Abstract
Arachidonic acid (AA) stimulates cell adhesion through a p38 mitogen activated protein kinase-mediated RhoA signaling pathway. Here we report that a proteomic screen following AA-treatment identified nucleolin, a multifunctional nucleolar protein, in a complex with the GTPase, RhoA, that also included the Rho kinase, ROCK. AA-stimulated cell adhesion was inhibited by expression of nucleolin-targeted shRNA and formation of the multiprotein complex was blocked by expression of dominant-negative RhoA. AA-treatment also induced ROCK-dependent serine phosphorylation of nucleolin and translocation of nucleolin from the nucleus to the cytoplasm, where it appeared to co-localize with RhoA. These data suggest the existence of a new signaling pathway through which the location and post-translational state of nucleolin are modulated.
Keywords: Nucleolin, RhoA, Rho kinase, Arachidonic acid, Cell adhesion
1. Introduction
Cell adhesion to the extracellular matrix is a critical feature of many cellular processes, including development, wound healing, and cancer cell metastasis. This adhesion depends on signaling pathways that modulate integrin activity [1]; thus, understanding these pathways and the environmental factors that modulate them allows for a rational approach to the development of therapies targeting cell adhesion [2]. One important class of environmental molecules is the eicosanoids. Arachidonic acid (AA) is a dietary n-6 polyunsaturated fatty acid that can be generated either by phospholipase-mediated release from cell membranes [3] or as a metabolite of linoleic acid, a fatty acid present at high levels in most Western diets [4]. These fatty acids and their bioactive products have been implicated in a variety of diseases, including cancer [5]. Previously, we observed that AA-stimulated adhesion of MDA-MB-435 human cancer cells to extracellular matrix molecules [6] in a manner that depended on several signaling pathways [7–10]. One of these pathways involves RhoA, a small GTPase, the regulation of which has been implicated in tumor metastasis [11]. In this paper, we report the results of a proteomic search for factors associated with RhoA in fatty acid-stimulated cell adhesion.
2. Materials and methods
2.1. Reagents and cell culture
AA (Cayman Biochemicals) was used as described [6]. Collagen type IV and poly-d-lysine were from BD Biosciences. Primary antibodies used were anti-Rho (from either Cytoskeleton Inc. or Santa Cruz Biotechnology), anti-HA (Upstate), anti-GAPDH (Upstate), anti-phosphotyrosine (Upstate), anti-phosphoserine (Pharmingen), anti-nucleolin (either Stressgen or Santa Cruz), and anti-ROCKII (Santa Cruz).
The DNA construct expressing the glutathione S-transferase-linked Rho binding domain of ROCK (GST-RBD) was a gift from John P. O’Bryan (University of Illinois). 3× HA-tagged wild-type RhoA, dominant-negative RhoA (T19N), and constitutively active RhoA (G12V) were from the University of Missouri-Rolla cDNA Resource Center (www.cdna.org). Sequences of a short hairpin RNA targeting nucleolin and a scrambled negative control are AAGAAGACAGTTACACCAGCC and AATCAACAGAGAAGCCTAGC, respectively. Expression cassettes containing the U6 RNA polymerase promoter sequence and terminator sequence were generated using the Silencer® Express kit (Ambion) and subcloned into the pSEC™ hygro vector (Ambion).
The human mammary adenocarcinoma cell line MDA-MB-435 was obtained from Dr. Janet Price (M.D. Anderson Cancer Center) and cultured as described [6]. Cell transfections were performed using FuGENE 6 (Roche Applied Science). Medium was changed 24 h after transfection to minimal essential medium (MEM) containing 200 µg/ml of hygromycin; stable populations were harvested and grown in hygromycin-containing medium.
