Abstract
Fusarium polyphialidicum caused a corneal ulcer in a Spanish man. Diagnosis was established by a histopathological study and repeated cultures. The isolate was clearly resistant in vitro to the antifungal agents tested. This is the first case of human or animal mycosis by this rare fungus.
CASE REPORT
A 65-year-old Spanish male was referred to the Ophthalmology Service of the Lozano Blesa University Hospital in Zaragoza on 5 June 2000 because of acute keratitis in his left eye. Approximately 1 month earlier, the patient suffered a nonpenetrating foreign-body injury when he was working on his farm. Since then, he had complained of increasing disturbance in the eye. On examination, he showed a central corneal ulcer of ca. 4 mm in diameter with irregular borders, slight infiltration, and a thinned weakness but with no risk of perforation. A hypopyon 1.2 mm high was also observed. Visual acuity was 1 to 200. The right eye showed no abnormality. Results of other local and systemic examinations were negative.
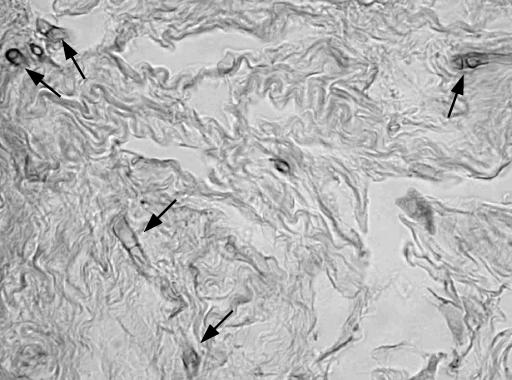
Deep corneal scrapings were collected with a sterile scalpel blade for fungal and bacteriological cultures. The corneal scrapings were inoculated directly onto Sabouraud glucose agar (Oxoid, Basingstoke, England) and incubated at 25 and 37°C. After 2 days, numerous small whitish colonies appeared on all cultures. All the colonies were apparently identical, and the fungus was identified as Fusarium sp. The results of routine bacteriological cultures were negative. Since the fungus was detected, treatment was initiated with 0.5% amphotericin B applied topically every hour. However, after a week, the condition of the eye worsened; the corneal infiltration and hypopyon increased. The ulcer became torpid and enlarged into a deepening corneal abscess and endophthalmitis. More corneal scrapings were collected for new cultures, which were again negative for bacteria and positive for the same fungus recovered previously. A corneal transplant was performed on 30 June. Cultures from a biopsy of the excised cornea again yielded the same Fusarium sp. Portions of the cornea were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Sections of the stained tissue revealed short, contorted, hyaline, septate, hyphal fragments (Fig. 1). The transplanted eye improved considerably, and therefore the patient was discharged. In September 2000, the patient presented a visual acuity of 20 to 40 in the affected eye.
FIG. 1.
Hematoxylin and eosin stain from the corneal tissue showing short hyphal fragments (arrows). Magnification, ×1,280.
The clinical strain was sent to the Faculty of Medicine of the Rovira i Virgili University in Reus, Spain, to be identified and to determine its antifungal susceptibility.
Mycological study and diagnosis.
