Abstract
Although age-related changes in cancellous bone structure in human are relatively well characterized, few studies have addressed changes in cortical bone. We have investigated age-related changes in iliac crest bone biopsy specimens from 54 normal subjects, 23 men and 31 women, aged 18–90 years. A significant decrease in cortical width and area was seen (P = 0.002 and < 0.001 respectively), with no difference between sexes. Haversian canal density increased significantly with age by approximately 9% per decade (P = 0.032) but Haversian canal area tended to be lower, resulting in no overall age-related difference in cortical porosity. Haversian canal area was significantly higher in the endosteal section than in the periosteal section of the cortex (P = 0.019) but the Haversian canal density was lower, resulting in similar overall porosity in the two sections. In conclusion, our results demonstrate an age-related decrease in iliac crest cortical width in men and women and an increase in Haversian canal density, but no overall change in cortical porosity.
Keywords: age-related changes, cortical bone, cortical porosity, cortical thickness, iliac crest
Introduction
Bone loss occurs throughout the skeleton with ageing and affects both cortical and cancellous bone (Riggs & Melton, 1986). In humans, histomorphometric analysis of the changes in bone remodelling and structure underlying age-related bone loss has mostly been conducted in iliac crest bone. Changes in bone structure vary according to the skeletal site and iliac crest bone, a non-weight-bearing site, may not always be representative of clinically relevant sites such as the vertebrae and proximal femur. Nevertheless, biopsy studies of the mechanisms of bone loss and gain in untreated and treated osteoporosis are almost exclusively confined to the iliac crest and reference data in normal subjects at this site are required for both cortical and cancellous bone.
Age-related bone loss differs in cancellous and cortical bone with respect to both its rate and structural consequences (Arnold, 1970; Parfitt, 1984). However, previous histomorphometric studies in healthy subjects have focused almost exclusively on cancellous bone and very few data are available for cortical bone. Brockstedt et al. (1993) have reported age- and sex-related changes in iliac crest cortical bone in 64 subjects, using a combination of biopsy and autopsy bone samples. A significant decrease in cortical width was demonstrated with ageing, whereas cortical porosity increased, at least in part due to an increase in the diameter of Haversian canals. In this study we report age-related changes in cortical bone structure in iliac crest biopsies from 54 normal healthy subjects. In addition to assessing cortical width and overall porosity, we also investigated the distribution within the periosteal and endosteal sections of the cortex of changes in Haversian canal density and area.
Materials and methods
Measurements were made in cortical bone from 54 biopsies obtained from healthy subjects, 23 males and 31 females aged 18–90 years, mean 47.7 years, whose details have been described in previous publications (Vedi et al. 1982, 1984). Trans-iliac biopsies were obtained with a Bordier trephine, internal diameter 6 mm, 1 inch below and posterior to the anterior superior iliac spine. These subjects had given written informed consent to undergo bone biopsy at the time of a general anaesthetic for a general surgical procedure. None had a history of illness or took medication affecting bone. The study was approved by the Local Ethics Committee. Of the 57 biopsies originally obtained in this study, three were omitted from the present study because the quality of the biopsy was inadequate for analysis of cortical bone. Four of the 54 biopsies included in the study had only one cortex for analysis. In 11 biopsies, only von Kossa-stained sections were available, whilst in the remaining 43, toluidine blue-stained sections were used. All sections were coded and assessed ‘blind’ by the same observer. Undecalcified sections of 7 μm, cut perpendicular to the longitudinal axis of the biopsy core from the upper, middle and lower thirds of each core, were prepared as previously described (Vedi & Compston, 2003).
Measurements were made using the Bioquant Osteo II image analysis system (version 8.11.20), which has an integrated QImaging camera, colour 12BIT, installed for capturing images from sections. For each biopsy a series of sequential images of stained sections is captured and saved in the BIF format (Bioquant Image Format), which records the X, Y and Z location of the image and the magnification of the image. These sequential images are loaded into the Image Montage Feature, which montages them into a single, large image ready for measurements to be made. Images are captured at a magnification of × 4, giving a resolution of 1.69 μm per pixel (MF, or magnification factor). The nomenclature for the measured variables is in accordance with that recommended by the ASBMR bone histomorphometry committee (Parfitt et al. 1987).
The following measurements were made.
