Abstract
The vomeronasal system is crucial for social and sexual communication in mammals. Two populations of vomeronasal sensory neurons, each expressing Gαi2 or Gαo proteins, send projections to glomeruli of the rostral or caudal accessory olfactory bulb, rAOB and cAOB, respectively. In rodents, the Gαi2- and Gαo-expressing vomeronasal pathways have shown differential responses to small/volatile vs. large/non-volatile semiochemicals, respectively. Moreover, early gene expression suggests predominant activation of rAOB and cAOB neurons in sexual vs. aggressive contexts, respectively. We recently described the AOB of Octodon degus, a semiarid-inhabiting diurnal caviomorph. Their AOB has a cell indentation between subdomains and the rAOB is twice the size of the cAOB. Moreover, their AOB receives innervation from the lateral aspect, contrasting with the medial innervation of all other mammals examined to date. Aiming to relate AOB anatomy with lifestyle, we performed a morphometric study on the AOB of the capybara, a semiaquatic caviomorph whose lifestyle differs remarkably from that of O. degus. Capybaras mate in water and scent-mark their surroundings with oily deposits, mostly for male–male communication. We found that, similar to O. degus, the AOB of capybaras shows a lateral innervation of the vomeronasal nerve, a cell indentation between subdomains and heterogeneous subdomains, but in contrast to O. degus the caudal portion is larger than the rostral one. We also observed that four other caviomorph species present a lateral AOB innervation and a cell indentation between AOB subdomains, suggesting that those traits could represent apomorphies of the group. We propose that although some AOB traits may be phylogenetically conserved in caviomorphs, ecological specializations may play an important role in shaping the AOB.
Keywords: chemical communication, Hydrochaeridae, hystricognathi, pheromones, semiochemicals, vomeronasal system
Introduction
The coordination of social and sexual interactions in rodents depends largely on the detection of semiochemicals by the vomeronasal system (VNS) and on the orchestration of neuroendocrine and behavioural responses by its amygdaloid and hypothalamic interconnections (Meisami & Bhatnagar, 1998; Halpern & Martinez-Marcos, 2003). The vomeronasal organ (VNO) of rodents is a blind-ended tubular structure located bilaterally at the base of the nasal septum and opens into the anterior nasal cavity through a narrow duct. Vomeronasal neurons located at the apical extent of the VNO neuroepithelium express receptors of the V1R family, the αi2 subunit of G-protein (Gαi2), and send axons that end in glomerular neuropil distributed on the rostral half of the accessory olfactory bulb (rAOB). Conversely, basally located vomeronasal neurons express V2R receptors, the Gαo protein subunit and project to glomeruli of the caudal half of the accessory olfactory bulb (cAOB) (Dulac & Axel, 1995; Halpern et al. 1995; Berghard & Buck, 1996; Jia & Halpern, 1996; Herrada & Dulac, 1997; Matsunami & Buck, 1997; Ryba & Tirindelli, 1997). Likewise, both AOB subdomains differ in their local connectivity and cell/axonal morphology (Larriva-Sahd, 2008), and in their projection patterns to the amygdala, the bed nucleus of the stria terminalis and the bed nucleus of the accessory olfactory tract (Martinez-Marcos & Halpern, 1999a; Mohedano-Moriano et al. 2007), as well as in their centrifugal afferences from amygdaloid nuclei (Martinez-Marcos & Halpern, 1999b).
Most research on the VNS comes from studies on laboratory strains of rats and mice, both species with a long history of human commensalism followed by inbreeding and artificial selection for biomedical purposes (Berdoy & Drickamer, 2007). The AOB of muroid rodents (e.g. mice, rats and hamsters) is a lentiform structure with rAOB and cAOB subdomains of similar form and size, and as all other mammals outside Caviomorpha (South American members of the Hystricognathi rodent sub-order), it receives vomeronasal innervation from the medial aspect (McCotter, 1912; Wekesa & Anholt, 1999; Suárez et al. 2009).
We recently described the AOB of the diurnal, social and semiarid-inhabiting caviomorph Octodon degus– common name degus – (Octodontidae), which shows rAOB-biased heterogeneities such as a twofold difference in overall volume and larger and more abundant glomeruli in the rAOB than cAOB (Suárez & Mpodozis, 2009). Moreover, the vomeronasal nerve arrives from the lateral aspect and a cell indentation coincides with the margin between AOB subdomains. We proposed in that study that the rostrally biased AOB pattern of degus might be related to aspects of their lifestyle, such as a putative long-range volatile form of chemosignalling (Soto-Gamboa, 2004), mostly due to scent-marking through dust-bathing behaviour (Ebensperger, 2000; Ebensperger & Caiozzi, 2002), and a prevalence of male–female over male–male nose-to-body sampling of semiochemicals, especially during the mating season (Soto-Gamboa et al. 2005).
