Abstract
An appropriate balance between proinflammatory (Th17 and Th1) and anti-inflammatory (regulatory T cells [Tregs] and Th2) subsets of T cells is critical to maintain homeostasis and avoid inflammatory disease. Type 2 diabetes (T2D) is a chronic inflammatory disease promoted by changes in immune cell function. Recent work indicates T cells are important mediators of inflammation in a mouse model of T2D. These studies identified an elevation in the Th17 and Th1 subsets with a decrease in the Treg subset, which culminates in inflammation and insulin resistance. Based on these data, we tested the hypothesis that T cells in T2D patients are skewed toward proinflammatory subsets. Our data show that blood from T2D patients has increased circulating Th17 cells and elevated activation of Th17 signature genes. Importantly, T cells required culture with monocytes to maintain Th17 signatures, and fresh ex vivo T cells from T2D patients appeared to be poised for IL-17 production. T cells from T2D patients also have increased production of IFN-γ, but produce healthy levels of IL-4. In contrast, T2D patients had decreased percentages of CD4+ Tregs. These data indicate that T cells in T2D patients are naturally skewed toward proinflammatory subsets that likely promote chronic inflammation in T2D through elevated cytokine production. Potential therapies targeted toward resetting this balance need to be approached with caution due to the reciprocal relationship between Th17 cells and Tregs. Understanding the unique aspects of T2D T cells is essential to predict outcomes of such treatments.
Tcells have been implicated in the pathogenesis of multiple inflammatory diseases, and many autoimmune and inflammatory disorders are driven by specific T cell subsets (1–5). Th17 cells are a major T cell subset implicated in the pathogenesis of multiple sclerosis, rheumatoid arthritis, and psoriasis, with elevated numbers of Th17 cells in sera and diseased tissues that likely explain elevated IL-17 levels (6). IL-17 promotes inflammation through a widely expressed family of IL-17 receptors, many of which trigger downstream NF-κB, thus cytokine production by monocytes, fibroblast, stromal, epithelial, and endothelial cells (7–9). IL-17 also induces mobilization, recruitment, and activation of granulocytes via induction of G-CSF (10). Similarly, IFN-γ–producing cells have been implicated in inflammatory bowel disease, lupus nephritis, multiple sclerosis, and a collagen-induced arthritis model of rheumatoid arthritis, all of which are characterized by the presence of IFN-γ–activated macrophages at the sites of inflammation (11). Overall, links between IL-17/IFN-γ downstream functions and chronic inflammatory conditions indicate an important role for T cells in the induction and exacerbation of multiple diseases.
Recent findings have unequivocally identified type 2 diabetes (T2D) as yet another example of a chronic inflammatory disease with changes in immune cell function (12–16). Monocytes from T2D patients constitutively and inducibly secrete elevated levels of IL-6, IL-8, TNF-α, and IL-1β (11, 15–18). Furthermore, B cells from T2D patients secrete elevated levels of IL-8 and decreased levels of the anti-inflammatory cytokine IL-10 (19). The resulting proinflammatory cytokine balance has been directly linked to T2D by in vivo studies that show inhibition of key inflammatory cytokines protects rodents from insulin resistance (20–25). Overall, these studies support the conclusion that elevated cytokine production specifically by immune cells both precedes and maintains insulin resistance in whole animals (21–23), thus adding mechanistic detail to the current paradigm of T2D as an inflammatory disease.
Several studies specifically demonstrate a role for T cell subset imbalance in mouse models of T2D inflammation. Regulatory T cells (Tregs) are naturally depleted in adipose tissue in an insulin-resistant model of obesity compared with adipose tissue from lean mice. Furthermore, ex vivo-expanded Tregs protect against inflammation, thus regulating insulin resistance (26). These data indicate Tregs inhibit T2D. In contrast, an elevated number of IFN-γ–producing cells occur in adipose tissue from obese mice and promote a loss in glucose homeostasis. Th2 and Tregs can reverse the Th1-mediated pathology (27), clearly indicating the importance of T cell compartment balance in the regulation of adipose tissue homeostasis. A companion study showed an IL-6-dependent increase in the number of IL-17-producing cells in spleen of obese mice, suggesting Th17s may also contribute to T2D inflammation and insulin resistance (28). However, similar studies have not been reported in T2D patients despite the compelling demonstrations that T cell subset imbalance promotes insulin resistance in animal models.
T2D patients have elevated serum levels of IL-6, IL-1β, and TGF-β, three cytokines known to induce Th17 differentiation (29–33). These data, in combination with the mouse studies outlined above, raise the possibility that elevated Th17-associated cytokines in T2D patients promote Th17 skewing and/or IL-17 secretion while potentially inhibiting Treg differentiation. Studies in this paper identify an increased percentage of circulating memory Th17 cells in T2D versus nondiabetic (ND) donors, and stimulation of PBMCs with T cell mitogens results in elevated IL-17 and IFN-γ secretion. Importantly, monocytes are required to maintain the proinflammatory T cell phenotype in T2D samples. In contrast, T2D patients have decreased percentages of circulating Tregs compared with ND donors, thus recapitulating findings in T2D mouse models. We conclude that T cells in T2D patients are skewed toward a proinflammatory phenotype that requires monocytes for maintenance and likely promotes chronic inflammation in T2D through elevated IFN-γ and IL-17 production.
Materials and Methods
Cells
Human samples were obtained following informed consent under a Boston University Institutional Review Board-approved protocol (Boston, MA). T2D patients were recruited from the Center for Endocrinology, Diabetes and Nutrition at the Boston University Medical Center (Boston, MA). Additional T2D and ND donors were recruited from the Clinical Research Center and the Boston University School of Medicine community. This study was cross-sectional and blinded. Characteristics of T2D and systemically healthy (ND) donors are shown in Tables I, II, and III. All donors were nonsmokers. Thirty to fifty milliliters peripheral blood was collected into heparinized tubes by venous puncture, and PBMCs or CD3+ T cells were purified by histopaque 1077 and negative selection with CD3 cell-excluding magnetic beads (Pan T cell kit, Miltenyi Biotec) as applicable. Only T cell preparations that were >97% pure as reanalyzed by flow cytometry for CD3 expression were used in all analyses requiring purified T cells. For fresh ex vivo ChIP assays, CD3+ T cells were analyzed after a 1 h rest on ice following negative isolation. CD14 positive selection was used to deplete monocytes (CD14−) from PBMCs for add-back experiments. Alternatively, T cells or monocytes were purified by negative selection and were cocultured at a 1:1 ratio (designated M+T) for 40 h in the presence or absence of stimuli.
Table I.
Description of all blood donors
| ND Median (Range) | T2D Median (Range) | |
|---|---|---|
| Age (y) | 47 (27–61) | 52 (31–66) |
| A1c (%) | NA | 7.7 (4.8–14.9) |
| BMI (kg/m2) | 26.6 (20.3–35) | 32 (17–65) |
| Glucose (ml/dl) | NA | 156 (79–474) |
| N (26 Total) | N (31 Total) | |
| Females | 12 | 17 |
| Males | 12 | 14 |
| Unknown sex | 2 | 0 |
| Race | ||
| White | 18 | 9 |
| African American | 3 | 13 |
| Native American | 0 | 1 |
| Hispanic | 1 | 5 |
| Asian | 1 | 0 |
| Unknown race | 3 | 3 |
A1c, hemoglobin A1c.
Table II.
Description of blood donors for CCR4/CCR6 analysis
| ND Median (Range)a | T2D Median (Range) | |
|---|---|---|
| Age (y) | 40 (27–61) | 51.5 (39–65) |
| A1c (%) | NA | 7.75 (6–10.5) |
| BMI (kg/m2) | 21 (21–35) | 30.4 (17–40) |
| Glucose (mg/dl) | NA | 153 (98–432) |
| N (10 Total) | N (11 Total) | |
| Females | 5 | 5 |
| Males | 4 | 6 |
| Unknown sex | 1 | 0 |
| Race | ||
| White | 5 | 5 |
| African American | 2 | 5 |
| Native American | 0 | 0 |
| Hispanic | 1 | 1 |
| Asian | 1 | 0 |
| Unknown race | 1 | 0 |
Age, sex, race, and BMI unknown for one ND donor.
A1c, hemoglobin A1c.
Table III.
