Abstract
Nasal carriage of Staphylococcus aureus is a major risk factor for invasive S. aureus disease. The aim of this study was to define factors associated with carriage. We conducted a prospective, longitudinal community-based study of infants and their mothers for a period of 6 months following delivery. The epidemiology of carriage was examined for 100 infant-mother pairs. Infant carriage varied significantly with age, falling from 40 to 50% during the first 8 weeks to 21% by 6 months. Determinants of infant S. aureus carriage included maternal carriage, breastfeeding, and number of siblings. Bacterial typing of S. aureus was performed by pulsed-field gel electrophoresis and multilocus sequence typing. The majority of individuals carried a single strain of S. aureus over time, and the mother was the usual source for colonizing isolates in infants. The effect of other components of the normal nasal flora on the development of S. aureus carriage was examined in 157 consecutive infants. Negative associations (putative bacterial interference) between S. aureus and other species occurred early in infancy but were not sustained. An increasing antistaphylococcal effect observed over time was not attributable to bacterial interference. S. aureus carriage in infants is likely to be determined by a combination of host, environmental, and bacterial factors, but bacterial interference does not appear to be an ultimate determinant of carrier status.
Staphylococcus aureus is a major cause of community-acquired and nosocomial sepsis (7, 23). The rising prevalence of antibiotic-resistant strains, such as methicillin-resistant S. aureus and S. aureus with reduced susceptibility to glycopeptides (15, 17, 22), increases the need to prevent disease through the identification and modification of risk factors. Three sets of observations indicate that nasal carriage of S. aureus is an important risk factor for sepsis with this organism: carriers have higher rates of infection than noncarriers (16, 29, 30, 31); the strain causing infection is usually the carriage strain in a given individual (16, 18, 28, 31); and eradication of carriage reduces nosocomial infection (2, 10, 20, 31). Temporary eradication of S. aureus in those at high risk of sepsis is desirable but relies on the unlikely premise of sustained susceptibility to antibiotics such as mupirocin. There is a need to develop alternative methods of S. aureus eradication, the success of which may depend on a detailed understanding of the determinants of carriage. The relative importance of host, bacterial, and environmental factors in determining S. aureus carriage is currently unknown. The aim of this study was to explore these factors during a prospective, longitudinal community-based study of carriage in mothers and their infants for a period of 6 months after delivery.
MATERIALS AND METHODS
Study design.
The study was conducted in two parts. Epidemiological factors influencing infant carriage were examined for a group of 100 consecutively recruited infants and their mothers who were derived from a larger birth cohort study of nasal and nasopharyngeal carriage flora conducted prospectively in Oxfordshire, United Kingdom between July 1999 and May 2001. Bacterial genotyping of S. aureus isolated from these individuals was performed by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). The study of the effect of bacterial interference by other members of the nasal flora on infant carriage of S. aureus was extended to a further 57 consecutively recruited infants. Ethical approval for the study was obtained from the Central Oxford Research Ethics Committee. Mothers were recruited through the antenatal registry from the John Radcliffe Hospital and contacted by letter 4 to 6 weeks prior to their expected delivery. Following delivery, trained nurses obtained nasal swabs from mothers and infants. Infants were swabbed at 2-week intervals during the first 3 months and at 1-month intervals for the next 3 months. Mothers were swabbed during the first visit (week 2) and the last 3 visits (weeks 16, 20, and 24). For each mother-infant pair, the sex, gestational age, and birth weight of the infant, the age of the mother and whether she smoked tobacco, number of siblings, and extent of breastfeeding were recorded.
Nasal swabs, bacterial isolates, and identification.
