Abstract
Activated phospholipase Cγ1 (PLC-γ1), produced in response to tyrosine phosphorylation, appears to play an important role during uterine contractions. These studies sought to determine which non-receptor protein tyrosine kinases (PTKs) are involved in the tyrosine phosphorylation and activation of PLC-γ1 in uterine tissue from the rat. In vitro uterine contraction studies were performed utilizing isoform specific PTK inhibitors. Western blots were performed utilizing antibodies to phosphotyrosine-PLC-γ1, total PLC-γ1, c-Src kinase and Lck kinase. Spontaneous, stretch-stimulated, and bpV(phen) (a tyrosine phosphatase inhibitor) enhanced uterine contractions were significantly suppressed in response to Damnacanthal (a Lck kinase inhibitor) and PP1 (a c-Src kinase inhibitor); whereas, several other PTK isoform inhibitors had no significant effect. Damnacanthal and PP1 also significantly suppressed bpV(phen)-enhanced tyrosine phosphorylation of PLC-γ1 compared to other PTK isoform inhibitors. Western blots confirmed expression of the Lck and c-Src kinases in uterine tissue. In conclusion, the Lck and c-Src kinases appear to play an important role in regulating tyrosine phosphorylation of PLC-γ1 and contractile activity in the rat uterus.
Keywords: Lck Kinase, c-Src Kinases, Phospholipase C-γ1, Phasic Myometrial Contractions, Uterine Stretch
Introduction
Activation of phospholipase C-γ (PLCγ) results in inositol trisphosphate (IP3) generation, stimulation of the phosphatidylinositol (PI) signaling pathway, and mobilization of intracellular calcium in various cell types including in uterine myocytes 1. Two isoforms of PLCγ have been previously reported: the PLCγ1 isoform is expressed in a wide range of cell types and animal tissues; whereas, the PLCγ2 isoform has been identified mainly in white blood cells and lymphoid tissues 2, 3. Western blot, reverse transcriptase polymerase chain reaction (RT-PCR), and immunohistochemical studies previously reported by our laboratory have confirmed the expression of both of these PLCγ isoforms in pregnant and non-pregnant rat myometrial tissue 4, 5. These previous studies using rat uterine tissue were consistent with those reported by Phaneuf et al.6 who utilized Western blots to demonstrate the expression of PLCγ1 and PLCγ2 in human myometrial cells.
PLCγ activation occurs by phosphorylation of tyrosine #783 in response to various membrane receptor tyrosine kinases and non-receptor protein tyrosine kinases (PTKs) 2, 3. Members of the Src family of non-receptor protein tyrosine kinases have been reported to produce tyrosine phosphorylation of PLCγ1 in various smooth muscle types, including in myometrium. Schmitz et al. 7 have reported that angiotensin II stimulates tyrosine phosphorylation of PLCγ through the activation of c-Src in vascular smooth muscle cells. Boulven et al. 8 demonstrated the ability of c-Src to generate phosphotyrosine-PLCγ1 in rat myometrial cells; an effect that was prevented by pretreatment of the tissue with the tyrosine kinase inhibitors genistein and PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine). In a previous report, we utilized bpV(phen) (potassium bisperoxo (1,10 phenanthroline) oxovanadate) to demonstrate the role of PLCγ1 and its tyrosine phosphorylation during phasic contractions of rat uterine tissue 1.
To date, at least 9 members of the Src family of non-receptor PTKs have been demonstrated in vertebrate cells. These Src family kinase isoforms include c-Src (the original member) along with the Blk, Fgr, Fyn, Hck, Lck, Lyn, Yes and Yrk isoforms; all have a common molecular structure, conserved Src-homology 2 (SH2) and Src-homology 3 (SH3) peptide domains, and similar molecular weights in the 52–62 kD range 9, 10. The Src kinases are activated through dephosphorylation of a tyrosine residue at their carboxy-terminal ends and protein-protein interactions (at their SH2 and SH3 domains), resulting in exposure of the catalytic domain. Several non-receptor PTKs, including c-Src, Lck, Fyn, Lyn, Hck and Syk (a non-Src family kinase), have been previously reported to produce tyrosine phosphorylation of PLCγ in various cell types 11–13. The goal of the present study was to determine if any of these PTKs play a role during tyrosine phosphorylation of PLCγ1 and the generation of spontaneous and bpV(phen)-enhanced phasic contractions of the rat uterus. In addition, we sought to determine if these PTK signaling events also contribute to the mechanisms underlying the stretch-stimulated phasic uterine contractions.
Materials & Methods
Uterine and other tissues were obtained for these studies from nonpregnant and timed-pregnant Sprague-Dawley rats using a protocol approved by the Animal Care and Utilization Committee at the University of Vermont College of Medicine. For the in vitro isometric contraction studies, uterine tissue was obtained from proestrus/estrus rats. These studies were performed using longitudinal segments of uterine tissue (6–8 mm relaxed length) in 3 mL muscle baths containing Earle’s balanced salt solution (EBSS) at 37° C as previously reported by our laboratory 1. Some contraction studies were performed using 20 μM potassium bisperoxo (1,10 phenanthroline) oxovanadate (bpV(phen)) (Calbiochem, San Diego, CA); a previously reported inhibitor of protein tyrosine phosphatases 1. Other contraction studies were performed with and without the addition of previously reported PTK inhibitors. PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; Biomol International, L.P. Plymouth Meeting, PA) or PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; Calbiochem, San Diego, CA) (60μM) were used to selectively inhibit c-Src kinase activity 8, 14, 15; Damnacanthal (Calbiochem, San Diego, CA) (60μM) was used to inhibit Lck kinase activity 16; and Piceatannol (Calbiochem, San Diego, CA) (60μM) to inhibit Syk kinase activity 17. Studies were also performed using SU6656 (Calbiochem, San Diego, CA) (100μM), an inhibitor of the Fyn, Yes and Lyn kinase isoforms, and which also weakly inhibits c-Src kinase 15, 18. Control studies were performed using comparable volumes of vehicle alone. The PTK inhibitor concentrations were based on the in vitro concentration-response effects of these inhibitors on contractile activity of uterine strips; the selected inhibitor concentrations were at or above the concentrations used in the previously referenced studies in an effort to optimize potential inhibitory effects.
