Abstract
In the present study we investigated the feasibility and effectiveness of a new biweekly schedule of fotemustine (FTM) in patients with recurrent glioblastoma, after at least one previous treatment. The primary endpoint was progression-free survival at 6 months; secondary objectives were clinical response, overall survival, disease-free survival, and toxicity. Forty patients (median age 52.8 years; median Karnofsky Performance Status at progression 90) underwent second-line chemotherapy with FTM. Selected patients were previously treated with a standard radiotherapy course with concomitant temozolomide (TMZ). After tumor relapse or progression proven by magnetic resonance imaging (MRI), all patients underwent chemotherapy with FTM, given intravenously at dose of 80 mg/m2 every 2 weeks for five consecutive administrations (induction phase), and then every 3 weeks at 100 mg/m2 as maintenance. A total of 329 infusions were administered; the median number of cycles administered was 8. All patients completed the induction phase, and 29 patients received at least one maintenance infusion. Response to treatment was assessed using MacDonald criteria. One complete response [2.5%, 95% confidence interval (CI): 0–10%], 9 partial responses (22.5%, 95% CI: 15–37%), and 16 stable diseases (40%, 95% CI: 32–51%) were observed. Median time to progression was 6.7 months (95% CI: 3.9–9.1 months). Progression-free survival at 6 months was 61%. Median survival from beginning of FTM chemotherapy was 11.1 months. The schedule was generally well tolerated; the main toxicities were hematologic (grade 3 thrombocytopenia in two cases). To the best of our knowledge, this is the first report specifically dealing with the use of a biweekly induction schedule of FTM. The study demonstrates that FTM has therapeutic efficacy as single-drug second-line chemotherapy with a favorable safety profile.
Keywords: Fotemustine, Relapsing glioblastoma, Primary brain tumors, Radiotherapy, Second-line therapy
Introduction
Glioblastoma [glioblastoma multiforme (GBM)] is the most common type of adult primary central nervous system tumor. Median survival of patients with conservatively treated GBM is 14 weeks; by surgical resection alone, 20 weeks; by surgery and radiation, 36 weeks; and with addition of chemotherapy, 40–50 weeks [1]. The gold-standard first-line treatment for GBM is based on a combination of radiotherapy and temozolomide (TMZ), as derived from the study by Stupp et al. [2]. In that study, patients were randomized to radiotherapy alone or radiotherapy plus TMZ. The hazard ratio (HR) for death among patients treated with radiotherapy plus TMZ, when compared with those who received radiotherapy alone, was 0.63 (95% confidence interval, 0.52–0.75; P = 0.001), thus indicating use of this combination for treatment of GBM [2]. The 5-year survey of that study confirmed an advantage for combined therapy, with overall survival (OS) at 5 years of 9.8% (6.4–14.0%) with TMZ versus 1.9% (0.6–4.4%) with radiotherapy alone (HR 0.6, 95% CI: 0.5–0.7; P = 0.0001) [3]. Despite the aggressive first-line therapy, and the recent results with surgery, radiation therapy, and adjuvant chemotherapy, tumors invariably recur and median survival is 15 months [2]; the most favorable patient group with methylated O6-methylguanine-DNA methyltransferase (MGMT) promoter treated with this combination had median survival of 22 months [4].