Nasal swabs were performed with a cotton swab moistened with sterile phosphate-buffered saline, which was rubbed over the anterior nares of both nostrils. The tip of the swab was broken into a bottle containing 1 ml of phosphate-buffered saline and stored at 4°C until returned to the laboratory. The sample was vortexed, and 100 μl was spread onto blood agar and mannitol salt agar plates, which were incubated at 37°C in air for 48 h. Yellow colonies on mannitol salt agar (mannitol fermenters) were plated to purity on blood agar and incubated at 37°C in air for 24 h. Bacteria were presumptively classified on the basis of colonial morphology and Gram stain results. Isolates were identified as S. aureus if positive for Staphaurex and DNase tests. A single colony of S. aureus was analyzed for each positive specimen. Identification of Corynebacterium spp., coagulase-negative staphylococci (CoNS), and alpha-hemolytic streptococci (hereafter called streptococci) present on the blood agar plate was performed by standard laboratory techniques. CoNS were identified to the species level by species-specific PCR of the 16S rRNA gene. This differentiated between S. epidermidis, S. haemolyticus, S. warneri, S. saprophyticus, S. hominis, S. sciuri, S. schleiferi, and S. capitis. Primer sequences and PCR cycling conditions were kindly provided by T. Pitt, Staphylococcal Reference Laboratory, Central Public Health Laboratory, London, United Kingdom (personal communication). Corynebacteria were identified to the species level with the API System (CORYNE), as were 26 of 342 CoNS isolates which were negative on PCR (API ID32 STAPH; bioMerieux Ltd.).
PFGE.
Preparation of chromosomal DNA, SmaI digestion, and PFGE were performed as previously described (5). Stored gel images were analyzed with BioNumerics, version 2.0 (Applied Maths, Kortrijk, Belgium). Using two sets of criteria, each isolate was assigned two PFGE types on the basis of their DNA banding patterns (24). In the first, isolates differing at fewer than 4 bands were considered closely related and assigned the same PFGE4 type. In the second, a separate PFGE1 type was assigned for each unique banding pattern, so that isolates had to possess identical banding patterns to be assigned the same PFGE1 type. Hence, PFGE1 types refer to unique genotypes, and PFGE4 types refer to related genotypes.
MLST.
Chromosomal DNA was extracted by using a Puregene DNA extraction kit (Gentra Systems), with the modification that lysostaphin (30 μg ml−1) (Sigma) was added at the cell lysis step. MLST was carried out with the method described by Enright et al. (8). In brief, fragments of 7 unlinked housekeeping genes were amplified by the PCR. Amplified products were precipitated and both strands were sequenced by using BigDye fluorescent terminators and the primers used in the initial PCR amplification. The sequences obtained were assigned allele numbers following comparison of the DNA sequence with results from previously typed strains found at the MLST web site (http://mlst.net/). For each isolate, the allele numbers at each of the seven loci defined the allelic profile, or sequence type (ST). New alleles were confirmed by repeat sequencing.
Statistical analysis.
Analysis of the epidemiology of carriage and the effects of factors such as maternal carriage, infant and maternal age, and breastfeeding was carried out by considering mother-infant pairs as the units of analysis. Mixed-effects logistic regression models were used to examine the effects of explanatory factors on infant carriage at any one time (using the time series analysis facilities of Stata 7.0 [Stata Corp., College Station, Tex.]). In this way, we attempted to take account of any correlation in carriage between observation and minimize any loss of efficiency due to missing data. Analysis of the effects of bacterial genotype was carried out by using isolates as the units of analysis. For each individual, one isolate of each genotype was selected for analysis. Nominal variables were analyzed by Fisher's exact or chi-squared tests, and continuous variables were compared between groups by the Kruskal-Wallis test. Bacterial antagonism was assessed by mixed-effects logistic regression modeling.
RESULTS
Epidemiology of carriage.
One hundred consecutive infants, paired with 99 mothers (there was one set of twins), were studied. Ninety-two (92%) of the infants had all 9 nasal swabs, with a further 5 infants missing only 1 swab. One infant had 2 swabs, and 4 infants had only a single swab and then withdrew from the study. Of the mothers, 56 (57%) had all 4 swabs and a further 25 (26%) had 3 swabs. Eight mothers had 2 swabs, and 9 mothers had only a single swab. Demographic and lifestyle details are summarized in Table 1.