Aiming to search for AOB traits that could be related to lifestyle, we performed a morphometric study in the AOB of capybaras, a caviomorph with a strikingly different lifestyle to that of degus. Capybaras (Hydrochaeris hydrochaeris; Hydrochaeridae) inhabit flooded terrains of tropical South America and scent-mark their territory, mostly for signalling male hierarchy, by depositing oily substances on twigs and bushes (Herrera & MacDonald, 1994).
We found that the AOB of capybaras shares some traits with that of degus and other caviomorphs, distinguishing them from all other mammals studied to date. However, the AOB glomerular morphometric patterns contrast in capybaras and degus, possibly reflecting differences in aspects of their lifestyle. This study provides the first description of the AOB of a semiaquatic rodent and introduces Caviomorpha as a group to study the anatomical diversity of the vomeronasal system.
Materials and methods
All experimental procedures were performed following the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, 1996), and were consistent with Brazilian and Chilean legislation on animal care. The number of individuals used was kept to a minimum and all efforts were made to minimize animal suffering.
For the morphometric study, we employed five adult capybaras, Hydrochaeris hydrochaeris (three males and two females, 1–2 years old; 30–45 kg), obtained from the Profauna Farm (São Paulo, Brazil), under a licence granted by the Brazilian Institute of the Environment (IBAMA 1-35-93-0848-0). Food (Nutricobaia pellets; Purina) and water were provided ad libitum.
To assess whether the lateral innervation and cell indentation were present in other caviomorphs, we obtained olfactory bulb tissue of four different species from a brain bank where they were stored following unrelated anatomical and physiological studies: Ctenomys talarum (Ctenomyidae, n = 3) and Spalacopus cyanus (Octodontidae, n = 3), both species diurnal and subterranean; and Octodon lunatus (Octodontidae, n = 3) (Vega-Zúñiga, 2009) and Chinchilla laniger (Chinchillidae, n = 2) (Delano et al. 2010), both species nocturnal and solitary.
Capybaras were deeply anaesthetized with an overdose of pentobarbitone (80 mg kg−1, i.v.). The carotid artery and jugular vein were exposed by making a skin incision along the neck, and 100 mL of 0.1 m phosphate-buffered saline (PBS) at pH 7.4 was perfused through a cannula inserted into the left common carotid artery, followed by 500 mL of 4% formaldehyde in PBS. The olfactory bulbs were carefully dissected out and kept in the same fixative solution until further processing (within 2 months after perfusion). The tissue was then submerged for cryoprotection in a 30% sucrose solution (w/v) in PBS until it sank. We obtained sagittal sections 60 μm thick using a sliding microtome (Leitz-Wetzlar 1400; Leitz, Germany) equipped with a freezing stage (BFS-30MP; Physitemp, Clifton, NJ, USA). Every other section was mounted and Nissl-stained with cresyl violet. The remaining sections were used for immunohistochemistry as follows: we put them in vials containing PBS with 0.05% Triton X-100 (PBST) and 0.3% H2O2 for 30 min, followed by 5% normal goat serum (NGS) in PBST for 1 h. We then incubated them in mouse monoclonal antibodies raised against Gαi2 (1 : 200, cat no. sc-13534; Santa Cruz Biotechnology) or Gαo (1 : 200, cat no. sc-13532; Santa Cruz Biotechnology) with 3% NGS in PBST for 18 h at 25 °C. Next, the sections were washed in PBS and incubated in biotinylated goat anti-mouse immunoglobulins (1 : 200, cat no. sc-2039; Santa Cruz Biotechnology) for 2 h and processed with avidin-biotin (ABC Elite kit; Vector Laboratories) for 1 h. Finally, the tissue was reacted in PBS with 0.6 mg mL−1 of 3,3-diaminobenzidine (Sigma) and 0.03% H2O2 for 20–120 s.
All other caviomorph brain tissue was conserved in 4% paraformaldehyde in PBS until its use (up to 18 months). After cryoprotection in sucrose 30% (w/v), we obtained 40-μm sagittal sections and stained them with cresyl violet. The olfactory bulbs of one specimen of O. lunatus were immunolabelled against Gαi2 with the same protocols described above for capybaras, before being counterstained with cresyl violet.
