Abstract
The aim of the present study was to determine the effects and molecular mechanisms by which AMP-activated protein kinase (AMPK) regulates smooth muscle contraction and blood pressure in mice. In cultured human vascular smooth muscle cells (HMSC), we observed that activation of AMPK by AICAR (5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside) inhibited agonists-induced phosphorylation of myosin light chain (MLC) and myosin phosphatase targeting subunit 1 (MYPT1). Conversely, AMPK inhibition with pharmacologic or genetic means potentiated agonists-induced the phosphorylation of MLC and MYPT1 while inhibited both Ras homolog gene family, member A (RhoA) and Rho-associated kinase (ROCK) activity. Further, AMPK activation or ROCK inhibition with Y27632 abolished agonists-induced phosphorylation of MLC and MYPT1. Gene silencing of p190-guanosine triphosphatase-activating protein (p190-GAP) abolished the effects of AMPK activation on MLC, MYPT1, and RhoA in HSMC. Ex vivo analyses revealed that agonist-induced contractions of the mesenteric artery and aortas were stronger in both AMPKα1−/− and AMPKα2−/− knockout mice than in wild-type mice. Inhibition of ROCK with Y27632 normalized agonist-induced contractions of AMPKα1−/− and AMPKα2−/− vessels. AMPKα2−/− mice had higher blood pressure along with decreased serine phosphorylation of p190-guanosine triphosphatase-activating protein (p190-GAP). Finally, inhibition of the RhoA/ROCK pathway with Y27632, which suppressed MYPT1 and MLC phosphorylation, lowered blood pressure in AMPKα2−/− mice. In conclusion, AMPK decreases vascular smooth muscle cell contractility by inhibiting p190-GAP-dependent RhoA activation, indicating AMPK may be a new therapeutic target in lowering high blood pressure.
Keywords: AMPK, Rho A, ROCK, p190-GAP, blood pressure
Introduction
AMP-activated protein kinase (AMPK) is a key component of an energy-sensing/signaling system that allows cells to sense changes in their energy status 1, 2. AMPK consists of three subunits, designated α, β, and γ. The α subunit contains the catalytic kinase domain, which transfers phosphate from ATP to the target protein. Phosphorylation of Thr172 on the α subunit by upstream signaling kinases activates AMPK 3, and AMP allosterically promotes phosphorylation of this residue 4. Activated AMPK phosphorylates a number of target proteins, resulting in increased glucose uptake, increased metabolism, and increased fatty acid oxidation. At the same time, AMPK activation inhibits hepatic lipogenesis, cholesterol synthesis, and glucose production 5. Because AMPK activation has beneficial metabolic consequences for patients with metabolic syndrome and diabetes, it is a new target for the treatment of obesity and type 2 diabetes 6.
Recent studies found that AMPK-mediated cellular functions have protective effects that counteract many cardiovascular diseases7–10. In addition, both AICAR (5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside; an AMPK agonist) and resveratrol (a botanical phytoallexin AMPK agonist) is reported to lower BP in insulin-resistant and obese rats 11, 12. However, these studies did not establish the direct effects of AMPK on BP because AMPK activation is known to improve insulin resistance and metabolic anomalies in these models. Whether or not AMPK regulates vascular tone and/or blood pressure remains unknown. Since vessel smooth muscle function is directly related to vascular tone 13 and abnormal VSMC relaxation or contraction is known to induce hypertension in both humans and in animal models 14, we hypothesized that activation of AMPK activation might lower BP by inhibiting vessel contraction. The aim of the present study was to elucidate the mechanisms by which AMPK activation attenuates agonist-induced contraction of smooth muscle and the physiological functions of AMPK in the regulation of BP in mice.
Methods and Materials
A full description of materials, animals, and methods used, including cell culture, gene transfection of cells, transfection of siRNA into cells, western blot analysis, rhotekin pull-down assay for RhoA activation, ROCK activity assay, measurement of vessel tension in mice, blood pressure measurement and statistical analysis, can be found in the online-only Data Supplement. Please see http://hyper.ahajournals.org.
Results
AICAR inhibits agonists-induced MLC phosphorylation in a time- and dose-dependent manner
The vessel tone is determined by the status of MLC (phosphorylation or dephosphorylation at ser19) in VSMCs15. We first determined whether AMPK activation could affect PE-induced phosphorylation of MLC. To test this, we treated HSMC with agonists and measured MLC phosphorylation by immunoblot analysis. Treatment of HSMC with PE (1 µM, 30 min) significantly increased the phosphorylation of MLC (Figure 1A and 1B) and AMPK phosphorylation at Thr172 (Figure S1A and S1B). As expected, pre-treatment with AICAR (2 mM), a known AMPK activator, increased PE-induced AMPK-Thr172 phosphorylation in a time- and dose-dependent manner in HSMC (Figure S1A and S1B). However, AICAR pretreatment abolished PE-induced MLC phosphorylation (Figure 1A and 1B).
Figure 1. Activation of AMPK inhibits agonist-induced MLC phosphorylation in VSMCs.
