Abstract
Research has shown that Pan and Homo have similar ectocranial suture synostosis patterns and a similar suture ontogeny (relative timing of suture fusion during the species ontogeny). This ontogeny includes patency during and after neurocranial expansion with a delayed bony response associated with adaptation to biomechanical forces generated by mastication. Here we investigate these relationships for Gorilla by examining the association among ectocranial suture morphology, cranial volume (as a proxy for neurocranial expansion) and dental development (as a proxy for the length of time that it has been masticating hard foods and exerting such strains on the cranial vault) in a large sample of Gorilla gorilla skulls. Two-hundred and fifty-five Gorilla gorilla skulls were examined for ectocranial suture closure status, cranial volume and dental eruption. Regression models were calculated for cranial volumes by suture activity, and Kendall's tau (a non-parametric measure of association) was calculated for dental eruption status by suture activity. Results suggest that, as reported for Pan and Homo, neurocranial expansion precedes suture synostosis activity. Here, Gorilla was shown to have a strong relationship between dental development and suture activity (synostosis). These data are suggestive of suture fusion extending further into ontogeny than brain expansion, similar to Homo and Pan. This finding allows for the possibility that masticatory forces influence ectocranial suture morphology.
Keywords: cranial suture, cranial volume, dental eruption, function, gorilla
Introduction
It is well established that cranial sutures, the fibrous joints of the skull, respond to forces from the expanding brain early in their ontogeny (Weidenreich, 1941; Moss & Young, 1960; Moss, 1969; Moss & Salentijn, 1969; Enlow & Hans, 1996; Cohen & MacLean, 2000; Mooney et al. 2002; Richtsmeier et al. 2006; Sardi et al. 2007; Mooney & Richtmeier, in press) and from mastication throughout the ontogeny of the suture prior to osseous fusion (Herring & Mucci, 1991; Herring, 1993, 2008; Herring & Teng, 2000; Herring et al. 2001; Mao, 2002; Fong et al. 2003; Byron et al. 2004, 2008; Alaqeel et al. 2006; Wu et al. 2007; Yu et al. 2009). However, research suggests ectocranial suture activity, defined as osteoblastic and osteoclastic activity that results in bone formation across the suture, is not temporally related to neurocranial expansion in Pan and Homo, but is related to mastication, which extends later into ontogeny (Cray et al. 2010).
The cyclic strains of mastication have a high degree of influence on ectocranial suture morphology and fusion (Herring, 1993, 2008; Byron et al. 2004, 2006). Compensatory bone growth and remodeling occurs at the suture osteogenic fronts in response to both tensile forces and compressive forces (Herring & Teng, 2000; Mao, 2002; Alaqeel et al. 2006; Herring, 2008). Sutures adapt under compressive forces, resulting in more interdigitation, thicker bones and narrower sutures. Widening and bone elongation in sutures are results of tension and cyclic strain forces (Fong et al. 2003; Wu et al. 2007; Herring, 2008). It has been demonstrated that the cranial sutures respond to strain throughout ontogeny by increasing complexity. Wave patterns and interdigitations form, and a relatively constant sutural width is maintained by bony adaption to strain prior to osseous fusion (Yu et al. 2009). Thus, strain magnitudes would have to increase throughout ontogeny to have additive effects on bone morphology.
The Gorilla skull exhibits many bony adaptations to enlarged temporal and masseter musculature. These adaptations have been considered allometric (Shea, 1983; Daegling, 1989, 2001, 2007; Daegling & Grine, 1991; Leigh & Shea, 1996; Daegling & Hylander, 1997, 2000; Daegling & Jungers, 2000; Taylor, 2002), the result of dietary influence (Hylander, 1979, 1984; Shea, 1983; Daegling & Hylander, 1997; Hylander et al. 1998, 2000; Daegling & Hotzman, 2003; Daegling, 2007; Taylor et al. 2008) and/or the result of evolutionary pressures (Willoughby, 1978; Leigh & Shea, 1996; Taylor, 2002). The most impressive bony response to these adaptations is the presence of the sagittal crest. Of all extant hominoids, gorillas may be characterized as exhibiting the most robust masticatory apparatus of all the extant hominoids, including the greatest hypertrophy of the temporal and masseter muscles (Willoughby, 1978; Shea, 1983; McCollum et al. 2006).
Despite their close phylogenetic relationship, Pan, Homo and Gorilla suture fusion patterns (suture site fusion progression) have been shown to differ slightly. The suture sites most susceptible to the mechanical influences of the temporal and masseter muscles, the lateral anterior (portions of the coronal, sphenotemporal and sphenofrontal sutures) exhibit similar patterns of fusion for all three species. Gorilla, however, exhibits a strong posterior to anterior gradient for vault sutures, suggesting an independent ectocranial suture pattern (Meindl & Lovejoy, 1985; Cray et al. 2010). It is unknown whether the differences in ectocranial suture morphology and fusion pattern observed for Gorilla are due to underlying genetic differences between these species, or to influences of the bony adaptation to the hypermuscular Gorilla form.