We compared morphometric values between the rAOB and cAOB in Nissl-stained sections of capybaras following the same rationale and methods previously used for degus (Suárez & Mpodozis, 2009). Briefly, we recorded the area of the AOB in series of nine sagittal 60-μm sections, spaced 120 μm each and centred in the slice with the maximum AOB area. We excluded the farthest medial and lateral portions of the AOB because cell layers curve up in the sagittal plane, making it difficult to outline the borders between cell layers and subdomains. For each section we measured the area of the overall AOB (from the vomeronasal nerve layer to the lateral olfactory tract) and of the glomerular layer only, in both rAOB and cAOB. We computed volume values by multiplying section areas by their thickness. The volume of the alternated slices was estimated as the average values between the volumes of flanking slices. We also scored, for each section and AOB subdivision, the total number of glomeruli, the mean diameter of glomeruli, determined by measuring 10 randomly chosen glomeruli at each subdivision, and the mean number of mitral/tufted cells inside a 0.01-mm2 area randomly placed at the mitral/tufted cell layer within each AOB subdivision. Each measurement, with the exception of glomeruli diameter, was the mean of two to four scoring replicates. All observations were made using a BX60 Olympus optical microscope (BX60; Olympus Optical, Thornwood, NY, USA), and all measurements and photographs were obtained using the SPOT digital camera and software (Spot Advanced; Diagnostic Instrument, Sterling Heights, MI, USA). Figures were prepared for presentation purposes using Adobe CS3 Photoshop and Illustrator (Adobe Systems, San Jose, CA, USA).
To compare morphometric values between rAOB and cAOB we employed the Wilcoxon-matched paired test using statistica 6.0 software (StatSoft Inc., Tulsa, OK, USA). Differences were considered significant when P < 0.05. Data are presented as the mean value ± 1 SE.
Results
The AOB of capybaras
Similar to what has been described in all mammals with a functional vomeronasal system, the AOB of capybaras is located at the dorso-caudal extent of the main olfactory bulb (Fig. 1A). It is a prominent structure consisting of several layers: the vomeronasal nerve (VN), glomerular (Gl), external plexiform (EP), mitral/tufted cell (MT) and granule cell (Gr) layers (Fig. 1B). The VN arrives from the lateral aspect, in contrast to the medial innervation observed in most mammals studied so far. It contains axons that terminate in glomeruli arranged 4–7 in depth at the Gl layer. Mitral/tufted cells are mostly distributed in a compact band at the MT layer; however, some scattered MT cells can be observed throughout the internal aspect of the EP layer (arrowheads in Fig. 1C). Gr cells were distinguished from MT cells by their small size and granular shape (Fig. 1C). The lateral olfactory tract (LOT) passes underneath the MT layer and within granule cells (Fig. 1B–C). The organization of granule cells differs from that of other rodents studied so far: although their small somata show the characteristic tangential sheet clustering beneath and within the LOT (Fig. 1B–C), they also show a scattered distribution reaching up to more superficial layers (arrows in Fig. 1C).
Fig. 1.
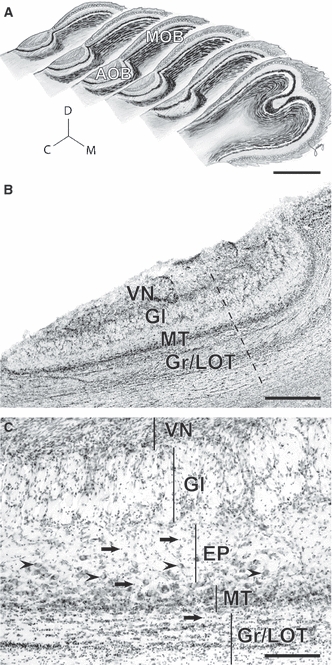
The accessory olfactory bulb of capybaras. (A) Sagittal representations throughout the olfactory bulb show that the accessory olfactory bulb (AOB) is located laterally. Note the size relation between the AOB and the main olfactory bulb (MOB). C, caudal; M, medial; D, dorsal. (B) Nissl-stained section revealing the cellular indentation between rostral and caudal subdivisions (dashed line) and AOB cell layers: VN, vomeronasal nerve; Gl, glomerular; MT, mitral/tufted cells; Gr/LOT, granule cells/lateral olfactory tract. (C) The extent of each layer is shown here with vertical lines. MT cells are densely packed at the MT layer and scatter in the inner portions of the external plexiform (EP) layer (arrowheads). Granule cells cluster at the Gr/LOT layer but also reach towards more superficial layers (arrows). Scale bars: 5 mm (A), 1 mm (B), 200 μm (C).
