycle sequencing Ready Reaction; Applied Biosystems, Courtaboeuf, France), with second-round PCR primers. Sequence reaction products were precipitated and subjected to electrophoresis on a 6% polyacrylamide gel containing 6 M urea and 1× Tris-borate-EDTA, and the sequences were analyzed with an ABI 377 automatic sequencer (Applied Biosystems). The sequences were compared by using Sequence Navigator software.
Statistical analysis.
The two groups of patients were compared for BDV positivity by using a chi-square test with Prism 2.0 software (GraphPad Software, San Diego, Calif.).
RESULTS
Cell yields in peripheral blood samples.
An average of 3 × 106 white cells per ml of peripheral blood was obtained. Up to 10% of these cells did not exclude Trypan blue. The number of white blood cells present in each extraction sample was never lower than 10 × 106 (mean, 3 × 107), and each RT assay was performed with the RNA extracted from approximately 5 × 106 white blood cells.
Threshold of detection.
The sensitivity of the RT-PCR technique for BDV p40 detection was first investigated with RNA extracted from serial dilutions of BDV-infected C6 cells. Viral RNA was detected in reaction tubes containing as little as 0.075 infected cell. When we attempted to test the sensitivity with the RNA transcribed from p40 INS, we consistently detected a signal using 1,000 copies of it (the detection of 100 copies was inconsistent).
Detection of BDV RNA and controls.
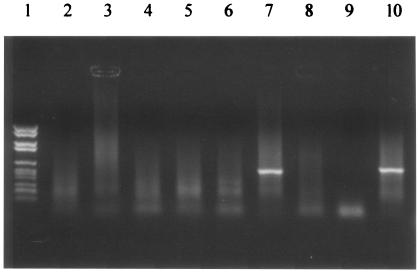
BDV p40 RNA was detected in the peripheral blood leukocytes of 11 of the 82 HIV-infected patients (13.41%). Their CD4 counts ranged from 1 to 724 (mean = 279; median = 199), and 6 of them had CD4 counts equal to or greater than 200/mm3. Four had recently experienced encephalitis, either opportunistic (Toxoplasma gondii and Cryptococcus neoformans infections, progressive multifocal leukoencephalopathy) or HIV induced; these had the lowest CD4 counts (1 to 18 CD4/mm3). Figure 1 illustrates the results of agarose gel electrophoresis for various HIV-infected patients.
FIG. 1.
Agarose gel staining after electrophoresis for peripheral blood leukocyte samples collected in various HIV-infected patients. A positive signal for BDV p40 RT-nested PCR was observed at the expected size (528 bp) in lane 7, whereas lanes 2 to 6 were negative. Lane 1, molecular weight marker; lane 8, negative control (water); lane 9, negative control (positive control in the absence of reverse transcriptase); lane 10, positive control in the presence of reverse transcriptase (p40 INS transcribed into RNA).
Only one of the 80 patients under therapeutic immunosuppression was positive (1.25%); he was a liver graft recipient treated with tacrolimus and corticosteroids who presented no biological feature of immunodepression at the time of blood sampling.
The difference between HIV-infected and HIV-negative immunosuppressed patients for the detection of BDV RNA was significant (χ2 = 8.73, P = 0.003).
BDV p24 RNA was detected in only one of the twelve p40-positive patients; he belonged to the HIV-infected group.
Negative controls showed the absence of DNA contamination in RNA extracts and of cross-amplification for either MV or VSV.
BDV sequence analysis.
Nucleotide sequencing showed complete identity between the BDV p40 DNA fragments amplified from 11 clinical samples and BDV-infected C6 cells. The sequence obtained from the twelfth (HIV-infected) patient demonstrated a single 120-bp deletion, as shown in Fig. 2. All sequences differed from the p40 INS-derived amplified products.
FIG. 2.
Sequence alignments for the BDV p40 amplification products obtained from the RNA-transcribed plasmid p40 INS containing a 56-bp insertion (line 1), an HIV-infected patient with a BDV containing a 120-bp deletion (line 2), an immunocompromised HIV-negative patient (line 3), and BDV-infected C6 cells (line 4).