2.2. Adhesion and small G protein activation assays
Adhesion assays were carried out essentially as described [6]. When appropriate, cells were treated with ROCK inhibitors Y27632 or H1152 (Calbiochem) for 30 min. For G protein activation, cells were harvested, washed and allowed to recover in serum- free MEM for 30 min. After treatment with 30 µM AA, cells were washed with ice-cold phosphate buffered saline (PBS) and lysed with 50 mM HEPES, pH 7.5, 1.5 mM MgCl2, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 0.25% sodium deoxycholate, 1 mM EGTA, 100 mM NaF, 10 µg/ml of each aprotinin and leupeptin, and 1 mM Na3VO4. Lysates were clarified and incubated for 1 h with GST-RBD bound to glutathione–Sepharose beads (Amersham). Samples were washed with lysis buffer and bound proteins were eluted by boiling in LDS NuPage® sample buffer (Invitrogen), separated by electrophoresis on a 4–12% Bis–Tris NuPage® gel and immunoblotted with appropriate antibodies.
2.3. Immunoprecipitations
Cells were transfected overnight with a 3× HA-tagged wild type Rho DNA construct, harvested, washed, allowed to recover in serum-free MEM for 30 min, and treated with 30 µM AA, prior to being lysed with 50 mM Tris–HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 10 µg/ml aprotinin, and 10 µg/ml leupeptin). Lysates were clarified by centrifugation, then precleared for 1 h with protein G agarose beads (Santa Cruz) and normal mouse serum, followed by incubation for 1 h with primary antibody bound to protein G beads. Precipitates were washed with lysis buffer and analyzed by SDS–PAGE and immunoblotting.
2.4. Mass spectrometry and confocal microscopy
Active RhoA was generated by treating MDA-MB-235 cells with 30 µM AA for 20 min as described [10]. GTP-bound RhoA was then pulled down and analyzed by SDS–polyacrylamide gel electrophoresis. In-gel digestion and mass spectrometry were performed essentially as described [12].
For confocal microscopy, cells were cultured overnight at 2 × 104 cells/collagen IV-coated glass coverslip. After treatment, cells were washed with PBS, fixed in 4% paraformaldehyde for 45 min, permeabilized with 0.3% Triton X-100 in PBS for 10 min and incubated for 45 min in 4% donkey serum in 0.1% gelatin blocking solution. Primary antibodies against nucleolin (diluted 1:500) and Rho (diluted 1:200) were added for 45 min, then cells were blocked overnight at 4 °C, and secondary antibodies [Alexa Fluor® 488 donkey anti-rabbit and Alexa Fluor® 594 donkey anti-mouse secondary antibodies (Invitrogen), diluted 1:2000], were added for 45 min. Slides were mounted using Prolong Gold anti-fade with DAPI (Invitrogen).
3. Results and discussion
3.1. Nucleolin is found in a complex with Rho and ROCK
We set out to identify proteins that associate with active Rho after AA stimulation. MDA-MB-435 human tumor cells were treated with 30 µM AA and active RhoA was immunoprecipitated as described (Section 2). Proteins in the immunoprecipitates were separated by electrophoresis and identified by mass spectrometry. Among the proteins identified, a 90 kDa species was observed in the AA-treated lysates that was not present in the untreated lysates (Fig. 1). This band, upon digestion and analysis by MALDITOF mass spectrometry, yielded 23 unique peptides, corresponding to 36% sequence coverage, identifying it as nucleolin, a nucleolar phosphoprotein that functions in nucleogenesis [13,14]. The data yielded a Mowse-based MASCOT score of 135 with a GPS assigned confidence index of 100% (Supplementary Table 1). We validated the mass spectrometry results by performing Rho pull-down assays and identifying nucleolin in the precipitate by electrophoresis and immunoblotting with nucleolin-specific antibody (Fig. 2A). We then reversed the experiment, testing for and observing an increase in RhoA in immunoprecipitates of nucleolin specifically after fatty acid treatment of these cells (Fig. 2B).
Fig. 1.
Proteins associated with Rho-GTP identified by mass spectrometry. GST-RBD was used to pull down Rho-GTP from cell lysates that were untreated or treated with arachidonic acid for 5 min. Proteins separated by SDS–PAGE were cut out and identified by mass spectrometry as: nucleolin (arrow), HSP70 (*), HSP90β (+) and nebulin (>). The data shown are representative of two experiments.