(A and B) Western blot analysis of Thr172-phosphorylated AMPK and Ser19-phoshorylated MLC in PE-stimulated HSMC pretreated with or without AICAR. Cells were pretreated with varying (A) concentrations or (B) times of AICAR and then stimulated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. control, #P<0.05 vs. PE alone. (C) Western blot analysis of phosphorylated MLC in cells incubated with AICAR (2 mM) for 1 h and then challenged with U46619 (30 nM) or IBOP (1 nM) for 30 min. n=3, *P<0.05 vs. basal, #P<0.05 vs. U46619 or IBOP alone. (D) MLC phosphorylation in cells transduced with adenovirus vectors encoding GFP, AMPK-DN (DN), or AMPK-CA (CA) for 48 h and then treated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. GFP alone, #P<0.05 vs. AMPK-DN alone. (E) MLC phosphorylation in HSMC transfected with control siRNA, AMPKα1 siRNA, or AMPKα2 siRNA for 48 h and then incubated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. control siRNA alone, #P<0.05 vs. PE plus control siRNA. (F) MLC phosphorylation in primary mouse VSMCs from WT, AMPKα1−/−, or AMPKα2−/− mice aortas treated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. WT alone, #P<0.05 vs. PE plus WT.
Next, we investigated if AICAR pretreatment altered MLC phosphorylation caused by other agonists, we assayed the effects of AICAR on PE-unrelated agonists induced MLC phosphorylation in HSMC. As depicted in Figure 1C, U46619 or IBOP, two structurally related thromboxane receptor activators, markedly increased the levels of p-MLC. Importantly, AICAR significantly lowered U46619- or IBOP- enhanced MLC phosphorylation.
PE-induced MLC phosphorylation is dependent on AMPK
To exclude potential off-target effects of AICAR, we next investigated whether genetic inhibition of AMPK by adenovirus-mediated expression of dominant-negative AMPK (AMPK-DN) affected PE-induced MLC activation. As expected, PE induced MLC phosphorylation in GFP-expressing control HSMC (Figure 1D). In AMPK-DN-expressing HSMC, PE-induced MLC phosphorylation was greater than those in GFP-expressing cells; conversely, MLC phosphorylation was absent in AMPK-CA-expressing HSMC (Figure 1D). Taken together, these data suggest that AMPK negatively regulates PE-induced MLC activation.
To investigate the roles of endogenous AMPKα in MLC phosphorylation, HSMC that had been transfected with AMPKα-specific siRNA or primary MSMCs isolated from AMPKα1−/− or AMPKα2−/− mice were exposed to PE. Western blot analysis revealed that either AMPKα1- or α2-targeted siRNA reduced the levels of AMPKα1 or α2, respectively (Figure S2A). As shown in Figure 1E, PE-induced MLC phosphorylation was greater in cells receiving AMPKα1 or AMPKα2-targeted siRNA than in WT or non-targeted control siRNA-transfected cells. Consistently, PE-induced MLC phosphorylation was significantly higher in AMPKα1- or α2-null cells compared to those in WT (Figure 1F). These experiments further support that AMPK negatively regulates PE-induced MLC activation in HSMC.