No investigations have been conducted concerning the timing of ectocranial suture activity in Gorilla. Research has shown that biomechanical forces may have a greater influence on ectocranial suture activity because masticatory forces and suture fusion continue further into the life cycle than neural expansion (Meindl & Lovejoy, 1985; Cray et al. 2008, 2010). The current study was designed to test the relationship, in Gorilla, between suture fusion activity and neurocranial expansion and dental development as a proxy for the onset and influence of masticatory forces. In particular, we investigated suture morphology (commencement and termination of osseous activity at the suture site) and its relationships to both cranial volume (as a proxy for brain growth) and dental eruption (as a proxy for the age of the individual and the length of time that it has been masticating hard foods and exerting such strains on the cranial vault). Gorilla represents a hyper-masticatory model and may have earlier masticatory influence on the sutures compared with Pan and Homo due to their growth differences in ontogeny. If suture adaptations are observed to extend later in ontogeny after brain growth is completed, then they likely result from biomechanical forces generated by the masticatory apparatus.
Materials and methods
Two-hundred and fifty-five Gorilla gorilla skulls (all wild-shot), housed at the Cleveland Museum of Natural History, were examined at 10 ectocranial suture sites by the primary author. Seven of the suture sites were classified as cranial vault sutures: midlambdoid, lambda, obelion, anterior sagittal, bregma, midcoronal, pterion; and three of the sutures were classified as lateral–anterior sutures (two from the cranial vault were classified as both): midcoronal, pterion, sphenofrontal inferior sphenotemporal, and superior sphenotemporal (Fig. 1; Meindl & Lovejoy, 1985; Cray et al. 2008). Each suture site was examined to determine suture synostosis activity and dichotomously scored for these two categories: (i) commencement of activity – defined as the earliest onset of bone formation activity within the fibrous joint (scored as a zero if not commenced or one if commenced); or (ii) termination of activity/obliteration – defined as the cessation of that activity or synostosis, i.e. the fibrous joint is replaced by bone (scored as a zero if not completely obliterated or one if obliterated; Fig. 2. The scores were then added to obtain a score for the sum of active suture sites for commencement (0–7 for vault and 0–5 for lateral anterior analyses) and the sum of fused/obliterated suture sites for termination (0–7 for vault and 0–5 for lateral anterior analyses; Meindl & Lovejoy, 1985; Cray et al. 2008). In this study, the primary author observed each skull twice, on separate days. Intraobserver reliability was 98.5%, meaning that over 98% of the suture site observations across study specimens were the same at each of the two observation times.
Fig. 1.

Suture observation sites: Gorilla gorilla. Illustrates anthropometric suture sites used in analysis: 1, midlambdoid; 2, lambda; 3, obelion; 4, anterior sagittal; 5, bregma; 6, midcoronal; 7, pterion; 8, sphenofrontal; 9, inferior sphenotemporal; 10, superior sphenotemporal. Reproduced from Cray et al. (2008).
Fig. 2.

Stages of suture fusion. (A) Displays an open suture site with 0% bony bridging, this site has not commenced activity. (B) Displays < 50% bony bridging at a suture site, this site has commenced activity. (C) Displays > 50% bony bridging at a suture site, this site has commenced activity but has not yet reached termination of activity. (D) Displays a closed or obliterated suture site, this site has reached termination of activity. (University of Pittsburgh, Department of Anthropology, comparative anatomy collection), reproduced from Cray et al. (2010).
The direct determination of cranial volume (in cm3) and sex determinations were provided by the Cleveland Museum of Natural History as part of their database of information on skeletal specimens. Cranial volume determination followed the methodology of Simmons (1942), including sand, water and anthropometric techniques.
As a proxy for ontogeny and masticatory muscle influence, dental eruption status was also scored for each skull and assigned to the following categories according to the sequence of permanent dental eruption in accordance with Krogman (1930):
deciduous;
first permanent molar eruption (defined as erupted into the occlusal plane);
permanent incisors and premolars eruption (defined as erupted into the occlusal plane);
second permanent molar eruption (defined as erupted into the occlusal plane);
permanent canines and last molars eruption (defined as erupted into the occlusal plane);
wear of age.
To determine if the pattern of differences in cranial volume vary by the sum of suture activity scores (commencement and termination for vault and lateral anterior suture sites) by sex (to account for the great sexual dimorphism in Gorilla), a two-way anova was conducted for each analysis to assess the significance of the interaction term. This analysis elucidated whether there were sex-specific patterns in neurocranial expansion as related to suture morphology.
To assess the statistical relationship between suture activity and cranial volume, a linear regression model, using a single predictor, was used to assess the relationship between cranial volume, and the sums of cranial vault and lateral anterior suture commencement and termination (0–7 for vault, and 0–5 for lateral anterior) were treated as the independent variables, respectively. Cranial volume was treated as the dependent variable or predicted variable. Pearson's product moment correlation coefficient (r) was used as a measure of linear association (Vittinghoff et al. 2005). This analysis allowed for the determination of whether neurocranial expansion was related to suture activity, bridging and synostosis in Gorilla ontogeny.
To assess the statistical relationship between the sum of active suture sites score (an ordinal variable) and dental status (an ordinal variable), a Kendall's tau (τ) was calculated (Ferguson, 1976). Sexual dimorphism was assessed for the sum of active and terminated suture sites for vault and lateral anterior sutures, as well as dental status. This analysis allowed for the determination of whether suture activity was related to dental eruption as a proxy for ontogeny, and the length of time that it has been masticating hard foods and exerting such strains on the cranial vault. All analyses were conducted using spss 15 (Chicago, IL, USA).
Results
Demographics
Demographics were provided by the Cleveland Museum of Natural History as part of their database of information on skeletal specimens. Of the 255 skulls that were analyzed, 69 were classified as subadults and 186 as adults. Of the subadults, 17 were classified as unknown sex, 27 males and 25 females. There were 117 males and 69 females in the adult classification group.