Description of blood donors for IL-17, IFN-γ, and IL-4 secretion
| ND Median (Range) | T2D Median (Range) | |
|---|---|---|
| Age (y) | 47 (30–57) | 51.5 (31–66) |
| A1c (%) | NA | 7.7 (7–14.9) |
| BMI (kg/m2) | 25.5 (21–29) | 33 (23–65) |
| Glucose (mg/dl) | NA | 153 (79–285) |
| N (16 Total) | N (18 Total) | |
| Females | 10 | 12 |
| Males | 6 | 6 |
| Unknown sex | 0 | 0 |
| Race | ||
| White | 12 | 5 |
| African American | 1 | 8 |
| Native American | 0 | 1 |
| Hispanic | 1 | 4 |
| Asian | 1 | 0 |
| Unknown Race | 1 | 0 |
A1c, hemoglobin A1c.
Flow cytometry
A total of 100 µl whole blood was incubated with fluorescently labeled Abs for 30 min at 4˚C. RBCs were lysed with 2 ml 1× FACS Lysing Solution (BD Pharmingen) for 30 min at room temperature. Stained cells were washed with 0.2% BSA/PBS and resuspended in PBS for analysis of CD3/CCR4/CCR6. The following Abs were purchased from eBioscience: FITC-labeled anti-CD3, PE-labeled anti-CCR4, PE-Cy5–labeled anti-CCR6, FITC-labeled anti–IL-17A, PE-labeled IFN-γ; and the human Foxp3 staining kit: PE-labeled Foxp3, allophycocyanin-labeled CD25, and FITC-labeled CD4. Alternatively, PBMCs were incubated with plate-bound anti-CD3 (2 µg/ml anti-CD3 in PBS incubated in plate for 1 h at 37˚C) and soluble anti-CD28 (2 µg/ml) or PHA (3 µg/ml) for 40 h with 3 µg/ml brefeldin A (eBioscience) added during the last 5 h of culture. Cells were stained for intracellular cytokine expression with PE-Cy5–labeled anti-CD3 then treated with 1× permeabilization buffer (eBioscience) and stained with FITC-labeled anti–IL-17A or PE-labeled IFN-γ. Freshly isolated PBMCs were used for Foxp3 staining following the eBioscience protocol. Cells were analyzed on an FACSCalibur or an LSRII (BD Biosciences) run by FACSDiva and FlowJo software was used for analyses (Tree Star).
Biochemistry
Cytokines were quantified in 40 h supernatants by ELISA or cells were analyzed by chromatin immunoprecipitation (ChIP) either fresh ex vivo or following a 40-h stimulation. ChIPs have been described in detail (34). For IL-17 secretion analyses, supernatants were alternatively collected from cultures 4 d following stimulation. Precipitated DNA was quantitatively amplified with primers specific to IL-17A: 5′-GTGGTTGACCCGGAGTTAC-TG-3′ and 5′-CCTTCGGGAAATGGAATAAAAA-3′; or retinoic acid-related orphan receptor C (RORC): 5′-CCCAGCACACAGTAAGTGAT-TAATAAA-3′ and 5′-TGGAGCCTTGAGGAGAAACAG-3′. For mono-cyte mRNA analyses, negatively isolated monocytes were rested for 1 h at 37˚C followed with LPS (100 ng/ml) stimulation for 0.5, 1, 2, 3, 4, 5, and 6 h. Cells were lysed with Buffer RLT (Qiagen) containing 1% 2-ME. RNA isolation was performed with the RNAeasy kit (Qiagen). RNA was DNAase treated for 20 min at 37˚C, and the enzyme was inactivated for 10 min at 65˚C. Reverse transcription was performed using 0.5 U AMV RT (Promega) for 1 h at 42˚C. No RT controls were universally blank. cDNA was quantitatively amplified with primers specific to IL-1β (34) or TNF-α: 5′-GGCCTACAGCTTTGATCCCTG-3′ and 5′-AAAGGCTCCCTGGTC-TCCAG-3′. Cytokine levels in cell-culture supernatants were measured by ELISA or multiplex assays (Invitrogen) and analyzed with the Softmax program (Molecular Devices) or Bioplex Manager 5 (BioRad), respectively.
Statistical analysis
The Mann-Whitney U test or an unpaired t test was used for comparisons of group means as indicated in the figure legends. A Pearson test was used to identify correlations for all applicable panels. A linear regression was used to identify the relationship indicated in Fig. 7E. A two-way ANOVA was used to identify differences shown in Fig. 5D. A p value <0.05 established statistical significance. Analyses were performed on Prism (GraphPad).
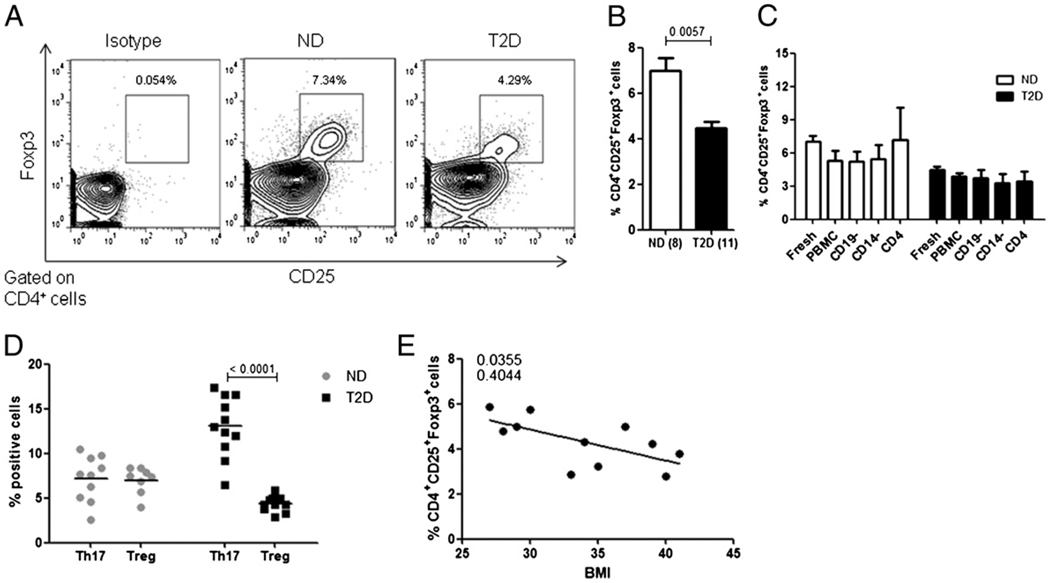
FIGURE 7. The percentage of anti-inflammatory Tregs is decreased in T2D patients.
A, One representative of eight ND samples or eleven T2D samples stained for CD4+CD25+Foxp3+ cells in fresh ex vivo PBMCs as indicated. Isotype control is from the T2D sample shown rightmost. B, Summary of percentage of CD4+CD25+Foxp3+ T cells in ND (white bars) or T2D (black bars) donors. The p value shown above bars was calculated by a Mann–Whitney U test. C, Summary of percentage of CD4+CD25+Foxp3+ T cells in fresh ex vivo PBMCs or from the following samples after 40 h (unstimulated) in culture: PBMCs, B cell-depleted PBMCs (CD19−), monocyte-depleted PBMCs (CD14−), or CD4+ (negatively selected) T cells as indicated. For B and C, bars show average and SEM and n = 5–11. Analysis of CD4+Foxp3+ T cells gave similar results (not shown). D, Comparison of percentage of CD3+CCR4+CCR6+ T cells (Th17s) and CD4+CD25+Foxp3+ (Tregs) in ND (gray dots) and T2D (black squares) PBMCs. Each point represents one donor. The p value shown was calculated by a Mann–Whitney U test. E, Linear regression between BMI (x-axis) and percentage of CD4+CD25+Foxp3+ T cells (y-axis) in T2D patients. The p value is indicated on the graph, and the r2 value is shown below the p value.