AMPK regulates Thr696 phosphorylation of MYPT1, which lies upstream of MLC
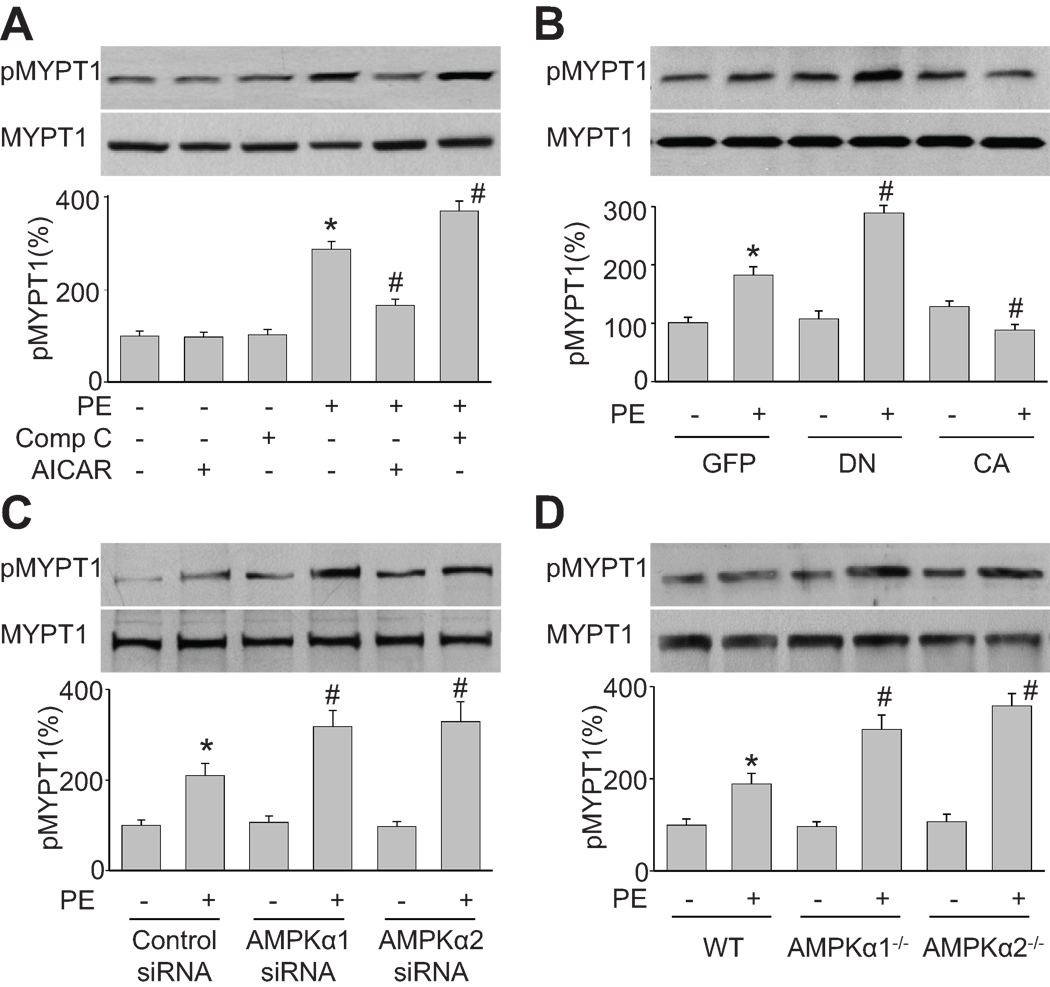
MLCP suppresses smooth muscle contractions by dephosphorylating its substrate MLC, which is bound to myosin heavy chain. MYPT1, as a subunit of MLCP complex, through the MYPT1 NH2 terminus, reduces the MLCP complex catalytic activity for myosin II. Western blot analysis revealed that PE-induced MYPT1 phosphorylation at Thr696 was significantly greater in HSMC treated with compound C or expressing AMPK-DN than in control cells (Figure 2A and 2B). Conversely, either AICAR treatment or AMPK-CA expression attenuated PE-induced MYPT1 phosphorylation. The effects of AMPK inhibition on PE-induced MYPT1 phosphorylation were further confirmed by siRNA-mediated AMPKα knockdown and gene-specific knockout mice. Knockdown of AMPKα1 or α2 by siRNA significantly increased PE-induced MYPT1 phosphorylation at Thr696 (Figure 2C). Similarly, MYPT1 phosphorylation at Thr696 was significantly higher in VSMC isolated from AMPKα1 or α2 when compared to those in WT (Figure 2D). Taken together, these data indicate that AMPK negatively regulates Thr696 phosphorylation of MYPT1, an upstream regulator of MLC.
Figure 2. AMPK regulates Thr696 phosphorylation of MYPT1, an upstream regulator of MLC.
(A) Western blot analysis of Thr696-phosphorylated MYPT1 in HSMC pretreated with AICAR (2 mM) or compound C (20 µM) for 2 h and then incubated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. basal, #P<0.05 vs. PE alone. (B) Thr696 phosphorylation of MYPT1 in HSMC transduced with adenovirus vectors encoding GFP, AMPK-DN (DN), or AMPK-CA (CA) for 48 h and then treated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. GFP alone, #P<0.05 vs. GFP plus PE. (C) Thr696 phosphorylation of MYPT1 in HSMC transfected with control siRNA, AMPKα1 siRNA, or AMPKα2 siRNA for 48 h and then incubated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. control siRNA alone, #P<0.05 vs. PE plus control siRNA. (D) Effect of PE (1 µM, 30 min) on Thr696 phosphorylation of MYPT1 in primary mouse VSMCs from WT, AMPKα1−/−, or AMPKα2−/− aortas. n=3, *P<0.05 vs. WT alone, #P<0.05 vs. PE plus WT.
ROCK participates in the regulation of MYPT1 by AMPK
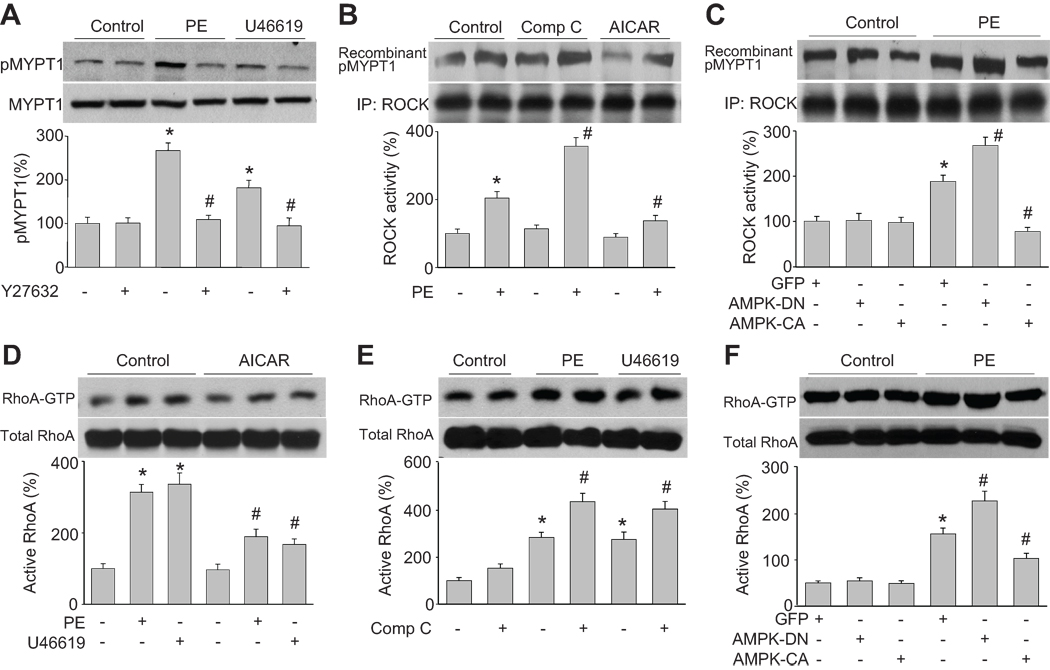
The ROCK-dependent signal pathway is essential in mediating agonists-induced smooth muscle vessel contraction16, 17. We next determined the effect of the ROCK inhibitor, Y27632, on MYPT1 phosphorylation. As shown in Figure 3A, Y27632 treatment reversed both PE- and U46619-induced MYPT1 phosphorylation in HSMC, implying that ROCK mediates PE- and U46619- induced MYPT1/MLC activation.