Cranial volume
The sample was culled (n = 150) to include only those skulls that had data for cranial volume analysis. A 2 × 8 between-subjects anova was performed on cranial volume as a function of sex (male, female) and vault suture commencement and termination score (0–7), respectively. The assumption of normality was met for all groups, P> 0.05 for each analysis, and the assumption of homogeneity of variance was met for each analysis, P> 0.05. The interaction terms demonstrate there is no significant pattern of difference on cranial volume as a function of sex and vault suture commencement score, F5,137 = 0.921, P = 0.470, or as a function of sex and vault suture termination score, F7,134 = 1.116, P = 0.357.
A 2 × 6 between-subjects anova was performed on cranial volume as a function of sex (male, female) and lateral anterior suture commencement and termination score (0–5), respectively. The assumption of normality was met for all groups, P> 0.05 for each analysis, and the assumption of homogeneity of variance was met for each analysis, P> 0.05. No significant pattern of difference on cranial volume as a function of sex and lateral anterior commencement score, F5,138 = 0.465, P = 0.802, or as a function of sex and vault termination score, F5,138 = 0.866, P = 0.506 was found, as demonstrated by the interaction terms.
The resulting regression model for cranial volume predicted by the sum of vault suture commencement score was not significant (r2 = 0.019, P = 0.078), cranial volume = 463.582 + 4.931 × vault suture commencement score. Likewise, the regression model for cranial volume predicted by the sum of vault suture termination score was not significant (r2 = 0.007, P = 0.275), cranial volume = 485.631 + 1.868 × vault suture termination score. A regression model for cranial volume predicted by the sum of lateral anterior suture commencement score was not significant (r2 = 0.017, P = 0.100), cranial volume = 474.188 + 5.004 × lateral anterior suture commencement score. The regression model for cranial volume predicted by the sum of lateral anterior suture termination score was again not significant (r2 = 0.002, P = 0.621), cranial volume = 491.589 + 1.121 × lateral anterior suture termination score. Figure 3 represents the resulting regression models with constants included.
Fig. 3.

Regression models with constant for suture activity as a predictive variable for cranial volume. The regression models were not significant P> 0.05.
Dental eruption
All 255 specimens were included in the dentition analysis. Sex was not significantly correlated with dental status (τ = −0.152, P = 0.12), nor was it correlated with sum of vault suture commencement score (τ = −0.079, P = 0.210), sum of lateral anterior suture commencement score (τ = −0.063, P = 0.304), sum of vault suture termination score (τ = −0.093, P = 0.114) or lateral anterior suture termination score (τ = −0.045, P = 0.455). Figures 4 and 5 exhibit the vault and lateral anterior suture activity as a function of dental status. Mean vault and lateral anterior commencement appear as early as deciduous dentition to permanent incisor and canine eruption, but it is at third molar eruption where it appears most suture sites have commenced activity. Mean vault termination first appears to occur after the incisor and canines emerge, but exhibit variability. A mean value > 1 is not observed for vault termination until third molar eruption. Mean lateral anterior termination does not begin until third molar eruption and proceeds thereafter.
Fig. 4.
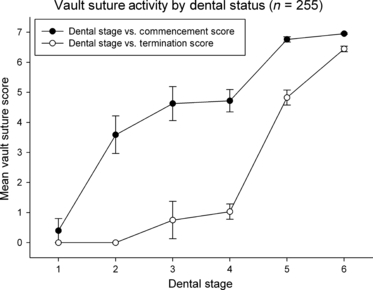
Vault suture activity scores (mean ± SE mean) for commencement and termination of activity plotted against dental stages (1, deciduous; 2, first permanent molar eruption; 3, permanent incisors and premolars eruption; 4, second permanent molar eruption; 5, permanent canines and last molars eruption; 6, wear of age). Shaded circles denote vault commencement, unshaded circles denote vault termination.
Fig. 5.

Lateral anterior suture activity scores (mean ± SE mean) for commencement and termination of activity plotted against dental stages (1, deciduous; 2, first permanent molar eruption; 3, permanent incisors and premolars eruption; 4, second permanent molar eruption; 5, permanent canines and last molars eruption; 6, wear of age). Shaded circles denote lateral–anterior commencement, unshaded circles denote lateral–anterior termination.
Dental status was significantly correlated with sum of vault suture commencement score (r = 0.721, P < 0.001), sum of lateral anterior suture commencement score (r = 0.774, P < 0.001), sum of vault suture termination score (r = 0.839, P < 0.001) and sum of lateral anterior suture termination score (r = 0.601, P < 0.001). All analyses exhibited highly significant positive correlations, suggesting a strong association between advanced dental status and osseous suture bridging and synostosis.
Discussion
Gorilla does not have a strong association between suture activity and brain volume, similar to what has been reported for Pan (Cray et al. 2010) and Homo (Meindl & Lovejoy, 1985; Cohen & MacLean, 2000). Our regression models for cranial volume and suture activity represent a positive association. However, the resulting models were not significant and r2-values were very low, suggesting a poor predictive model. These data suggest that neurocranial expansion is completed before sutures begin to adapt to biomechanical forces (suture serration and bridging or fusion in non-pathological cases). Thus, the expanding brain continues to be the predominant force that shapes the neurocranium early in ontogeny (Weidenreich, 1941; Moss, 1969; Moss & Salentijn, 1969; Enlow & Hans, 1996; Mooney et al. 2002; Richtsmeier et al. 2006), even in this truncated ontogeny where Gorilla display relatively early suture adaptation activity. Suture patency appears to persist at least through the evolutionarily selected neurocranial expansion for Pan, Homo and Gorilla.