DISCUSSION
BDV infections, well studied in various animal species, remain controversial in humans (25, 31, 38, 40), although the wide host range of this virus rather suggests such a possibility. The present study indicated that “BDV-like” RNA could be detected by RT-PCR in the peripheral white blood cells of a significant number of immunocompromised patients. To our knowledge, such results were not yet available in France, although similar data were previously published elsewhere (9, 22, 25).
Because scientific doubts were repeatedly expressed on this topic, this study was conducted with constant attention to any source of contamination; this went as far as performing the BDV-infected C6 cell cultures in a level 3 containment laboratory otherwise dedicated to HIV culture diagnostic procedures and located in a building distant from the laboratory where patients samples were analyzed. Negative controls included “RT-minus,” RT-PCR MV and VSV because of the structural similarities among Mononegavirales, and none were positive. In addition, sequencing of our PCR products never indicated any contamination with the plasmid used as a positive control. On the other hand, the RT-nested PCR technique used in this study was reasonably sensitive, since it allowed the detection of 0.075 in vitro-infected cell (such cells were shown to contain approximately 0.0008 infectious BDV each [11]) or 1,000 copies of the RNA-transcribed plasmid. mRNAs of the first gene to be transcribed, p40, represent most of the viral RNA species present in infected cells and were therefore chosen as the main RT-nested PCR target. Finally, hybridization of the amplified DNA fragments enhanced the specificity of the detection because a specific probe was used.
The BDV p40 sequence detected in one patient presented an unusual deletion. Unfortunately, p24 RNA could not be amplified from this sample, which precluded any phylogenetic comparison.
Although this study was not designed to investigate the prevalence of BDV infection, the percentage of positive results in HIV-infected patients (13.41%) was comparable to previously published serological results (3, 7, 8). Another study did not detect any BDV RNA in 27 HIV-infected patients (4): the limited number of patients and the use of a single PCR could account for this discrepancy. Of note, BDV RNA was detected in only one of the 80 patients subjected to immunosuppressive treatments (1.25%), a result similar to those obtained with control patients (7, 8, 23). HIV-associated and drug-induced immunosuppressive conditions present important qualitative differences with regard to the immune system, which could account for the significant difference observed between the two groups of patients in the detection of the BDV RNA. In comparison, human cytomegalovirus infection in these two populations of patients differs in both frequency and clinical expression. There is not yet a definitive explanation for this, but it could depend on subtle differences in the host immune capacity of defense against cytomegalovirus. Other viruses have a preferential expression in particular immunocompromised subjects too, like JC virus in HIV-infected patients or Epstein-Barr virus in some but not all patients with cellular immunodeficiencies. BDV infection could depend on similar mechanisms. On one hand, HIV-infected patients may be altogether more susceptible to BDV infection than other immunocompromised patients, and on the other hand, HIV-specific functional abnormalities in T-cell-mediated immunity could favor BDV reactivation in latently infected individuals, thus improving its detection.
Interestingly, none of the patients investigated in this study showed acute psychiatric symptoms or was treated for a chronic psychiatric disease. Indeed, it was recently suggested that most BDV infections could occur asymptomatically (31, 38). In animals, symptomatic Borna disease seems to result predominantly from immunopathologic mechanisms; BDV-induced neuronal destruction results from a strong host cellular immune response to the virus, including the recruitment of CD8+ and CD4+ T cells (39). Therefore, the relative impairment of cellular immune responses in HIV-infected patients could diminish the risk of clinical expression and bring about an asymptomatic carriage of the virus. Moreover, in the present study, there was no clinical indication that BDV could act as an opportunistic pathogenic agent.