Figure 3. AMPK negatively regulates agonist-upregulated ROCK activity and RhoA activation.
(A) Western blot analysis of Thr696-phosphorylated MYPT1 in cultured HSMC pretreated with Y27632 (10 µM) for 2 h and then incubated with PE (1 µM) or U46619 (30 nM) for 30 min. n=3, *P<0.05 vs. control, #P<0.05 vs. PE or U46619 alone. (B) ROCK activity in cells were treated with AICAR (2 mM) or compound C (20 µM) for 2 h and then incubated with PE for 30 min. Levels of Thr696-phosphorylated recombinant MYPT1 served as an index of ROCK activity. n=3, *P<0.05 vs. control, #P<0.05 vs. PE alone. (C) ROCK activity in cells transduced with adenovirus vectors encoding GFP, AMPK-DN (DN), or AMPK-CA (CA) for 48 h and then treated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. GFP alone, #P<0.05 vs. GFP plus PE. (D) RhoA activation, as measured by Rhotekin pull-down, in cells were pretreated with AICAR (2 mM) for 2 h and then incubated with PE (1 µM) or U46619 (30 nM) for 30 min. n=3, *P<0.05 vs. control, #P<0.05 vs. PE or U46619 alone. (E) RhoA activation in cells pretreated with compound C (20 µM) for 2 h and then incubated with PE (1 µM) or U46619 (30 nM) for 30 min. n=3, *P<0.05 vs. control, #P<0.05 vs. PE or U46619 alone. (F) RhoA activation in cells transduced with endovirus vectors encoding GFP, AMPK-DN (DN), or AMPK-CA (CA) for 48 h and then treated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. GFP alone, #P<0.05 vs. GFP plus PE.
It was important to determine the effects of AMPK on ROCK activity. To this end, ROCK activity was assayed in cells treated with AICAR (AMPK activator) or compound C (AMPK inhibitor). As depicted in Figure 3B, AICAR reversed PE-induced ROCK activity while compound C enhanced this activity. Consistently, genetic activation of AMPK by overexpressing AMPK-CA significantly lowered PE-induced up regulation of ROCK activity (Figure 3C) whereas AMPK-DN enhanced PE-induced upregulation of ROCK activity. Overall, these data suggest that PE-induced ROCK activation is AMPK dependent.
AMPK negatively regulates PE-induced RhoA activation
Smooth muscle MYPT1 is a G protein-regulated enzyme 18. RhoA belongs to the Ras low-molecular-weight G protein superfamily. RhoA binds GTP to activate ROCK, and this in turn leads to MYPT1 phosphorylation, MLC activation, and smooth muscle cell contraction. As shown in Figure 3D and 3E, both PE and U46619 activated RhoA by increasing the amount of GTP-bound RhoA. AICAR inhibited RhoA activation, but compound C increased its activation. These results were confirmed by transduction of cells with AMPK-CA or AMPK-DN and measurement of the RhoA activation status. PE-induced RhoA activation was lower in AMPK-CA-expressing cells that in GFP-expressing control cells (Figure 3F); however, AMPK-DN enhanced PE-induced RhoA activation. Thus, these data suggest that PE-induced RhoA activation is AMPK dependent.
Activation of AMPK inhibits the binding of p190-guanosine triphosphatase-activating protein (GAP) to RhoA in agonist-stimulated VSMCs
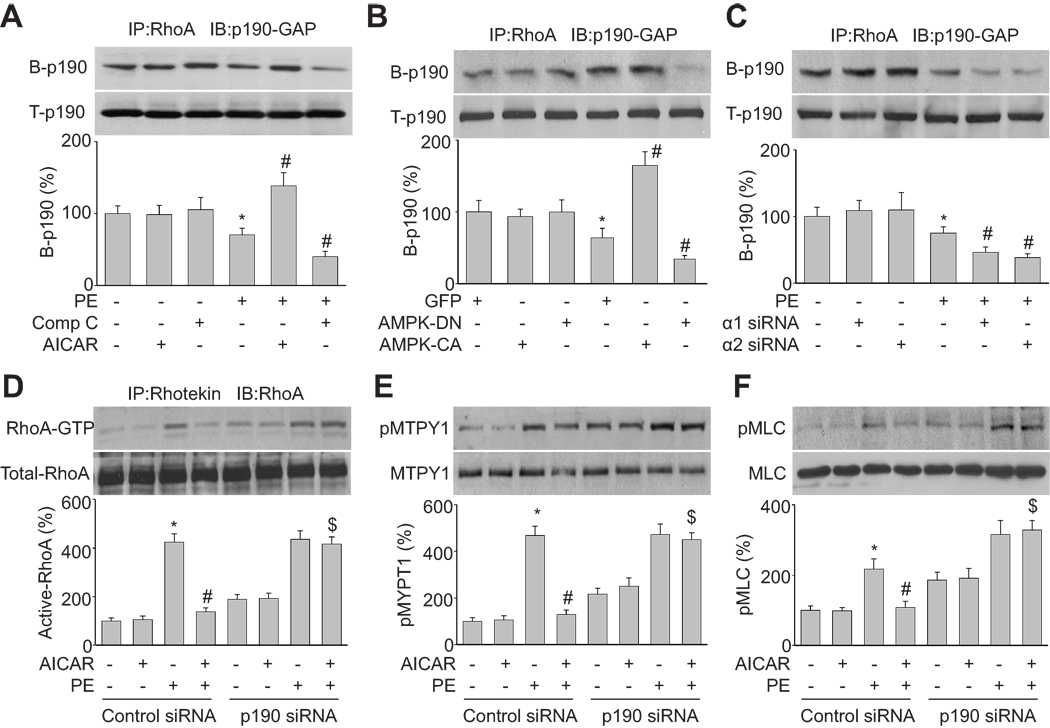
RhoA cycles between an active GTP-bound state and an inactive GDP-bound state through nucleotide exchange and intrinsic guanosine triphosphatase activity. The RhoA activation status is controlled by guanine nucleotide exchange factors (GEFs) 19 and GAPs, including the p190-GAP. We tested whether AMPK inactivates RhoA by promoting the association of RhoA with p190-GAP. We found that the binding of p190-GAP to RhoA was significantly increased by AICAR (2 mM) in PE-stimulated HSMC, but inhibition of AMPK with compound C (20 µM) significantly decreased RhoA/p190-GAP complex formation (Figure 4A). Overexpression of AMPK-CA mimicked the effect of AICAR on p190-GAP/RhoA binding in PE-treated HSMC (Figure 4B). Similarly, the effect of compound C was mimicked by over expression of AMPK-DN or AMPKα knockdown using AMPKα-specific siRNA (Figure 4B and 4C). Taken together, these data suggest that activation of AMPK promotes the binding of p190-GAP to RhoA in agonists-stimulated HSMC.