Additional studies were performed to assess the effects of Damnacanthal, PP1, Piceatannol and PAO (phenylarsine oxide, a PLCγ inhibitor 1 ) on uterine stretch. For these studies, the tissue was preincubated with these reagents for 20 minutes followed by rapid stretch to 4 grams of tension; the resulting contractile activity was then compared to that observed for uterine strips pretreated with vehicle (dimethylsulfoxide (DMSO)) alone. As previously reported from our laboratory 1, the analog contraction data were computer digitized, analyzed to determine the total contractile area in selected 5-minute intervals, normalized for tissue cross-sectional area, and reported as the percent of spontaneous contractile activity (or for the stretch studies: as contractile activity in grams tension generated per milligram tissue per minute).
For the Western blots, uteri and other rat tissues were homogenized in a protease/phosphatase inhibitor solution (containing 50 mM Tris, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 5 μg/mL aprotinin, 3 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate). Homogenate solutions were centrifuged at 800 X g to remove the cellular debris; and the protein concentrations of the supernatant solutions (crude tissue homogenates) were determined by BCA protein assay. Subsequently, 50 μg protein aliquots were resolved using 10% SDS-PAGE gels and the Bio-Rad Mini-PROTEAN 3 Electrophoresis system (Bio-Rad Laboratories, Hercules, CA). The resolved proteins were then electophoretically transferred to nitrocellulose membranes. The nitrocellulose membranes were blocked with 5% powdered milk in Tween-20/Tris buffered saline (TTBS), then incubated overnight in the primary antibody solution containing anti-phosphotyrosine-PLCγ1 polyclonal antibodies (Cell Signaling Technology, Danvers, MA), or with anti-PLCγ1 monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for total PLCγ1 protein. Additional Western blots were performed using anti-Src (Calbiochem, San Diego, CA) and anti-Lck (Santa Cruz Biotechnology; Santa Cruz, CA) monoclonal antibodies. Visualization of the chemiluminescent protein bands was performed by exposing Hyperfilm (Amersham, Piscataway, NJ) to the nitrocellulose membranes. The relative density of the bands was then determined using the Kodak 1-D Digital Science Electrophoresis Documentation and Analysis System (Eastman Kodak Co, Rochester, NY).