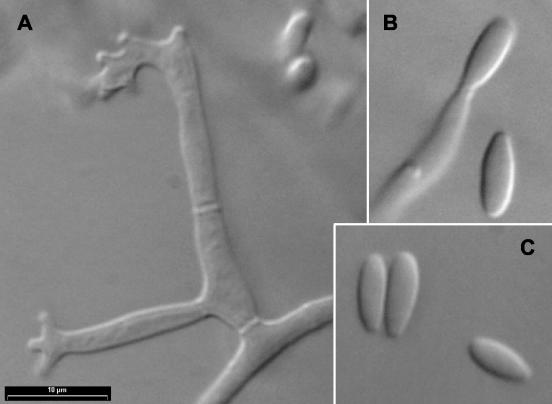
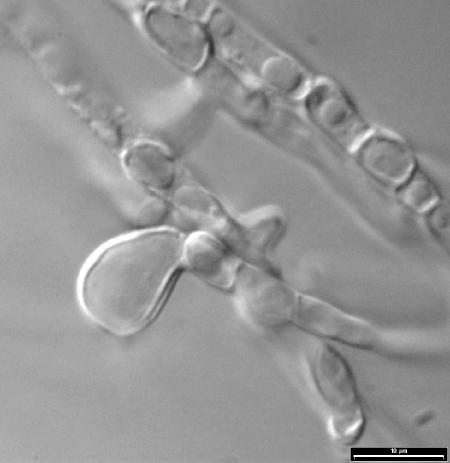
For identification purposes, the fungus was subcultured on potato dextrose agar (PDA; Difco Laboratories, Detroit, Mich.) and oatmeal agar (30 g of oat flakes, 1 g of MgSO4 · 7H2O, 1.5 g of KH2PO4, 15 g of agar, 1,000 ml of tap water) and incubated at 25, 37, and 40°C in darkness. After 12 days at 25°C, the colonies on PDA attained a diameter of 68 to 70 mm and those on oatmeal agar covered the whole agar surface. Colony morphologies were very similar on both media: white, cottony, and floccose towards the edge, with a colorless or pale yellow reverse. Microscopically, mono- and polyphialidic conidiogenous cells, which were hyaline and measured 10 to 48 μm long by 2.5 to 3.5 μm wide, were observed (Fig. 2A and B). Polyphialidic conidiogenous cells were predominant and usually presented 2 to 5 conidiogenic loci. Only microconidia were produced (Fig. 2C). They were abundant, grouped in slimy masses, hyaline, usually nonseptate, fusiform, or subclavate and measured 5 to 14 by 2 to 3 μm. Intercalary, smooth- and thick-walled chlamydospores, up to 15 μm in diameter, were observed, although only on PDA (Fig. 3). Colonies on PDA at 37°C attained a diameter of 12 to 14 mm after 5 days. They were white and floccose, with a yellowish reverse and without sporulation. The fungus did not grow at 40°C.
FIG. 2.
F. polyphialidicum FMR 7804. (A) Polyphialidic conidiogenous cells. (B and C) Monophialidic conidiogenous cells and microconidia.
FIG. 3.
F. polyphialidicum FMR 7804. Chlamydospores are shown.
The combination of the morphological features indicated above is not typical of any fusarial species described as pathogenic to humans (5). On the basis of the macroscopic features, the abundant production of polyphialidic conidiogenous cells, and the microconidial morphology, we tentatively identified the case strain as Fusarium polyphialidicum. However, the fungus could not be definitively identified because macroconidia and rough-walled chlamydospores (typical of that species) were never developed by the strain. To confirm the identification, the internal transcribed spacer (ITS) region was sequenced. DNA extraction, amplification, and sequencing were performed as described by Solé et al. (18). A search of the GenBank database (1) revealed a high level of similarity (99%) with the sequence of another F. polyphialidicum strain (GenBank accession no. X94172). Living cultures were deposited in the Faculty of Medicine, Reus, Spain (FMR 7804), in the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS 111770), and in the Institute of Hygiene and Epidemiology, Brussels, Belgium (IHEM 19748).
Antifungal susceptibility testing.
The susceptibility of the fungus to eight antifungal drugs (amphotericin B, ketoconazole, fluconazole, flucytosine, itraconazole, terbinafine, ravuconazole, and voriconazole) was determined by a previously described microdilution method (16), mainly following the guidelines of the National Committee for Clinical Laboratory Standards for molds (13). The tests were performed by using RPMI 1640 medium buffered to pH 7 with 0.165 M morpholinepropanesulfonic acid, an inoculum of 1.7 × 104 CFU/ml, an incubation temperature of 32°C, an incubation time of 48 h, and an additive drug dilution procedure. MICs were >128 μg of flucytosine/ml, 64 μg of fluconazole/ml, 16 μg of ketoconazole/ml, 8 μg of itraconazole and voriconazole/ml, 4 to 8 μg of terbinafine/ml, 2 to 4 μg of amphotericin B/ml, and 2 μg of ravuconazole/ml.
Discussion.