Figure 4. p190-GAP mediates AMPK suppression of RhoA activation.
(A) Coimmunoprecipitation/western blot analysis of p190-GAP/RhoA binding in cells treated with AICAR (2 mM) or compound C (20 µM) for 2 h and then incubated with PE for 30 min. n=3, *P<0.05 vs. control, #P<0.05 vs. PE alone. (B) p190-GAP/RhoA binding in cells transduced with adenovirus vectors encoding GFP, AMPK-DN (DN), or AMPK-CA (CA) for 48 h and then treated with PE (1 µM) for 30 min. n=3, *P<0.05 vs. GFP alone, #P<0.05 vs. GFP plus PE. (C) p190-GAP/RhoA binding in HSMC transfected with AMPKα1 siRNA or AMPKα2 siRNA for 48 h and then incubated with PE (1 µM) for 30 min. Control siRNA is served as control. n=3, *P<0.05 vs. basal, #P<0.05 vs. PE plus control siRNA. (D, E, and F) Cells were transfected with control siRNA and p190-GAP siRNA for 48 h and then incubated with PE (1 µM) for 30 min in presence of AICAR (2 mM). Cell lysates were subjected to rhotekin pull-down assays to measure RhoA activation (D). Alternatively, they were subjected to western blot analysis of phosphorylated MYPT1 (E) or phosphorylated MLC (F). n=3, *P<0.05 vs. control, #P<0.05 vs. PE alone in control siRNA-transfected cells. $P<0.05 vs. PE plus AICAR in control siRNA-transfected cells.
p190-GAP silencing abolishes AICAR suppression of RhoA activation, MYPT1 phosphorylation, and MLC phosphorylation in VSMCs
To establish if p190-GAP mediated the effects of AMPKα on RhoA inactivation, VSMC were transfected with p190-GAP-targeted siRNA or non-targeted control siRNA. Silencing of p190-GAP expression was confirmed by western blot analysis (Figure S2B). As expected, in PE-stimulated HSMC cells that were transfected with non-targeted control siRNA, AICAR treatment significantly reduced the levels of active RhoA (Figure 4D), phosphorylated MYPT1 (Figure 4E), and phosphorylated MLC (Figure 4F). In contrast, gene silencing of p190-GAP abolished the effects of AICAR, implying that p190-GAP is required for AMPK -mediated RhoA inhibition.
Inhibition of AMPK potentiates agonist-induced contraction of mesenteric and aortic vessels in mice
As peripheral resistance is determined by the contractile status of VSMCs in small arterioles 20, we then determined vascular smooth muscle function in resistance vessels from WT, AMPKα1−/−, and AMPKα2−/− mice. Both PE and U46619 were used to induce contraction of mouse mesenteric artery ring explants in an organ bath (Figure S3A and S3B). Both PE and U46619 significantly increased mesenteric artery contraction in WT, AMPKα1−/−, and AMPKα2−/− explants. However, explants from AMPKα1−/− mice had a stronger mesenteric artery contraction force than explants from WT mice, which is in line with an earlier report21. Explants from AMPKα2−/− mice also had a stronger mesenteric artery contraction force than explants from WT mice. Further, AMPK deletion also enhanced the contraction of conduit vessels in mouse aortas (Figure S4A).
Next, we determined the effect of AICAR (a potent AMPK activator) or compound C (a potent AMPK inhibitor) on PE-induced contraction of aortic rings. Vessel rings were pre-treated with AICAR (2 mM) or compound C (20 µM) for 2 h, and contractions were then induced by PE, as above. Vessel contraction was significantly inhibited by AICAR but was increased by compound C (Figure S4B). These data suggest that activated AMPK suppresses agonist-induced vasoconstriction.
AMPKα-deletion-enhanced vessel contraction is ROCK dependent
Next, we tested whether ROCK mediates the effect of AMPK on vessel contraction. Aortas and mesenteric arteries from AMPKα1−/− and AMPKα2−/− mice were pre-treated with Y27632 (a selective ROCK inhibitor, 2 µM) for 30 min, and this significantly inhibited agonist-induced increases in contraction (Figure S3A, S3B and Figure S4C). These data show that inhibition of AMPK potentiates agonist-induced contraction of murine conduit and resistance vessels, likely through regulation of ROCK.