Statistical analyses for these studies were performed using the t-test, Mann-Whitney Rank Sum test, or the Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks, followed by multiple comparisons tests (including the Student-Newman-Keuls or Dunn test) where appropriate. Statistical significance was considered attained when p < 0.05.
Results
In vitro contraction studies
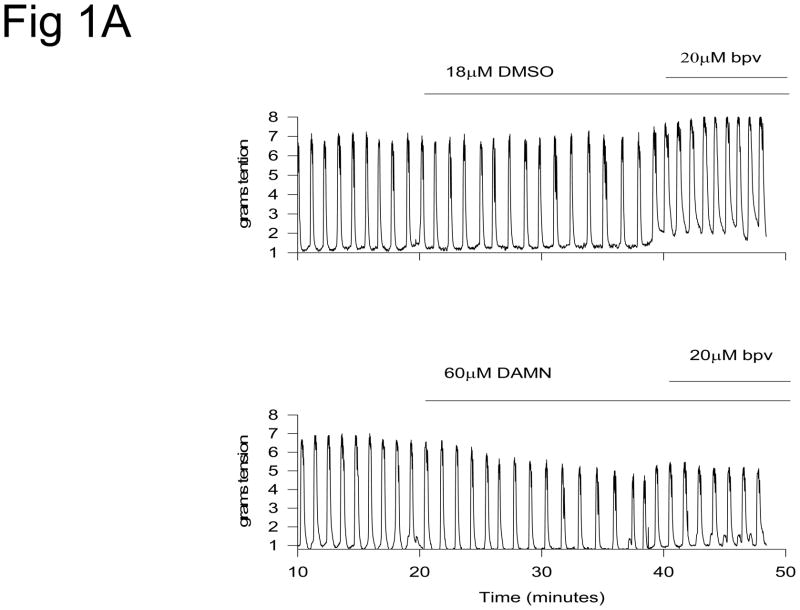
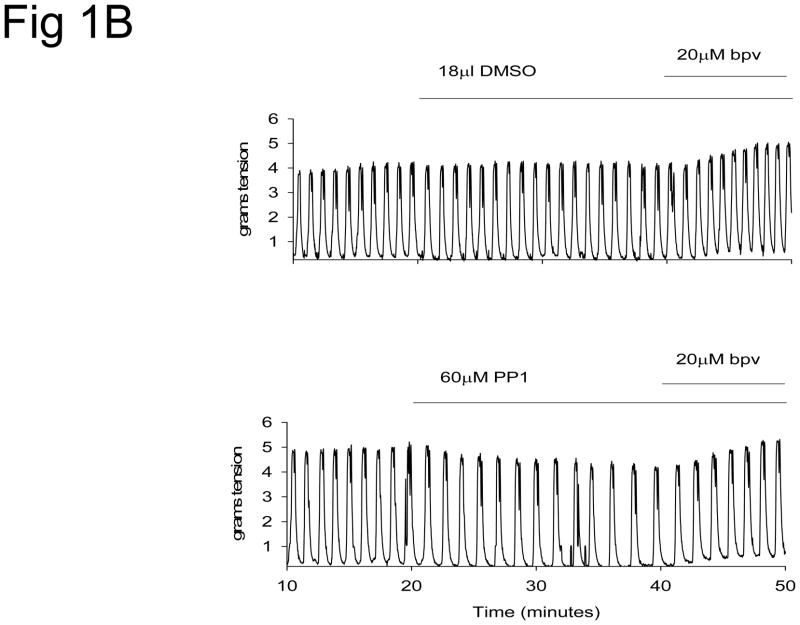
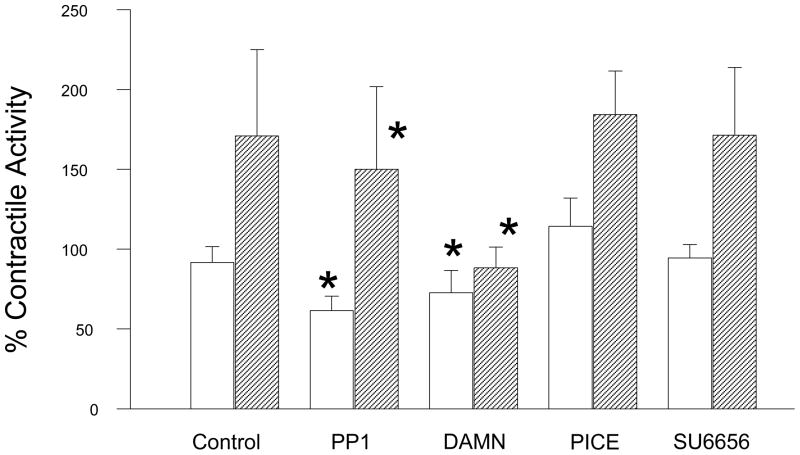
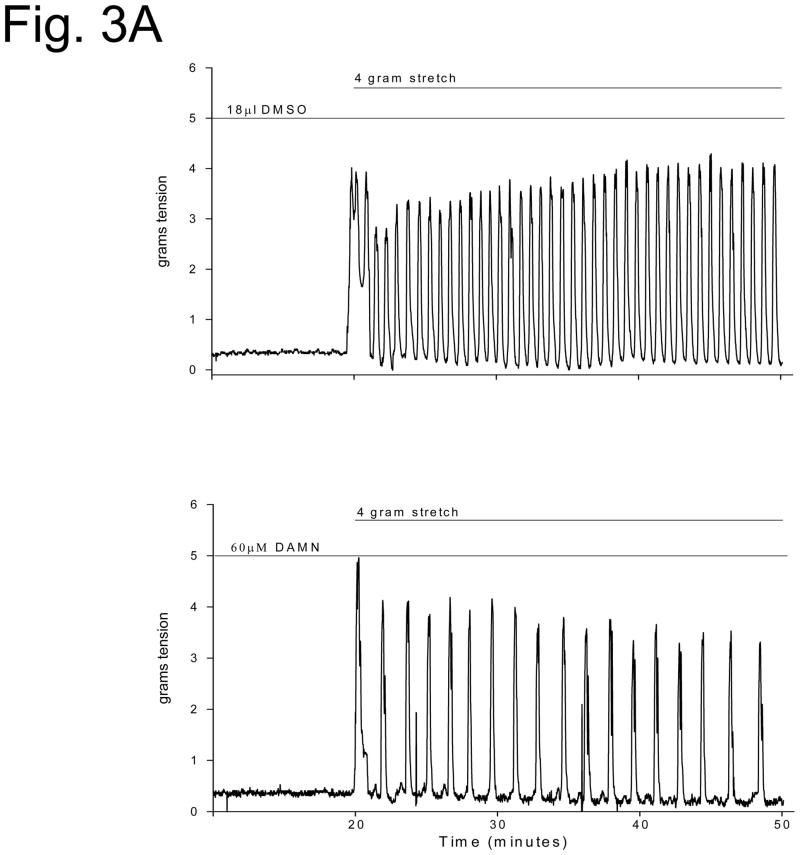
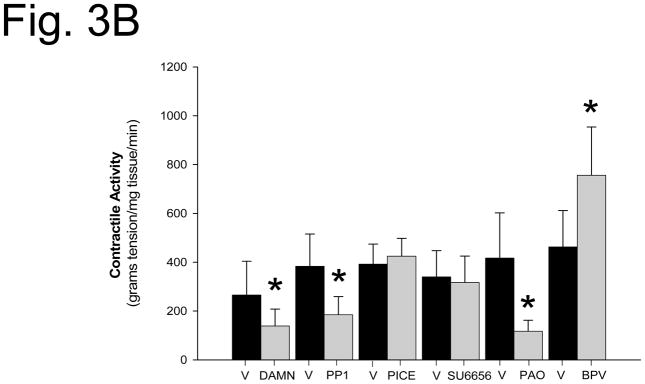
Spontaneous uterine contractions were significantly enhanced in response to bpV(phen), a protein tyrosine phosphatase inhibitor, as observed in the vehicle (DMSO) control strips in Figure 1. Damnacanthal (an inhibitor of the Lck kinase isoform) significantly suppressed spontaneous and bpV(phen)-enhanced uterine contractile activity (p<0.01, N = 7–8) (Figure 1A and 2). PP1 (an inhibitor of c-Src kinase) also significantly suppressed spontaneous and bpV(phen)-enhanced contractile activity (p< 0.05, N = 8–9) (Figure 1B and 2). In contrast, Piceatannol (a Syk kinase inhibitor) had no effect on spontaneous or bpV(phen)-enhanced contractile activity (Figure 2). Similarly, SU6656 (an inhibitor of the Fyn, Yes and Lyn kinase isoforms, and a weak inhibitor for c-Src) failed to suppress spontaneous and bpV(phen)-enhanced contractions (Figure 2). These data (as observed in Table I) support a potential role for the Lck and c-Src kinases expressed in the uterus during spontaneous and bpV(phen)-enhanced phasic uterine contractions. As observed in Figure 3 (top panel), acute stretch of uterine strips results in the rapid generation of robust phasic contractions; this response was significantly suppressed in those strips pretreated with the Lck inhibitor Damnacanthal (p<0.05, N = 12) and the c-Src inhibitor PP1 (p<0.05, N = 5) (Figure 3B). In contrast, pretreatment Piceatannol failed to suppress stretch stimulated contractile activity, thereby suggesting that Syk kinase does not play an important role during stretch mediated contractions. SU6656 also failed to suppress the stretch-stimulated contractile activity. Stretch studies performed using PAO (a PLC-γ inhibitor that has been previously reported to inhibit bpV(phen)-enhanced myometrial contractions 1) also significantly suppressed stretch-stimulated contractile activity as shown in Figure 3B (p<0.01, N = 6). In contrast, pretreatment with bpV(phen) significantly enhanced stretch-stimulated contractions (p<0.01, N = 8) (Figure 3B); a result that is consistent with its ability to maintain PLC-γ phosphorylation leading to enhanced spontaneous contractions.