In contrast, the data presented for suture scores and dental status exhibit statistically significant positive relationships. Gorilla displays early suture activity unlike that reported for both Pan and Homo, where suture activity has a strong relationship with third molar eruption (Cray et al. 2010). This relationship is interpreted as the result of the allometrically larger masticatory apparatus for Gorilla (Hylander, 1979; Shea, 1983, 1985; Daegling, 1989, 2007; Taylor, 2002; Daegling & Hotzman, 2003; Taylor et al. 2008). However, even with earlier suture activity, it appears that the ectocranial suture sites do not fuse until after third molar eruption, similar to that reported for Pan and Homo (Meindl & Lovejoy, 1985; Cray et al. 2010). It is observed that mean vault termination first appears to occur after the incisor and canines emerge, but exhibit variability. In fact, the mean values for stages 3 and 4 are both < 1, suggesting a propensity of the individuals observed had not begun termination, and those cases that had were only observed for on average one suture site. Thus, statistically, it appears the onset of termination is not observed for most specimens until eruption of the third molar. This relationship is observed for lateral anterior termination as well, which does not begin until third molar eruption and proceeds thereafter. The extended delay observed between dental eruption and cessation of suture activity is not as pronounced in the Gorilla as in Pan and Homo (Cray et al. 2010). However, there was no suture fusion observed before the third molar eruption in Gorilla. This earlier display of suture adaptation appears to be due to the increased masticatory musculature and apparatus of the Gorilla. Thus, mastication appears to effect ectocranial suture activity of Gorilla much more than the expanding neurocranium.
Later suture adaptations to biomechanical adaptations are further evidence suggesting an overall strong selection force for the patency of the sutures to allow for extended neurocranial expansion (Cheverud et al. 1990; Gilbert et al. 2005; Ponting & Jackson, 2005; Bakewell et al. 2007). There is also a wealth of research suggesting masticatory biomechanical forces increase sutural adaptations including interdigitation and eventual fusion (Moss & Young, 1960; Riesenfeld, 1967; Herring, 1972, 1993, 2008; Byron et al. 2004; Sun et al. 2004; Shibazaki et al. 2007; Wu et al. 2007). Calvarial sutures respond to masticatory strains through bony growth and remodeling at the osteogenic fronts and increases in complexity, waveform pattern and interdigitation before osseous fusion (Herring & Teng, 2000; Mao, 2002; Fong et al. 2003; Byron et al. 2004, 2006, 2008; Lieberman et al. 2004; Alaqeel et al. 2006; Byron, 2006). This response to strain supported by removal of force by muscle resection (Moss, 1961) or suture immobilization (Persson et al. 1979) results in a loss of interdigitation. The increased masticatory forces observed in the adult ontogeny have been shown to correlate with increases in tension across the cranial sutures (Herring & Teng, 2000; Sun et al. 2004; McCollum et al. 2006; Shibazaki et al. 2007).
In contrast, it has been shown that compressive forces dissipate with suture remodeling in rodents where sutures remain patent (Sun et al. 2004; Shibazaki et al. 2007). In addition, Henderson et al. (2004) modeled early sutural strain in humans with results that suggested that tissue level strains were likely too small to effect osteoblast biology. These contrasting data are suggestive of a lack of effect for biomechanical forces on the suture. The data presented here support the model that adaptation in suture morphology is temporally linked to the development of the masticatory apparatus and later chewing. The gorilla has the most robustly developed masticatory muscles of the hominoids. We observe few changes to the morphology of the calvarial sutures prior to the completion of neurocranial expansion similar to the pattern observed for both Homo and Pan (Cray et al. 2010). In fact, these data suggest that suture fusion in the gorilla is only temporally related to eruption of the third molar (dental maturity). It is important to note that the data presented here were abstracted from dry skull morphological investigation. The direct causation of adaptation in suture morphology and eventual fusion likely involves biomechanical adaptation, tension- and compression-related changes to the extracellular matrix and cells of the perisuture area, as well as mechanotransduction of cells eliciting the expression of growth factor gradients and cellular expression related to osteoblast and osteoclast activity.
What may be puzzling about the effects of biomechanics on the calvarial sutures, is the relatively higher strains affecting the facial sutures (Adab et al. 2002; Mao, 2002; Rafferty et al. 2003; Al-Mubarak et al. 2005). The facial sutures in most hominoids do not fuse except in rare cases very late in ontogeny (Todd & Lyon, 1925a,b,c; Krogman, 1930). It has been suggested that facial suture patency may be adaptive to protect the thin bones of the face during dynamic loading, thus acting as a type of shock absorber (Jaslow, 1990; Rafferty & Herring, 1999; Rafferty et al. 2003). It is likely similar growth factors are at work in the morphogenesis, growth and maintenance of both cranial and facial sutures, transforming growth factors (TGFβs), bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), twist transcription factor (TWIST), Msh Homeobox 2 (MSX2), Runt-related transcription factor (RUNX2). It may be, however, that these growth factors and others, matrix metalloproteinase (MMP1 and 2) and insulin-like growth factor (IGF1 and 2), for example, have differential expression or different metabolisms between cranial and facial sutures (Rafferty & Herring, 1999; Adab et al. 2002; Rafferty et al. 2003; Al-Mubarak et al. 2005). There is no consensus concerning facial suture patency, and further research is required.