Cortical area (Ct.Ar.; μm2)
Using the cursor, an outline of each cortex was drawn to separate them from the cancellous bone. These were thresholded to give two parallel coloured cortices with holes in them. Using the dilate threshold icon, these holes were filled as much as possible and on the Automeasure box, the areas of both cortices were measured separately.
After thresholding the section to distinguish bone from marrow, the cursor was used to draw around each cortex, starting at the periosteal surface. The endosteal surface of the cortex was identified subjectively to exclude trabecular bone and include only bone with a typical cortical osteonal structure.
Periosteal perimeter (Ps.Pm.; μm)
The periosteal surface of the biopsy is the outer surface of each cortex attached to muscle and other connective tissue. It was measured on a single shrunk field of view of each montaged section. Using the autoline icon, the upper and lower limits of the periosteal surface were marked and the length of this edge then recorded with the automeasure icon.
Endosteal perimeter (Ec.Pm.; μm)
The endosteal surface is the inner surface of each cortex at its junction with cancellous bone. It was measured in the same way as the periosteal perimeter by marking the upper and lower limits of the surface and recording the length.
Cortical width (Ct.Wi.; μm)
Cortical width is the mean perpendicular distance between the periosteal surface and the endosteal surface. Once the cortical area had been measured, the autoline button outlines the cortex and the endpoints define the start and finish points of the edges. The computer then automatically creates parallel equidistant lines, from which the average width of the cortex was estimated.
Haversian canal area (Ha.Ca.Ar; μm2)
The cortex was divided into half by drawing a line through the approximate middle to define periosteal and endosteal portions of the cortex.
The number, location (X–Y co-ordinates) and area of each Haversian canal (Ha.Ca.Ar) were recorded. The area of each Haversian canal was measured by drawing the surface of the canal using a cursor in manual measure mode and the area was measured automatically. The distance of each Haversian canal from the nearest cortical surface (endosteal or periosteal) was measured by drawing a perpendicular line from the centre of the canal to the nearest surface.
The following parameters were derived from the above indices.
Cortical porosity (Ct.Po; %)
This was calculated as the total Haversian canal area as a percentage of the total cortical area.
Haversian canal density (Ha.Ca.Dn; n μm−2)
This is a measure of total number of Haversian canals as a fraction of the total cortical area.
Ha.Ca.Dn = Total canal no./Ct.Ar.
Although all measurements were made separately on each cortex, the mean value for both cortices was used for statistical analysis.
Statistical analysis
Bland–Altman plots (Bland & Altman, 1986) were used to assess whether the two staining methods (toluidine blue and von Kossa) gave concordant measurements of bone histomorphometric parameters based on data from seven samples that were measured using both staining methods. A multivariable linear mixed regression model was used to assess the associations between cortical width, porosity and Haversian canal density with age and sex, as well as modelling the association between individual Haversian canal areas and distances from periosteal or endosteal surface. As the distributions of Haversian canal areas, cortical porosity and Haversian canal density were positively skewed, approximately normal distributions were achieved by natural log-transformation of these parameters in the regression modelling. The linear mixed model included a random intercept term at the subject level to account for the correlation between repeated measures provided by each subject (i.e. left and right side for porosity and density, and multiple Haversian canals for modelling of the association of canal size and distance from surface). The use of logarithmic transformation meant that the regression coefficients β estimated on the log-scale could be interpreted with exponentiation (i.e. eβ) as the proportional change in the response variable in the original scale.
Results
Table 1 provides some descriptive statistics, by sex and overall, of the histomorphometric measurements made on the iliac crest biopsies from 54 participants (23 males and 31 females), mean age = 47.7 years (SD = 15.6). Of the 54 samples contributing to the analyses, 43 were stained using the toluidine blue method and 11 using the von Kossa method. No systematic differences were seen between these two staining methods with regard to measurements of cortical area, width, porosity and Haversian canal density based on data from seven samples that were measured using both methods (i.e. the 95% CI for the difference includes zero for all four).
Table 1.