Figure 1.
A. In vitro contraction study demonstrating the effect of vehicle (DMSO), Damnacanthal (DAMN, a Lck kinase inhibitor), and bpV(phen) (a protein phosphatase inhibitor) on spontaneous contractions of the proestrus/estrus rat uterine strips. The uterine strips were incubated with these agents for the time periods indicated by the horizontal bars. Contractile activity reported in grams of tension generated. B. In vitro contraction study demonstrating the effect of vehicle (DMSO), PP1 (a c-Src kinase inhibitor) and bpV(phen) on contractile activity. The uterine strips were incubated with these agents for the time periods indicated by the horizontal bars. Contractile activity reported in grams of tension generated.
Figure 2.
Bar graph demonstrating the effects of PP1 (a c-Src kinase inhibitor), Damnacanthal (DAMN, a Lck kinase inhibitor), Piceatannol (PICE, a Syk kinase inhibitor) and SU6656 (an inhibitor of the Fyn, Yes, and Lyn kinases, and a weak c-Src inhibitor) on spontaneous (open bars) and bpV(phen)-enhanced (hatched bars) contractile activity. Contractile activity reported as the percent spontaneous (pretreatment) contractile activity. Each bar = mean ± S.D. N = 4–9 experiments. (✳) p<0.05 for comparison to vehicle treated strips using the t-test or Mann-Whitney Rank Sum test.
Table I.
| Spont. CTX + Vehicle | Spont CTX + Agent | bpV(phen) + Vehicle | bpV(phen) + Agent | (N) | |
|---|---|---|---|---|---|
| Damnacanthal | 97.9 ± 10.8 | 72.7 ± 14.0* | 157.5 ± 27.0 | 88.3 ± 13.0* | (7–8) |
| PP1 | 85.8 ± 9.0 | 61.5 ± 9.1* | 214.5 ± 69.2 | 150.0 ± 51.9* | (8–9) |
| Piceatannol | 93.8 ± 11.7 | 114.2 ± 17.9 | 165.1 ± 35.2 | 184.4 ± 27.2 | (4) |
| SU6656 | 90.8 ± 4.4 | 94.5 ± 8.4 | 132.4 ± 24.6 | 171.3 ± 42.4 | (6) |
Notes:
Spont. CTX = spontaneous contractions
Mean ± standard deviation
(✳) = p<0.05 compared to vehicle treated strips
Figure 3.
A. In vitro contraction study demonstrating the effect of vehicle (DMSO) and Damnacanthal (DAMN, a Lck kinase inhibitor) on stretch-stimulated contractile activity. The uterine strips were incubated with these agents for the time periods indicated by the horizontal bars and then stretched to 4 grams of tension, as noted. Contractile activity recorded and reported in grams of tension generated. B. Bar graph demonstrating the effects of Damnacanthal (DAMN, a Lck inhibitor), PP1 (a c-Src inhibitor), Piceatannol (PICE, a Syk inhibitor), SU6656 (an inhibitor of the Fyn, Yes, and Lyn kinases, and a weak c-Src inhibitor), PAO (a PLCγ inhibitor) and bpV(phen) (a protein phosphatase inhibitor) on stretch stimulated contractions (gray bars) compared to vehicle treated strips (black bars). Contractile activity in grams tension/mg tissue/minute. Each bar = mean ± S.D. N = 5–12 experiments. (✳) p<0.05 for comparison to vehicle treated strips using the t-test or Mann-Whitney Rank Sum test.
Western blots for phosphotyrosine PLC-γ1
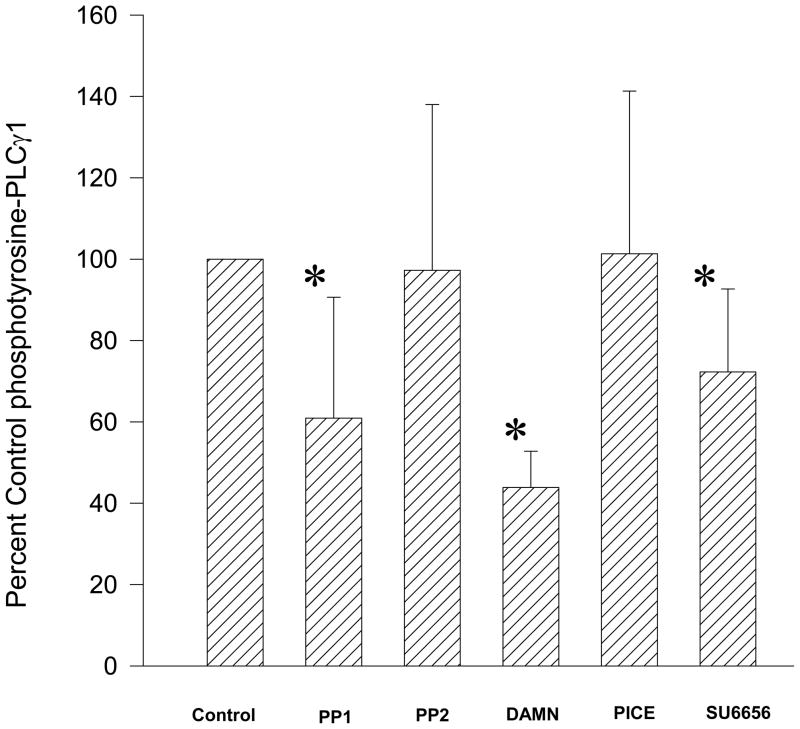
Western blots were performed using proteins isolated from uterine tissue pretreated for 20 minutes with PTK inhibitors, followed by treatment with 10 μM bpV(phen) for 10 minutes. These studies demonstrated that PP1 or Damnacanthal significantly suppressed bpV(phen)-enhanced tyrosine phosphorylation (Figure 4). Interestingly, SU6656 also produced a modest, but significant reduction of bpV(phen)-enhanced tyrosine phosphorylation of PLC-γ1. In contrast, PP2 (another c-Src kinase inhibitor) failed to suppress phosphotyrosine-PLC-γ1 levels in uterine tissue during this series of experiments (Figure 4). Studies performed using Piceatannol similarly failed to demonstrate significant suppression of bpV(phen)-enhanced tyrosine phosphorylation of PLC-γ1 in uterine tissue (Figure 4).