TABLE 1.
Baseline characteristics
| Characteristic | Result |
|---|---|
| Mean maternal age (yr) (95% CI) | 33.0 (31.6-34.5) |
| No. (%) of smoking mothers | 32 (32) |
| Median no. of siblings (IQR,a range) | 1 (0-2, 0-6) |
| No. (%) of male infants | 50 (50) |
| Mean gestational age at birth (days) | 280 (279-282, 266-294) |
| (95% CI, range) | |
| Mean birth wt (kg) (95% CI, range) | 3.48 (3.39-3.58, 2.44-4.99) |
| No. (%) of breastfed infants | 75 (75) |
IQR, interquartile range.
Infants.
Seventy-one (71%) infants had at least one nasal swab that tested positive for S. aureus at some stage during the study. Carriage rates were between 40 and 50% during the first 8 weeks but fell thereafter to 21% by 6 months of age (Fig. 1). This downward trend was statistically highly significant (P < 0.0001) and remained so after exclusion of those children (n = 8) who had missed one or more swabs. Modeling the change in infant carriage over time showed an approximately 5% reduction in carriage rate with each 2-week period from 6 weeks onwards (Fig. 1).
FIG. 1.
Breastfeeding and nasal carriage of S. aureus by mothers and their infants over a period of 6 months following delivery. Data are shown as percentages (standard errors [SE]) of individuals carrying S. aureus at each time point sampled.
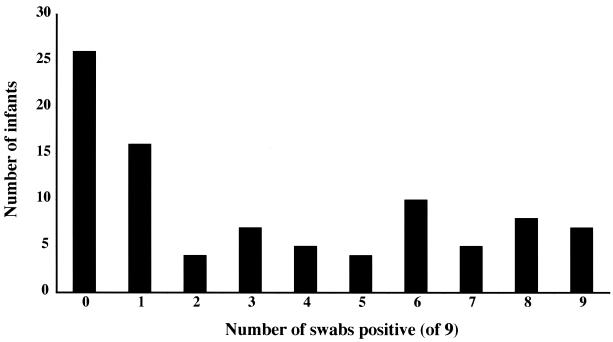
Three patterns of carriage have been described in adults, with approximately 20% of individuals being termed persistent noncarriers, 20% termed persistent carriers, and the remainder termed intermittent carriers (11). The pattern of carriage over the first 6 months of life was examined for infants in this study (Fig. 2). Of the 92 infants who had all 9 swabs, just over one-quarter were never positive for S. aureus. The remainder did not segregate into a bimodal distribution consistent with a model of intermittent and persistent carriers.
FIG. 2.
The number of swabs positive (of a possible total of 9 swabs taken) from 96 infants over a period of 6 months following delivery.
Mothers.
Fifty-one mothers (52%) had at least one swab that tested positive for S. aureus at some stage during the study. The mean prevalence of carriage at any one time point on a single swab was 30% (Fig. 1). There was no variation in the carriage rate with time (P = 0.5). Of the 82 mothers who had 3 or more nasal swabs taken, 10 (12%) were positive for S. aureus on all swabs, 30 (37%) were intermittently positive with 1 or 2 swabs positive, and 42 (51%) were never positive.
Interactions between maternal and infant carriage.
There was a striking degree of concordance for S. aureus carriage between mothers and infants, with 67 concordant pairs (68%). In 45 pairs, both mother and infant carried at some stage. In only 6 pairs was the infant a noncarrier despite the mother being a carrier, and in 26 pairs, the infant carried at some stage despite persistent noncarriage by the mother (P = 0.0005, by McNemar's test). At 24 weeks, after the decline in infant carriage from its initial peak, there were only 8 pairs in which the infant was a carrier while the mother was not. The crude odds ratio (OR) for infant carriage at any time given maternal carriage at any time was 6.5 (95% confidence interval [CI], 2.3 to 18; P = 0.0001). However, as mothers were swabbed less frequently than infants, the chance of them being identified as intermittent or occasional carriers is lower. In a mixed-effects logistic regression model stratifying for mother-infant pairs and considering only those 4 time points at which both were swabbed, the OR for infant carriage given maternal carriage at any one time was 3.7 (95% CI, 1.7 to 8.0; P < 0.001).