BDV detection in humans has raised a sustained controversy, possibly because of its alleged implication in the physiopathology of psychiatric disorders. BDV load in circulating blood could be very low (one infected cell out of 5 × 106 mononucleated cells in rats) (17). Therefore, the cell yields tested in molecular assays could represent a critical factor: an insufficient cellular input could lead to chance-driven loss of the virus and subsequent false-negative results in certain sample aliquots. Since mononucleated cells may not be the exclusive viral carriers (28), the present study focused on total circulating blood cells, after exclusion of erythrocytes. The analysis of large cell pellets presented technical drawbacks: most commercially available extraction devices were not designed to analyze large amounts of cells and RNA. A classical extraction method was therefore chosen. Molecular techniques including an internal standard should certainly be preferred in future investigations (24, 41). Questions about the best available method for achieving optimal detection of BDV RNA in blood and definitively eliminating false-negative results are still unanswered.
This work, performed in two initially BDV-naive medical laboratories trained for routine molecular diagnostic procedures, under strict experimental conditions, confirmed the presence of BDV or BDV-like sequences in asymptomatic immunocompromised human beings. Defining the prevalence of BDV infection and confirming the putative link with a particular cellular immunosuppression should now be attempted.
Acknowledgments
This work would not have been possible without the generous gifts of Christian Sauder (Abteilung Virologie, Institut für Medizinische Mikrobiologie und Hygiene, University of Freiburg, Freiburg, Germany) and Kazoyoshi Ikuta (Department of Virology, Research Institute for Microbial Diseases, Osaka, Japan), who provided us, respectively, with the DNA plasmid p40 INS and the p40 and p24 recombinant proteins and the appropriate control proteins for serological methods. We express our thanks to Daniel Gonzalez-Dunia (Pasteur Institute, Paris, France), who introduced us to this research field and provided us with indispensable cell lines, and to Juan Carlos de la Torre (The Scripps Research Institute, La Jolla, Calif.), who kindly discussed our preliminary results. We are indebted in Françoise Latapie-Tisnedebat and Annick Guilhaume for their kind, constant, administrative and bibliographic assistance.
REFERENCES
- 1.Allmang, U., M. Hofer, S. Herzog, K. Bechter, and P. Staeheli. 2001. Low avidity of human serum antibodies for Borna disease virus antigens questions their diagnostic value. Mol. Psychiatry 6:329-333. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam, J. D., A. Winokur, W. Dyson, S. Herzog, F. Gonzalez, R. Rott, and H. Koprowski. 1985. Borna disease virus. A possible etiologic factor in human affective disorders? Arch. Gen. Psychiatry 42:1093-1096. [DOI] [PubMed] [Google Scholar]
- 3.Auwanit, W., P. I. Ayuthaya, T. Nakaya, S. Fujiwara, T. Kurata, K. Yamanishi, and K. Ikuta. 1996. Unusually high seroprevalence of Borna disease virus in clade E human immunodeficiency virus type 1-infected patients with sexually transmitted diseases in Thailand. Clin. Diagn. Lab. Immunol. 3:590-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, S., P. Caplazi, M. Fischer, F. Ehrensperger, and R. W. Cone. 1999. Lack of association between Borna disease virus infection and neurological disorders among HIV-infected individuals. J. Neurovirol. 5:190-195. [DOI] [PubMed] [Google Scholar]