Fig. 2.
Nucleolin forms a complex with Rho. (A) GST-RBD was used to pull down active Rho in cell lysates that were treated with arachidonic acid. The precipitates, and whole cell lysates used in the pull-down assay, were separated by SDS–PAGE and immunoblotted for nucleolin, Rho A and GAPDH. The data shown are representative of four experiments. (B) Whole cell lysates were prepared from cells exposed to arachidonic acid (AA) (30 µM) or vehicle (V) (ethanol/NaCl/KOH) for 20 min. Nucleolin was immunoprecipitated from the lysates and analyzed by immunoblotting for RhoA and nucleolin. (C) Nucleolin was immunoprecipitated from whole cell lysates as above and analyzed by immunoblotting for ROCK and nucleolin.
Rho-associated kinase (ROCK) is frequently found associated with Rho-GTP [15,16]; thus, we tested for its presence in the complex by immunoprecipitating nucleolin from AA-treated and untreated lysates and observed an increase in co-immunoprecipitation of ROCK with nucleolin in lysates of AA-treated cells (Fig. 2C), suggesting a novel multiprotein signaling complex that includes Rho, ROCK and nucleolin.
3.2. Nucleolin is critical for AA-induced adhesion on type IV collagen
AA has been previously shown to alter a variety of signaling pathways that are important for cell adhesion [7–10]. Furthermore, AA-treated MDA-MB-435 cells that adhere to collagen type IV show significant changes in cytoskeletal structures, including increased cortical actin and vinculin-containing focal adhesions (unpublished data). RhoA activity is critical for this AA-induced cell adhesion to type IV collagen [10]. To test whether nucleolin is important for this process, we transfected MDA-MB-435 cells with nucleolin-targeted shRNA and control shRNA vectors. Two populations of cells expressing the nucleolin-targeted shRNA exhibited a significant reduction of nucleolin protein (63% and 82%, respectively) (Fig. 3A) and both populations were completely blocked in AA-induced adhesion when compared to control shRNA-containing cells (Fig. 3B). However, we observed that nucleolin protein reduction did not block the stimulation of RhoA activity by AA (Fig. 3C). The simplest interpretation of this data is that nucleolin functions downstream of RhoA activation. Other model systems have provided evidence for a role of nucleolin in adhesion [17–19], but our data suggest a novel mechanism in which nucleolin is regulated by a key signaling pathway.
Fig. 3.
Nucleolin is critical for arachidonic acid-induced adhesion on type IV collagen. (A) Cell lysates from MDA-MB-435 cells (M), cells with stable expression of the scrambled shRNA (S), and two cell lines with stable expression of the shRNA targeting nucleolin (K1 and K2) were separated by SDS–PAGE and immunoblotted for nucleolin and GAPDH. (B) Cell adhesion to collagen IV was assayed in the cells lines listed above, treating with vehicle (□) or 30 µM arachidonic acid (AA) (■). Values were compared to non-transfected cells (P). Statistically significantly differences between transfected and non-transfected, vehicle-treated cells [as determined by Student’s t-test (P < 0.05)] are indicated (*). The data shown are representative of three experiments. (C) GST-RBD was used to pull down active Rho from cell lysates from cells listed above that were treated with arachidonic acid. Precipitating proteins and whole cell lysates were analyzed by SDS–PAGE and immunoblotted for nucleolin, RhoA, and GAPDH. The data shown are representative of two experiments.