Demographic factors and breastfeeding.
Maternal age and smoking, and infant birth weight, gestational age, and sex and had no significant effect on infant carriage of S. aureus. Breastfeeding at the time of each individual swab was positively associated with infant (but not maternal) S. aureus carriage (OR, 4.0; 95% CI, 1.8 to 9.2; P = 0.001); this association appeared to be independent of both maternal carriage status and infant age (both breastfeeding and infant carriage fall with time) (Fig. 1). Breastfeeding intensity (number of feeds per day) added nothing further to this association. There was a weak positive association between infant S. aureus carriage and the number of older siblings (OR, 1.5; 95% CI, 1.0 to 2.1) for each additional child (P < 0.04). This effect was independent of maternal carriage.
S. aureus carriage and bacterial typing.
A total of 412 isolates of S. aureus were identified from 1,206 nasal swabs from 100 infant-mother pairs, comprising 98 isolates from mothers (332 swabs), and 314 isolates from infants (874 swabs). PFGE typing of the 412 isolates demonstrated 33 PFGE4 types and 68 PFGE1 types. MLST was performed on the first S. aureus isolate from all mothers and infants and on any isolates obtained from subsequent swabs that differed by one or more PFGE bands from the original strain in a given individual (i.e., had differing PFGE1 types). This makes the assumption that consecutive isolates from the same individual with identical PFGE1 types are the same organism. MLST of 147 isolates from 50 mothers and 71 infants demonstrated 42 STs. Six isolates had STs that were identical to others in the same individual despite differing PFGE1 patterns. Two of these were related (differing by one to three bands and therefore possessing the same PFGE4 type), and four were unrelated by PFGE (differing by four or more bands, and hence having different PFGE1 and PFGE4 types). Within mother-infant pairs, there was excellent concordance between PFGE types and MLST STs. There were no cases of isolates sharing the same PFGE4 type within a mother-infant pair but possessing different STs, although in four pairs, isolates bearing the same ST varied by a single band and therefore had different PFGE1 types. Because isolates were selected for MLST on the basis of differing PFGE patterns, it was not possible to assess to what degree STs vary within identical PFGE1 and PFGE4 types, although results from a recent study suggest that such differences may exist but are few in number (19).
Interactions between carriage and MLST.
The population genetic structure of S. aureus carried by mothers was compared with that for isolates carried by infants. The maternal and infant isolate populations were similarly diverse, as assessed by the distributions of STs (Fig. 3). Thus, infants did not appear to acquire a set or subset of isolates (for example, adapted and successful hospital strains) that were not represented in the community. These data argue against the idea that S. aureus population genetic structure shifts from one human generation to the next.
FIG. 3.
Graphical representation of S. aureus multilocus STs distributed between mother and infant isolate groups. Each ST is represented by a unique color so that the relative proportions in each group can be seen.
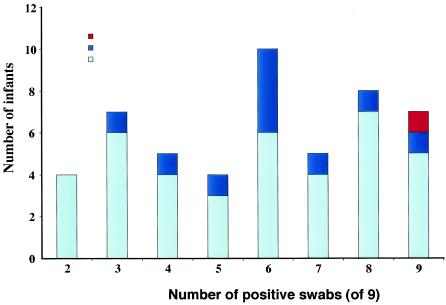
Previous longitudinal studies of S. aureus carriage in adults have reported that persistent carriers were colonized with a single clone of S. aureus (9, 27) and that intermittent carriers were colonized with genetically unrelated strains of S. aureus isolates over time (27). This was not reproduced in the present study in which the majority of individuals appeared to carry a single ST, regardless of whether or not carriage was intermittent or persistent during the study period (Fig. 4). More than one ST was found in only 2 mothers (4%) and 11 infants (15%). There were 43 mother-infant pairs in which both mother and infant were carriers. Comparison of STs between these pairs demonstrated that bacteria with the same ST were recovered from both in 36 pairs (84%). In addition, one pair was found to carry bacteria of two STs in both mother and infant (bacteria with different STs being isolated on separate occasions). Hence, there were 38 instances of STs being shared between mother and infant. These data suggest that the mother is the usual source for the primary colonizing isolate in the infant.