Interestingly, the AOB of capybaras resembles that of degus in that a cell indentation, most evident at the MT layer, is observed between subdomains (dashed line in Fig. 1B). These regions appear as two lobular structures of distinct shape that can be easily observed with Nissl staining; whereas the cAOB is elongated, the rAOB is rounded (Fig. 1B). Furthermore, as also described in degus, the cell indentation coincides with the segregated pattern of expression of Gαi2 and Gαo proteins (Fig. 2). Immunohistochemistry for Gαo protein labels the VN and Gl layers of the cAOB, as well as the olfactory nerve and glomeruli of the main olfactory bulb (Fig. 2A). On the other hand, Gαi2 is expressed in the VN and Gl layers of the rAOB only (Fig. 2B).
Fig. 2.
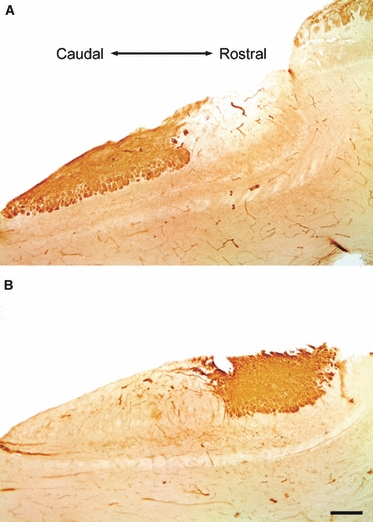
Pattern of G-protein expression in the AOB of capybaras. (A) Immunolabeling of Gαo-protein is observed at the superficial (vomeronasal nerve and glomerular) layers of the caudal AOB (lower left) and main olfactory bulb (upper right). (B) Expression of Gαi2-protein is observed in the vomeronasal and glomerular layers of the rostral AOB only (upper right). Note that the expression of both markers coincides with the cell indentation. Dorsal is to the top. Scale bar: 1 mm.
Caudal-biased glomerular heterogeneities in the AOB of capybaras
We compared morphometric measurements between rostral and caudal AOB subdomains and found several significant differences. The overall AOB volume (i.e. from the VN to the LOT) is larger in the cAOB than in the rAOB (Z = 2.021, df = 5, P < 0.03; Fig. 3A). This heterogeneity most likely reflects glomerular differences, as the Gl layer alone is larger in the cAOB than in the rAOB (Z = 2.021, df = 5, P < 0.03; Fig. 3B). Indeed, the caudally biased AOB heterogeneities are expressed in terms of number (Z = 2.021, df = 5, P < 0.03; Fig. 3C) but not mean diameter of glomeruli (Z = 1.753, df = 5, P = 0.08; Fig. 3D). Interestingly, following a similar pattern to degus, the density of mitral/tufted cells is significantly larger in the rAOB than in the cAOB (Z = 1.992, df = 5, P < 0.05; Fig. 3E). Although sexual dimorphisms in AOB measurements may well be expected to occur in capybaras, in particular considering their highly dimorphic habits, our sampling number did not allow us to make comparisons between the sexes. However, we observed the cAOB-biased heterogeneities in all specimens, irrespective of sex, in a similar way to what we described in degus (Suárez & Mpodozis, 2009).
Fig. 3.
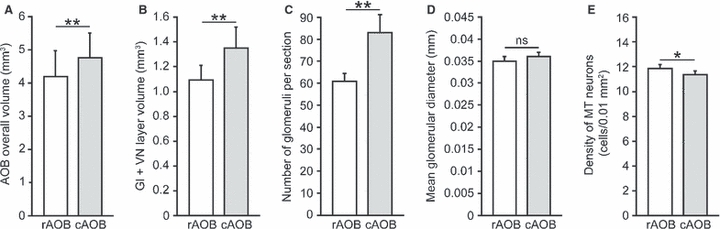
Anatomical heterogeneities between rostral and caudal AOB subdomains. The caudal AOB (cAOB) shows larger values than the rostral AOB (rAOB) in overall volume (A), in the volume of the superficial portion of the AOB, including vomeronasal (VN) and glomerular (Gl) layers (B), and in the number of glomeruli per section (C). (D) The size of glomeruli did not differ between subdomains. (E) The density of MT cells, however, was higher in the rAOB than cAOB. *P < 0.05, **P < 0.03.
AOB of other caviomorph species
With the aim of investigating whether the AOB traits shared by degus and capybaras are also present in other caviomorphs, we made observations on sagittal sections through the olfactory bulb of C. talarum (Fig. 4A), S. cyanus (Fig. 4B), O. lunatus (Fig. 4C) and C. laniger (Fig. 4D). In all cases, the AOB appears in lateral sections (asterisks in Fig. 4A–E, left panels), but disappears medially (right panels). The precise site of arrival of the vomeronasal nerve can be seen in a lateral section of O. lunatus counterstained against Gαi2 (arrows in Fig. 4C). Interestingly, the cell indentation at the margin between AOB subdomains is also evident in all caviomorphs examined, expanding throughout cell layers (arrowheads in Fig. 4A–E).