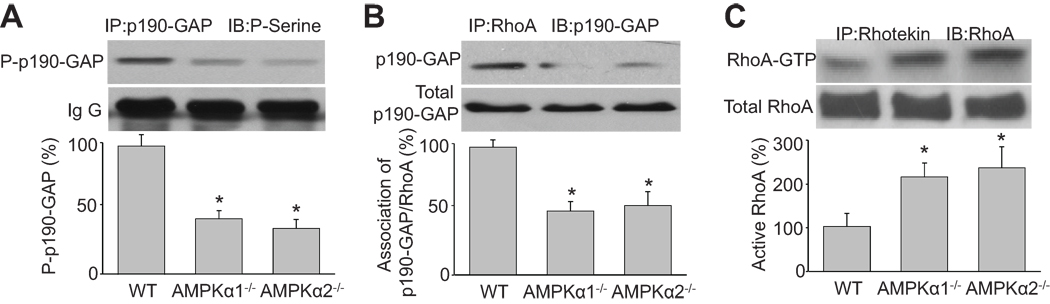
Deletion of AMPK suppresses p190-GAP serine phosphorylation and binding to RhoA in vivo
p190-GAP activity is regulated in a serine or tyrosine phosphorylation-dependent manner 22. We hypothesized AMPK, which is a serine/threonine kinase, directly phosphrylates p190-GAP. Because no site-specific phosphoserine (P-serine) p190-GAP antibodies are available, we relied upon immunoprecipitation (IP) of total p190-GAP and then western blot analysis using a general P-serine antibody (Figure 5). IP isolated p190-GAP in cells from WT mice was detected by the P-serine antibody; however deletion of either AMPKα1 or α2 dramatically suppressed the serine phosphorylation of p190-GAP (Figure 5A), suggesting that AMPKα deletion may suppress p190-GAP activity by preventing its phosphorylation. Further, deletion of AMPKα1 or α2 also inhibited the binding of RhoA by p190-GAP (Figure 5B) and this in turn increased RhoA activation (Figure 5C).
Figure 5. Inhibition of AMPK elevates the p190-GAP dependent RhoA activation in vivo.
Aortic tissue from WT, AMPKα1−/−, and AMPKα2−/− mice was subjected to coimmunoprecipitation/western blot to analyze (A) serine phosphorylation of p190-GAP, (B) p190-GAP/RhoA binding, and (C) rhotekin pull-down assays to determine levels of active RhoA. n=6, *P<0.05 vs. WT.
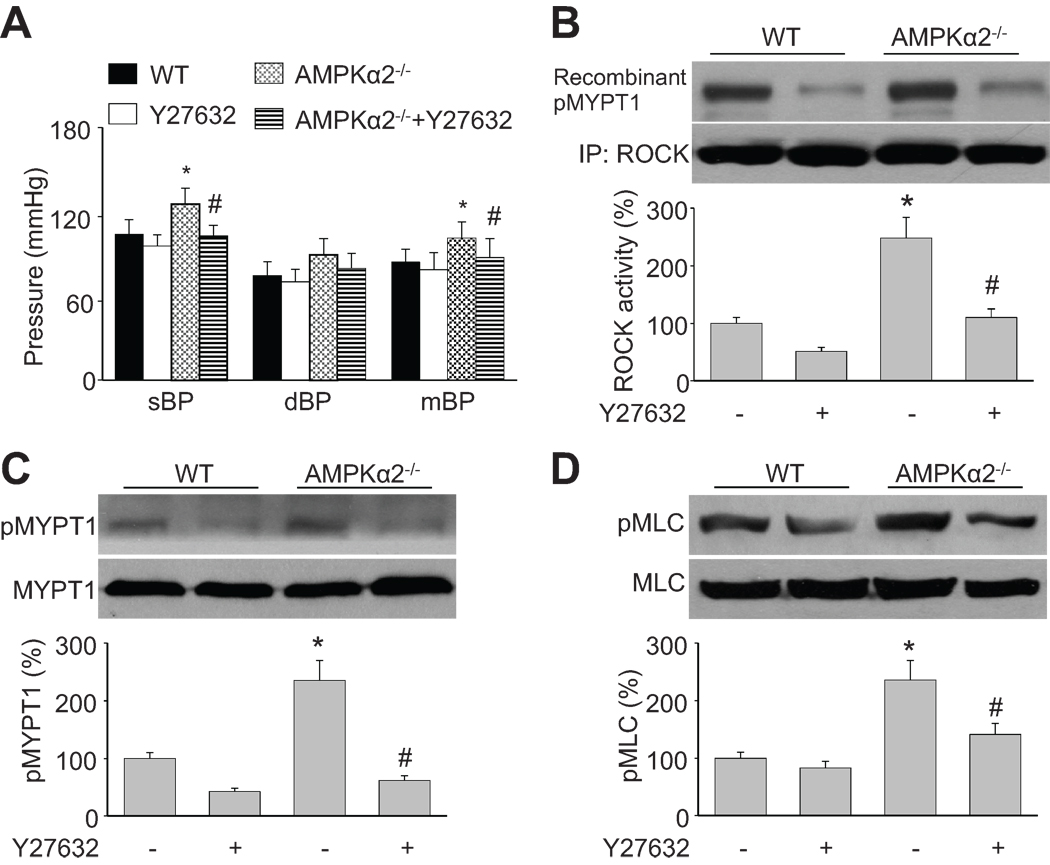
Y27632-inhibitable hypertension in AMPKα2−/− mice
We previously reported that AMPKα1−/− mice exhibit splenomegaly and moderate anemia 23, and were therefore not suitable for BP studies. Thus, we determined the systolic blood pressure (sBP), diastolic blood pressure (dBP), and mean blood pressure (mBP) in WT and AMPKα2−/− mice. As depicted in Figure 6A, all of the BP measurements (sBP, dBP, and mBP) in AMPKα2−/− mice were higher than those in WT mice under basal conditions. Importantly, Y27632 (10 mg/kg, 4 h, given intraperitoneally) significantly reduced the BP values (Figure 6A, sBP, dBP, and mBP), ROCK activity (Figure 6B), MYPT1 phosphorylation (Figure 6C), and MLC phosphorylation (Figure 6D) in AMPKα2−/− mice. Collectively, these data indicate that upregulation of the p190-GAP-dependent RhoA/ROCK/MYPT1/MLC pathway contributes to hypertension in AMPKα2−/− mice.