The problem of unsatisfactory local control of recurrence continues to exist even after application of relatively high-dose radiotherapy; in fact, tumor progression usually starts again after 20–27 weeks. There are no clearly established chemotherapy regimens for treatment of recurrent GBM. TMZ is the best documented agent and has shown a single-agent response rate of 5–8% in GBM, and of 35% in anaplastic astrocytoma [5]. Trials in recurrent GBM have used platinum, procarbazine (PCB), enzastaurin (alone or in combination with carboplatin), and different combinations of these drugs. The response rates reported are up to 20–30%, with short progression-free intervals and median survival rarely exceeding 6 months. In general, studies about pharmacological treatment of recurrent GBM have given controversial results [6]. Recent phase II trials have demonstrated activity of nitrosoureas such as fotemustine (FTM) in recurrent GBM [7–10]. FTM is a third-generation nitrosourea with an alkylating cytotoxic activity, characterized by a phosphoalanine carrier group grafted onto the nitrosourea radical, which gives it high lipophilicity that allows it to cross the blood–brain barrier [11, 12]. The drug, developed some 20 years ago, has been employed in treatment of metastatic melanoma, hematological malignancies, and on the basis of its pharmacokinetic properties, in brain tumors, either primitive or metastatic [7]. FTM showed both in vitro and in vivo marked antineoplastic activity on human GBM and medulloblastoma cell lines [8, 9]. In a phase I study, Khayat et al. [13] detected the dose of FTM to be used in clinical practice: 100 mg m2 in i.v. infusion of 1 h, administered on days 1, 8, and 15 (induction), to be repeated after 4–5 weeks every 21 days (maintenance). In another two phase II trials, FTM activity was confirmed to be 15.5–26% in recurrent GBM [10, 14] if administered with similar schedules. Myelosuppression represented the most relevant side-effect, being reported also in more than 30% of the subpopulation pretreated with chemotherapy. In the last months, several phase II studies have evaluated both efficacy and safety of FTM as second-line chemotherapy in patients previously treated with TMZ plus radiotherapy as adjuvant treatment in 160 patients with recurrent GBM. The treatment was active, with a promising disease control rate (DCR) and a favorable safety profile. These data are interesting if we consider that most of the patients had poor performance status with compromised hematological status [7]. In the trial by Brandes et al. patients with progressive GBM after radiotherapy plus concomitant and/or adjuvant TMZ received 3-weekly doses (100–75 mg/m2) of FTM followed, after 5 weeks rest, by FTM (100 mg/m2) every 3 weeks. Forty-three patients were enrolled. Progression-free survival at 6 months was 20.9%; 3 patients (7.1%) had partial response (PR), and 15 (34.9%) had disease stabilization (SD). Median survival was 6 months (95% CI: 5–7 months) [15]. It is noteworthy that, after the inclusion of the first three patients, the protocol was amended to reduce FTM dosage during induction therapy to 75 mg/m2 due to occurrence of grade 4 thrombocytopenia. In another study, on 40 patients with recurrent pretreated GBM, the authors confirmed that low-dose FTM at 65–75 mg/m2 (induction phase) had activity comparable to that of the conventional schedule [16]. In another two phase II studies, FTM, at a similar induction schedule, was used for treatment of recurrent GBM after previous combination therapy with radiotherapy and TMZ [17, 18] with favorable results in terms of both response rate and progression-free survival at 6 months (PFS-6). Global incidence of acute toxicity effects was 40% (all grades) during treatment with FTM. Preclinical evidence suggests that the MGMT repair protein is involved in resistance to alkylating agents including FTM [19]. However, there is little information available regarding the clinical correlation between MGMT promoter methylation status and the anticancer activity of FTM.
On the bases of all the previous considerations, we performed a phase II trial enrolling 40 patients with relapsing GBM, pretreated with radiotherapy plus TMZ, in order to assess both efficacy and the safety profile of a new schedule of FTM administrated at low chronic doses followed by a maintenance phase. The objectives of the trial are evaluation of: PFS-6, response rate, toxicity, and any correlation of the latter with MGMT gene promoter methylation status.
Patients and methods
Patient eligibility criteria
Adult patients with recurrent or progressive, histologically confirmed GBM following surgery, and radiotherapy and chemotherapy with TMZ, for at least three cycles according to Stupp protocol, were enrolled in the trial. Progression was documented by MRI or computed tomography (CT) scans at least 3 months after the end of radiotherapy or evidence of progressive disease (PD) on two consecutive radiologic investigations. Patients were required to have proven evidence of tumor recurrence or progression and Karnofsky Performance Status >70 at the moment of starting FTM chemotherapy. Patients needed to have: minimum life expectancy of 3 months; measurable disease with contrast enhancement using MRI and/or CT scans, assessed within 2 weeks before study entry; and at least one unidimensionally measurable lesion of 2 cm in diameter by MRI. Other eligibility criteria included adequate hematologic function with white cell count >2 × 109/l, platelets count >100,000/mm2, and hemoglobin >8 g/dl, renal function with creatinine level <2 mg/dl, and adequate liver function with aspartate aminotransferase level <1.5× the upper limit of normal. The Institutional Ethical Committee approved the protocol, and patients were required to provide informed consent before beginning the treatment.