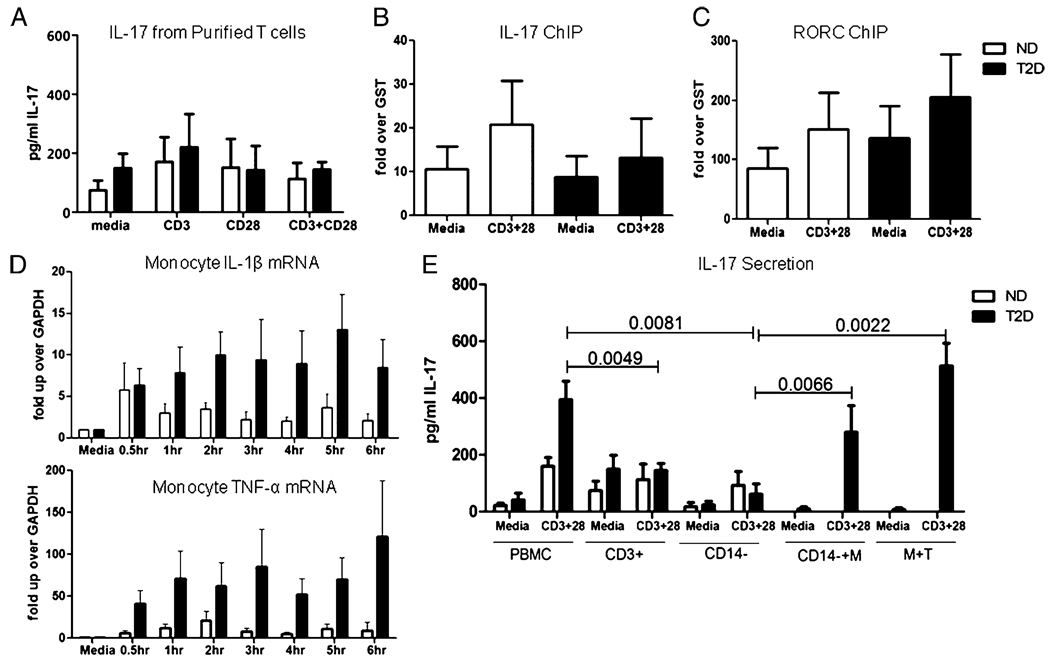
FIGURE 5. T cells require monocyte coculture to maintain Th17 phenotype.
A, Purified T cells (>97% pure) were incubated with the indicated stimuli for 40 h, then secreted IL-17 protein was measured by ELISA. n = 11 to 12 for each donor cohort. ChIP measuring association of acetylated histone H3 to the IL-17 (B) or RORC (C) promoter in purified T cells from ND (white bars) or T2D (black bars) donors. Cells were unstimulated or stimulated as indicated for 40 h. n = 4 for each donor cohort. D, Purified monocytes from ND (white bars) or T2D (black bars) donors were stimulated with Escherichia coli LPS for indicated time and analyzed by RT-PCR for expression of IL-1β (top panel) or TNF-α (bottom panel) mRNA. The p value indicating a significant difference between ND and T2D cohorts (p < 0.0001) was calculated by two-way ANOVA. n = 9 for ND donors; n = 8 for T2D patients. E, PBMCs, purified T cells, or CD14-depleted PBMCs from ND (white bars) or T2D (black bars) donors were incubated for 40 h in the presence or absence of anti-CD3 + anti-CD28 as indicated. Alternatively, CD14-depleted PBMCs + CD14+ monocytes (CD14−+M) or purified T cells and monocytes (M+T) from T2D donors were cocultured for 40 h with or without anti-CD3 plus anti-CD28. Supernatants were analyzed for IL-17. Bars show average and SEM. p values were calculated by Mann–Whitney U test and are shown above bars that are significantly different (p < 0.05). All other comparisons were insignificantly different (p > 0.05).
Results
Th17 cells are elevated in T2D patients
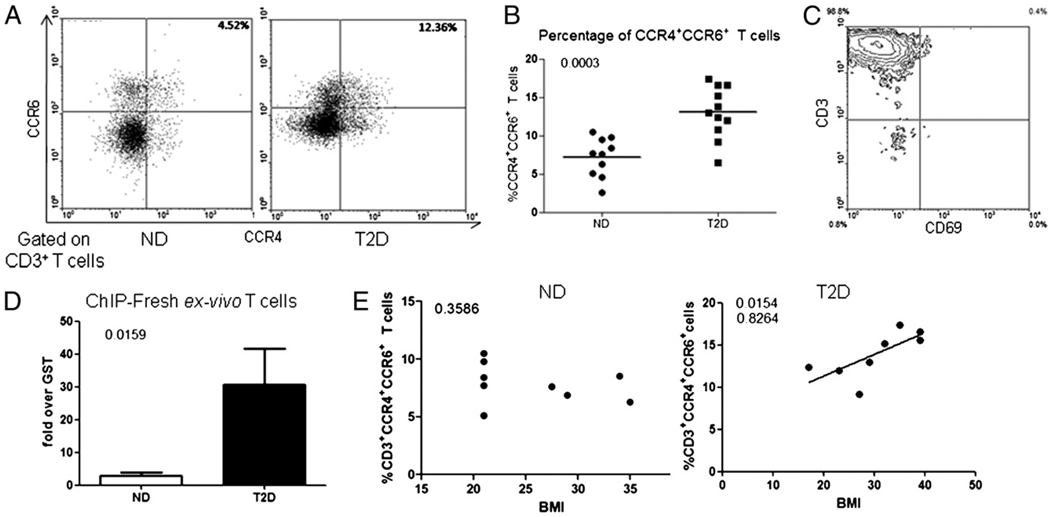
T2D patients have elevated serum levels of cytokines implicated in human Th17 differentiation (30–33). To test the possibility that these cytokines promote inflammation in T2D patients through increased Th17 differentiation, we took advantage of the demonstration that CCR4/CCR6 double-positive staining identifies human memory Th17 cells (35). We quantified the percentage of CD3+ CCR4+ CCR6+ T cells in whole blood of T2D and ND donors as shown in Fig. 1A. T cells were identified first by gating of the lymphocyte population based on forward and side scatter (not shown) followed by identification of the CD3+ population (not shown). On average, an increased percentage of T cells in T2D patients coexpress CCR4 and CCR6 compared with percentages measured in ND donors (13.03 ± 3.4% or 7.19 ± 2.5%, respectively; p < 0.01; Fig. 1A, right panel, 1B). To independently verify these findings, we purified T cells from T2D or ND donors (Fig. 1C) and measured molecular signatures of IL-17 gene activation in fresh ex vivo-purified T cells. Fig. 1D shows that only T cells from T2D patients package the IL-17 promoter into active chromatin structures as evidenced by significant association of acetylated histone H3 measured by ChIP.
FIGURE 1. T2D patients have elevated percentages of circulating Th17 cells.
A, One representative flow plot of 10 ND and 11 T2D whole blood samples stained for CD3+ CCR4+ CCR6+ cells. Blood donors for these studies are described in Table II. T cells were identified based on forward and side scatter and CD3 staining (not shown) and plotted to show CCR4 (x-axis) and CCR6 (y-axis) staining. Note that slight elevation of CCR6 expression in lower left quadrant was inconsistent. Percent double-positive cells are indicated in the top right quadrant. B, Summary of flow cytometric analyses of percentage CCR4/CCR6 double-positive T cells in whole blood. Each dot represents one individual. n = 10 for ND or 11 for T2D samples. C, Representative flow plot of T cell purity for fresh ex vivo T cells used in purified T cell analyses. T cells in this sample are 98.8% pure based on CD3 expression (top left quadrant). CD69 is shown on the x-axis, and CD3 is displayed on y-axis, the former to indicate low T cell activation levels. D, ChIP measuring constitutive association of acetylated histone H3 with the IL-17 promoter in fresh ex vivo T cells from ND (white bar) or T2D (black bar) donors. Bars show average signal (± SEM) compared with control immunoprecipitations with anti-GST Ab. p values calculated by Mann–Whitney U test highlight the significant difference between ND and T2D samples. n = 4 to 5 independent determinations. E, Relationship between BMI (x-axis) and percentage of CD3+ CCR4+ CCR6+ cells (y-axis) in ND (left panel) or T2D (right panel) cohorts. Correlation was calculated by a Pearson’s test. Pearson’s r value is shown below the significant (<0.05) p value.
Obesity and T2D are tightly linked; therefore, either condition could explain the elevated percentage of CCR4+CCR6+ T cells in our generally obese T2D cohort. To test whether obesity plays a major role in determining levels of circulating Th17 cells, we calculated the relationship between body mass index (BMI), a standard measure of obesity, and percentage of CD3+CCR4+CCR6+ T cells for each donor. We found a positive correlation between BMI and percentage of CD3+CCR4+CCR6+ T cells only in the T2D cohort (Fig. 1E, right panel). This analysis indicates that obesity likely plays a minor role in determining levels of circulating Th17 cells. Overall, we conclude that the chronic inflammatory milieu of T2D patients associates with elevated percentages of circulating Th17 cells.