Fig. 4.
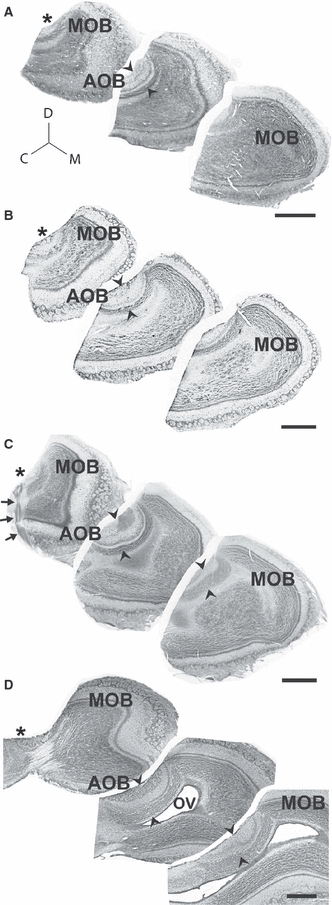
Lateral innervation and cell indentation in the AOB of caviomorph rodents. Panels A–D show Nissl-stained sagittal sections through the olfactory bulbs, from lateral portions on the left to progressively more medial portions on the right, of Ctenomys talarum (A), Spalacopus cyanus (B), Octodon lunatus (C) and Chinchilla laniger (D). The vomeronasal nerve arrives into AOB glomeruli from the lateral aspect (asterisks). Towards medial sections, all AOB glomeruli disappear (right). Note that a cell indentation is evident in all species (arrowheads). Nissl stain combined with immunohistochemistry for Gαi2-protein in O. lunatus shows the arrival of the vomeronasal nerve (arrows) and the coincidence of the cell indentation with rostral AOB expression of Gαi2-protein (C). Although the olfactory ventricle (OV) is collapsed in most caviomorphs, in chinchillas it is remarkably large. C, caudal; D, dorsal; M, medial; MOB, main olfactory bulb. Scale bar: 1 mm.
Discussion
AOB of caviomorph rodents
In all non-caviomorph mammals studied to date the vomeronasal nerve arrives at the AOB after following a medial course between the olfactory bulbs. This has been reported in opossums, tenrecs, rabbits, mice, rats, sheeps and dogs (McCotter, 1912; Imamura et al. 1985; Wekesa & Anholt, 1999; Suárez et al. 2009). Moreover, due to its homogeneous lentiform structure, the boundary between AOB subdomains has only been possible to determine using histochemistry and/or immunolabeling (Halpern et al. 1998; Dudley & Moss, 1999). In this study we extend our previous findings in degus by showing that capybaras and other caviomorphs also show a vomeronasal innervation from the lateral aspect and a cell indentation across layers at the boundary between AOB subdomains. Both traits are present in all caviomorphs examined so far, irrespective of their different lifestyles, and may represent apomorphies of Caviomorpha. Whether these traits are also present in African–Asian Hystricognaths, such as Old World porcupines (Hystricidae), mole rats (Bathyergidae) or cane rats (Thryonomyidae) is an interesting possibility that deserves further investigation.
In spite of the traits shared by caviomorphs, the AOBs of capybaras and degus show a contrasting pattern: while degus have more glomeruli in the rAOB than in the cAOB, in capybaras the cAOB is larger and has more glomeruli than the rAOB. We propose that these differences may be related with differences of lifestyle between these species, as described below.
Functional differences between AOB subdomains in rodents
Two lines of evidence suggest that the segregated vomeronasal pathways of rodents are operationally distinct. First, the ligand-binding domains of the vomeronasal receptors vary significantly between receptor families. While V1Rs have a minute extracellular N-domain (Herrada & Dulac, 1997; Matsunami & Buck, 1997) and a remarkably high affinity for small molecules, such as urinary and gland-derived volatiles (Leinders-Zufall et al. 2000; Sugai et al. 2006), receptors of the V2R family have much larger extracellular domains (Herrada & Dulac, 1997; Matsunami & Buck, 1997; Ryba & Tirindelli, 1997) and a high affinity for larger molecules, such as urinary proteins and exocrine gland-secreted peptides (Leinders-Zufall et al. 2004; Kimoto et al. 2005; Leinders-Zufall et al. 2009). Secondly, analysis of early-gene expression have shown that male and female rodents sniffing opposite-sex conspecifics show more activation of neurons at the rAOB than the cAOB, whereas same-sex chemoinvestigation, most evident in male–male interactions, shows more activated neurons at the cAOB than at the rAOB (Dudley & Moss, 1999; Inamura et al. 1999; Kumar et al. 1999; Matsuoka et al. 1999; Halem et al. 2001). The activation of AOB subdomains in distinct behavioural contexts may also bear a relation to the segregated interconnections between some of AOB recipient nuclei and hypothalamic structures differentially involved in reproductive vs. defensive behaviours (Choi et al. 2005).