There has been much research concerning expression of gene product and growth factors before or after calvarial suture fusion in rodent models (Cohen & MacLean, 2000; Opperman & Ogle, 2002; Poisson et al. 2004; Rawlins & Opperman, 2008; Yu et al. 2009). It has been suggested that factors such as Collagen II, MMP, and tissue inhibitor of matrix metalloproteinases (TIMP) increase under tensile forces. These factors in turn increase proliferation of osteoblasts and fibroblasts as well as promoting collagen fiber synthesis (Meikle et al. 1980, 1984; Green et al. 1990; Movassaghi et al. 1995; Ikegame et al. 2001; Sasaki et al. 2002; Kopher & Mao, 2003; Kopher et al. 2003; Hirukawa et al. 2005). Concerning those factors known to be important to the morphogenesis and maintenance of the calvarial sutures, IGF-1 and IGF-1R (Tokimasa et al. 2003; Hirukawa et al. 2005), TGFB1 (Sawada & Shimizu, 1996), FGF2 (Yu et al. 2001; and BMP4 (Ikegame et al. 2001 #233) increase under stress to the sutures. Transcription factors Twist, MSX2, RUNX2 and T-box transcription factor (Tbx2) also show increases under force, whereas connexin 43 has been shown to decrease expression (Borke et al. 2003).
Finally, it is likely that Gorilla, Pan and Homo exhibit heritable age-related changes to the sutures due to heritability of perisutural growth factor concentration gradients, including the TGFβ, FGFs and the Ets-2 CAM-kinase pathways (Cohen & MacLean, 2000; Opperman & Ogle, 2002; Poisson et al. 2004; Rawlins & Opperman, 2008; Yu et al. 2009). It is also likely that the earlier suture remodeling observed in the Gorilla may be related to the increased size of the masticatory muscles and apparatus observed for this species.
Conclusion
In conclusion, like Pan and Homo the expanding neurocranium is unrelated to the onset or cessation of suture fusion activity in Gorilla. However, the Gorilla model does exhibit earlier suture response to the age of the individual and the length of time that it has been masticating hard foods and exerting such strains on the cranial vault sutures. This causes earlier, biomechanically mediated adaptation to the suture during Gorilla ontogeny. Only after neurocranial expansion is complete does this occur, suggesting the expanding brain still is the important driving force for early suture patency. Future research should continue to explore the relationship of ectocranial suture activity among and between hominoids, and primates in general, and the causal mechanism of suture synostosis.
Acknowledgments
The authors would like to thank Lymann Jellema of the Cleveland Museum of Natural History for access to the study specimens. We would also like to thank Emily Lensie Durham for critical reading of this work.
References
- Adab K, Sayne JR, Carlson DS, et al. Tgf-beta1, Tgf-beta2, Tgf-beta3 and Msx2 expression is elevated during frontonasal suture morphogenesis and during active postnatal facial growth. Orthod Craniofac Res. 2002;5:227–237. doi: 10.1034/j.1600-0544.2002.02227.x. [DOI] [PubMed] [Google Scholar]
- Alaqeel SM, Hinton RJ, Opperman LA. Cellular response to force application at craniofacial sutures. Orthod Craniofac Res. 2006;9:111–122. doi: 10.1111/j.1601-6343.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- Al-Mubarak R, Da Silveira A, Mao JJ. Expression and mechanical modulation of matrix metalloproteinase-1 and -2 genes in facial and cranial sutures. Cell Tissue Res. 2005;321:465–471. doi: 10.1007/s00441-005-1136-2. [DOI] [PubMed] [Google Scholar]
- Bakewell MA, Shi P, Zhang J. More genes underwent positive selection in chimpanzee evolution than in human evolution. Proc Natl Acad Sci USA. 2007;104:7489–7494. doi: 10.1073/pnas.0701705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borke JL, Yu JC, Isales CM, et al. Tension-induced reduction in connexin 43 expression in cranial sutures is linked to transcriptional regulation by TBX2. Ann Plast Surg. 2003;51:499–504. doi: 10.1097/01.SAP.0000067964.14122.3E. [DOI] [PubMed] [Google Scholar]
- Byron CD. Role of the osteoclast in cranial suture waveform patterning. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:552–563. doi: 10.1002/ar.a.20322. [DOI] [PubMed] [Google Scholar]
- Byron CD, Borke J, Yu J, et al. Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:676–684. doi: 10.1002/ar.a.20055. [DOI] [PubMed] [Google Scholar]
- Byron CD, Hamrick MW, Wingard CJ. Alterations of temporalis muscle contractile force and histological content from the myostatin and Mdx deficient mouse. Arch Oral Biol. 2006;51:396–405. doi: 10.1016/j.archoralbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Byron C, Maness H, Yu J, et al. Enlargement of the temporalis muscle and alterations in the lateral cranial vault. Int Comput Biol. 2008;48:338–344. doi: 10.1093/icb/icn020. [DOI] [PubMed] [Google Scholar]
- Cheverud JM, Falk D, Vannier M, et al. Heritability of brain size and surface features in rhesus macaques (Macaca mulatta) J Hered. 1990;81:51–57. doi: 10.1093/oxfordjournals.jhered.a110924. [DOI] [PubMed] [Google Scholar]