Figure 4.
Bar graph demonstrating the effects of PP1 and PP2 (both are c-Src kinase inhibitors), Damnacanthal (DAMN, a Lck kinase inhibitor), Piceatannol (PICE, a Syk kinase inhibitor) and SU6656 (an inhibitor of the Fyn, Yes, and Lyn kinases, and a weak c-Src inhibitor) on phosphotyrosine PLCγ1 levels. The band densities were normalized to total PLCγ1 and reported as the percent of the control (vehicle) band densities. Each bar = mean ± S.D. N = 3–13 experiments. (✳) p<0.05 for comparison to vehicle treated strips using ANOVA on Ranks.
Western blots for c-Src and Lck Expression
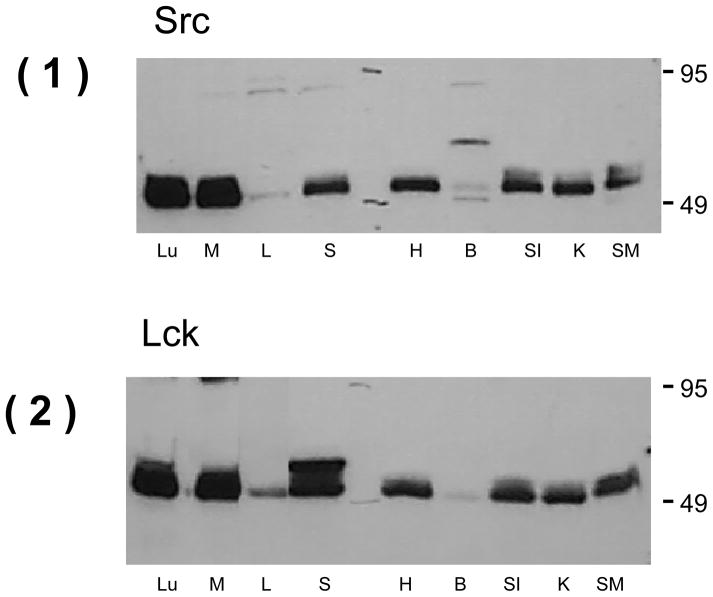
Additional Western blots were performed to qualitatively compare the expression of c-Src and Lck kinases in the uterus to multiple other rat tissues. As observed in Figure 5, these studies confirmed robust expression of c-Src kinase in uterine tissue at levels comparable to several other rat tissues including lung, spleen, heart, kidney, small intestine and skeletal muscle. These Western blots also demonstrated a similar pattern of expression of the Lck kinase in uterine tissue compared to the other rat tissues.
Figure 5.
(1) Western blots demonstrating c-Src kinase expression in several rat tissue (Lu = lung, M = myometrium (uterus), L = liver, S = spleen, H = heart, B = brain, SI = small intestine, K = kidney, and SM = skeletal muscle). (2) Another Western blot demonstrating Lck kinase expression in the spectrum of rat tissues noted above.
Comments
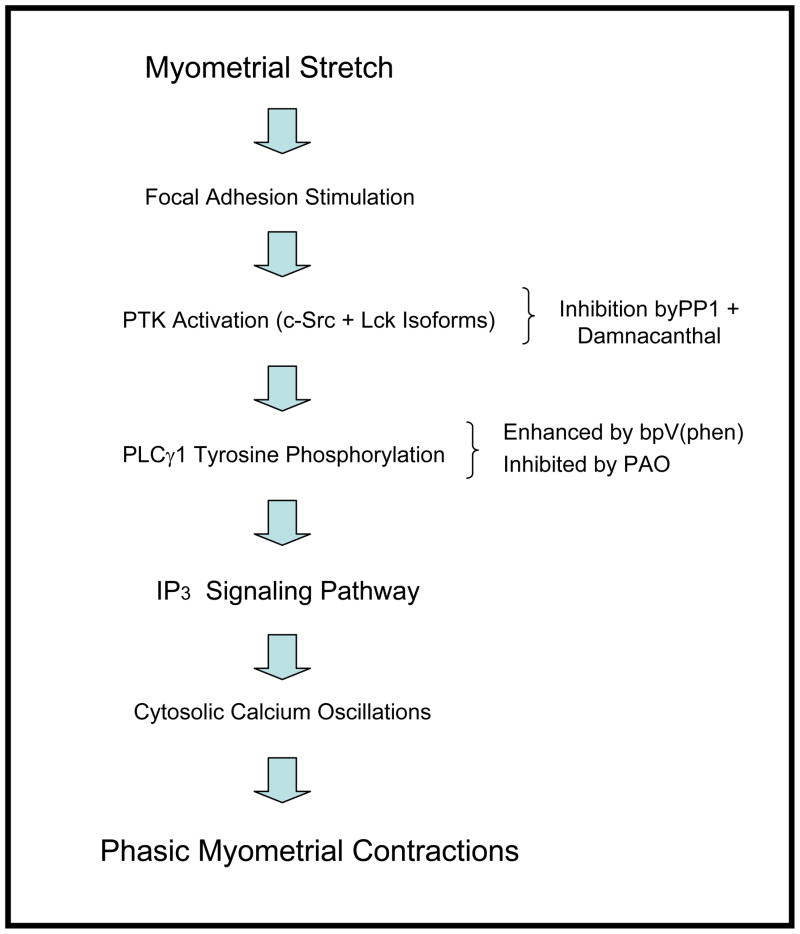
Confirming the important role for two members of the Src-family of non-receptor PTKs, these studies have demonstrated a significant decrease in spontaneous and bpV(phen)-enhanced contractions of rat uterine strips when treated with isoform specific inhibitors for the Lck and c-Src kinases. In contrast, inhibitors for other members of the Src-family and for Syk kinase did not significantly affect this contractile activity. Experiments performed to evaluate the effects of these PTK inhibitors on stretch-stimulated uterine contractions also demonstrated a significant decrease in contractile activity in response to the Lck and c-Src kinase inhibitors. The semi-quantitative tyrosine phosphorylation studies demonstrating a significant decrease in PLCγ1-phosphotyrosine levels provide support for the proposed mechanistic relationship between these PTKs and uterine contractions (see Figure 6). Although only descriptive, the Western blots demonstrating the robust expression of c-Src and Lck kinases in uterine tissue suggest a potential role for these two non-receptor PTKs during uterine function.