Contrasting AOB morphometric patterns in species with distinct lifestyles
The cAOB-biased glomerular heterogeneities we describe here in capybaras contrast with the rAOB-biased heterogeneities we described previously in degus (Suárez & Mpodozis, 2009) and may be related to divergent ecological specializations, such as in their habitat, their scent-marking strategies and/or the socio-sexual context in which semiochemical communication predominates.
Capybaras are the largest living rodents. They are crepuscular and social, and are distributed in flooded savannas and tropical forests rich in water sources they exploit for predator avoidance, foraging, thermoregulation and copulation. They form stable social groups composed of a dominant male, several females, subordinate males and young of both sexes (Herrera & MacDonald, 1989). These groups are polygynous; males maintain a strict linear hierarchy through aggressive interactions. Dominant males are heavier, occupy a central position within the group and mate more often than subordinate males (Herrera & MacDonald, 1993). Capybaras scent-mark their territory by leaving secretions from two distinct scent glands. The anal glands of males contain detachable hairs, known as osmetrichia, which are dry and rich in crystalline deposits. In contrast, anal glands of females lack detachable osmetrichia and are coated with a greasier smear. A second scent gland is located at the top of the snout: the morrillo gland. It secretes an oily substance and is prominent in males, but almost non-existent in females (MacDonald et al. 1984; Herrera & MacDonald, 1994). Scent-marking behaviour often involves rubbing the morrillo against a shrub or twig, followed by dragging the anal glands by straddling the plant. Male capybaras scent-mark up to four times more than females, and dominant males mark significantly more than subordinates, with both glands together and with the morrillo only (Herrera & MacDonald, 1994). Indeed, the size of the morrillo gland correlates with the size of testes (Herrera, 1992).
Accordingly, scent marking in capybaras seems to play an important role in the assessment of male hierarchies, perhaps involving non-volatile semiochemicals, as can be expected for semiaquatic chemosignalling. As argued above, both alternatives are consistent with an enhanced cAOB function. However, it should be noted that the cAOB could also be activated differentially by interspecific semiochemicals involved in anti-predatory and/or defensive/aggressive contexts, as shown in rats and mice (McGregor et al. 2004; Papes et al. 2010).
On the other hand, degus are found in semiarid environments of central Chile. They are diurnal caviomorphs and form groups lasting throughout the breeding season. They often perform dust-bathing behaviours near their burrows entrance by rubbing their flanks and ventrum against the soil (Fulk, 1976; Ebensperger & Caiozzi, 2002). Field observations suggest that during the breeding season male degus make nose-to-body contact with females only (Soto-Gamboa et al. 2005). Indeed, captive males can discriminate urinary volatiles of virgin vs. experienced males (Soto-Gamboa, 2004). Thus, we proposed that the rAOB-biased glomerular pattern might be related to a preferentially volatile form of chemosignalling and/or a predomination of male–female over male–male semiochemical appraisal (Suárez & Mpodozis, 2009).
Consequently, it is tempting to speculate that chemosignalling function in capybaras may be biased towards the assessment of male socio-sexual status and/or individual identity, by means of long-lasting but short-ranging molecules (as could be expected for humid environments), whereas in degus it may serve mainly in the assessment of female receptivity, by means of short-lasting but long-ranging molecules (as could be expected for arid environments) (Fig. 5). Under this scenario, it would be interesting to study whether similar cAOB heterogeneities occur in other semi-aquatic rodents, such as water rats/voles and muskrats (Cricetidae), beavers (Castoridae) or coypus (Myocastoridae).
Fig. 5.
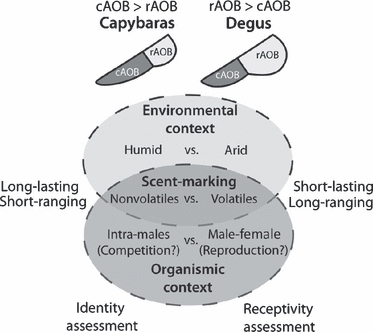
Interplay between environmental and organismic contingencies in the shaping the caviomorph AOB. We suggest that the opposite patterns of AOB heterogeneities found in capybaras (caudal-biased) and Octodon degus (rostrally biased) may reflect contextual differences in their habitat (humid vs. arid), their scent-marking strategies (non-volatiles vs. volatiles) and/or the social context in which semiochemical communication occurs (intra-males vs. male–female).