- 5.Berg, M., M. Johansson, H. Montell, and A. L. Berg. 2001. Wild birds as a possible natural reservoir of Borna disease virus. Epidemiol. Infect. 127:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bode, L., R. Durrwald, F. A. Rantam, R. Ferszt, and H. Ludwig. 1996. First isolates of infectious human Borna disease virus from patients with mood disorders. Mol. Psychiatry 1:200-212. [PubMed] [Google Scholar]
- 7.Bode, L., S. Riegel, W. Lange, and H. Ludwig. 1992. Human infections with Borna disease virus: seroprevalence in patients with chronic diseases and healthy individuals. J. Med. Virol. 36:309-315. [DOI] [PubMed] [Google Scholar]
- 8.Bode, L., S. Riegel, H. Ludwig, J. D. Amsterdam, W. Lange, and H. Koprowski. 1988. Borna disease virus-specific antibodies in patients with HIV infection and with mental disorders. Lancet 2:689. [DOI] [PubMed] [Google Scholar]
- 9.Bode, L., W. Zimmermann, R. Ferszt, F. Steinbach, and H. Ludwig. 1995. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat. Med. 1:232-236. [DOI] [PubMed] [Google Scholar]
- 10.Carbone, K. M. 2001. Borna disease virus and human disease. Clin. Microbiol. Rev. 14:513-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone, K. M., S. A. Rubin, A. M. Sierra-Honigmann, and H. M. Lederman. 1993. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J. Virol. 67:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 13.Cubitt, B., C. Oldstone, and J. C. de la Torre. 1994. Sequence and genome organization of Borna disease virus. J. Virol. 68:1382-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dauphin, G., V. Legay, C. Sailleau, S. Smondack, S. Hammoumi, and S. Zientara. 2001. Evidence of Borna disease virus genome detection in French domestic animals and in foxes (Vulpes vulpes). J. Gen. Virol. 82:2199-2204. [DOI] [PubMed] [Google Scholar]
- 15.Formella, S., C. Jehle, C. Sauder, P. Staeheli, and M. Schwemmle. 2000. Sequence variability of Borna disease virus: resistance to superinfection may contribute to high genome stability in persistently infected cells. J. Virol. 74:7878-7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galabru, J., M. F. Saron, M. Berg, A. L. Berg, S. Herzog, J. Labie, and S. Zientara. 2000. Borna disease virus antibodies in French horses. Vet. Rec. 147:721-722. [PubMed] [Google Scholar]
- 17.Gonzalez-Dunia, D. 1998. Le virus de la maladie de Borna. Virologie 2:191-198. [Google Scholar]
- 18.Gonzalez-Dunia, D., C. Sauder, and J. C. de la Torre. 1997. Borna disease virus and the brain. Brain Res. Bull. 44:647-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosztonyi, G., B. Dietzschold, M. Kao, C. E. Rupprecht, H. Ludwig, and H. Koprowski. 1993. Rabies and Borna disease. A comparative pathogenetic study of two neurovirulent agents. Lab. Investig. 68:285-295. [PubMed] [Google Scholar]
- 20.Igata-Yi, R., K. Yamaguchi, K. Yoshiki, S. Takemoto, H. Yamasaki, M. Matsuoka, and T. Miyakawa. 1996. Borna disease virus and the consumption of raw horse meat. Nat. Med. 2:948-949. [DOI] [PubMed] [Google Scholar]
- 21.Iwahashi, K., M. Watanabe, K. Nakamura, H. Suwaki, T. Nakaya, Y. Nakamura, H. Takahashi, and K. Ikuta. 1997. Clinical investigation of the relationship between Borna disease virus (BDV) infection and schizophrenia in 67 patients in Japan. Acta Psychiatr. Scand. 96:412-415. [DOI] [PubMed] [Google Scholar]
- 22.Jordan, I., and W. I. Lipkin. 2001. Borna disease virus. Rev. Med. Virol. 11:37-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishi, M., T. Nakaya, Y. Nakamura, M. Kakinuma, T. A. Takahashi, S. Sekiguchi, M. Uchikawa, K. Tadokoro, K. Ikeda, and K. Ikuta. 1995. Prevalence of Borna disease virus RNA in peripheral blood mononuclear cells from blood donors. Med. Microbiol. Immunol. (Berlin) 184:135-138. [DOI] [PubMed] [Google Scholar]
- 24.Legay, V., C. Sailleau, G. Dauphin, and S. Zientara. 2000. Construction of an internal standard used in RT nested PCR for Borna Disease Virus RNA detection in biological samples. Vet. Res. 31:565-572. [DOI] [PubMed] [Google Scholar]