Figure 6.
Model demonstrating the hypothesis that stretch-stimulated phasic contractions are mediated through activation of protein tyrosine kinase (PTK) activity produced by c-Src and Lck kinases (or inhibition in response to PP1 and Damnacanthal). This results in tyrosine phosphorylation of phospholipase Cγ1 (PLCγ1) (which is enhanced bpV(phen) or inhibited by PAO) leading to activation of the inositol trisphosphate (IP3) signaling pathway and ultimately resulting in cytosolic calcium oscillations that drive phasic myometrial contractions.
The Src kinases are a family of non-receptor protein tyrosine kinases consisting of at least 9 members. The members of this family are 52–62 kD proteins comprised of six structural domains including the Src homology domains; i.e. the SH2 domain and the SH3 domain 9, 10. Src-family tyrosine kinases are found associated with cell membranes, appear to produce their downstream effects through tyrosine phosphorylation, and have been implicated in the regulation of cell proliferation, differentiation, motility and adhesion. Src-family kinases function in pathways leading to PLC activation via direct phosphorylation of PLCγ itself and/or of linker proteins associated with PLCγ 19. Tyrosine kinase activity of Src proteins is regulated by their phosphorylation status: phosphorylation of Tyrosine residues #527 or #530 results in an interaction between the carboxy terminus and the SH2 domain of Src protein, thereby suppressing its tyrosine kinase activity. Conversely, dephosphorylation of this site relieves this inhibition and Src kinase becomes active. In addition, phosphorylation of Tyrosine residues #416 or #419 of c-Src results in an increase in its tyrosine kinase activity 10.
Lymphocyte specific kinase (Lck) is a Src-family tyrosine kinase isoform first demonstrated to be expressed in T-lymphocytes and implicated in the regulation of T cell receptor expression and in T cell selection 20. Like other Src-family kinases, the Lck isoform is regulated by tyrosine phosphorylation; the specific regulatory site is Tyrosine residue #505 20. Ramos-Morales, et al. 21 have demonstrated that the SH2 domain of Lck associates with other tyrosine and serine/threonine kinases; these phenomena suggest that tyrosine-phosphorylated Lck may act as a docking point for many other proteins that contain this SH2 domain resulting in complexes important in signal transduction. Lck has been implicated in PLCγ activation. Ozdener et al. 22 have suggested that activation of PLCγ-2 by Lck is mediated by phosphorylation of tyrosines located in the SH2–SH3 linker region. Veri et al. 23 have reported that Lck is necessary for phosphorylation of PLCγ-1 in lipid rafts. The results in our report suggest that the Lck kinases are involved in the phosphorylation of PLCγ-1 in the rat uterine myocytes.
Src kinases are widely expressed in many cell types, including smooth muscle cells. Not surprisingly, expression of Src kinase isoforms varies within the family; some kinases (Src, Fyn, Yes and Yrk) are expressed ubiquitously while the expression of other kinases (Lyn, Hck, Fgr, Blk and Lck) has been reported to be more limited 24. Our Western blot studies have demonstrated the robust expression of the c-Src and Lck kinase isoforms in several rat tissues including the uterus. Hollenberg has suggested that tyrosine kinase pathways may play a role in the regulation of smooth muscle contraction 25. The c-Src kinase isoform is highly expressed in vascular smooth muscle cells 26, is present in unstimulated smooth muscle, and c-Src constitutively modulates Ca2+ channels in smooth muscle 27. Recently, Che et al. 28 have demonstrated that Src family kinases are involved in angiotensin II-stimulated arterial contractions in rat. In 1996, Palmier et al. 29 showed that pervanadate (a tyrosine phosphatase inhibitor) enhances myometrial contractions through PLCγ-1 activation; these investigators also showed that inhibition of Src kinase activity suppressed this effect, thereby providing support for the hypothesis that Src activation is responsible for PLCγ-1 related myometrial contractions. In 2002, Boulven et al. 8 demonstrated that Src kinase activity was enhanced by treatment with pervanadate. These investigators also demonstrated that tyrosine phosphorylation of PLC-γ1 along with IP3 production were suppressed in response to treatment of rat myometrium with PP1; these observations led these investigators to conclude that c-Src kinase is responsible for the PLC-γ1 signaling events in rat myometrium. In our current study, suppression of contractile activity in response to inhibition of both the c-Src and Lck kinases suggests that both of these PTKs are involved in the signaling pathway contributing to uterine contractions. In contrast, other members of the Src-family and the Syk kinase (a non-Src family PTK) do not appear to play a role in spontaneous, stretch, or bpV(phen)-enhanced myometrial contractions despite their potential ability to tyrosine phosphorylate PLC-γ1.