In summary, although the lateral innervation of the vomeronasal nerve and the indentation of the cell layers at the margin between subdomains seem to be conserved traits within caviomorphs, other traits, such as the relative size and glomerular composition between AOB subdomains, show variability that may be related to distinct ecological niche specializations. Comparative studies combining genomic and anatomical data, together with a more comprehensive consideration of the ecology of species, will be highly valuable for our understanding of the evolution of the mammalian vomeronasal system.
Acknowledgments
We would like to thank Elisa Sentis, Solano Henríquez, Geraldine Vásquez and Pedro Fernández-Aburto (Universidad de Chile) for their expert technical assistance, and the anonymous reviewers for helpful comments on the manuscript. This work received financial support from Conicyt (Beca Término de Tesis Doctoral) to R.S., FAPESP 2005-53835-6 to A.A.C., and Fondecyt N 1080094 to J.M.
References
- Berdoy M, Drickamer LC. Comparative social organization and life history of Rattus and Mus. In: Wolff JO, Sherman PW, editors. Rodent Societies: An Ecological and Evolutionary Perspective. Chicago: The University of Chicago Press; 2007. pp. 380–392. [Google Scholar]
- Berghard A, Buck LB. Sensory transduction in vomeronasal neurons: evidence for Gαo, Gαi2, and adenylyl cyclase II as a major components of a pheromone signaling cascade. J Neurosci. 1996;16:909–918. doi: 10.1523/JNEUROSCI.16-03-00909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G, Dong H, Murphy A, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Delano P, Elgueda D, Ramirez F, et al. A visual cue modulates the firing rate and latency of auditory-cortex neurons in the chinchilla. J Physiol Paris. 2010;104:190–196. doi: 10.1016/j.jphysparis.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Moss RL. Activation of an anatomically distinct subpopulation of accessory olfactory bulb neurons by chemosensory stimulation. Neuroscience. 1999;91:1549–1556. doi: 10.1016/s0306-4522(98)00711-8. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Ebensperger LA. Dustbathing and intra-sexual communication of social degus, Octodon degus (Rodentia: Octodontidae) Rev Chil Hist Nat. 2000;73:359–365. [Google Scholar]
- Ebensperger LA, Caiozzi A. Male degus, Octodon degus, modify their dustbathing behavior in response to social familiarity of previous dustbathing marks. Rev Chil Hist Nat. 2002;75:157–163. [Google Scholar]
- Fulk G. Notes on the activity, reproduction, and social behavior of Octodon degus. J Mammal. 1976;57:495–505. [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Central forebrain Fos responses to familiar male odours are attenuated in recently mated female mice. Eur J Neurosci. 2001;13:389–399. [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Halpern M, Shapiro LS, Jia CP. Differential localization of G proteins in the opossum vomeronasal system. Brain Res. 1995;677:157–161. doi: 10.1016/0006-8993(95)00159-n. [DOI] [PubMed] [Google Scholar]
- Halpern M, Jia CP, Shapiro LS. Segregated pathways in the vomeronasal system. Microsc Res Tech. 1998;41:519–529. doi: 10.1002/(SICI)1097-0029(19980615)41:6<519::AID-JEMT7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Herrera E. Size of testes and scent glands in capybaras, Hydrochaeris hydrochaeris (Rodentia: Caviomorpha) J Mammal. 1992;73:871–875. [Google Scholar]
- Herrera E, MacDonald D. Resource utilization and territoriality in group-living capybaras (Hydrochoerus hydrochaeris) J Anim Ecol. 1989;58:667–679. [Google Scholar]
- Herrera E, MacDonald D. Aggression, dominance, and mating success among capybara males (Hydrochaeris hydrochaeris) Behav Ecol. 1993;4:114–119. [Google Scholar]
- Herrera E, MacDonald D. Social significance of scent marking in capybaras. J Mammal. 1994;75:410–415. [Google Scholar]
- Imamura K, Mori K, Fujita SC, et al. Immunochemical identification of subgroups of vomeronasal nerve fibers and their segregated terminations in the accessory olfactory bulb. Brain Res. 1985;328:362–366. doi: 10.1016/0006-8993(85)91050-9. [DOI] [PubMed] [Google Scholar]