PBMCs from T2D patients secrete elevated levels of IL-17
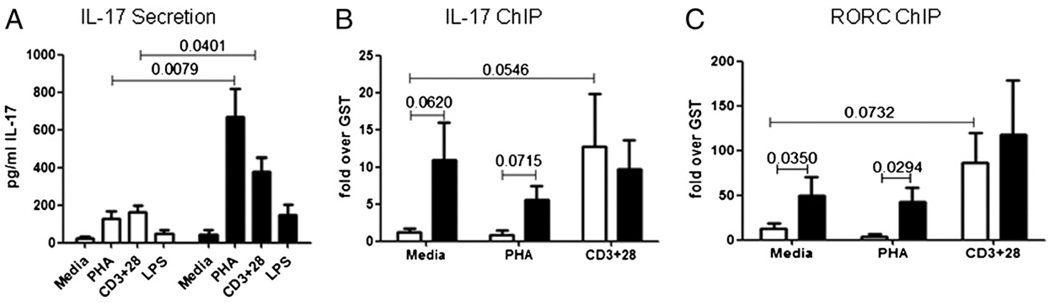
Based on the elevated percentages of CCR4+/6+ T cells and the activated IL-17 gene structure shown in Fig. 1, we predicted that T cells from T2D patients would secrete increased levels of the proinflammatory cytokine IL-17. We first tested the possibility that T cells in the context of PBMCs from T2D patients produce elevated levels of IL-17 constitutively and/or in response to stimuli. Constitutive IL-17 production by PBMCs from T2D donors was modestly, but statistically insignificantly, elevated compared with PBMCs from ND donors (Fig. 2A, Media). However, PHA stimulation resulted in significantly elevated IL-17 production by PBMCs from T2D patients compared with ND donor PBMCs (3.6-fold increase; p = 0.0079) or to unstimulated T2D PBMCs (15.5-fold increase; p = 0.0002). IL-17 production by stimulated PBMCs from T2D versus ND donors remained significantly elevated after 4 d in culture (data not shown). Anti-CD3 + anti-CD28–stimulated PBMCs from T2D patients also secreted elevated levels of IL-17 compared with stimulated PBMCs from ND donors (2.5-fold increase; p = 0.04) or to unstimulated T2D PBMCs (8.8-fold increase; p = 0.0008). Furthermore, the levels of IL-17 production in PHA-stimulated PBMCs is higher than in anti-CD3 + anti-CD28–stim-ulated PBMCs (1.75-fold) in the T2D patients, but this increase did not achieve statistical significance. Finally, LPS stimulation increased IL-17 secretion only in PBMCs from T2D patients (p = 0.01 compared with media); however, the increase was quantitatively modest. Overall, PBMCs from T2D patients secrete elevated levels of IL-17 in response to T cell stimuli compared with PBMCs from ND donors.
FIGURE 2. Elevated production of IL-17 by PBMCs from T2D patients.
A, PBMCs from ND (white bars) or T2D (black bars) donors were incubated for 40 h in media alone or with PHA, anti-CD3 plus anti-CD28, or LPS as indicated. Samples were analyzed for IL-17 by ELISA. Bars show average and SEM. p values were calculated by the Mann–Whitney U test and are shown above bars that are significantly different between ND and T2D donors (p < 0.05). Significant differences (not shown) within the ND cohort include: media versus PHA, p = 0.0116; and media versus CD3+28, p = 0.0019. Significant differences (not shown) within the T2D cohort include: media versus PHA, p = 0.0002; media versus CD3+28, p = 0.0008; and media versus LPS, p = 0.01. All other comparisons were statistically insignificant (p > 0.05). n = 8–11 for ND donors and n = 7–13 for T2D patients described in Table III. ChIP measuring association of acetylated histone H3 to the IL-17 (B) or RORC (C) promoter in PBMCs from ND (white bars) and T2D (black bars) donors. Cells were unstimulated or stimulated as indicated for 40 h. Bars show average signal compared with control immunoprecipitations with GST Ab ± SEM. n = 3–6. The p values that were significant (p < 0.05) or approached significance (p < 0.08) as measured by Mann–Whitney U test are indicated on graphs.
Th17 cells are characterized by activation of multiple genes, including the inflammatory cytokine IL-17 and the Th17 master regulator RORC (36–39). To independently verify elevated IL-17 production in Fig. 2A, we compared activation signatures on key Th17 genes in PBMCs from T2D and ND donors. Only PBMCs from T2D patients constitutively package the IL-17 (Fig. 2B) and RORC (Fig. 2C) promoters into active structures, as evidenced by increased association of acetylated histone H3. These findings support the likelihood that T cells from T2D patients are constitutively activated and therefore poised for IL-17 production, as further evidenced by analysis of fresh ex vivo T cells (Fig. 1D). In contrast, PBMCs from ND donors have an activated chromatin signature at the IL-17 and RORC genes only if they are stimulated with anti-CD3 + anti-CD28 (Fig. 2B, 2C, white bars). These data indicate activation of IL-17 signature genes requires a TCR-specific signal in T cells from ND donors. Despite quantitatively similar association of acetylated H3 with the IL-17 promoter in anti-CD3 + anti-CD28–stimulated ND and T2D cells, IL-17 protein production remains significantly lower in the ND donor samples (Fig. 2A). This comparison suggests multiple layers of regulation for IL-17 production in ND donors are lost in T2D patients. We conclude that T cells from T2D patients are poised to express IL-17, consistent with skewing of T cells to the proin-flammatory Th17 subset.
Molecular signatures of Th17 cells and elevated IL-17 production are specifically attributable to T cells
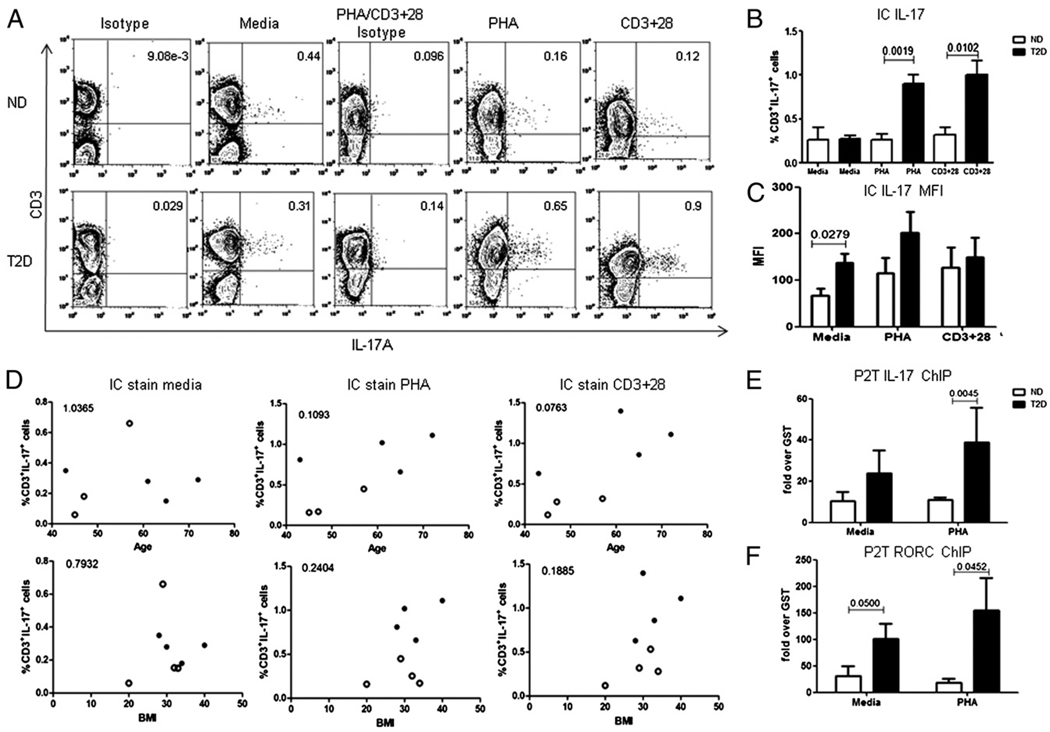
New evidence demonstrates that non-T cells can also secrete IL-17, albeit at much lower concentrations than Th17 cells (40). These data highlight the importance of specifically showing that T cells are the source of elevated IL-17 in T2D. Intracellular staining confirmed significant IL-17 protein was limited to the T cell population, with a statistically significant elevation in samples from T2D donors stimulated with PHA or anti-CD3 + anti-CD28 (Fig. 3A, 3B). Note that only T cells from T2D patients significantly elevated IL-17 production when stimulated with PHA and CD3+28 (Fig. 3B). Evaluation of mean fluorescence intensity (MFI) for IL-17 in CD3+ T cells indicated that the estimated number of IL-17 molecules per cell does not increase with stimulation, although each cell constitutively hyperproduced IL-17 (Fig. 3C). The percentage of IL-17+ T cells did not correlate with age or BMI in analyses of all samples used for these measures (Fig. 3D), although the relationship between the percentage of IL-17+ T cells and age trended toward significance in anti-CD + anti-CD28–stimulated samples (Fig. 3D, top right panel; p = 0.076). Regardless, the lack of strong correlation indicates that differences in age or BMI between ND and T2D donors (see below) do not play dominant roles in determining the percentage of circulating IL-17+ T cells.