- Cohen M, MacLean R. Craniosynostosis: Diagnosis, Evaluation, and Management. New York: Oxford Press; 2000. [Google Scholar]
- Cray J, Jr, Meindl RS, Sherwood CC, et al. Ectocranial suture closure in Pan troglodytes and Gorilla gorilla: pattern and phylogeny. Am J Phys Anthropol. 2008;136:394–399. doi: 10.1002/ajpa.20821. [DOI] [PubMed] [Google Scholar]
- Cray J, Jr, Mooney MP, Siegel MI. Timing of ectocranial suture activity in Pan troglodytes as related to cranial volume and dental eruption. Anat Rec (Hoboken) 2010;293:1289–1296. doi: 10.1002/ar.21167. [DOI] [PubMed] [Google Scholar]
- Daegling DJ. Biomechanics of cross-sectional size and shape in the hominoid mandibular corpus. Am J Phys Anthropol. 1989;80:91–106. doi: 10.1002/ajpa.1330800111. [DOI] [PubMed] [Google Scholar]
- Daegling DJ. Biomechanical scaling of the hominoid mandibular symphysis. J Morphol. 2001;250:12–23. doi: 10.1002/jmor.1055. [DOI] [PubMed] [Google Scholar]
- Daegling DJ. Relationship of bone utilization and biomechanical competence in hominoid mandibles. Arch Oral Biol. 2007;52:51–63. doi: 10.1016/j.archoralbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Grine FE. Compact bone distribution and biomechanics of early hominid mandibles. Am J Phys Anthropol. 1991;86:321–339. doi: 10.1002/ajpa.1330860302. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Hotzman JL. Functional significance of cortical bone distribution in anthropoid mandibles: an in vitro assessment of bone strain under combined loads. Am J Phys Anthropol. 2003;122:38–50. doi: 10.1002/ajpa.10225. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Hylander WL. Occlusal forces and mandibular bone strain: is the primate jaw ‘overdesigned’? J Hum Evol. 1997;33:705–717. doi: 10.1006/jhev.1997.0164. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Hylander WL. Experimental observation, theoretical models, and biomechanical inference in the study of mandibular form. Am J Phys Anthropol. 2000;112:541–551. doi: 10.1002/1096-8644(200008)112:4<541::AID-AJPA8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Jungers WL. Elliptical fourier analysis of symphyseal shape in great ape mandibles. J Hum Evol. 2000;39:107–122. doi: 10.1006/jhev.2000.0402. [DOI] [PubMed] [Google Scholar]
- Enlow D, Hans M. Essentials of Facial Growth. Ann Arbor: Needham Press; 1996. [Google Scholar]
- Ferguson G. Statistical Analysis in Psychology and Education. New York: McGraw-Hill; 1976. [Google Scholar]
- Fong KD, Nacamuli RP, Loboa EG, et al. Equibiaxial tensile strain affects calvarial osteoblast biology. J Craniofac Surg. 2003;14:348–355. doi: 10.1097/00001665-200305000-00013. [DOI] [PubMed] [Google Scholar]
- Gilbert SL, Dobyns WB, Lahn BT. Genetic links between brain development and brain evolution. Nat Rev Genet. 2005;6:581–590. doi: 10.1038/nrg1634. [DOI] [PubMed] [Google Scholar]
- Green DD, Hembry RM, Atkinson SJ, et al. Immunolocalization of collagenase and tissue inhibitor of metalloproteinases (TIMP) in mechanically deformed fibrous joints. Am J Orthod Dentofacial Orthop. 1990;97:281–288. doi: 10.1016/0889-5406(90)70100-Q. [DOI] [PubMed] [Google Scholar]
- Henderson JH, Longaker MT, Carter DR. Sutural bone deposition rate and strain magnitude during cranial development. Bone. 2004;34:271–280. doi: 10.1016/j.bone.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Herring SW. Sutures – a tool in functional cranial analysis. Acta Anat (Basel) 1972;83:222–247. doi: 10.1159/000143860. [DOI] [PubMed] [Google Scholar]
- Herring SW. Epigenetic and functional influences on skull growth. In: Hanken J, Hall B, editors. The Skull. Chicago: University of Chicago Press; 1993. pp. 153–206. [Google Scholar]
- Herring SW. Mechanical influences on suture development and patency. Front Oral Biol. 2008;12:41–56. doi: 10.1159/0000115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Mucci RJ. In vivo strain in cranial sutures: the zygomatic arch. J Morphol. 1991;207:225–239. doi: 10.1002/jmor.1052070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Teng S. Strain in the braincase and its sutures during function. Am J Phys Anthropol. 2000;112:575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Rafferty KL, Liu ZJ, et al. Jaw muscles and the skull in mammals: the biomechanics of mastication. Comp Biochem Physiol A Mol Integr Physiol. 2001;131:207–219. doi: 10.1016/s1095-6433(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Hirukawa K, Miyazawa K, Maeda H, et al. Effect of tensile force on the expression of IGF-I and IGF-I receptor in the organ-cultured rat cranial suture. Arch Oral Biol. 2005;50:367–372. doi: 10.1016/j.archoralbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hylander WL. The functional significance of primate mandibular form. J Morphol. 1979;160:223–240. doi: 10.1002/jmor.1051600208. [DOI] [PubMed] [Google Scholar]