- Inamura K, Kashiwayanagi M, Kurihara K. Regionalization of Fos immunostaining in rat accessory olfactory bulb when the vomeronasal organ was exposed to urine. Eur J Neurosci. 1999;11:2254–2260. doi: 10.1046/j.1460-9568.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- Jia CP, Halpern M. Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Giα2 and Goα) and segregated projections to the accessory olfactory bulb. Brain Res. 1996;719:117–128. doi: 10.1016/0006-8993(96)00110-2. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, et al. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dudley CA, Moss RL. Functional dichotomy within the vomeronasal system: distinct zones of neuronal activity in the accessory olfactory bulb correlate with sex-specific behaviors. J Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-20-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriva-Sahd J. The accessory olfactory bulb in the adult rat: a cytological study of its cell types, neuropil, neuronal modules, and interactions with the main olfactory system. J Comp Neurol. 2008;510:309–350. doi: 10.1002/cne.21790. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, et al. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmayer P, et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Ishii T, Mombaerts P, et al. Structural requirements for the activation of vomeronasal sensory neurons by MHC peptides. Nat Neurosci. 2009;12:1551–1558. doi: 10.1038/nn.2452. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Krantz K, Aplin R. Behavioural, anatomical and chemical aspects of scent marking amongst capybaras, Hydrochoeris hydrochaeris (Rodentia: Caviomorpha) J Zool (London) 1984;202:341–360. [Google Scholar]
- Martinez-Marcos A, Halpern M. Differential projections from the anterior and posterior divisions of the accessory olfactory bulb to the medial amygdala in the opossum, Monodelphis domestica. Eur J Neurosci. 1999a;11:3789–3799. doi: 10.1046/j.1460-9568.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Marcos A, Halpern M. Differential centrifugal afferents to the anterior and posterior accessory olfactory bulb. Neuroreport. 1999b;10:2011–2015. doi: 10.1097/00001756-199907130-00004. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Yokosuka M, Mori Y, et al. Specific expression pattern of Fos in the accessory olfactory bulb of male mice after exposure to soiled bedding of females. Neurosci Res. 1999;35:189–195. doi: 10.1016/s0168-0102(99)00082-6. [DOI] [PubMed] [Google Scholar]
- McCotter RE. The connection of the vomeronasal nerves with the accessory olfactory bulb in the opossum and other mammals. Anat Rec. 1912;6:299–318. [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, et al. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisami E, Bhatnagar KP. Structure and diversity in mammalian accessory olfactory bulb. Microsc Res Tech. 1998;43:476–499. doi: 10.1002/(SICI)1097-0029(19981215)43:6<476::AID-JEMT2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Úbeda-Bañón I, et al. Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25:2065–2080. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba NJP, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–379. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Soto-Gamboa M. Formación y Estabilidad de Estructuras Sociales en Micromamíferos, su Regulación Hormonal y la Importancia de las Interacciones Entre Machos. Santiago: Facultad de Ciencias Biológicas. Universidad Católica de Chile; 2004. pp. 1–133. [Google Scholar]
- Soto-Gamboa M, Villalon M, Bozinovic F. Social cues and hormone levels in male Octodon degus (Rodentia): a field test of the challenge hypothesis. Horm Behav. 2005;47:311–318. doi: 10.1016/j.yhbeh.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Suárez R, Mpodozis J. Heterogeneities of size and sexual dimorphism between the subdomains of the lateral-innervated accessory olfactory bulb (AOB) of Octodon degus (Rodentia: Hystricognathi) Behav Brain Res. 2009;198:306–312. doi: 10.1016/j.bbr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Suárez R, Villalón A, Künzle H, et al. Transposition and intermingling of Gαi2 and Gαo afferences into single vomeronasal glomeruli in the Madagascan lesser tenrec Echinops telfairi. PLoS ONE. 2009;4:e8005. doi: 10.1371/journal.pone.0008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai T, Yoshimura H, Kato N, et al. Component-dependent urine responses in the rat accessory olfactory bulb. Neuroreport. 2006;17:1663–1667. doi: 10.1097/01.wnr.0000239950.14954.59. [DOI] [PubMed] [Google Scholar]
- Vega-Zúñiga T. Plasticidad Filogenética Comparada de las Vías Visuales Principales en los Roedores Octodóntidos. Santiago: Facultad de Ciencias. Universidad de Chile; 2009. [Google Scholar]
- Wekesa KS, Anholt R. Differential expression of G proteins in the mouse olfactory system. Brain Res. 1999;837:117–126. doi: 10.1016/s0006-8993(99)01630-3. [DOI] [PubMed] [Google Scholar]