Treatment plan
Patients were treated with 1 h intravenous infusion of FTM according to the following schedule: induction phase with 80 mg/m2 FTM on days 1, 15, 30, 45, and 60 followed by a 4-week rest period. After this period, in nonprogressive patients, maintenance therapy was given with 80 mg/m2 FTM every 4 weeks until progression or unacceptable toxicity. In the case of toxicity occurrence, if treatment suspension was prolonged by more than 2 weeks beyond the next scheduled cycle of the planned treatment, the patient was permanently withdrawn from the study.
Response and toxicity assessment
Tumor evaluation was performed through brain MRI and clinical examination. Response to treatment was assessed at baseline, after the induction phase, before the maintenance schedule, and every three cycles thereafter, or whenever disease progression was clinically suspected. MacDonald et al. [20] criteria were uniformly adopted for response evaluation. According to MacDonald, the following four response categories can be identified: (1) complete response (CR): disappearance of all enhancing tumor on consecutive CT or MRI scans at least 1 month apart, off steroids, and neurologically stable or improved; (2) PR: −50% reduction in size of enhancing tumor on consecutive CT or MRI scans at least 1 month apart, steroids stable or reduced, and neurologically stable or improved; (3) progressive disease (PD): >25% increase in size of enhancing tumor or any new tumor on CT or MRI scans, or neurologically worse, and steroids stable or increased; (4) stable disease (SD): all other situations. Neurological status was assessed by considering signs and symptoms possibly correlated with progression, as compared with the previous examination; each variation in daily corticosteroids dosage was recorded. Toxicity was evaluated according to the National Cancer Institute (NCI) common toxicity criteria (CTC, version 3.0) during routine controls at 2-weekly intervals or, if clinically indicated, at weekly intervals. Monitoring of serum chemistry and blood cell counts was performed prior to each cycle of therapy at 2-weekly intervals during induction, and at 4-weekly intervals during maintenance. In case of hematological toxicity necessitating delay of chemotherapy administration, blood counts were performed at weekly intervals.
Study objectives and statistical analysis
The study is an open, multicenter, nonrandomized, single-arm, phase II study in recurrent GBM patients. The primary efficacy endpoint of the study was the percentage of patients free from disease, PFS-6. PFS was measured from initiation of FTM to progression or death due to any cause or last follow-up assessment, whichever came first. OS was measured from start of FTM to death for any reason, or last follow-up assessment. Secondary endpoints were the following: rate of best observed response, duration of objective response and stabilization, duration of CR, time to disease progression, OS, and toxicity. Other objectives were assessment of functional status, of the amount of symptomatic drug assumption, and evaluation of MGMT methylation and its correlation with clinical outcome. Drug activity was evaluated based on a one-stage Fleming [21] study design for determination of response rates based on a single treatment group. Median time to progression and median time to survival were also estimated with their 95% confidence interval; PFS-6 and OS were calculated using the Kaplan–Meier method [22] (Fig. 1). MGMT promoter methylation analysis was performed on tissues taken from the primary surgical specimen before treatment with radiotherapy and TMZ. Genomic DNA was isolated from one paraffin section of malignant glioma tissue; it was subjected to bisulfite treatment, and modified DNA was analyzed by methylation-specific protein C reactive (PCR) performed in a two-step approach.
Fig. 1.