FIGURE 3. Molecular signatures of Th17 cells and elevated IL-17 production are T cell specific.
A, One representative of eight samples stained for intracellular IL-17 in CD3+ T cells in PBMCs from ND (upper panels; n = 4) or T2D (lower panels; n = 4) donors. PBMCs were stimulated as indicated for 40 h. Brefeldin, PMA, and ionomycin were added 5 h before harvest. B, Summary of intracellular cytokine staining for IL-17 in CD3+ T cells from ND (white bars) and T2D (black bars) donors as shown in A (percent positive, top panels) or C (MFI). p values that were significant (p < 0.05) as measured by Mann–Whitney U test are shown on graph. n = 4 for each bar. Due to differences in isotype control Ab staining between media and PHA or CD3+28-stimulated samples, isotype staining in leftmost columns was used as a gating control for media-treated samples and PHA/CD3+28 isotype (middle columns) was used for PHA or CD3+28-stimulated samples (rightmost columns). In B, all comparisons between media (unstimulated) and stimulated values were statistically significant (p < 0.05) for the T2D cohort (media versus PHA, p = 0.0286; media versus CD3+28, p = 0.028). D, Intracellular IL-17 levels fail to correlate with age (top panels) or BMI (bottom panels) in all ND (○) and T2D (●) donors. p > 0.05 for all analyses as calculated by the Pearson test. Quantitation of active IL-17 (E) or RORC (F) promoters as measured by ChIP for DNA associated with hyperacetylated histone H3. P2T denotes PBMCs cultured in the presence or absence of indicated stimulus for 40 h, after which T cells were purified by negative selection (>98% pure) and analyzed. The non-T fraction had extremely low signals (fold GST control Ab <1: not shown). Data represent P2Ts from ND (white bars) and T2D (black bars) donors. Bars show average and SEM. n = 3–6. The p values above bars were calculated by Mann–Whitney U test.
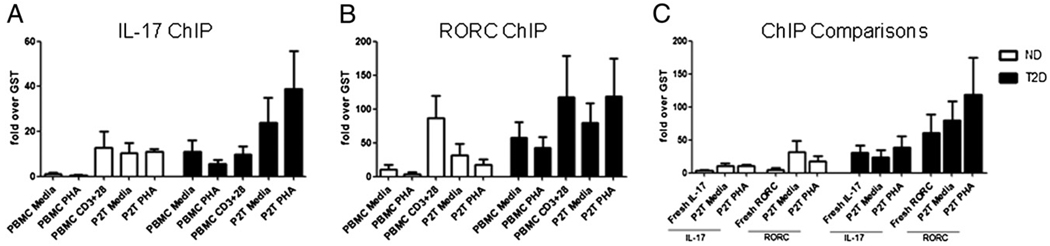
To verify that the molecular signatures of poised and activated Th17 cells implied in Fig. 2B and 2C are T cell specific, we cultured PBMCs from T2D patients and ND donors for 40 h and then isolated purified T cells (denoted P2T) and evaluated Th17 signature genes by ChIP. T cells from T2D patients have elevated association of acetylated histone H3 to both the IL-17 (Fig. 3E) and the RORC (Fig. 3F) promoters, although the former failed to reach significance in unstimulated samples (Fig. 3E, left). As expected, association signals in the P2Ts are quantitatively greater compared with the PBMCs, probably due to dilution of the T cell signal in the PBMC cultures (Fig. 4A, 4B). Additionally, comparison of molecular signatures in fresh ex vivo T cells and P2T T cells confirms that the in vivo Th17 signatures are stable in culture when T cells are maintained in the context of PBMCs (Fig. 4C, compare fresh ex vivo to P2T values, no statistically significant changes; p > 0.05). These data also indicate that P2T T cells recapitulate fresh ex vivo T cell attributes.
FIGURE 4. Molecular signatures of T cell activation are maintained in cultured PBMCs from ND and T2D patients.
Comparisons of ChIP analyses showing association of acetylated histone H3 with the IL-17 (A) or RORC (B) promoters in stimulated or unstimulated PBMCs and P2Ts (see text) as indicated. Signals in the non-T cells from the P2T assays were below the level of detection (not shown). C, Comparisons of ChIP analyses of association of acetylated histone H3 to the IL-17 or RORC promoters of fresh ex vivo T cells and unstimulated or stimulated P2Ts from ND (white bars) and T2D (black bars) donors as indicated. Bars show average and SEM. n = 3–6 for each donor cohort. All comparisons were insignificantly different (p > 0.05) by Mann–Whitney U test, indicating no changes in molecular signatures postculture for 40 h.
T cells require monocyte coculture to maintain skewed Th17 phenotype
To more rigorously verify that IL-17 production in the PBMCs largely originated from T cells, we purified T cells from ND and T2D donors, stimulated them with anti-CD3 + anti-CD28 and measured IL-17 secretion. Unexpectedly, purified T cells from T2D and ND donors secreted similar amounts of IL-17 (Fig. 5A). Furthermore, and in contrast to PBMCs or P2T samples, molecular signatures of IL-17 and RORC promoter activation were similar in purified T cells from T2D and ND donors (Fig. 5B, 5C). These data unveil a requirement for the inflammatory milieu or, more likely, non-T cells in the elevated production of IL-17 in T2D.
Previous studies demonstrated that monocytes are important for T cell skewing in type 1 diabetes (T1D) (41), and a similar role for monocytes in Th17 function in T2D patients is suggested by multiple studies (42, 43). Monocytes from T2D patients constitutively and inducibly produce elevated IL-1β and IL-6, as compared with monocytes from ND donors (17, 18, 34). These proinflammatory cytokines are known to induce IL-17 production by human central memory T cells (32, 33). We confirmed that monocytes from our T2D cohort transcribed elevated levels of IL-1β and TNF-α mRNA, indicating both proinflammatory and pro-Th17 functions of these cells (Fig. 5D; p < 0.001 between ND and T2D cohorts). Taken together, these studies suggest that monocytes indirectly promote elevated IL-17 production by T cells in T2D patients. To test this possibility, we depleted CD14+ monocytes from PBMCs (CD14−), then stimulated the cultures with anti-CD3 + anti-CD28 and measured IL-17 secretion. As we predicted, monocyte-depleted PBMCs from T2D donors constitutively secreted IL-17 at levels that approximated amounts secreted by PBMCs from ND donors, (Fig. 5E, CD14−). Preliminary intracellular flow cytometry confirmed IL-17 expression was limited to the CD3+CD4+ population of the CD14+ monocyte-depleted PBMCs (not shown). IL-17 production by PBMCs from T2D, but not ND, donors was decreased by monocyte depletion (p = 0.0081; compare PBMC CD3+28 to CD14−CD3+28), indicating changes in monocyte function underlie elevated Th17 function in T2D.
To independently confirm CD14+ monocytes played important roles in elevated T cell IL-17 production in T2D patients, we added back the positively selected CD14+ monocytes into the CD14 depleted PBMCs (CD14−+M) and measured IL-17 production. Reintroduction of autologous CD14+ monocytes rescued elevated IL-17 production by T cells from T2D donors (Fig. 5E; p = 0.0066). To most rigorously confirm a specific role for mono-cytes in inducing elevated IL-17 production by T cells in T2D patients, we negatively isolated T cells and monocytes from T2D donors and cocultured purified autologous cells in the presence or absence of anti-CD3 + anti-CD28 stimulation. Coculture of monocytes and T cells (M+T) rescued elevated IL-17 production by T cells from T2D donors, with levels indistinguishable from levels produced by intact PBMC cultures (Fig. 5E). Monocytes cultured alone had nearly undetectable levels of IL-17 (not shown). We conclude that monocytes play a critical role in elevated IL-17 production by T cells from T2D patients.