Keratomycosis is a relatively common disease that can rapidly lead to progressive corneal destruction and loss of vision in most cases (3). Numerous predisposing factors, such as use of corticosteroids or antibiotics, foreign bodies, postoperative infections, pre-existing eye diseases, contact lenses, systemic illness, age, sex, climate, and season, have been reported (3). The human cornea can be infected by multiple fungal species, of which Candida, Aspergillus, and Fusarium spp. are the most common. In most cases, Fusarium species have clearly been predominant. For example, in a review of 156 cases of mycotic keratitis performed in India, 32.3% were caused by Fusarium, 29% by Aspergillus, 12.9% by Alternaria, and 6.4% by Candida (20). However, species of other genera are regularly being added to the long list of agents able to cause keratomycosis. Recently, we reported three Brazilian cases caused by Curvalaria senegalensis (9), Phaeoisaria clematidis (8), and Sarcopodium oculorum (6), three species not previously reported as causing human keratomycoses.
The keratitis caused by Fusarium is clinically similar to that caused by other fungi, but its prognosis is worse (22). The presence of malignant glaucoma is a severe complication of fusarial keratitis (10). The most common Fusarium species that cause ocular infections is F. solani (5). This species has also been reported to cause keratitis related to contact lenses (19). Other species, such as F. dimerum, F. oxysporum, F. sacchari, and F. verticillioides, are more rarely involved in ocular infections (7, 22). Here, we describe a case of keratitis in an otherwise healthy patient caused by F. polyphialidicum. The present report is the first one to show that it can infect animals and humans.
F. polyphialidicum is a rare species that was described in 1984 from plant debris collected in South Africa (11). It is mainly characterized by its well-developed polyphialides, from which fusiform microconidia are produced in false heads; its long and falcate, usually 5-septate macroconidia with a distinct curved apical cell; its smooth or verrucose chlamydospores; and its cottony or floccose, fast-growing white colonies. Although an F. polyphialidicum isolate recovered from a man was deposited in the CBS culture collection (4), no case of human or animal infection by this fungus has been reported so far. F. polyphialidicum presents morphological features similar to those of other Fusarium species occasionally found in human infections, such as F. chlamydosporum and F. semitectum. However, these two species can be easily distinguished because of the colony color on PDA (white to pink to brown with a carmine red reverse in F. chlamydosporum and tan to brown with a reverse of the same color in F. semitectum) and because of the size of their macroconidia, which are shorter and narrower than those produced by F. polyphialidicum.
A molecular analysis of ribosomal ITS region sequences of several Fusarium species of medical interest demonstrated that the ITS1 sequences of F. napiforme, F. nygamai, F. oxysporum, F. proliferatum, F. sacchari, F. subglutinans, and F. verticillioides were very similar to one another, while the sequence of F. polyphialidicum was considerably different (21). This finding suggests that sequencing this region could be useful for distinguishing this fungus from other occasionally pathogenic species.
It has been suggested that the reason for the marked virulence of Fusarium for the cornea is that it produces substances that help it to penetrate or toxins that help to produce the lesions (10). It has also been suggested that the ocular virulence of Fusarium may be due to its capacity for angioinvasion, which occludes the intraocular vascular channels with secondary infarction, hemorrhage, and necrosis (15). The potential virulence for ocular structures of F. solani, the most frequent pathogenic Fusarium species, has been confirmed in different animal models. The inoculation of this fungus into the anterior chambers of rats (14) or intravenously in mice (12) caused severe endophthalmitis in both cases. The very quick clinical evolution of the infection in the present case seems to indicate that F. polyphialidicum is particularly virulent for ocular structures. However, further experimental studies are required if this is to be confirmed.
Although the in vitro antifungal susceptibility of F. polyphialidicum had not been previously tested, the low susceptibility shown by the case strain was partly expected because of the demonstrated resistance of Fusarium species to the available antifungal drugs (2, 17). The high MIC of amphotericin B for this isolate correlated with the clinical outcome of the patient.
Nucleotide sequence accession number.