- Hylander WL. Stress and strain in the mandibular symphysis of primates: a test of competing hypotheses. Am J Phys Anthropol. 1984;64:1–46. doi: 10.1002/ajpa.1330640102. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Ravosa MJ, Ross CF, et al. Mandibular corpus strain in primates: further evidence for a functional link between symphyseal fusion and jaw-adductor muscle force. Am J Phys Anthropol. 1998;107:257–271. doi: 10.1002/(SICI)1096-8644(199811)107:3<257::AID-AJPA3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Ravosa MJ, Ross CF, et al. Symphyseal fusion and jaw-adductor muscle force: an EMG study. Am J Phys Anthropol. 2000;112:469–492. doi: 10.1002/1096-8644(200008)112:4<469::AID-AJPA5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ikegame M, Ishibashi O, Yoshizawa T, et al. Tensile stress induces bone morphogenetic protein 4 in preosteoblastic and fibroblastic cells, which later differentiate into osteoblasts leading to osteogenesis in the mouse calvariae in organ culture. J Bone Miner Res. 2001;16:24–32. doi: 10.1359/jbmr.2001.16.1.24. [DOI] [PubMed] [Google Scholar]
- Jaslow CR. Mechanical properties of cranial sutures. J Biomech. 1990;23:313–321. doi: 10.1016/0021-9290(90)90059-c. [DOI] [PubMed] [Google Scholar]
- Kopher RA, Mao JJ. Suture growth modulated by the oscillatory component of micromechanical strain. J Bone Miner Res. 2003;18:521–528. doi: 10.1359/jbmr.2003.18.3.521. [DOI] [PubMed] [Google Scholar]
- Kopher RA, Nudera JA, Wang X, et al. Expression of in vivo mechanical strain upon different wave forms of exogenous forces in rabbit craniofacial sutures. Ann Biomed Eng. 2003;31:1125–1131. doi: 10.1114/1.1603259. [DOI] [PubMed] [Google Scholar]
- Krogman W. Studies in growth changes in the skull and face of anthropoids. II. Ectocranial and endocranial suture closure in anthropoids and old world apes. Am J Anat. 1930;46:315–353. [Google Scholar]
- Leigh SR, Shea BT. Ontogeny of body size variation in African apes. Am J Phys Anthropol. 1996;99:43–65. doi: 10.1002/(SICI)1096-8644(199601)99:1<43::AID-AJPA3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Krovitz GE, Yates FW, et al. Effects of food processing on masticatory strain and craniofacial growth in a retrognathic face. J Hum Evol. 2004;46:655–677. doi: 10.1016/j.jhevol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Mechanobiology of craniofacial sutures. J Dent Res. 2002;81:810–816. doi: 10.1177/154405910208101203. [DOI] [PubMed] [Google Scholar]
- McCollum MA, Sherwood CC, Vinyard CJ, et al. Of muscle-bound crania and human brain evolution: the story behind the MYH16 headlines. J Hum Evol. 2006;50:232–236. doi: 10.1016/j.jhevol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Meikle MC, Sellers A, Reynolds JJ. Effect of tensile mechanical stress on the synthesis of metalloproteinases by rabbit coronal sutures in vitro. Calcif Tissue Int. 1980;30:77–82. doi: 10.1007/BF02408610. [DOI] [PubMed] [Google Scholar]
- Meikle MC, Heath JK, Reynolds JJ. The use of in vitro models for investigating the response of fibrous joints to tensile mechanical stress. Am J Orthod. 1984;85:141–153. doi: 10.1016/0002-9416(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Meindl RS, Lovejoy CO. Ectocranial suture closure: a revised method for the determination of skeletal age at death based on the lateral-anterior sutures. Am J Phys Anthropol. 1985;68:57–66. doi: 10.1002/ajpa.1330680106. [DOI] [PubMed] [Google Scholar]
- Mooney M, Richtmeier J. Cranial sutures and calvaria: normal development and craniosynostosis. In: Mao J, Nah D, editors. Craniofacial Growth and Development. New York: Blackwell Munksgund Publishing; in press. [Google Scholar]
- Mooney M, Siegel M, Smith T, et al. Evolutionary changes in the cranial vault and base: establishing the primate form. In: Mooney M, Siegel M, editors. Understanding Craniofacial Anomalies: the Etiopathogenesis of Craniosynostosis and Facial Clefting. New York: John Wiley; 2002. pp. 275–293. [Google Scholar]
- Moss ML. Extrinsic determination of sutural area morphology in the rat calvaria. Acta Anat (Basel) 1961;44:263–272. doi: 10.1159/000141726. [DOI] [PubMed] [Google Scholar]
- Moss ML. The differential roles of periosteal and capsular functional matrices in oro-facial growth. Rep Congr Eur Orthod Soc. 1969:193–205. [PubMed] [Google Scholar]
- Moss ML, Salentijn L. The capsular matrix. Am J Orthod. 1969;56:474–490. doi: 10.1016/0002-9416(69)90209-7. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Movassaghi K, Altobelli DE, Zhou H. Frontonasal suture expansion in the rabbit using titanium screws. J Oral Maxillofac Surg. 1995;53:1033–1042. doi: 10.1016/0278-2391(95)90121-3. Discussion 1042–1043. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Ogle RC. Molecular studies of craniosynostosis: factors affecting cranial suture morphogenesis and patency. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies: the Etiopathogenesis of Craniosynostosis and Facial Clefting. New York: John Wiley; 2002. pp. 497–518. [Google Scholar]
- Persson KM, Roy WA, Persing JA, et al. Craniofacial growth following experimental craniosynostosis and craniectomy in rabbits. J Neurosurg. 1979;50:187–197. doi: 10.3171/jns.1979.50.2.0187. [DOI] [PubMed] [Google Scholar]
- Poisson E, Sciote JJ, Koepsel R, et al. Transforming growth factor-beta isoform expression in the perisutural tissues of craniosynostotic rabbits. Cleft Palate Craniofac J. 2004;41:392–402. doi: 10.1597/02-140.1. [DOI] [PubMed] [Google Scholar]