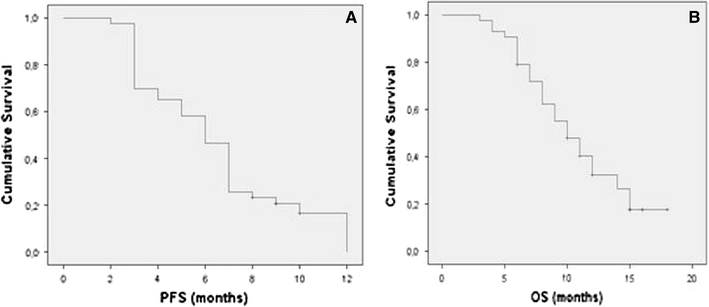
Kaplan–Meier estimates of progression-free (a) and overall (b) survival time from study entry for the patients enrolled in the study
Results
Patient characteristics
Between October 2006 and December 2008, 40 patients were enrolled. Main patient and tumor characteristics are summarized in Tables 1, 2, and 3. Median age was 52.8 years (range 31–75 years) and median KPS was 90. All 40 patients had histologically proven recurrent GBM. Twenty-three patients underwent surgery with macroscopically radical resection. Each patient had completed external-beam radiation therapy (60 Gy/30 fractions) concurrently and/or followed by adjuvant TMZ. Median number of TMZ cycles was 6 (range 3–24). No patient underwent a second surgical procedure at the time of progression following TMZ administration. Median time interval between surgery and recurrence was 9.5 months (range 3.3–34.2 months); median time interval between diagnosis and FTM induction was 11.6 months (range 5.7–42 months).
Table 1.
Demographic characteristics of patients enrolled in the trial
| n (%) | |
|---|---|
| Sex | |
| Female | 11 (27.5) |
| Male | 29 (72.5) |
| Age (years) | |
| Median | 52.8 |
| Range | 30–75 |
| KPS | |
| Median | 90 |
| Range | 70–100 |
| Radicality of surgical resection | |
| Macroscopically radical | 23 (57.5) |
| Partial | 10 (25) |
| Biopsy | 7 (17.5) |
| Initial grade | |
| Glioblastoma | 40 (100) |
| Radiotherapy + TMZ | |
| 60 Gy RT + TMZ | 40 (100) |
| Interval between completion of TMZ and FTM initiation | |
| Within 3 months | 14 (35) |
| Beyond 3 months | 26 (65) |
| MGMT status | |
| Methylated | 7 (17.5) |
| Unmethylated | 17 (42.5) |
| Unknown | 16 (40) |
Table 2.
Clinical activity of the schedule
| Overall response | No. of patients (%) |
|---|---|
| CR | 1 (2.5) |
| PR | 9 (22.5) |
| Overall response rate | 10 (25) |
| No change | 16 (40) |
| DCR | 26 (65) |
| PD | 14 (35) |
| Total | 40 (100) |
Table 3.
Incidence of drug-related adverse events (safety population), by grade of severity, during induction and maintenance
| Adverse event | Induction (n = 40) | Maintenance (n = 29) |
|---|---|---|
| Leukopenia | ||
| Grade 1–2 | 7 (17.5) | 4 (14) |
| Grade 3 | 1 (2.5) | 1 (3.5) |
| Grade 4 | – | – |
| Neutropenia | ||
| Grade 1–2 | 8 (20) | 4 (14) |
| Grade 3 | 1 (2.5) | 2 (7) |
| Grade 4 | – | – |
| Thrombocytopenia | ||
| Grade 1–2 | 9 (22.5) | 7 (24) |
| Grade 3 | 2 (5) | 2 (7) |
| Grade 4 | – | – |
| Anemia | ||
| Grade 1–2 | 6 (15) | 2 (7) |
| Grade 3 | 1 (2.5) | – |
| Grade 4 | – | – |
| Nausea | ||
| Grade 1–2 | 5 (12.5) | 3 (10) |
| Grade 3 | – | – |
| Grade 4 | – | – |
| Vomiting | ||
| Grade 1–2 | 4 (10) | 3 (10) |
| Grade 3 | – | – |
| Grade 4 | – | – |
| Cutaneous rush | ||
| Grade 1–2 | 2 (5) | – |
| Grade 3 | – | – |
| Grade 4 | – | – |
| Metabolic/laboratory AST/ALT | ||
| Grade 1–2 | 3 (7.5) | 7 (24) |
| Grade 3 | – | 3 (10) |
| Grade 4 | – | – |
AST aspartate transaminase, ALT alanine transaminase
Clinical activity evaluation
All patients included in the study were assessable for response. Median follow-up time was 18 months. Among the 40 assessable patients, 1 had CR (2.5%, 95% CI: 0–10%) and 9 had PR (22.5%, 95% CI: 15–37%). SD was noted in 16 patients (40%, 95% CI: 32–51%), and median duration of SD in these 16 patients was 4.9 months (95% CI: 1.9–9.1 months). On the other hand, median PFS of responders was 9.0 months (95% CI: 7.98–10.02 months). However, clinical benefit (CR + PR + SD lasting 12 weeks) accounted for 65% (95% CI: 59.7–81.9%), while 14 patients (35%) experienced disease progression. All responses were confirmed by independent centralized review, and stable or decreased steroid dosage was confirmed in all patients at the time of recording response.