Summary characteristics by sex and overall
| Parameter | Male (n = 23) mean (SD) or median (IQR) | Female (n = 31) mean (SD) or median (IQR) | Total (n = 54) mean (SD) or median (IQR) |
|---|---|---|---|
| Age (years) | 48.2 (15.7) | 47.3 (15.8) | 47.7 (15.6) |
| Ct.Ar (μm2) | 4200696 (1831175) | 5060844 (2304058) | 4694485 (2140357) |
| Ps.Pm (μm) | 5059 (578) | 5163 (1277) | 5119 (1032) |
| Ec.Pm (μm) | 5425 (735) | 5520 (1195) | 5480 (1017) |
| Ct.Wi (μm) | 935 (366) | 1162 (574) | 1065 (505) |
| Ct.Po (%) | 4.67 (2.45, 7.58) | 4.59 (3.2, 7.48) | 4.67 (3.2, 7.48) |
| Ha.Ca.Dn (n mm−2) | 7.25 (5.35, 10.92) | 8.63 (6.08, 11.05) | 8.15 (5.76, 10.92) |
| Ha.Ca.Ar (μm2) | 1534 (1333, 1954) | 1632 (1276, 2114) | 1597 (1299, 1999) |
| Ct.Po%, periosteal | 5.96 (4.73, 7.28) | 5.29 (3.12, 8.62) | 5.52 (3.56, 7.79) |
| Ct.Po%, endosteal | 5.36 (3.04, 7.25) | 4.65 (3.13, 7.17) | 5.10 (3.13, 7.17) |
| Ha.Ca.Dn, periosteal | 9.89 (6.81, 10.83) | 10.52 (6.42, 14.11) | 10.06 (6.77, 12.59) |
| Ha.Ca.Dn, endosteal | 7.24 (5.72, 10.29) | 6.99 (5.54, 9.46) | 7.12 (5.72, 9.78) |
| Ha.Ca.Ar (μm2), periosteal | 1632 (1109, 3355) | 2045 (942, 3763) | 1836 (1080, 3355) |
| Ha.Ca.Ar (μm2), endosteal | 2063 (914, 3413) | 2201 (816, 6562) | 2132 (914, 5108) |
The regression modelling results for cortical area, cortical width, overall cortical porosity and overall Haversian canal density are detailed in Table 2. Model 1 assessed any statistically significant age*sex interactions in addition to the effects of age, sex and staining method. As none of the age*sex interactions attained statistical significance, only main effects were retained in the more parsimonious Model 2 for ease of interpretability of coefficients.
Table 2.
Multivariable models for the association of cortical bone parameters with age and sex overall
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Outcome\multivariable model variables | Beta (95% CI) | P | Beta (95% CI) | P |
| Ct.Ar (μm2) | ||||
| Age (per year) | −60 483 (−104 156, −16 809) | 0.007 | −51 944 (−79 890, −23 997) | < 0.0001 |
| Stain (von Kossa vs. toluidine blue) | −9487 (−890 309, 871 336) | 0.983 | −19 615 (−895 653, 856 423) | 0.965 |
| Sex (female vs. male) | 520 215 (−320 655, 1 361 084) | 0.225 | 487 908 (−338 086, 1 313 901) | 0.247 |
| Age*(sex = female vs. male) | 14 099 (−41 216, 69 414) | 0.617 | - | - |
| Constant | 4 470 705 (3 790 116, 5 151 293) | < 0.0001 | 4 490 738 (3 819 329, 5 162 147) | < 0.0001 |
| Ct.Wi (μm) | ||||
| Age (per year) | −10 (−19, −1) | 0.032 | −9 (−15, −3) | 0.002 |
| Stain (von Kossa vs. toluidine blue) | 50 (−137, 237) | 0.601 | 49 (−137, 235) | 0.605 |
| Sex (female vs. male) | 176 (−2, 353) | 0.053 | 172 (−2, 346) | 0.053 |
| Age*(sex = female vs. male) | 2 (−10, 13) | 0.801 | - | - |
| Constant | 945 (801, 1089) | < 0.0001 | 946 (805, 1088) | < 0.0001 |
| Loge Ct.Po (%) – overall | ||||
| Age (per year) | 0.011 (−0.007, 0.028) | 0.238 | 0.005 (−0.006, 0.017) | 0.345 |
| Stain (von Kossa vs. toluidine blue) | 0.141 (−0.203, 0.485) | 0.422 | 0.148 (−0.195, 0.491) | 0.399 |
| Sex (female vs. male) | 0.134 (−0.207, 0.476) | 0.441 | 0.154 (−0.184, 0.491) | 0.372 |
| Age*(sex = female vs. male) | −0.009 (−0.031, 0.014) | 0.454 | - | - |
| Constant | 1.553 (1.279, 1.828) | < 0.0001 | 1.541 (1.269, 1.813) | < 0.0001 |
| Loge Ha.Ca.Dn (n mm−2) – overall | ||||
| Age (per year) | 0.009 (−0.004, 0.021) | 0.168 | 0.009 (0.001, 0.016) | 0.032 |
| Stain (von Kossa vs. toluidine blue) | 0.135 (−0.102, 0.372) | 0.265 | 0.135 (−0.100, 0.370) | 0.260 |
| Sex (female vs. male) | 0.113 (−0.124, 0.350) | 0.349 | 0.114 (−0.119, 0.346) | 0.338 |
| Age*(sex = female vs. male) | 0.000 (−0.015, 0.015) | 1.000 | - | - |
| Constant | 2.020 (1.829, 2.210) | < 0.0001 | 2.019 (1.832, 2.207) | < 0.0001 |
The interaction effects represent the difference in regression coefficients between groups, e.g. for cortical width, the coefficient of 2 (−10, 13) for age*(sex = female vs. male) implies that the rate of cortical width decline was about 2 μ per year higher in females than in males (P = 0.801).