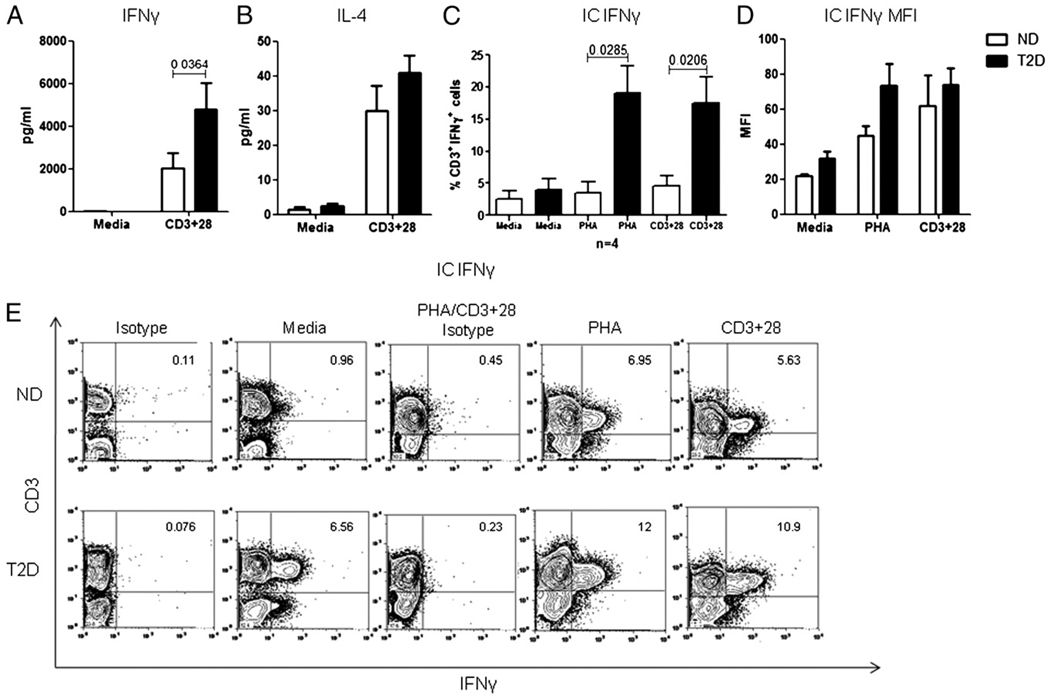
IFN-γ but not IL-4 is elevated in PBMCs and T cells from T2D patients
Based on the relationship between IL-17/Th17 cells and IFN-γ/Th1 cells shown in published work (44), we predicted that IFN-γ production would also be elevated in T cells from T2D patients. PBMCs from T2D patients stimulated with anti-CD3 + anti-CD28 secrete elevated levels of IFN-γ (Fig. 6A) but not the generally anti-inflammatory cytokine IL-4 (Fig. 6B) compared with PBMCs from ND donors. These data are consistent with elevated IFN-γ production by T cells in a mouse model of T2D (26, 27). Intracellular staining confirmed that IFN-γ production is T cell specific and was significantly elevated upon PHA and CD3 plus CD28 stimulation (Fig. 6C, 6E). Note that although the percentage of IFN-γ+ T cells was elevated in T2D samples, MFI was statistically indistinguishable in ND versus T2D T cells (Fig. 6D), indicating that more cells are recruited into the IFN-γ+ pool, but secretion by each cell differs insignificantly. Interestingly, PHA stimulation trends toward elevated IFN-γ secretion in T cells from T2D patients (Fig. 6D, middle bars; p < 0.08), suggesting that costimulation of both T cells and APCs by this lectin may increase IFN-γ secretion by T2D versus ND T cells on a per-cell basis. Importantly, IL-17+IFN-γ+ T cells were absent (data not shown); thus, different T cell populations produce IL-17 and IFN-γ. Finally, comparison of IL-2 secretion by unstimulated versus anti-CD3 + anti-CD28–stimulated PBMCs confirmed relatively equal levels of overall T cell activation between T2D patients and ND donors (Supplemental Fig. 1). We conclude two pathogenic T cell cytokines, IL-17 and IFN-γ, likely promote chronic inflammation in T2D patients.
FIGURE 6. Th1 but not Th2 cytokines are elevated in T cells from T2D patients.
A and B, PBMCs from ND (white bars) or T2D (black bars) donors were incubated for 40 h in the presence or absence of anti-CD3 plus anti-CD28. Samples were analyzed for IFN-γ (A) or IL-4 (B). Bars show average and SEM. p values were calculated by Mann–Whitney U test and are shown above bars that are significantly different (p < 0.05). All other comparisons were insignificantly different (p > 0.05). n = 12 for each donor cohort described in Table III. C, Summary of intracellular staining of IFN-γ in CD3+ T cells in PBMCs from ND (white bars) or T2D (black bars) donors as detailed for A (left). Comparisons between media and stimulated were statistically significant (p < 0.05) for the T2D cohort (media versus PHA, p = 0.0155; media versus CD3+28, p = 0.0209). D, MFI for IFN-γ levels in T cells from ND (white bars) or T2D (black bars) donors. Comparisons between media and stimulated MFIs were statistically significant (p < 0.05) for both cohorts (ND: media versus PHA, p = 0.0182; media versus CD3+28, p = 0.0411; T2D: media versus PHA, p = 0.0207; media versus CD3+28, p = 0.0069). No comparisons between ND and T2D cohorts were statistically significant for MFI. n = 4 for each donor cohort. E, One representative of four samples stained for intracellular IFN-γ in CD3+ T cells from ND (upper panels) or T2D (lower panels) PBMCs. Cells were stimulated as indicated for 40 h. CD3− cellular staining for IFN-γ in the media control was not consistent. Brefeldin, PMA, and ionomycin were added 4 h before harvest. n = 4. Isotype controls are as explained in Fig. 3.
The percentage of anti-inflammatory Tregs is decreased in T2D patients
Multiple groups have indicated that Tregs and Th17 cells have a reciprocal developmental relationship demonstrated by an expansion of Th17 cells coupled with decreased Tregs, the latter being strongly implicated in protection from T2D inflammation and insulin resistance (26, 45). Therefore, we hypothesized that the elevation of Th17 cells in T2D patients associates with decreases in the Treg compartment. To test this possibility, we measured the percentage of CD4+CD25+Foxp3+ cells in fresh ex vivo PBMCs from ND and T2D patients (Fig. 7A). We found a statistically significant reduction in the percentage of circulating Tregs in T2D patients compared with ND donors (4.321 ± 0.37% or 7.33 ± 0.5%, respectively; Fig. 7B), despite the fact that we detected no difference in IL-2 (per Supplemental Fig. 1), a cytokine that supports Treg differentiation (46–48). This result contrasts with analysis of T1D samples, which have similar percentages of CD4+ CD25+Foxp3+ T cells compared with samples from ND donors (49–51). Our data therefore show significant differences in Treg presence between T1D and T2D patients for the first time, to our knowledge. Furthermore, decreased percentages of Tregs in T2D PBMCs remained stable over time in culture and did not require the continued presence of B cells or monocytes for stability (CD19− or CD14−, respectively; Fig. 7C). Importantly, and in contrast to loss of elevated IL-17/Th17 function in purified T cells from T2D patients (Fig. 5A–C), purified CD4+ T cells from ND donors maintained an elevated percentage of Foxp3+ Tregs in culture (Fig. 7C, compare CD4 white and black bars).
The balance between the proinflammatory Th17 and anti-inflammatory Treg compartments is essential to maintain homeostasis. To evaluate the physiological impact of an elevated Th1/Th17 axis and decreased Treg percentages in T2D patients, we compared percentages of Th17 cells (CD3+CCR4+CCR6+) to percentages of Tregs (CD4+CD25+Foxp3+) in T2D and ND donors. This comparison showed a highly significant difference in percentage of proinflammatory Th17s versus protective Tregs in T2D but not ND donors (Fig. 7D). This dramatic shift in the CD4+ T cell compartment highlights the skewed proinflammatory T cell phenotype in T2D patients.
Previous reports have indicated a negative correlation between BMI and Tregs (26, 27). Consistent with these reports, we found a negative correlation between BMI and Tregs (CD4+CD25+Foxp3+) in the T2D cohort (Fig. 7E). This finding perhaps indicates that with increasing obesity in T2D patients, protective Tregs decrease, further skewing these patients toward a proinflammatory phenotype.