In the present trial we enrolled 14 patients with PD recorded after or during TMZ adjuvant treatment. In detail, six patients were in progression while receiving adjuvant TMZ before completion of six cycles of therapy, and the eight remaining patients progressed after completion of adjuvant TMZ and a treatment-free interval smaller than 3 months. We did not observe any clinical response in these 14 patients, and only 4 of them achieved SD. Among the 24 patients with data available regarding MGMT promoter methylation status, DCR was greater in methylated (3/7, 42%) than in unmethylated (6/17, 35%) MGMT patients, but the difference was not statistically significant (P = 0.24). On the other hand, DCR was significantly greater in patients who started FTM at least 3 months after TMZ administration had been concluded (16% versus 9%, P = 0.004). Median PFS was 6.7 months (95% CI: 3.9–9.1 months). Median PFS-6 was 39%. Median OS from beginning FTM chemotherapy was 11.1 months. No significant differences were found between median PFS, evaluated using log-rank test, in relation to KPS (P = 0.42, using cutoff value of KPS ≥80), age (P = 0.62, using cutoff value of ≥65 years) or methylated or unmethylated status of MGMT promoter (P = 0.18). Efficacy of this treatment was confirmed by the decreased requirement for medication to palliate neurologic symptoms. At baseline, 37 (92.5%) patients required corticosteroids, and 13 (32.5%) opioids. After 2 months of FTM treatment, these percentages progressively decreased. In fact, at 2 months, 23 (57.5%) patients required corticosteroids, and 6 (15%) opioids. These data confirm the clinical benefit of the schedule used in the current study in this subset of patients.
Toxicity evaluation
Overall, 40 patients received a total of 329 infusions; the median number of cycles administered was 8. All 40 patients completed the induction phase as planned. During the induction phase the major toxicity was thrombocytopenia, which developed in 11 (27.5%) patients, 2 (5%) of them with NCI-CTC grade 3 intensity, and neutropenia, which developed in 9 (22.5%) patients, but only one (2.5%) with grade 3 intensity. In our series we did not observe any cases of lymphopenia. The most commonly reported grade 3 nonhematological toxicities were nausea and vomiting, in six (12.5%) and five (10%) patients, respectively, without any grade 3 toxicity. Twenty-nine (72.5%) patients started maintenance chemotherapy and received a median of 4 cycles (range 2–12). During this period we observed an increase of incidence of hepatic toxicity, with a rise in ALT and AST values in ten patients (34%), three of whom (10%) had NCI CTC grade 3 toxicity. Grade 2 and 3 thrombocytopenia was documented in seven (24%) and two (7%) patients, respectively. Overall, the toxicity incidence rate of FTM increased with the number of completed cycles. FTM was generally well tolerated, and treatment interruption due to toxicity was not observed in our series; three patients had a delay of infusion of 1 week because of hepatic toxicity, and another two patients required dose reduction of 25% during maintenance.
Discussion
For most patients with newly diagnosed GBM, postoperative radiotherapy plus TMZ has become the standard of care [2]. Assuming that TMZ is moving rapidly into the first-line setting, the question arises whether it should be given again in relapsed patients.
The choice of chemotherapy depended on whether the patient had previously received chemotherapy as first-line therapy. Frequently, treatment decisions have to be made based on case-by-case evaluation, depending on prior therapy, time to relapse, tumor grade, and performance status [23]. Several phase II studies have shown that chemotherapy approaches can provide some encouraging results in terms of efficacy. Nitrosoureas, PCB, paclitaxel, TMZ, and cisplatin were tested, although the impact on OS was minimal [24–26]. In a large randomized, multicentre, open-label phase II study that compared TMZ and PCB in 225 patients with GBM at first relapse, Yung et al. [27] demonstrated that the 6-month PFS rate for patients who received TMZ was 21%, with an improvement in patient health-related quality of life. Antiangiogenic therapy has been demonstrated to represent a promising novel approach for treatment of malignant brain tumors. Recent clinical trials targeting vascular endothelial growth factor (VEGF) signaling with bevacizumab plus irinotecan have shown promising radiographic and clinical responses, while also confirming adequate safety in recurrent GBM patients [28]. We have also to consider the high impact on national health systems of the pharmaco-economic aspects of this regimen, as both irinotecan and bevacizumab are extremely expensive agents.