Cortical area and width decreased significantly with age (P < 0.002) but no statistically significant differences were found in the magnitude of decrease between sexes (P > 0.617, in model 1). There was a borderline significant sex difference in mean cortical width, with females having thicker cortices than males (difference (95% CI) = 172(−2, 346) μ, P = 0.053). There were no statistically significant associations found between overall cortical porosity and age and sex, but overall Haversian canal density increased with age by about 9% per decade (i.e. e10*0.009, P = 0.032) (Fig. 1, Table 2).
Fig 1.
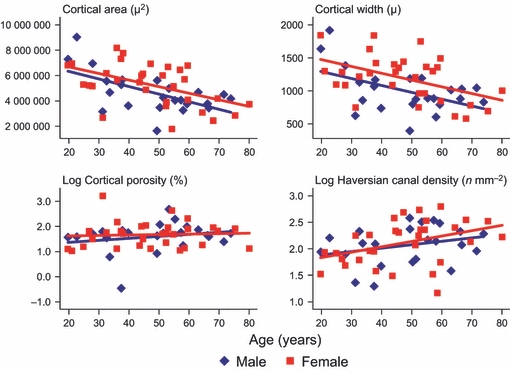
Scatter plots for associations between cortical area, width, porosity and Haversian canal density with age by sex.
As shown in Table 3, when the calculations were done separately for the two halves of the cortex with respect to the nearest surface (i.e. the periosteal surface or the endosteal surface, assuming that the cortex was split into two halves and half of the total cortical area was used to define the porosity with respect to the nearest surface) it was found that cortical porosity in the endosteal section was marginally significantly lower than cortical porosity with respect to the periosteal section [difference (95% CI) in log porosity = −0.218 (−0.441, −0.004), P = 0.055]. Equivalently, cortical porosity was about 20% (i.e. e−0.218) lower in the endosteal section of the cortex. Similarly the Haversian canal density in the endosteal section was lower than that in the periosteal section [difference (95% CI) in log Haversian canal density = −0.255 (−0.385, −0.126), P < 0.0001]. Equivalently, the Haversian canal density was 23% (i.e. e−0.255) lower in the endosteal section of the cortex. The increase in Haversian canal density with ageing was, however, not significantly different between the endosteal vs. periosteal regions of the cortex (P = 0.568 for age*cortex interaction in Model 1, Table 3).
Table 3.