Previous studies have suggested that activated ion channels are directly involved in stretch-induced contractions in smooth muscle 30, and that stretch-induced contractions in uterine tissue are dependent on an influx of Ca2+ from the extracellular space 31. Modulation of voltage dependent channels leads to the influx of Ca2+ contributing to stretch induced contraction; these phenomena also appear to involve IP3, diacyglycerol and/or protein kinase C activation32. In 1994, Tanaka et al. 33 demonstrated that the IP3 concentration in coronary arteries was three fold higher than basal after undergoing stretch; that a PLC inhibitor abolished stretch-induced IP3 production; and that this PLC inhibitor partially suppressed stretch induced contraction. These investigators suggested that stretching may increase IP3 formation through a mechanism involving PLC activation 33. A report by Matsumoto et al. 34 has demonstrated that stretch induced contractions are related to Ca2+ entry through cation channels, that PLC appears to be activated by stretch, that PLC is involved in the cellular response to stretch, and that stretch induced PLC activation is linked to Ca2+ influx through cation channels. Oeckler et al.35 have reported the involvement of Src family kinases during stretch induced contraction of the bovine coronary artery. In 2002, Oldenhof et al. 36 showed that stretching rat myometrial cells induced rapid and transient activation of mitogen-activated protein kinase (MAPK), extracellular-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK); that upstream tyrosine kinase activity is necessary in the pathway leading to stretch induced MAPK activation; and that Src kinase activity may be involved in MAPK activation. In a recently published report, Li et al.37 have described stretch-induced Src kinase activity, increased focal adhesion signaling including ERK phosphorylation, and enhanced in vitro contractile activity of myometrial smooth muscle from near term pregnant rats. All of these effects of myometrial stretch were markedly suppressed by inhibition of Src kinase activity. Consistent with this report by Li et al.37, our current report has demonstrated that two Src-family PTKs (i.e. c-Src and Lck kinase) appear to be necessary and important mediators in the signaling pathway leading to stretch-induced myometrial contractions. These observations have provided support for the hypothesis that stretch-induced myometrial contractions are mediated, in part, through activation of PLCγ signaling pathway as demonstrated in Figure 6.
In summary, these studies have provided insight into the molecular mechanisms occurring during myometrial contractions of the rat uterus, and more specifically they have helped to elucidate the signaling pathway related to phosphotyrosine-PLCγ-1 mediated contractions of the uterus. In this report, we have demonstrated that the Src family kinases c-Src and Lck are expressed in rat myometrium, and appear to play a role during spontaneous, bpV(phen) (a tyrosine phosphatase inhibitor) enhanced, and stretch-induced myometrial contractions. Understanding of these intracellular signaling pathways will ultimately allow the development of more effective pharmaceutical modalities to treat abnormalities of human parturition leading to ineffective contractions (that result in the increasing need for Cesarean section deliveries) and/or inappropriate contractions (that result in preterm labor and delivery).
Acknowledgments
Funding for this research from the National Institute of Child Health and Human Development (HD28506 and HD44747)
References
- 1.Phillippe M, Sweet LM, Engle D. The role of phospholipase Cgamma1 tyrosine phosphorylation during phasic myometrial contractions. Am J Obstet Gynecol. 2007;196:179, e1–7. doi: 10.1016/j.ajog.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekiya F, Bae YS, Rhee SG. Regulation of phospholipase C isozymes: activation of phospholipase C-gamma in the absence of tyrosine-phosphorylation. Chem Phys Lipids. 1999;98:3–11. doi: 10.1016/s0009-3084(99)00013-4. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter G, Ji Q. Phospholipase C-gamma as a signal-transducing element. Exp Cell Res. 1999;253:15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- 4.Bieber E, Stratman T, Sanseverino M, Sangueza J, Phillippe M. Phosphatidylinositol-specific phospholipase C isoform expression in pregnant and nonpregnant rat myometrial tissue. Am J Obstet Gynecol. 1998;178:848–54. doi: 10.1016/s0002-9378(98)70502-2. [DOI] [PubMed] [Google Scholar]
- 5.Kim TT, Saunders T, Bieber E, Phillippe M. Protein expression of phospholipase C in pregnant and nonpregnant rat uterine tissue. Am J Obstet Gynecol. 2001;185:1191–7. doi: 10.1067/mob.2001.118143. [DOI] [PubMed] [Google Scholar]
- 6.Phaneuf S, Carrasco MP, Europe-Finner GN, Hamilton CH, Lopez Bernal A. Multiple G proteins and phospholipase C isoforms in human myometrial cells: implication for oxytocin action. J Clin Endocrinol Metab. 1996;81:2098–103. doi: 10.1210/jcem.81.6.8964834. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz U, Ishida M, Berk BC. Angiotensin II stimulates tyrosine phosphorylation of phospholipase C-gamma-associated proteins. Characterization of a c-Src-dependent 97-kD protein in vascular smooth muscle cells. Circ Res. 1997;81:550–7. doi: 10.1161/01.res.81.4.550. [DOI] [PubMed] [Google Scholar]
- 8.Boulven I, Robin P, Desmyter C, Harbon S, Leiber D. Differential involvement of Src family kinases in pervanadate-mediated responses in rat myometrial cells. Cell Signal. 2002;14:341–9. doi: 10.1016/s0898-6568(01)00269-8. [DOI] [PubMed] [Google Scholar]