FTM represents an evolution of nitrosourea that was synthesized to facilitate passage through the blood–brain barrier by the addition of a phosphoalanine vector. The use and efficacy of this drug, using the conventional schedule [14] in patients with recurrent GBM, are known but limited by considerable toxicity. Hematological toxicity is observed in nearly 40% of patients receiving FTM, and thrombocytopenia and leukopenia are more frequent and significant in pretreated patients.
Myelosuppression was the most common adverse event that occurred, mainly during the induction phase of treatment. We cannot ignore that the traditional schedule of FTM including the induction phase achieved hopeful clinical results. On the basis of these considerations, we planned a new treatment that maintains the global dosage/time ratio but with different fractionation, similarly to our experience reported for low protracted dose of TMZ [29]. In fact, metronomic administration of cytotoxic drugs is proven to be less toxic than acute administration, causing also important and different biological effects on tumor cells. In the traditional schedule, patients received 400 mg/m2 FTM during an 8-week period (days 1, 8, and 15 every 28–35 days), with considerable toxicity that frequently does not allow completion of the schedule. In the present trial, the patients received, during the same period, 400 mg/m2 FTM fractionated to days 1, 15, 30, 45, and 60, and we did not observe any serious adverse events that compromised the dose density of this treatment. In our opinion, this could be the explanation for the promising results obtained in our series. The results of the present study show that fractionated FTM monotherapy is able to achieve response in 25% of patients, with an overall DCR of 65%. These results are remarkable, since they were observed in a pretreated population that had received one previous line of chemotherapy. However, our favorable data also have to be interpreted on the basis of “pseudoprogression” occurrence that could overestimate the results obtained in the present trial. We tried to reduce the influence of the occurrence of such a pseudoprogression effect by excluding patients who experienced progression within 3 months from radiation therapy according to NCI of Canada recommendations [30]. Moreover, none of the 14 patients enrolled in the study and who ended adjuvant TMZ treatment in the 3 months achieved an objective response during or after FTM therapy. However, we cannot exclude that some of the recorded responses could be resolution of radionecrosis, since we do not have data from amino-acid positron emission tomography (PET) and/or MRI spectroscopy demonstrating the specific features of tumor tissue. In addition, pretreatment with an alkylating agent could positively select for patients who will respond to alkylating therapy in relapse. The results regarding the activity of our schedule are in line with those reported in the literature with single-agent FTM at the conventional schedule of 100 mg/m2 [10, 14]. However, these previous experiences were characterized by important hematological toxicity, as recently confirmed by Brandes et al. [15]. In the present study, we confirmed these results in a larger population of patients, with a better DCR and toxicity profile. Our efficacy data (PFS-6) are similar to those reported in two other trials, by Scoccianti et al. [18] and Fabrini et al. [17], but with a lower rate of grade 3–4 hematological toxicities, likely due to the longer period of rest between each induction phase. This is reasonable if we consider that we use a similar intensive dose for induction phase, and probably it may represent an explanation for the better results than those obtained by Brandes et al. [15], even if comparison across trials is always challenging. The crucial role of the induction phase for the efficacy of this drug was recently shown in two studies exploring FTM in combination with either dacarbazine or PCB [31, 32]. In these two trials the lower observed efficacy, considered as response rate (3–11%), is probably due to the different schedule that, in our case, includes the weekly induction phase. Our data confirmed the absence of cross-resistance between FTM and TMZ, since all responses were observed in TMZ-pretreated patients. PFS-6, the primary endpoint of the protocol, was 61% and represents an encouraging result compared with other experience with this drug or with other chemotherapeutic or targeted agents [33] or using the combination of irinotecan and bevacizumab. The treatment was well tolerated, underlining the lower toxicity than the usual schedule of FTM. In fact, in our series we recorded few grade 3 toxicities, preserving patient quality of life (QoL), which remains a crucial goal for these patients who currently cannot be cured but only palliated. Moreover, the patients who started FTM at least 3 months after completion of TMZ administration had a significantly higher response rate than patients who started FTM immediately after TMZ completion. These findings are in line with similar observations reported by others [15, 33]. MGMT promoter methylation status represents an important prognostic factor in newly diagnosed GBM patients, and it can influence the efficacy of TMZ therapy [4]. However, after primary treatment for newly diagnosed GBM, changes may occur in the status of MGMT promoter methylation [34]. In our study, we found a higher rate of disease control in patients with methylated MGMT, and a trend toward prolonged PFS-6, without achieving statistical significance. This result reflects the limited statistical power due to the relatively small number of cases enrolled in the present trial. To the best of our knowledge, this is the first report specifically dealing with use of protracted low FTM doses for induction phase. The considerable activity and lack of toxicity of this schedule open a new avenue for investigation of the possible use of FTM in combination with targeted therapy agents.