IL-17 protein secretion positively correlates with T2D severity
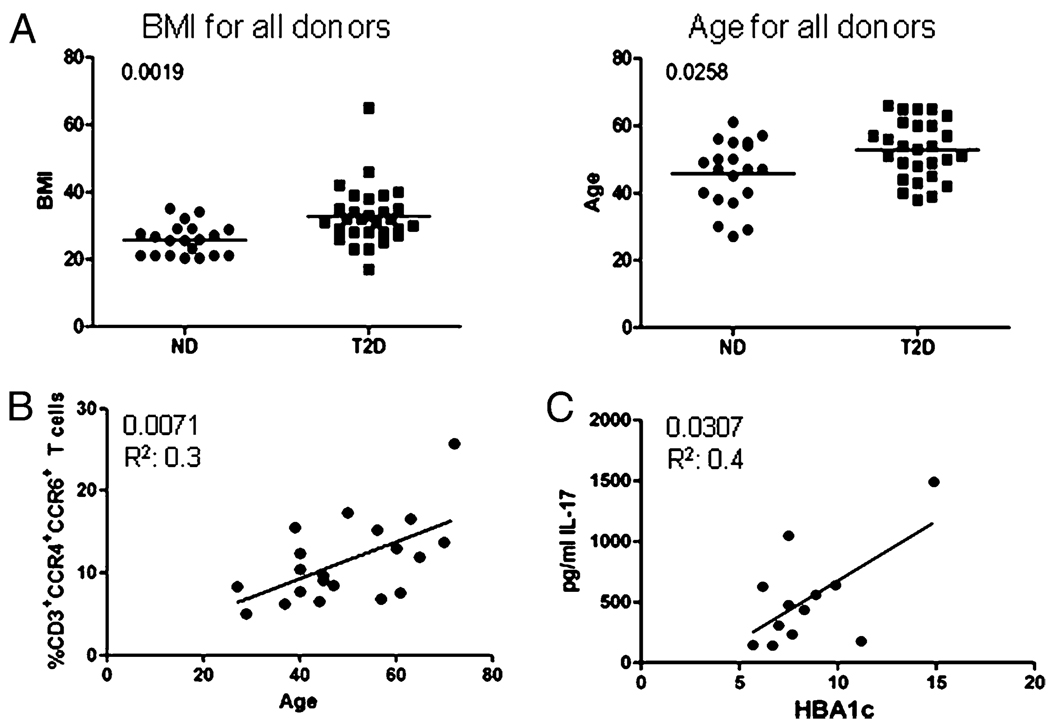
Because our study was cross-sectional and blinded, it was important to more rigorously determine if our data depicted alterations in T cell subset distribution and function that were solely attributed to T2D. Our T2D and ND cohorts statistically differed in both BMI and age (Fig. 8A, Table I). To determine whether differences in age or BMI explained elevated Th17 cells in the T2D cohort, we determined the relationships between either percentages of Th17 cells (based on CCR4/6) or IL-17 secretion and clinical parameters. This analysis showed a positive relationship between percentage of memory Th17 cells and age (Fig. 8B). However, we found no relationship between age and IL-17, IFN-γ, or IL-4 secretion (Supplemental Fig. 2, top row) in analysis of all donors tested. These data could indicate that although there is an increase in proinflammatory Th17 cells, other regulatory elements that prevent proinflammatory cytokine secretion in aging individuals are lost in T2D patients, further supporting a unique chronic inflammatory state in T2D patients. Furthermore, we found no relationship among IL-17, IFN-γ, or IL-4 secretion with BMI, race, or sex (Supplemental Fig. 2, rows 2–4). Taken together, we conclude differences in BMI or age of our overall patient cohorts are unlikely to significantly contribute to differences in T cell function identified in our assays.
FIGURE 8. IL-17 secretion correlates with T2D disease severity.
A, Comparison of all ND and T2D donors described in Table I based on BMI (left panel) or age (right panel). Significant differences between ND and T2D cohorts were calculated by the Mann–Whitney U test. B, Relationship between age (x-axis) and percentage of CD3+CCR4+CCR6+ cells (y-axis). C, Relationship between hemoglobin A1c, a marker of diabetes severity (HBA1c; x-axis) and secreted IL-17 protein (y-axis). The p values indicated in B and C were calculated using Pearson’s correlation; r2 values are shown below p values.
Hemoglobin A1c (HbA1c) is the most common marker used in the clinic to measure diabetes severity. We found a positive relationship trending toward significance between HbA1c and BMI in our T2D cohort (Supplemental Fig. 3; p = 0.0664), consistent with previous reports (52, 53). Due to the positive correlation between memory Th17 cells and BMI in the T2D cohort (Fig. 1E), we predicted IL-17 production may associate with T2D severity. To test this prediction, we determined the relationship between percentage of HbA1c from T2D patients and IL-17 production. We found a positive correlation between percentage of HbA1c and IL-17 protein secretion in the T2D cohort (Fig. 8C). Lack of percentage of HbA1c information on ND donors prevented this analysis in the control cohort. We conclude that it is unlikely that T2D-independent differences between the ND and T2D cohorts explain proinflammatory T cell imbalances in our study. Importantly, a positive correlation between the percentage of HbA1c and IL-17 secretion highlights a potential role for Th17 cells in T2D previously suggested by analysis in model organisms (28).
Discussion
Our data show T2D patients have an increase in the proinflammatory Th17/Th1 axis that is supported by monocytes, a cell type more traditionally associated with inflammation in T2D. This conclusion is supported by the increase in circulating Th17 cells and elevated secretion of IL-17 and IFN-γ by T cells from T2D patients. These findings are independently confirmed by an increase in molecular signatures that characterize Th17 cells, including active structures at the IL-17 and RORC promoters in fresh ex vivo T cells and in T cells incubated in the context of PBMCs from T2D patients. Increased proinflammatory T cell function is compounded by the decrease of the CD4+ Treg compartment. Importantly, our data show for the first time, to our knowledge, a loss in homeostasis in T cell subset balance that most likely promotes the chronic inflammation characterizing T2D patients. Our findings also identify a new role for monocytes in T2D: support of proinflammatory T cell function.
Several groups have recently identified important roles for T cells in adipose tissue inflammation and insulin resistance in a mouse model of T2D. Predictably, inflammatory IL-17+ and IFN-γ+ cells are elevated in at least some adipose depots of obese/insulin-resistant mice (26–28). Additionally, previous work shows that IFN-γ downregulates the insulin response in adipocytes (54), thus identifying a mechanism that links proinflammatory T cell function to a more traditional diabetogenic cell type. Complementary studies showed Tregs play important roles in inhibiting insulin resistance, and loss of Tregs promoted inflammation (26). Our data critically extend the conclusions of these animal studies into humans and demonstrate that a focus on T cells represents a clinically important area of T2D research. Although our new studies did not specifically define a role for Th1 and Th17 cells in adipose tissue inflammation, the data provide evidence for a role of IL-17– and IFN-γ–producing T cells in systemic T2D inflammation, which likely parallels adipose-associated events.
Multiple pieces of evidence indicate a potential proinflammatory feed-forward loop between adipocytes and T cells in obesity and T2D. Over nutrition and obesity induce adipose tissue to secrete proinflammatory factors such as IL-6, a cytokine that induces Th17 differentiation (33, 55, 56). Increased secretion of IL-6, along with IL-1β and TGF-β (29–33) can lead to systemic inflammation and a pro-Th17 skewing milieu, which in turn results in elevated levels of IL-17. T2D patients also have elevated levels of serum IL-12 (57, 58), a cytokine that promotes Th1 differentiation and elevated IFN-γ production (59–62). Conversely, adipocytes are potential targets of proinflammatory T cell cytokines. Adipocytes express significant levels of the IL-17 receptors IL-17RA and IL-17RC (63) and respond to IL-17 by secreting IL-6, which may reinforce the IL-6 produced under hypercaloric conditions (63). Similarly, adipocytes respond to IFN-γ by attenuating JAK/STAT activation, hence insulin signaling (54), which leads to insulin resistance. Taken together, these studies support the possibility that adipocytes and T cells synergize to establish a feed-forward loop of chronic inflammation. Additional work on the specific roles of Th1 and Th17 cells in exacerbating chronic inflammation (perhaps via adipocytes) would further our understanding of the complex interplay between two seemingly unrelated cell types in T2D.
Two chemokine receptors, CCR4 and CCR6, identify IL-17–producing memory Th17 cells (35). Later reports found CCR4 and CCR6 on the surface of Tregs as well, although these were primarily in the tonsil (64), which, by its nature, is chronically inflamed when excised from patients. Based on the reciprocal relationship between Th17 cells and Tregs, it is possible that CCR4 and CCR6 could be yet another set of features that these functionally opposed cell types share, at least under some conditions. Importantly, elevated secretion of IL-17 in T2D patients independently confirmed conclusions from the initial CCR4/CCR6 studies, regardless of whether these chemokine receptors uniquely identified Th17 cells in T2D patients. An alternative explanation for the elevated percentages of CCR4+CCR6+ cells, along with elevated secretion of IL-17, is that in the inflammatory milieu found in T2D patients, Foxp3+ cells could be induced to produce IL-17. Foxp3/IL-17A double-positive T cells identified by published studies on ND T cells (65–67) demonstrate that generally anti-inflammatory Tregs may have proinflammatory activities under some conditions. CCR4/6 expression on Tregs and Th17 cells may explain the seeming contradictory findings that the percentage of CCR4+CCR6+ T cells, but not IL-17 secretion, positively correlates with age (Fig. 8B, Supplemental Fig. 2). Likewise, IL-17 secretion but not the percentage of CCR4/6 T cells correlated with T2D severity as measured by HbA1c (Fig. 8C, Supplemental Fig. 2). Future studies will identify the mechanistic underpinnings of these related but biologically discernible cell populations.