- Ponting C, Jackson AP. Evolution of primary microcephaly genes and the enlargement of primate brains. Curr Opin Genet Dev. 2005;15:241–248. doi: 10.1016/j.gde.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW. Craniofacial sutures: morphology, growth, and in vivo masticatory strains. J Morphol. 1999;242:167–179. doi: 10.1002/(SICI)1097-4687(199911)242:2<167::AID-JMOR8>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW, Marshall CD. Biomechanics of the rostrum and the role of facial sutures. J Morphol. 2003;257:33–44. doi: 10.1002/jmor.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins JT, Opperman LA. Tgf-beta regulation of suture morphogenesis and growth. Front Oral Biol. 2008;12:178–196. doi: 10.1159/000115038. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, Aldridge K, DeLeon VB, et al. Phenotypic integration of neurocranium and brain. J Exp Zoolog B Mol Dev Evol. 2006;306:360–378. doi: 10.1002/jez.b.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenfeld A. Biodynamics of head form and cranio-facial relationships. Homo. 1967;18:233–251. [Google Scholar]
- Sardi ML, Ventrice F, Ramirez Rozzi F. Allometries throughout the late prenatal and early postnatal human craniofacial ontogeny. Anat Rec (Hoboken) 2007;290:1112–1120. doi: 10.1002/ar.20581. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sugiyama H, Tanaka E, et al. Effects of sutural distraction osteogenesis applied to rat maxillary complex on craniofacial growth. J Oral Maxillofac Surg. 2002;60:667–675. doi: 10.1053/joms.2002.33117. [DOI] [PubMed] [Google Scholar]
- Sawada M, Shimizu N. Stimulation of bone formation in the expanding mid-palatal suture by transforming growth factor-beta 1 in the rat. Eur J Orthod. 1996;18:169–179. doi: 10.1093/ejo/18.2.169. [DOI] [PubMed] [Google Scholar]
- Shea BT. Size and diet in the evolution of African ape craniodental form. Folia Primatol (Basel) 1983;40:32–68. doi: 10.1159/000156090. [DOI] [PubMed] [Google Scholar]
- Shea BT. On aspects of skull form in African apes and orangutans, with implications for hominoid evolution. Am J Phys Anthropol. 1985;68:329–342. doi: 10.1002/ajpa.1330680304. [DOI] [PubMed] [Google Scholar]
- Shibazaki R, Dechow PC, Maki K, et al. Biomechanical strain and morphologic changes with age in rat calvarial bone and sutures. Plast Reconstr Surg. 2007;119:2167–2178. doi: 10.1097/01.prs.0000260705.70329.38. [DOI] [PubMed] [Google Scholar]
- Simmons K. Cranial capacities by both plastic and water techniques with cranial linear measurements of the reserve collection: white and negro. Hum Biol. 1942;14:473–498. [Google Scholar]
- Sun Z, Lee E, Herring SW. Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AB. Masticatory form and function in the African apes. Am J Phys Anthropol. 2002;117:133–156. doi: 10.1002/ajpa.10013. [DOI] [PubMed] [Google Scholar]
- Taylor AB, Vogel ER, Dominy NJ. Food material properties and mandibular load resistance abilities in large-bodied hominoids. J Hum Evol. 2008;55:604–616. doi: 10.1016/j.jhevol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Todd T, Lyon D. Cranial suture closure: its progress and relationship. IV. Ectocranial suture closure of adult males of negro stock. Am J Phys Anthropol. 1925a;8:149–168. [Google Scholar]
- Todd T, Lyon D. Cranial suture closure: its progress and age relationship. II. Ectocranial closure of males of white stock. Am J Phys Anthropol. 1925b;8:23–43. [Google Scholar]
- Todd T, Lyon D. Cranial suture closure: its progress and age relationship. III. Endocranial suture closure. Its progress and age relationship. Am J Phys Anthropol. 1925c;8:47–71. [Google Scholar]
- Tokimasa C, Kawata T, Fujita T, et al. Effects of insulin-like growth factor-I on the expression of osteoclasts and osteoblasts in the nasopremaxillary suture under different masticatory loading conditions in growing mice. Arch Oral Biol. 2003;48:31–38. doi: 10.1016/s0003-9969(02)00161-9. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, Glidden D, Shiboski S, et al. Regression Methods in Biostatistics. New York: Springer; 2005. [Google Scholar]
- Weidenreich F. The brain and its role in the phylogenetic transformation of the human skull. Trans Am Phil Soc. 1941;31:321–442. [Google Scholar]
- Willoughby DP. All About Gorillas. South Brunswick, NJ: A.S. Barnes; 1978. [Google Scholar]
- Wu YD, Chien CH, Chao YJ, et al. Fourier analysis of human sagittal sutures. Cleft Palate Craniofac J. 2007;44:482–493. doi: 10.1597/06-122.1. [DOI] [PubMed] [Google Scholar]
- Yu JC, Lucas JH, Fryberg K, et al. Extrinsic tension results in FGF-2 release, membrane permeability change, and intracellular Ca++ increase in immature cranial sutures. J Craniofac Surg. 2001;12:391–398. doi: 10.1097/00001665-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Yu JC, Chen JR, Lin CH, et al. Tensile strain-induced Ets-2 phosphorylation by CaMKII and the homeostasis of cranial sutures. Plast Reconstr Surg. 2009;123:83S–93S. doi: 10.1097/PRS.0b013e318191c029. [DOI] [PubMed] [Google Scholar]