- 25.Lieb, K., and P. Staeheli. 2001. Borna disease virus-does it infect humans and cause psychiatric disorders? J. Clin. Virol. 21:119-127. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 27.Matsuzono, Y., M. Narita, N. Ishiguro, and T. Togashi. 1994. Detection of measles virus from clinical samples using the polymerase chain reaction. Arch. Pediatr. Adolesc. Med. 148:289-293. [DOI] [PubMed] [Google Scholar]
- 28.Planz, O., C. Rentzsch, A. Batra, T. Winkler, M. Buttner, H. J. Rziha, and L. Stitz. 1999. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J. Virol. 73:6251-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Planz, O., H. J. Rziha, and L. Stitz. 2000. Bornavirus isolates of human origin. Lancet 355:656-657. [DOI] [PubMed] [Google Scholar]
- 30.Richt, J. A., R. C. Alexander, S. Herzog, D. C. Hooper, R. Kean, S. Spitsin, K. Bechter, R. Schuttler, H. Feldmann, A. Heiske, Z. F. Fu, B. Dietzschold, R. Rott, and H. Koprowski. 1997. Failure to detect Borna disease virus infection in peripheral blood leukocytes from humans with psychiatric disorders. J. Neurovirol. 3:174-178. [DOI] [PubMed] [Google Scholar]
- 31.Richt, J. A., and R. Rott. 2001. Borna disease virus: a mystery as an emerging zoonotic pathogen. Vet J. 161:24-40. [DOI] [PubMed] [Google Scholar]
- 32.Rott, R., S. Herzog, K. Bechter, and K. Frese. 1991. Borna disease, a possible hazard for man? Arch. Virol. 118:143-149. [DOI] [PubMed] [Google Scholar]
- 33.Rott, R., S. Herzog, B. Fleischer, A. Winokur, J. Amsterdam, W. Dyson, and H. Koprowski. 1985. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science 228:755-756. [DOI] [PubMed] [Google Scholar]
- 34.Salvatore, M., S. Morzunov, M. Schwemmle, and W. I. Lipkin. 1997. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet 349:1813-1814. [DOI] [PubMed] [Google Scholar]
- 35.Sauder, C., A. Muller, B. Cubitt, J. Mayer, J. Steinmetz, W. Trabert, B. Ziegler, K. Wanke, N. Mueller-Lantzsch, J. C. de la Torre, and F. A. Grasser. 1996. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J. Virol. 70:7713-7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider, P. A., T. Briese, W. Zimmermann, H. Ludwig, and W. I. Lipkin. 1994. Sequence conservation in field and experimental isolates of Borna disease virus. J. Virol. 68:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwemmle, M., C. Jehle, S. Formella, and P. Staeheli. 1999. Sequence similarities between human bornavirus isolates and laboratory strains question human origin. Lancet 354:1973-1974. [DOI] [PubMed] [Google Scholar]
- 38.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 39.Stitz, L., B. Dietzschold, and K. M. Carbone. 1995. Immunopathogenesis of Borna disease. Curr. Top. Microbiol. Immunol. 190:75-92. [DOI] [PubMed] [Google Scholar]
- 40.Taieb, O., J. M. Baleyte, P. Mazet, and A. M. Fillet. 2001. Borna disease virus and psychiatry. Eur. Psychiatry 16:3-10. [DOI] [PubMed] [Google Scholar]
- 41.Vahlenkamp, T. W., H. K. Enbergs, and H. Muller. 2000. Experimental and natural Borna disease virus infections: presence of viral RNA in cells of the peripheral blood. Vet. Microbiol. 76:229-244. [DOI] [PubMed] [Google Scholar]
- 42.Waltrip, R. W., II, R. W. Buchanan, W. T. Carpenter, Jr., B. Kirkpatrick, A. Summerfelt, A. Breier, S. A. Rubin, and K. M. Carbone. 1997. Borna disease virus antibodies and the deficit syndrome of schizophrenia. Schizophr Res. 23:253-257. [DOI] [PubMed] [Google Scholar]
- 43.Waltrip, R. W., II, R. W. Buchanan, A. Summerfelt, A. Breier, W. T. Carpenter, Jr., N. L. Bryant, S. A. Rubin, and K. M. Carbone. 1995. Borna disease virus and schizophrenia. Psychiatry Res. 56:33-44. [DOI] [PubMed] [Google Scholar]