Acknowledgment
This work was partially supported by grants from Lega Italiana per la Lotta contro i Tumori (LILT).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s11060-010-0415-2
References
- 1.Huncharek M, Muscat J. Treatment of recurrent high grade astrocytoma: results of a systematic review of 1415 patients. Anticancer Res. 1998;18(2B):1303–1311. [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 5.Nieder C, Adam M, Molls M, Grosu AL. Therapeutic options for recurrent highgrade glioma in adult patients: recent advances. Crit Rev Oncol Hematol. 2006;60(3):181–193. doi: 10.1016/j.critrevonc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Addeo R, De Santo SM, Del Prete S, Caraglia M. Fotemustine and recurrent glioblastoma: possible new opportunities for an old drug. Cancer Chemother Pharmacol. 2009;64:863–866. doi: 10.1007/s00280-009-1086-6. [DOI] [PubMed] [Google Scholar]
- 7.De Rossi A, Rossi L, Laudisi A, et al. Focus on fotemustine. J Exp Clin Cancer Res. 2006;25(4):461–468. [PubMed] [Google Scholar]
- 8.Filippeschi S, Colombo T, Bassani D, De Francesco L, Arioli P, D’Incalci M, Bartosek I, Guaitani A. Antitumor activity of the novel nitrosourea S10036 in rodent tumors. Anticancer Res. 1988;8:1351–1354. [PubMed] [Google Scholar]
- 9.Fischel JL, Formento P, Etienne MC, Gioanni J, Frenay M, DeloVre P, Bizzari JP, Milano G. In vitro chemosensitivity testing of Fotemustine (S 10036), a new antitumor nitrosourea. Cancer Chemother Pharmacol. 1990;25:337–341. doi: 10.1007/BF00686233. [DOI] [PubMed] [Google Scholar]
- 10.Malhaire JP, Lucas B, Simon H, Person H, Dam-Hieu P, Labat JP. Fotemustine (Muphoran) in 22 patients with relapses of high-grade cerebral gliomas. Bull Cancer. 1999;86:289–294. [PubMed] [Google Scholar]
- 11.Meulemans A, Giroux B, Hannoun P, Robine D, Henzel D. Comparative diffusion study of two nitrosoureas: carmustine and fotemustine in normal rat brain, human and rat brain biopsies. Chemotherapy. 1991;37:86–92. doi: 10.1159/000238838. [DOI] [PubMed] [Google Scholar]
- 12.Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem. 1980;23:682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- 13.Khayat D, Lokiec F, Bizzari JP, et al. Phase I clinical study of the new amino acidlinked nitrosourea, S 10036, administered on a weekly schedule. Cancer Res. 1987;47(24 Pt 1):6782–6785. [PubMed] [Google Scholar]
- 14.Frenay M, Giroux B, Khoury S, Derlon JM, Namer M. Phase II study of fotemustine in recurrent supratentorial malignant gliomas. Eur J Cancer. 1991;27:852–856. doi: 10.1016/0277-5379(91)90133-X. [DOI] [PubMed] [Google Scholar]
- 15.Brandes AA, Tosoni A, Franceschi E, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Cancer Chemother Pharmacol. 2009;64(4):769–775. doi: 10.1007/s00280-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabi A, Metro G, Russillo M, et al. Treatment of recurrent malignant gliomas with fotemustine monotherapy: impact of dose and correlation with MGMT promoter methylation. BMC Cancer. 2009;9:101. doi: 10.1186/1471-2407-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabrini MG, Silvano G, Lolli I, Perrone F, Marsella A, Scotti V, Cionini L. A multiinstitutional phase II study on secondline fotemustine chemotherapy in recurrent glioblastoma. J Neurooncol. 2009;92(1):79–86. doi: 10.1007/s11060-008-9739-6. [DOI] [PubMed] [Google Scholar]