Multivariable models for the association of cortical bone parameters with age and sex including contrast by cortex region
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Outcome\multivariable model variables | Beta (95% CI) | P | Beta (95% CI) | P |
| Loge Ct.Po (%) – by cortex | ||||
| Age (per year) | 0.012 (−0.005, 0.030) | 0.168 | 0.003 (−0.007, 0.014) | 0.567 |
| Cortex (endosteal vs. periosteal) | −0.237 (−0.463, −0.011) | 0.039 | −0.218 (−0.441, 0.004) | 0.055 |
| Stain (von Kossa vs. toluidine blue) | 0.080 (−0.227, 0.386) | 0.611 | 0.087 (−0.219, 0.393) | 0.579 |
| Sex (female vs. male) | 0.156 (−0.160, 0.473) | 0.332 | 0.177 (−0.135, 0.490) | 0.266 |
| Age*(cortex = endosteal vs. periosteal) | −0.008 (−0.023, 0.007) | 0.308 | - | - |
| Age*(sex = female) | −0.009 (−0.030, 0.011) | 0.375 | - | - |
| Constant | 1.531 (1.254, 1.807) | < 0.0001 | 1.508 (1.234, 1.782) | < 0.0001 |
| Loge Ha.Ca.Dn (n mm−2) – by cortex | ||||
| Age (per year) | 0.010 (−0.003, 0.022) | 0.130 | 0.008 (0.001, 0.016) | 0.030 |
| Cortex (endosteal vs. periosteal) | −0.261 (−0.393, −0.130) | < 0.0001 | −0.255 (−0.385, −0.126) | < 0.0001 |
| Stain (von Kossa vs. toluidine blue) | 0.130 (−0.069, 0.330) | 0.200 | 0.131 (−0.067, 0.329) | 0.196 |
| Sex (female vs. male) | 0.112 (−0.119, 0.343) | 0.341 | 0.112 (−0.114, 0.338) | 0.331 |
| Age*(cortex = endosteal vs. periosteal) | −0.003 (−0.011, 0.006) | 0.568 | - | - |
| Age*(sex = female) | −0.000 (−0.015, 0.015) | 0.995 | - | - |
| Constant | 2.119 (1.925, 2.312) | < 0.0001 | 2.115 (1.925, 2.306) | < 0.0001 |
| Loge Ha.Ca.Ar (log μm2) | ||||
| Age (per year) | 0.003 (−0.005, 0.012) | 0.480 | −0.001 (−0.007, 0.005) | 0.817 |
| Distance to Haversian canal (per 1000 μm) | 0.335 (0.095, 0.575) | 0.006 | 0.332 (0.093, 0.572) | 0.007 |
| Cortex (endosteal vs. periosteal) | 0.162 (0.026, 0.297) | 0.019 | 0.162 (0.027, 0.298) | 0.019 |
| Stain (von Kossa vs. toluidine blue) | −0.171 (−0.298, −0.045) | 0.008 | −0.167 (−0.294, −0.041) | 0.009 |
| Sex (female vs. male) | −0.038 (−0.203, 0.127) | 0.652 | −0.022 (−0.186, 0.141) | 0.788 |
| Age*(cortex = endosteal vs. periosteal) | −0.005 (−0.010, −0.000) | 0.039 | −0.005 (−0.010, −< 0.0001) | 0.040 |
| Distance*(cortex = endosteal vs. periosteal) | −0.409 (−0.731, −0.086) | 0.013 | −0.408 (−0.730, −0.085) | 0.013 |
| Age*(sex = female) | −0.006 (−0.017, 0.004) | 0.232 | - | - |
| Constant | 7.600 (7.449, 7.751) | < 0.0001 | 7.591 (7.441, 7.742) | < 0.0001 |
See text and footnote on Table 2 for interpretation of the interaction effects.
Discussion
The results of this study demonstrate an age-related decrease in iliac crest cortical width and area both in men and women. Although Haversian canal density increased with age, Haversian canal area tended to decrease with age, resulting in no overall age-related change in cortical porosity. Haversian canal area was significantly higher in the endosteal section than in the periosteal section of the cortex but the Haversian canal density was lower, resulting in similar overall porosity in the two sections.
The age-related decrease in cortical width observed in this study is consistent with data reported by others both in the iliac crest and at other skeletal sites including the femur, rib, metacarpals and vertebrae (Atkinson, 1964; Smith & Walker, 1964; Adams et al. 1970; Arnold, 1970; Melsen et al. 1978; Laval-Jeantet et al. 1983; Brockstedt et al. 1993). This reduction in cortical width is likely to be due to endosteal resorption, which may be partly compensated for by increased periosteal bone formation at some skeletal sites (Takahashi & Frost, 1966; Garn et al. 1967). Whether periosteal expansion occurs in iliac crest bone is uncertain, but our results and those of others are consistent with net loss of cortical width with ageing. In the present study a significant decrease with age was demonstrated both in females and males; in contrast, Brockstedt et al. (1993) reported a significant age-related decrease only in women, although there was a non-significant trend towards a decrease with age in men.