3.3. Rho signaling is critical for complex formation
We next asked if RhoA signaling was critical for its association with nucleolin. Cells were transfected with a wild-type HA-tagged RhoA, a constitutively active HA-tagged RhoAG12V or a dominant negative HA-tagged RhoAT19N. We observed an increase in the association of both wild-type RhoA and constitutively active RhoA with nucleolin in AA-treated cell lysates (Fig. 4A). In contrast, dominant-negative RhoA did not associate with nucleolin, even in AA-treated cells, indicating that Rho activation is critical for the association with nucleolin (Fig. 4B). However, the constitutively active RhoA did not show increased association with nucleolin in vehicle-treated cells, indicating that RhoA activity alone is not sufficient for recruitment of nucleolin to the complex. Recently, nucleolin has been identified as a binding partner to the small GTPase K-Ras4B and a nuclear guanine nucleotide exchange factor, BIG [20,21]. It has also been shown that this binding is independent of GTP binding to K-ras [22]. Our data complement these findings, and suggest that (1) the interaction of Rho and nucleolin may be a general phenomenon of importance to this signaling pathway, and (2) at least in the MDA-MB-435 cells, other factors activated by exposure to AA are critical for formation of this complex.
Fig. 4.
Rho signaling is critical for complex formation. (A) MDA-MB-435 cells were transfected with HA-tagged wild type (RhoA) or constitutively active (RhoAG12V) RhoA for 24 h. Nucleolin was precipitated from arachidonic acid-treated (AA) and vehicle-treated (V) lysates. The precipitates were analyzed by SDS–PAGE and immunoblotted for the HA-tag and nucleolin. Quantification was performed using ImageJ and expressed as a ratio vs. wild-type transfected, vehicle-treated cells and normalized to the amount of precipitated nucleolin. Whole cell lysates used in the immunoprecipitation were separated by SDS–PAGE and immunoblotted for the HA-tag and GAPDH. (B) Nucleolin was immunoprecipitated from cell lysates of HA-tagged wild-type (RhoA) or dominant negative (RhoAT19N) RhoA expressing cells treated with vehicle (V) or arachidonic acid (AA). The precipitates were immunoblotted for the HA-tag and nucleolin. Whole cell lysates were separated by SDS–PAGE and immunoblotted for the HA-tag and GAPDH. The data shown are representative of three experiments. ROCK was also found to be present by immunoblotting in whole cell lysates used in both panels A and B (data not shown).
3.4. AA stimulates ROCK-dependent nucleolin serine phosphorylation
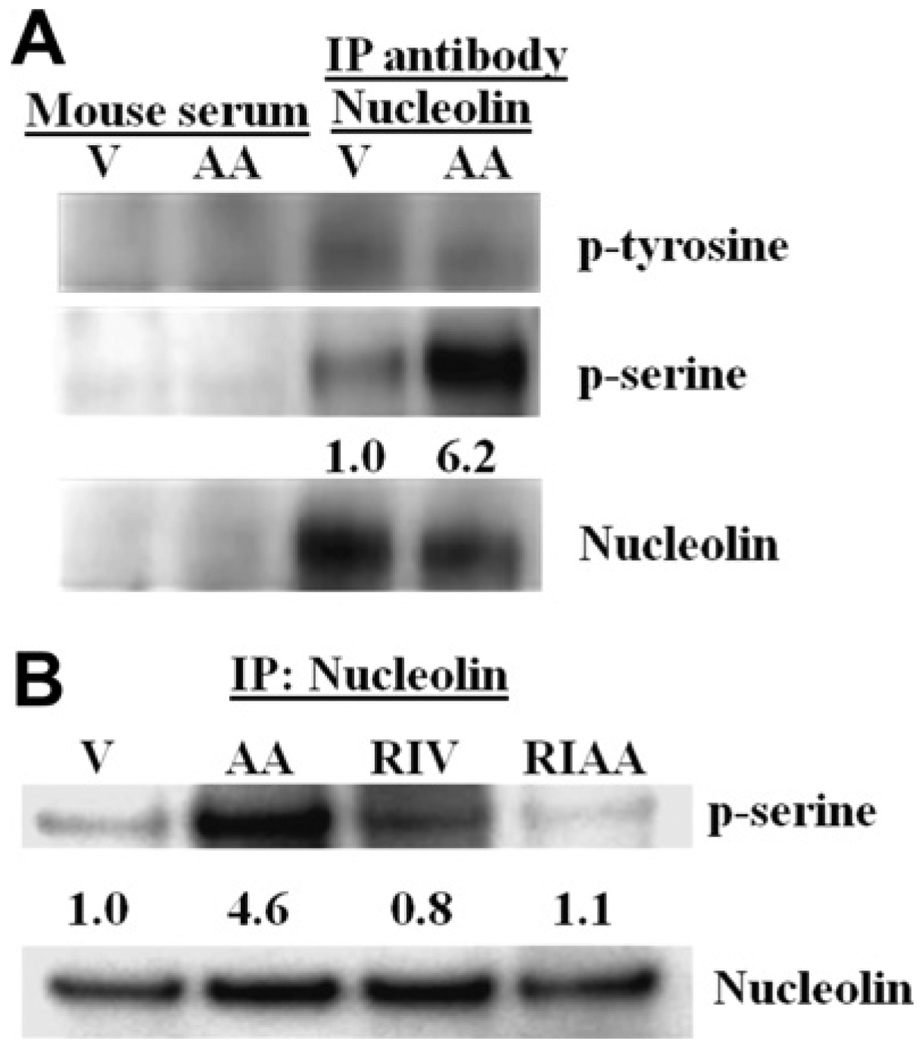