Chemokine receptors such as CCR4 and CCR6 are critical for recruitment of appropriate immune cells to sites of inflammation. Preliminary mRNA analysis indicates expression of CCL20, the ligand for CCR4, in human adipose-associated stromal vascular fractions (S. Fried and D. Gong, unpublished observations). Interestingly, because Tregs and Th17 cells share trafficking receptors, both cell types could be recruited to adipose tissue. These findings raise the possibility that Tregs recruited to a pro-Th17 milieu in the adipose tissue through CCR4 and CCR6 are subsequently skewed toward the Th17 phenotype. The plasticity between Tregs and Th17 cells indicate a potential for phenotypic changes based on signals from the environment, which in a non-disease state would be beneficial for a balanced immune response, but highly detrimental when homeostasis is lost.
Because inflammatory cytokines induce insulin resistance, reducing inflammation is an important outcome for T2D treatments. Our data indicate that one potential approach toward this goal would be to reset the balance between the proinflammatory and anti-inflammatory T cell subsets, Th1/Th17 and Tregs, respectively, perhaps by identifying factors that drive BMI-mediated elevations in CCR4+CCR6+ T cells that are present in T2D donors but absent in ND donors (Fig. 1E). Alternatively, identifying mechanisms that explain BMI-dependent decreases in Tregs from T2D samples (Fig. 7E) may offer therapeutic options. Finally, our work shows monocyte manipulation is a viable indirect method for altering T cell function in T2D. An intimate understanding of the molecular mechanisms behind the reciprocal Th17/Treg relationship is essential to be able to safely manipulate T cell subset balance to re-establish homeostasis in a disease environment. Targeting the imbalance in T cells as a treatment for T2D patients could be used as an Achilles heel to reduce inflammation leading to improved glucose tolerance and reduced insulin resistance.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01AI54611 and R21DK089270 and an American Diabetes Association Research Grant (to B.S.N.).
We thank Gerald Denis, Annabel Belkina, and the Boston University Medical Center flow core facility for expertise in flow cytometry and in use of the LSRII and Katherine Bossart for the use of the Bio-Rad multiplex protein analyzer. We also thank Susan Fried, DaWei Gong, and the Nutrition Obesity Research Centers of Maryland and Boston for the mRNA data and Dr. Bill Cruikshank for valuable insights and comments on the manuscript.
Abbreviations used in this article
- BMI
body mass index
- ChIP
chromatin immunoprecipitation
- HbA1c
hemoglobin A1c
- MFI
mean fluorescence intensity
- ND
nondiabetic
- RORC
retinoic acid-related orphan receptor C
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- Treg
regulatory T cell
Footnotes
Supplementary Data http://www.jimmunol.org/content/suppl/2010/12/17/jimmunol.1002615.DC1.html
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun. Rev. 2007;6:169–175. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 4.Galicia G, Kasran A, Uyttenhove C, De Swert K, Van Snick J, Ceuppens JL. ICOS deficiency results in exacerbated IL-17 mediated experimental autoimmune encephalomyelitis. J. Clin. Immunol. 2009;29:426–433. doi: 10.1007/s10875-009-9287-7. [DOI] [PubMed] [Google Scholar]
- 5.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 6.Pe`ne J, Chevalier S, Preisser L, Vénéreau E, Guilleux MH, Ghannam S, Mole`s JP, Danger Y, Ravon E, Lesaux S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 7.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 8.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spranger J, Kroke A, Mo¨hlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 14.Grimble RF. Inflammatory status and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:551–559. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 16.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 17.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res. Clin. Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin. Exp. Immunol. 2006;146:443–447. doi: 10.1111/j.1365-2249.2006.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, Kantarci A, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 21.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 22.de Roos B, Rungapamestry V, Ross K, Rucklidge G, Reid M, Duncan G, Horgan G, Toomey S, Browne J, Loscher CE, et al. Attenuation of inflammation and cellular stress-related pathways maintains insulin sensitivity in obese type I interleukin-1 receptor knockout mice on a high-fat diet. Proteomics. 2009;9:3244–3256. doi: 10.1002/pmic.200800761. [DOI] [PubMed] [Google Scholar]
- 23.Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl. Acad. Sci. USA. 2009;106:13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reimers JI. Interleukin-1 beta induced transient diabetes mellitus in rats. A model of the initial events in the pathogenesis of insulin-dependent diabetes mellitus? Dan. Med. Bull. 1998;45:157–180. [PubMed] [Google Scholar]
- 25.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, Dosch HM. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 29.Herder C, Zierer A, Koenig W, Roden M, Meisinger C, Thorand B. Transforming growth factor-beta1 and incident type 2 diabetes: results from the MONICA/KORA case-cohort study, 1984–2002. Diabetes Care. 2009;32:1921–1923. doi: 10.2337/dc09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andriankaja OM, Barros SP, Moss K, Panagakos FS, DeVizio W, Beck J, Offenbacher S. Levels of serum interleukin (IL)-6 and gingival crevicular fluid of IL-1beta and prostaglandin E(2) among non-smoking subjects with gingivitis and type 2 diabetes. J. Periodontol. 2009;80:307–316. doi: 10.1902/jop.2009.080385. [DOI] [PubMed] [Google Scholar]
- 31.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine. 2008;44:141–148. doi: 10.1016/j.cyto.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 34.Liang MD, Zhang Y, McDevit D, Marecki S, Nikolajczyk BS. The interleukin-1beta gene is transcribed from a poised promoter architecture in monocytes. J. Biol. Chem. 2006;281:9227–9237. doi: 10.1074/jbc.M510700200. [DOI] [PubMed] [Google Scholar]
- 35.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 36.Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, Querci V, Fambrini M, Liotta F, Levings MK, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur. J. Immunol. 2010;40:2174–2181. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 37.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 38.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J. Immunol. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW, Taams LS. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. USA. 2009;106:6232–6237. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwamoto S, Iwai S, Tsujiyama K, Kurahashi C, Takeshita K, Naoe M, Masunaga A, Ogawa Y, Oguchi K, Miyazaki A. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J. Immunol. 2007;179:1449–1457. doi: 10.4049/jimmunol.179.3.1449. [DOI] [PubMed] [Google Scholar]
- 44.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, Gonzalez J, Krueger JG, Lowes MA. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J. Invest. Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int. Immunol. 2009;21:489–498. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur. J. Immunol. 2008;38:912–915. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 47.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 48.Murawski MR, Litherland SA, Clare-Salzler MJ, Davoodi-Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Ann. N. Y. Acad. Sci. 2006;1079:198–204. doi: 10.1196/annals.1375.031. [DOI] [PubMed] [Google Scholar]
- 49.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J. Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 51.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 52.Norberg M, Eriksson JW, Lindahl B, Andersson C, Rolandsson O, Stenlund H, Weinehall L. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. J. Intern. Med. 2006;260:263–271. doi: 10.1111/j.1365-2796.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 53.Haimoto H, Sasakabe T, Umegaki H, Wakai K. Acute metabolic responses to a high-carbohydrate meal in outpatients with type 2 diabetes treated with a low-carbohydrate diet: a crossover meal tolerance study. Nutr. Metab. (Lond) 2009;6:52. doi: 10.1186/1743-7075-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, Smyth EM, Reilly MP. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J. Biol. Chem. 2009;284:31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 56.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 57.Wegner M, Winiarska H, Bobkiewicz-Kozłowska T, Dworacka M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine. 2008;42:312–316. doi: 10.1016/j.cyto.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J. Interferon Cytokine Res. 2004;24:381–387. doi: 10.1089/1079990041535665. [DOI] [PubMed] [Google Scholar]
- 59.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu CY, Demeure C, Kiniwa M, Gately M, Delespesse G. IL-12 induces the production of IFN-gamma by neonatal human CD4 T cells. J. Immunol. 1993;151:1938–1949. [PubMed] [Google Scholar]
- 61.Yssel H, Fasler S, de Vries JE, de Waal Malefyt R. IL-12 transiently induces IFN-gamma transcription and protein synthesis in human CD4+ allergen-specific Th2 T cell clones. Int. Immunol. 1994;6:1091–1096. doi: 10.1093/intimm/6.7.1091. [DOI] [PubMed] [Google Scholar]
- 62.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf SF, Belardelli F, Gessani S. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J. Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 63.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates proinflammatory responses in adipocytes. Biochem. Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J. Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 65.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor ROR-gamma t. Proc. Natl. Acad. Sci. USA. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 67.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.