Figure 6. Inhibition of AMPK elevates the p190-GAP/RhoA/ROCK pathway in vivo.
WT and AMPKα2−/− mice were intraperitoneally injected with Y27632 (10 mg/kg). Four hours later, the BP was measured by left carotid catheter (A). Aortic tissues were collected for analysis of (B) ROCK activity, (C) phosphorylated MYPT1, and (D) phosphorylated MLC. n=6, *P<0.05 vs. WT alone (basal), #P<0.05 vs. control AMPKα2−/− alone.
Discussion
The major finding of this study is that AMPK activation lowers blood pressure by suppressing VSMC contractility via the inhibition of MYPT1/MLC phosphorylation. Mechanistically, we found that AMPK decreases vascular smooth muscle cell contractility by inhibiting p190-GAP-dependent RhoA activation.
One of the most important findings of this study is that AMPK activation suppresses VSMC contractility by inhibiting MYPT1/MLC phosphorylation. This appears to occur indirectly through MYPT1-Thr696 dephosphorylation, which in turn inhibits MLCP activity. This conclusion is supported by several findings. First, deletion of AMPKα1 or α2 enhanced agonist-induced contraction in explanted murine mesenteric arteries, and inhibition of AMPK with a pharmacological inhibitor (compound C) reproduced this effect. Second, activation of AMPK by AICAR attenuated agonist-induced contraction. Third, systemic BP was higher in AMPKα2−/− mice than in WT mice under normal physiological conditions. In addition, angiotensin-II caused higher BP in AMPKα2−/− mice than in WT mice. Although AMPKα1−/− mice were excluded from this study because knockout of this gene results in splenomegaly and moderate anemia 23, 24, we expect that the hypertensive phenotype observed in the AMPKα2−/− mice would also be observed in AMPKα1−/− mice. Finally, AMPK-CA or AICAR treatment abolished PE-induced phosphorylation of MYPT1 at Thr696 and MLC at Ser19. Conversely, AMPK-DN or compound C treatment potentiated PE-induced activation of MYPT1 and MLC. Taken together, our results support a direct inhibitory effect of AMPK on VSMC contractility.
RhoA/ROCK signaling plays an important role in cell proliferation, cell cycle progression, and cell survival following vascular injury 25. As AMPK activation promotes the binding of p190-GAP to RhoA and in this way blocks RhoA activity, AMPK might also be important for other physiological processes, such as angiogenesis or tube formation after vascular damage, in which RhoA/ROCK signaling is involved 26. This finding is consistent with a recent study demonstrating that the AMPK activator, AICAR, inhibits ROCK activity in osteoblastic cells in a dose-dependent manner 27. The regulation of the RhoA/ROCK pathway by AMPK appears to be cell type-dependent. It has been recently shown that AMPK activation enhances MLC phosphorylation through the RhoA/ROCK signaling pathway in epithelial cells 28. Taken together, these studies support a novel mechanism of AMPK action whereby activation of AMPK suppresses the PE-induced signaling pathway by reducing RhoA/ROCK activity via p190-GAP. This, in turn, inhibits MYPT1/MLC phosphorylation in smooth muscle cells, leading to decreased vessel contraction (Figure S5). In combination, these effects contribute to the regulation of BP in mice.
Perspectives
Activation of the RhoA/ROCK cell signaling pathway is known to play an essential role in hypertension 29. Thus, inhibition of RhoA/ROCK by AMPK might be important mechanisms for lowering blood pressure. In addition to its suppression of VSMC contractility, AMPK can also lower blood pressure by improving NO bioactivity and endothelial cell function. AMPK might increase NO release by increasing phosphorylation and activation of endothelial nitric oxide (NO) synthase (eNOS) at Ser1177 and Ser633 or by decreasing NO inactivation by reactive oxygen species. Indeed, there is a growing body of evidence that supports the notion that AMPK suppresses ROS from mitochondria and/or NAD(P)H oxidase9, 30. Interestingly, depressed AMPK activation, or AMPK deregulation, has been observed in arteries of rodent models where vascular dysfunction exists, including in streptozotocin-induced diabetes, Zucker diabetic fatty rats, aged rats, and Otsuka Long Evans Tokushima Fatty (OLETF) rats31. Further, adiponectin-AMPK signaling becomes depressed in insulin-sensitive tissues of hypertensive rats32. Consistently, AMPK activation by AICAR or resveratrol (a botanical phytoallexin AMPK agonist) lowered BP in insulin-resistant and obese rats11, 12. Importantly, metformin, one of the mostly widely used anti-diabetic drugs which is reported to activate AMPK in vivo, are reported to lower blood pressures in various models of hypertensive animals and in patients with or without diabetes. Interestingly, recent studies report that telmisartan and losartan, selective angiotensin II receptor blockers, is reported to inhibit VSMC proliferation33 and glucose uptake by activating AMPK34. Taken together, AMPK inhibition might contribute to the initiation and progression of hypertension and AMPK is a novel therapeutic target for effective treatments of hypertension, especially in metabolic syndrome such as obesity and diabetes.