The GenBank accession number for the sequence (522-bp long) obtained from the case strain is AJ538042.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, S. Nangia, and J. H. Rex. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens-Baumann, W. 1999. Mycosis of the eye and its adnexa. In W. Behrens-Baumann (ed.), Developments in ophthalmology, vol. 32. Karger, Basel, Switzerland.
- 4.Centraalbureau voor Schimmelcultures. 2001. List of cultures, 35th ed. Fungal Biodiversity Center, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 5.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 6.Guarro, J., A. M. Höfling-Lima, J. Gené, D. de Freitas, P. Godoy, M. L. Zorat-yu, L. Zaror, and O. Fischman. 2002. Corneal ulcer caused by the new fungal species, Sarcopodium oculorum. J. Clin. Microbiol. 40:3071-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarro, J., and J. Gené. 1995. Opportunistic fusarial infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 14:741-754. [DOI] [PubMed] [Google Scholar]
- 8.Guarro, J., L. A. Vieira, D. de Freitas, J. Gené, L. Zaror, A. L. Hofling-Lima, O. Fischman, C. Zorat-Yu, and M. J. Figueras. 2000. Phaeoisaria clematidis as a cause of keratomycosis. J. Clin. Microbiol. 38:2434-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarro, J., T. Akiti, R. Almada-Horta, L. A. M. Leite-Filho, J. Gené, S. Ferreira-Gomes, C. Aguilar, and M. Ortoneda. 1999. Mycotic keratitis due to Curvularia senegalensis and in vitro antifungal susceptibilities of Curvularia spp. J. Clin. Microbiol. 37:4170-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuriakose, T., and P. A. Thomas. 1991. Keratomycotic malignant glaucoma. Indian J. Ophthalmol. 39:118-121. [PubMed] [Google Scholar]
- 11.Marasas, W. F. O., P. E. Nelson, T. A. Toussoun, and P. S. van Wyk. 1986. Fusarium polyphialidicum, a new species from South Africa. Mycologia 78:678-682. [Google Scholar]
- 12.Mayayo, E., J. Guarro, and I. Pujol. 1998. Endogenous endophthalmitis by Fusarium solani: an animal experimental model. Med. Mycol. 36:249-253. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.O'Day, D. M., P. L. Akrabawi, L. D. Richmond, B. R. Jones, and Y. Clayton. 1979. An animal model of Fusarium solani endophthalmitis. Br. J. Ophthalmol. 63:277-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel, A. S., R. K. Hemady, M. Rodrigues, S. Rajagopalan, and M. J. Elman. 1994. Endogenous Fusarium endophthalmitis in a patient with acute lymphocytic leukemia. Am. J. Ophthalmol. 117:363-368. [DOI] [PubMed] [Google Scholar]
- 16.Pujol, I., J. Guarro, C. Llop, L. Soler, and J. Fernández. 1996. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob. Agents Chemother. 40:2106-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujol, I., J. Guarro, J. Gené, and J. Sala. 1997. In vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrob. Chemother. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 18.Solé, M., J. Cano, and J. Guarro. 2002. Molecular taxonomy of Amauroascus, Auxarthron and other related genera of the Onygenales. Mycol. Res. 106:388-396. [Google Scholar]
- 19.Strelow, S. A., H. D. Kent, R. C. Eagle, and E. S. Cohen. 1992. A case of contact lens related Fusarium solani keratitis. Contact Lens Assoc. Ophthalmol. J. 18:125-127. [PubMed] [Google Scholar]
- 20.Vajpayee, R. B., S. K. Gupta, U. Bareja, and K. Kishore. 1990. Ocular atopy and mycotic keratitis. Ann. Ophthalmol. 22:369-372. [PubMed] [Google Scholar]
- 21.Waalwijk, C., J. R. A. de Koning, R. P. Baayen, and W. Gams. 1996. Discordant groupings of Fusarium spp. from sections Elegans, Liseola and Dlaminia based on ribosomal ITSI and ITS2 sequences. Mycologia 88:361-368. [Google Scholar]
- 22.Zapater, R. C. 1986. Opportunistic fungus infections. Fusarium infections (keratomycosis by Fusarium). Jpn. J. Med. Mycol. 27:68-69. [Google Scholar]