Phosphorylation of nucleolin has been linked to both its activity and cellular localization [23]. We hypothesized that AA treatment, which is known to activate multiple protein kinase pathways, may stimulate nucleolin phosphorylation. To test this hypothesis, we immunoprecipitated nucleolin from AA-treated and untreated MDA-MB-435 cell lysates and observed that nucleolin immunoprecipitated from AA-treated cells was serine phosphorylated, but not tyrosine phosphorylated (Fig. 5A). A ROCK inhibitor significantly decreased the AA-stimulated increase in serine phosphorylation of nucleolin (Fig. 5B). We were unable to demonstrate direct phosphorylation of nucleolin by ROCK in in vitro kinase assays (data not shown), suggesting that nucleolin may be phosphorylated by a downstream kinase whose activity depends on ROCK.
Fig. 5.
Arachidonic acid stimulates ROCK-dependent serine phosphorylation of nucleolin. (A) Lysates from vehicle-treated (V) and arachidonic acid-treated (AA) cells were precipitated for nucleolin and immunoblotted for phosphoserine, phosphotyrosine and nucleolin. (B) Nucleolin was immunoprecipitated from lysates of cells treated with vehicle (V), arachidonic acid (AA), vehicle plus H1152 ROCK inhibitor (I) and arachidonic acid plus H1152 (IAA). The precipitates were immunoblotted for nucleolin and phosphoserine. Data are representative of three individual experiments. Quantifications of phosphoserine bands were performed using ImageJ, normalized to nucleolin and expressed as a ratio vs. the bands from vehicle-treated cells.
3.5. AA induces ROCK-dependent translocation of nucleolin from the nucleus into the cytoplasm
Nucleolin has been shown to localize in multiple cell compartments [23] and to appear on the cell surface, potentially facilitating cell adhesion [19,24].We used confocal microscopy to show that in vehicle-treated MDA-MB-435 cells, nucleolin was located primarily in the nucleus, with some in the cytoplasm (Fig. 6A and C), whereas Rho was located only in the cytoplasm and did not colocalize with nucleolin (Fig. 6B and D). In cells treated with AA, the majority of nucleolin appeared in the cytoplasm, with a small amount remaining in the nucleus (Fig. 6E). Rho localization did not change with AA treatment and nucleolin appeared to co-localize with Rho in the cytoplasm (Fig. 6F and H). [Several different antibodies to nucleolin and RhoA were tested and each yielded similar staining (data not shown), suggesting the translocation is not due to an artifact of the particular antibody. Furthermore, the staining for nucleolin disappeared when cells carrying the nucleolin-specific shRNA were used, indicating the staining is specific for nucleolin.] Treatment of the cells with Y27632, a ROCK inhibitor, had no effect on the localization of Rho or nucleolin in cells not exposed to AA (Fig. 6I–L). The ROCK inhibitor did successfully block the translocation of nucleolin from the nucleus to the cytoplasm in AA-treated cells (Fig. 6M–P), suggesting that ROCK activity is critical for nucleolin translocation.