Haversian canal areas were significantly larger in the endosteal section of the cortex than in the periosteal section [difference (95% CI) in log areas = 0.162 (0.026, 0.298), P = 0.019] or equivalently 18% larger areas in the endosteal region of the cortex. There was a general decrease in Haversian canal areas with ageing, but this was only significant in the endosteal section of the cortex [coefficient (95%) per year decrease in log areas = −0.001 (−0.007, 0.005), P = 0.817 in the periosteal region and −0.006 (−0.016, −0.001), P = 0.023 in the endosteal region], a difference of P = 0.040 (Table 3). A statistically significant interaction was found in the association of individual Haversian canal areas and their distance from the periosteal surface [coefficient (95% CI) mm−1 increase in distance 0.332 (0.093, 0.572), P = 0.007] but not in the endosteal region [−0.073 (−0.303, 0.156) P = 0.531], the difference being −0.408 (−0.730, −0.085), P = 0.013.
In contrast to the results reported by Brockstedt et al. (1993), no significant changes in cortical porosity were demonstrated in the present study. Cortical porosity may change as a result of a change in the number of pores (Haversian canal density) or in their size (Haversian canal area). We found that although there was a trend towards increased Haversian canal density with age, canal area decreased with age, resulting in no overall change in cortical porosity. In the study of Arnold (1970), an age-related increase in Haversian canal diameter was demonstrated and cortical porosity, separately assessed using an eye-piece graticule, also increased significantly with age. Other studies have reported age-related increases in cortical porosity in the iliac crest and at other skeletal sites (Jowsey, 1960; Wu et al. 1967; Melsen et al. 1978; Martin et al. 1980; Brockstedt et al. 1993). The lack of change in cortical porosity in the present study may reflect methodological differences and/or insufficient sample size. In addition, because of increasing trabecularization of cortical bone with age, identification of the endosteal margin of the cortex is often difficult and largely subjective (Brown et al. 1987). Inclusion of partially trabecularized cortical bone in the measurements will favour the finding of increased porosity with age, whereas its exclusion will reduce the likelihood of this finding.
The Haversian canal diameter and area depend on the remodelling balance achieved within individual remodelling units. An increase in Haversian area may thus result from increased resorption depth, decreased wall width or a combination of the two. Several previous studies support a negative remodelling balance in older individuals, resulting in increased Haversian canal diameter, although this finding is not universal and may vary between skeletal sites (Atkinson, 1964; Thompson, 1980; Broulik et al. 1982; Brockstedt et al. 1993; Thomas et al. 2006). In the present study no significant age-related changes were found in Haversian canal area in the whole cortex; the apparent age-related decrease in the endosteal section may reflect the exclusion of larger canals from measurement because of the process of trabecularization of the cortex associated with ageing. Canal area in the endosteal section of the cortex was greater than in the periosteal section, consistent with trabecularization of the endosteal cortex. In the periosteal section of the cortex the canal area increased with increasing distance from the periosteal surface but canal area did not vary significantly in the endosteal section of the cortex.
Our study has some limitations. Although our series of biopsies from healthy controls is large relative to most other series, the sample size is relatively small for a study of age-related changes, particularly when men and women are considered separately, and thus the statistical power to show significant changes may have been inadequate for some parameters. Inclusion of autopsy samples in some studies has been used to increase the sample size; however, information on medical history prior to death is often limited in these subjects and diseases or life-style variables affecting bone metabolism cannot be excluded. Secondly, as in other studies the approach to the definition of the endosteal margin of the cortex was to some extent subjective and may have affected estimates of porosity and Haversian canal area. Because of the necessarily subjective nature of the definition, it is not possible to assess whether different approaches could explain differences between studies. Thirdly, as this was a cross-sectional study we cannot exclude the possibility that secular changes in cortical dimensions and architecture may have influenced our results.
In conclusion, our study in iliac crest biopsies from healthy subjects demonstrates an age-related decrease in cortical width and area in both men and women with a trend towards increased number of Haversian canals but no overall increase in cortical porosity. The density of the Haversian canal was lower and the area higher in the endosteal than in the periosteal section of the cortex. Our results extend the limited reference data available on cortical bone structure in the iliac crest and provide a broader base for comparison in studies of untreated and treated bone disease.
Acknowledgments
The authors have no conflicts of interest. J.E.C. acknowledges support from the Cambridge Biomedical Research Centre and National Institute for Health Research (NIHR).