Supplementary Material
Acknowledgements
We are grateful for Dr. Najeeb Shirwany for his editorial support.
Sources of Funding
This work was supported by National Institutes of Health grants (HL079584, HL074399, HL080499, HL089920, HL096032, and HL105157) as well as research awards from the American Diabetes Association and Warren Chair in Diabetes Research, University of Oklahoma Health Sciences Center. Dr. M. H. Zou is a recipient of the National Established Investigator Award of the American Heart Association.
Abbreviations
- AMPK
AMP-activated protein kinase
- Ad-AMPK-CA
adenovirus encoding constitutively active AMPK mutants
- Ad-AMPK-DN
adenovirus encoding AMPK dominant negative mutants
- AICAR
5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside
- BP
blood pressure
- dBP
diastolic blood pressure
- CaM
calmodulin
- GAP
guanosine triphosphatase-activating protein
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- GTP
guanosine triphosphate
- mBP
mean blood pressure
- MLC
myosin light chain
- MLCK
MLC kinase
- MLCP
MLC phosphatase
- MYPT1
myosin phosphatase targeting subunit 1
- PE
phenylephrine
- PKC
protein kinase C
- RhoA
Ras homolog gene family, member A
- ROCK
Rho-associated kinase
- sBP
systolic blood pressure
- U46619
9,11-dideoxy-11-,9-epoxy-methano-prostaglandin F2
- VSMC
vascular smooth muscle cell
- WT
wild type
- Y27632
ROCK inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Lee-Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP, Wasserman DH. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem. 2009;284:23925–23934. doi: 10.1074/jbc.M109.021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 3.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5'-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 5.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 6.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, Zou MH. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2009;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 12.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci U S A. 2008;105:6702–6707. doi: 10.1073/pnas.0802128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 16.Sward K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J Physiol. 2000;522(Pt 1):33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 19.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 20.McVeigh GE, Plumb R, Hughes S. Vascular abnormalities in hypertension: cause, effect, or therapeutic target? Curr Hypertens Rep. 2004;6:171–176. doi: 10.1007/s11906-004-0065-x. [DOI] [PubMed] [Google Scholar]
- 21.Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MP, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008;283:18505–18512. doi: 10.1074/jbc.M802053200. [DOI] [PubMed] [Google Scholar]
- 22.Fordjour AK, Harrington EO. PKCdelta influences p190 phosphorylation and activity: events independent of PKCdelta-mediated regulation of endothelial cell stress fiber and focal adhesion formation and barrier function. Biochim Biophys Acta. 2009;1790:1179–1190. doi: 10.1016/j.bbagen.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foretz M, Hebrard S, Guihard S, Leclerc J, Do Cruzeiro M, Hamard G, Niedergang F, Gaudry M, Viollet B. The AMPKgamma1 subunit plays an essential role in erythrocyte membrane elasticity, and its genetic inactivation induces splenomegaly and anemia. FASEB J. 2011;25:337–347. doi: 10.1096/fj.10-169383. [DOI] [PubMed] [Google Scholar]
- 25.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroll J, Epting D, Kern K, Dietz CT, Feng Y, Hammes HP, Wieland T, Augustin HG. Inhibition of Rho-dependent kinases ROCK I/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am J Physiol Heart Circ Physiol. 2009;296:H893–H899. doi: 10.1152/ajpheart.01038.2008. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am J Physiol Endocrinol Metab. 2009;296:E139–E146. doi: 10.1152/ajpendo.90677.2008. [DOI] [PubMed] [Google Scholar]
- 28.Miranda L, Carpentier S, Platek A, Hussain N, Gueuning MA, Vertommen D, Ozkan Y, Sid B, Hue L, Courtoy PJ, Rider MH, Horman S. AMP-activated protein kinase induces actin cytoskeleton reorganization in epithelial cells. Biochem Biophys Res Commun. 396:656–661. doi: 10.1016/j.bbrc.2010.04.151. [DOI] [PubMed] [Google Scholar]
- 29.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 30.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez A, Catalan V, Becerril S, Gil MJ, Mugueta C, Gomez-Ambrosi J, Fruhbeck G. Impaired adiponectin-AMPK signalling in insulin-sensitive tissues of hypertensive rats. Life Sci. 2008;83:540–549. doi: 10.1016/j.lfs.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Kim JE, Choi HC. Losartan Inhibits Vascular Smooth Muscle Cell Proliferation through Activation of AMP-Activated Protein Kinase. Korean J Physiol Pharmacol. 2010;14:299–304. doi: 10.4196/kjpp.2010.14.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida D, Higashiura K, Shinshi Y, Satoh K, Hyakkoku M, Yoshida H, Miyazaki Y, Ura N, Shimamoto K. Effects of angiotensin II receptor blockade on glucose metabolism via AMP-activated protein kinase in insulin-resistant hypertensive rats. J Am Soc Hypertens. 2009;3:3–8. doi: 10.1016/j.jash.2008.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.