Fig. 6.
Arachidonic acid induces ROCK-dependent translocation of nucleolin from the nucleus into the cytoplasm. Panels A–D: MDA-MB-435 cells treated with vehicle (V); panels E–H: cells treated with arachidonic acid (AA); panels I–L: cells treated with vehicle plus Y27632, an inhibitor of ROCK (I); and panels M–P: cells treated with arachidonic acid plus inhibitor (IAA). Adherent cells on collagen IV-coated cover slips were probed for nucleolin (red) and Rho (green) as described, and DAPI (blue) was used to identify cell nuclei. Co-localization of Rho and nucleolin is displayed in merged images (panels D, H, L and P) as yellow. A section of each merged image is enlarged in the lower left corner of each panel D, H, L, and P (enclosed in the dotted lines). Images are representative of three separate experiments. When used, the ROCK inhibitor was applied at a concentration of 10 µM, 30 min prior to addition of arachidonic acid or vehicle.
The mechanism by which nucleolin interacts with this complex of signaling proteins is not yet clear. However, this work identifies nucleolin as a required cofactor in fatty acid-stimulated cell adhesion and places its role downstream of Rho/ROCK, implicating nucleolin, or its interacting partners, as attractive targets for intervention in altering cell adhesion for therapeutic purposes.
Supplementary Material
Acknowledgements
We thank Drs. Paul Wade, David Armstrong, and Steven Akiyama for a careful reading of the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health through the National Institute of Environmental Health Sciences.
Abbreviations
- AA
arachidonic acid
- GST-RBD
glutathione S-transferase-linked Rho binding domain
- MEM
minimal essential medium
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.febslet.2011.01.035.
References
- 1.Rathinam R, Alahari S. Important role of integrins in the cancer biology. Cancer Metast. Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 2.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakanishi M, Rosenberg DW. Roles of cPLA(2)alpha and arachidonic acid in cancer. Biochem. Biophys. Acta. 2006;1761:1335–1343. doi: 10.1016/j.bbalip.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou L, Nilsson A. Sources of eicosanoid precursor fatty acid pools in tissues. J. Lipid Res. 2001;42:1521–1542. [PubMed] [Google Scholar]
- 5.Bardon S, Benelli C, Bernard-Gallon D, Blottiere H, Demarquoy J, Duee PH, Forest C. Dietary fatty acids and cancer: potential cellular and molecular mechanisms. Bull. Cancer. 2005;92:697–707. [PubMed] [Google Scholar]
- 6.Palmantier R, Roberts JD, Glasgow WC, Eling TE, Olden K. Regulation of the adhesion of a human breast carcinoma cell line to type IV collagen and vitronectin: roles for lipoxygenase and protein kinase C. Cancer Res. 1996;56:2206–2212. [PubMed] [Google Scholar]
- 7.Paine E, Palmantier R, Akiyama SK, Olden K, Roberts JD. Arachidonic acid activates mitogen-activated protein (MAP) kinase-activated protein kinase 2 and mediates adhesion of a human breast carcinoma cell line to collagen type IV through a p38 MAP kinase-dependent pathway. J. Biol. Chem. 2000;275:11284–11290. doi: 10.1074/jbc.275.15.11284. [DOI] [PubMed] [Google Scholar]
- 8.Palmantier R, George MD, Akiyama SK, Wolber FM, Olden K, Roberts JD. Cis-polyunsaturated fatty acids stimulate beta1 integrin-mediated adhesion of human breast carcinoma cells to type IV collagen by activating protein kinases C-epsilon and -mu. Cancer Res. 2001;61:2445–2452. [PubMed] [Google Scholar]