- 18.Scoccianti S, Detti B, Sardaro A, et al. Second-line chemotherapy with fotemustine in temozolomide-pretreated patients with relapsing glioblastoma: a single institution experience. Anticancer Drugs. 2008;19(6):613–620. doi: 10.1097/CAD.0b013e3283005075. [DOI] [PubMed] [Google Scholar]
- 19.Kaina B, Mühlhausen U, Piee-Staffa A, et al. Inhibition of O6-methylguanine-DNA methyltransferase by glucose-conjugated inhibitors: comparison with nonconjugated inhibitors and effect on fotemustine and temozolomide-induced cell death. J Pharmacol Exp Ther. 2004;311(2):585–593. doi: 10.1124/jpet.104.071316. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 21.Fleming TR. One sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. doi: 10.2307/2530297. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 23.Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms—an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 24.Pipas JM, Meyer LP, Rhodes CH, et al. A phase II trial of paclitaxel and topotecan with filgrastim in patients with recurrent or refractory glioblastoma multiforme or anaplastic astrocytoma. J Neurooncol. 2005;71(3):301–305. doi: 10.1007/s11060-004-2026-2. [DOI] [PubMed] [Google Scholar]
- 25.Silvani A, Eoli M, Salmaggi A, Lamperti E, Maccagnano E, Broggi G, Boiardi AJ. Phase II trial of cisplatin plus temozolomide, in recurrent and progressive malignant glioma patients. Neurooncol. 2004;66(1–2):203–208. doi: 10.1023/B:NEON.0000013479.64348.69. [DOI] [PubMed] [Google Scholar]
- 26.Brandes AA, Tosoni A, Cavallo G, Bertorelle R, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from Gruppo Italiano Cooperativo di Neuro-oncologia (GICNO) Br J Cancer. 2006;95(9):1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yung WKA, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 29.Addeo R, De Rosa C, Faiola V, et al. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for non small cell lung cancer and breast cancer patients with brain metastases. Cancer. 2008;113(9):2524–2531. doi: 10.1002/cncr.23859. [DOI] [PubMed] [Google Scholar]
- 30.Pirzkall A, McGue C, Saraswathy S, et al. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2009;11(6):842–852. doi: 10.1215/15228517-2009-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fazeny-Dörner B, Veitl M, Wenzel C, et al. Second-line chemotherapy with dacarbazine and fotemustine in nitrosourea-pretreated patients with recurrent glioblastoma multiforme. Anticancer Drug. 2003;14:437–442. doi: 10.1097/00001813-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Silvani A, Lamperti E, Gaviani P, et al. Salvage chemotherapy with procarbazine and fotemustine combination in the treatment of temozolomide treated recurrent glioblastoma patients. J Neurooncol. 2008;87:143–151. doi: 10.1007/s11060-007-9427-y. [DOI] [PubMed] [Google Scholar]
- 33.Perry JR, Rizek P, Cashman R, Morrison M, Morrison T. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the rescue approach. Cancer. 2008;113:2152–2157. doi: 10.1002/cncr.23813. [DOI] [PubMed] [Google Scholar]
- 34.Parkinson JF, Wheeler HR, Clarkson A. Variation of O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation in serial samples in glioblastoma. J Neurooncol. 2008;87:71–78. doi: 10.1007/s11060-007-9486-0. [DOI] [PubMed] [Google Scholar]