- 9.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 10.Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–35. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- 11.Tokmakov AA, Sato KI, Iwasaki T, Fukami Y. Src kinase induces calcium release in Xenopus egg extracts via PLCgamma and IP3-dependent mechanism. Cell Calcium. 2002;32:11–20. doi: 10.1016/s0143-4160(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 12.Pisegna S, Zingoni A, Pirozzi G, et al. Src-dependent Syk activation controls CD69-mediated signaling and function on human NK cells. J Immunol. 2002;169:68–74. doi: 10.4049/jimmunol.169.1.68. [DOI] [PubMed] [Google Scholar]
- 13.Liao F, Shin HS, Rhee SG. In vitro tyrosine phosphorylation of PLC-gamma 1 and PLC-gamma 2 by src-family protein tyrosine kinases. Biochem Biophys Res Commun. 1993;191:1028–33. doi: 10.1006/bbrc.1993.1320. [DOI] [PubMed] [Google Scholar]
- 14.Nakao F, Kobayashi S, Mogami K, et al. Involvement of Src family protein tyrosine kinases in Ca(2+) sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ Res. 2002;91:953–60. doi: 10.1161/01.res.0000042702.04920.bf. [DOI] [PubMed] [Google Scholar]
- 15.Knock GA, Shaifta Y, Snetkov VA, et al. Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery. Cardiovasc Res. 2008;77:570–9. doi: 10.1093/cvr/cvm073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki K, Parent A, Zhang J. Mechanism of damnacanthal-induced [Ca(2+)](i) elevation in human dermal fibroblasts. Eur J Pharmacol. 2000;387:119–24. doi: 10.1016/s0014-2999(99)00811-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee HM, Won KJ, Kim J, et al. Endothelin-1 induces contraction via a Syk-mediated p38 mitogen-activated protein kinase pathway in rat aortic smooth muscle. J Pharmacol Sci. 2007;103:427–33. doi: 10.1254/jphs.fp0070039. [DOI] [PubMed] [Google Scholar]
- 18.Blake RA, Broome MA, Liu X, et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–27. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gervais FG, Chow LM, Lee JM, Branton PE, Veillette A. The SH2 domain is required for stable phosphorylation of p56lck at tyrosine 505, the negative regulatory site. Mol Cell Biol. 1993;13:7112–21. doi: 10.1128/mcb.13.11.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos-Morales F, Doute M, Fischer S. P56lck: a transducing protein that binds to SH2 containing proteins and to phosphotyrosine containing proteins. Cell Mol Biol (Noisy-le-grand) 1994;40:695–700. [PubMed] [Google Scholar]
- 22.Ozdener F, Dangelmaier C, Ashby B, Kunapuli SP, Daniel JL. Activation of phospholipase Cgamma2 by tyrosine phosphorylation. Mol Pharmacol. 2002;62:672–9. doi: 10.1124/mol.62.3.672. [DOI] [PubMed] [Google Scholar]
- 23.Veri MC, DeBell KE, Seminario MC, et al. Membrane raft-dependent regulation of phospholipase Cgamma-1 activation in T lymphocytes. Mol Cell Biol. 2001;21:6939–50. doi: 10.1128/MCB.21.20.6939-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 25.Hollenberg MD. Tyrosine kinase-mediated signal transduction pathways and the actions of polypeptide growth factors and G-protein-coupled agonists in smooth muscle. Mol Cell Biochem. 1995;149–150:77–85. doi: 10.1007/BF01076566. [DOI] [PubMed] [Google Scholar]
- 26.Oda Y, Renaux B, Bjorge J, Saifeddine M, Fujita DJ, Hollenberg MD. cSrc is a major cytosolic tyrosine kinase in vascular tissue. Can J Physiol Pharmacol. 1999;77:606–17. [PubMed] [Google Scholar]
- 27.Hu XQ, Singh N, Mukhopadhyay D, Akbarali HI. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J Biol Chem. 1998;273:5337–42. doi: 10.1074/jbc.273.9.5337. [DOI] [PubMed] [Google Scholar]
- 28.Che Q, Carmines PK. Src family kinase involvement in rat preglomerular microvascular contractile and [Ca2+]i responses to ANG II. Am J Physiol Renal Physiol. 2005;288:F658–64. doi: 10.1152/ajprenal.00392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmier B, Leiber D, Harbon S. Pervanadate mediated an increased generation of inositol phosphates and tension in rat myometrium. Activation and phosphorylation of phospholipase C-gamma 1. Biol Reprod. 1996;54:1383–9. doi: 10.1095/biolreprod54.6.1383. [DOI] [PubMed] [Google Scholar]
- 30.Kirber MT, Walsh JV, Jr, Singer JJ. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflugers Arch. 1988;412:339–45. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- 31.Kasai Y, Tsutsumi O, Taketani Y, Endo M, Iino M. Stretch-induced enhancement of contractions in uterine smooth muscle of rats. J Physiol. 1995;486 ( Pt 2):373–84. doi: 10.1113/jphysiol.1995.sp020819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka Y, Hata S, Ishiro H, Ishii K, Nakayama K. Stretching releases Ca2+ from intracellular storage sites in canine cerebral arteries. Can J Physiol Pharmacol. 1994;72:19–24. doi: 10.1139/y94-004. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka Y, Hata S, Ishiro H, Ishii K, Nakayama K. Quick stretch increases the production of inositol 1,4,5-trisphosphate (IP3) in porcine coronary artery. Life Sci. 1994;55:227–35. doi: 10.1016/0024-3205(94)00884-1. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto H, Baron CB, Coburn RF. Smooth muscle stretch-activated phospholipase C activity. Am J Physiol. 1995;268:C458–65. doi: 10.1152/ajpcell.1995.268.2.C458. [DOI] [PubMed] [Google Scholar]
- 35.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res. 2003;92:23–31. doi: 10.1161/01.res.0000051860.84509.ce. [DOI] [PubMed] [Google Scholar]
- 36.Oldenhof AD, Shynlova OP, Liu M, Langille BL, Lye SJ. Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol. 2002;283:C1530–9. doi: 10.1152/ajpcell.00607.2001. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Gallant C, Malek S, Morgan KG. Focal adhesion signaling is required for myometrial ERK activation and contractile phenotype switch before labor. J Cell Biochem. 2007;100:129–40. doi: 10.1002/jcb.21033. [DOI] [PubMed] [Google Scholar]