- 9.Kennett SB, Roberts JD, Olden K. Requirement of protein kinase C mu activation and calpain-mediated proteolysis for arachidonic acid-stimulated adhesion of MDA-MB-435 human mammary carcinoma cells to collagen type IV. J. Biol. Chem. 2004;279:3300–3307. doi: 10.1074/jbc.M305734200. [DOI] [PubMed] [Google Scholar]
- 10.Garcia MC, Ray DM, Lackford B, Rubino M, Olden K, Roberts JD. Arachidonic acid stimulates cell adhesion through a novel p38 MAPK–RhoA signaling pathway that involves heat shock protein 27. J. Biol. Chem. 2009;284:20936–20945. doi: 10.1074/jbc.M109.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. BioEssays. 2005;27:602–613. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- 12.Choi JH, Williams J, Cho J, Falck JR, Shears SB. Purification, sequencing, and molecular identification of a mammalian PP-InsP(5) kinase that is activated when cells are exposed to hyperosmotic stress. J. Biol. Chem. 2007;282:30763–30775. doi: 10.1074/jbc.M704655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuteja R, Tuteja N. Nucleolin: a multifunctional major nucleolar phosphoprotein. Crit. Rev. Biochem. Mol. Biol. 1998;33:407–436. doi: 10.1080/10409239891204260. [DOI] [PubMed] [Google Scholar]
- 14.Medina FJ, Gonzalez-Camacho F, Manzano AI, Manrique A, Herranz R. Nucleolin, a major conserved multifunctional nucleolar. J. Appl. Biomed. 2010;8:141–150. [Google Scholar]
- 15.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. P160 (ROCK), a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 16.Amano M, Nakayama M, Kaibuchi K. Rho-Kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrucea S, et al. Cellular adhesion mediated by factor J, a complement inhibitor – evidence for nucleolin involvement. J. Biol. Chem. 1998;273:31718–31725. doi: 10.1074/jbc.273.48.31718. [DOI] [PubMed] [Google Scholar]
- 18.Turck N, Lefebvre O, Gross I, Gendry P, Kedinger M, Simon-Assmann P, Launay JF. Effect of laminin-1 on intestinal cell differentiation involves inhibition of nuclear nucleolin. J. Cell. Physiol. 2006;206:545–555. doi: 10.1002/jcp.20501. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Reyes EM, Akiyama SK. Cell-surface nucleolin is a signal transducing P-selectin binding protein for human colon carcinoma cells. Exp. Cell Res. 2008;314:2212–2223. doi: 10.1016/j.yexcr.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birchenall-Roberts MC, Fu T, Kim SG, Huang YK, Dambach M, Resau JH, Ruscetti FW. K-Ras4B proteins are expressed in the nucleolus: interaction with nucleolin. Biochem. Biophys. Res. Commun. 2006;348:540–549. doi: 10.1016/j.bbrc.2006.07.094. [DOI] [PubMed] [Google Scholar]
- 21.Padilla PI, Uhart M, Pacheco-Rodriguez G, Peculis BA, Moss J, Vaughan M. Association of guanine nucleotide-exchange protein BIG1 in HepG2 cell nuclei with nucleolin, U3 snoRNA, and fibrillarin. Proc. Natl. Acad. Sci. USA. 2008;105:3357–3361. doi: 10.1073/pnas.0712387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inder KL, et al. Nucleophosmin and nucleolin regulate K-Ras plasma membrane interactions and MAPK signal transduction. J. Biol. Chem. 2009;284:28410–28419. doi: 10.1074/jbc.M109.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava M, Pollard HB. Molecular dissection of nucleolin’s role in growth and cell proliferation: new insights. FASEB J. 1999;13:1911–1922. [PubMed] [Google Scholar]
- 24.Hovanessian AG, Puvion-Dutilleul F, Nisole S, Svab J, Perret E, Deng JS, Krust B. The cell-surface-expressed nucleolin is associated with the actin cytoskeleton. Exp. Cell Res. 2000;261:312–328. doi: 10.1006/excr.2000.5071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.