References
- Adams P, Davies GT, Sweetnam P. Osteoporosis and the effects of aging on bone mass in elderly men and women. Q J Med. 1970;39:602–605. [PubMed] [Google Scholar]
- Arnold JS. Focal extensive endosteal resorption in aging and senile osteoporosis. In: Barzel US, editor. Osteoporosis. Grune & Stratton: New York; 1970. pp. 80–100. [Google Scholar]
- Atkinson PJ. Quantitative analysis of osteoporosis in cortical bone. Nature. 1964;201:373–375. doi: 10.1038/201373a0. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- Brockstedt H, Kassem M, Eriksen EF, et al. Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone. 1993;14:681–691. doi: 10.1016/8756-3282(93)90092-o. [DOI] [PubMed] [Google Scholar]
- Broulik P, Kragstrup J, Mosekilde L, et al. Osteon cross sectional size in the iliac crest. Acta Pathol Microbiol Immunol Scand A. 1982;90:339–344. [PubMed] [Google Scholar]
- Brown JP, Delmas PD, Arlot M, et al. Active bone turnover of the cortico-endosteal envelope in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1987;64:954–959. doi: 10.1210/jcem-64-5-954. [DOI] [PubMed] [Google Scholar]
- Garn SM, Rohmann CG, Wagner B, et al. Continuing bone growth throughout life. A general phenomenon. Am J Phys Anthropol. 1967;26:313–317. doi: 10.1002/ajpa.1330260306. [DOI] [PubMed] [Google Scholar]
- Jowsey J. Age changes in human bone. Clin Orthop. 1960;17:210–217. [Google Scholar]
- Laval-Jeantet AM, Bergot C, Caroll R, et al. Cortical bone senescence and mineral bone density of humerus. Calcif Tissue Int. 1983;35:268–272. doi: 10.1007/BF02405044. [DOI] [PubMed] [Google Scholar]
- Martin RB, Pickett JC, Zinaich S. Studies of skeletal remodeling in aging men. Clin Orthop. 1980;149:268–282. [PubMed] [Google Scholar]
- Melsen F, Melsen B, Mosekilde L, et al. Histomorphometric analysis of normal bone from the iliac crest. Acta Pathol Microbiol Scand. 1978;86:70–81. doi: 10.1111/j.1699-0463.1978.tb02014.x. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Age-related structural changes in trabecular and cortical bone. Cellular mechanisms and biomechanical consequences. Calcif Tissue Int. 1984;36:123–128. doi: 10.1007/BF02406145. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry nomenclature, symbols and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ. Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Smith RW, Walker RP. Femoral expansion in aging women: implications for osteoporosis and fractures. Science. 1964;145:156–157. doi: 10.1126/science.145.3628.156. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Frost HM. Age and sex related changes in the amount of cortex in normal human ribs. Acta Orthop Scand. 1966;37:122. doi: 10.3109/17453676608993272. [DOI] [PubMed] [Google Scholar]
- Thomas CDL, Feik SA, Clement JG. Increase in pore area, and not pore density, is the main determinant in the development of porosity in human cortical bone. J Anat. 2006;209:219–230. doi: 10.1111/j.1469-7580.2006.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DD. Age changes in bone mineralisation, cortical thickness and Haversian canal area. Calcif Tissue Int. 1980;31:5–11. doi: 10.1007/BF02407161. [DOI] [PubMed] [Google Scholar]
- Vedi S, Compston J. Bone histomorphometry. Methods Mol Med. 2003;80:283–298. doi: 10.1385/1-59259-366-6:283. [DOI] [PubMed] [Google Scholar]
- Vedi S, Compston JE, Webb A, et al. Histomorphometric analysis of biopsies from the iliac crest of normal British subjects. Metab Bone Dis Relat Res. 1982;4:231–236. doi: 10.1016/0221-8747(82)90032-7. [DOI] [PubMed] [Google Scholar]
- Vedi S, Compston JE, Webb A, et al. Histomorphometric analysis of dynamic parameters of trabecular bone formation from the iliac crest of normal British subjects. Metab Bone Dis Relat Res. 1984;5:69–74. doi: 10.1016/0221-8747(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Wu K, Jett S, Frost HM. Bone resorption rates in rib in physiological, senile, and postmenopausal osteoporosis. J Lab Clin Med. 1967;69:810–818. [PubMed] [Google Scholar]