FIG. 4.
Graphical representation of the number of S. aureus STs carried over time by infants according to number of positive swabs over 6 months.
The subset of infants that carried more than one ST over time was examined to determine how replacement strains in the infant related to those carried by the mother. There were 11 infants with more than one ST over time. In five of these, the first ST appeared to be permanently replaced by a new ST, which in 3 cases was the dominant ST carried by the mother. In the remaining 6 infants, there was a switch from the first ST to a second ST and then a switch back to the first ST. The first ST was the dominant ST of the mother in four of these cases. These data suggest that the mother is the usual source for strain replacement. The maternal strain also appeared to be the source for recolonization in intermittent carriers, although this could also be explained by persistent carriage where isolation of S. aureus intermittently fell below the level of detection.
The relationship was also explored between ST and both transmissibility and duration of carriage. The approach taken was to divide the isolates into two groups according to the frequency with which they colonized the study group. STs were referred to as common if they were found in more than one mother-infant pair or sporadic if occurring in only a single mother-infant pair. Of the 141 isolates, 103 (74%) were represented by 12 common STs and 38 isolates segregated into 30 sporadic STs. The group of isolates with common STs were more likely to occur in both mother and infant than in only one of a pair (49 versus 27%, P < 0.05), suggesting increased transmissibility within pairs. However, the duration of carriage in an individual was not significantly different between common and sporadic STs in either infants or mothers.
Effect of other members of the nasal flora on S. aureus carriage.
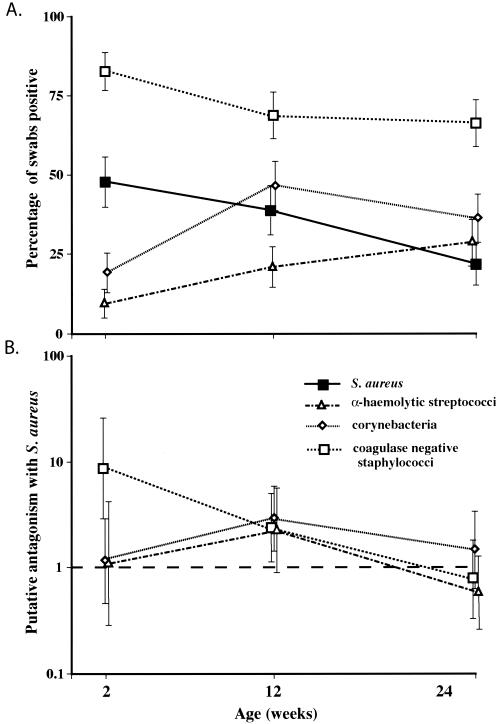
The effect of other members of the nasal flora on S. aureus carriage was examined for 157 infants by using swabs taken at the 2-, 12-, and 24-week time points, which were selected to allow feasible identification of the development of carriage over the full study period. Overall carriage rates for S. aureus and the three other bacterial groups all varied significantly with time (P < 0.002 in all cases) (Fig. 5a), indicating that the development of the normal nasal flora is a dynamic process during the first 6 months of life.
FIG. 5.
(A) Percentage of infants colonized with each species or genus at three time points; (B) antagonism between S. aureus and the other bacterial groups. Antagonism is represented by the OR for not being colonized with S. aureus if positive for a particular bacterial group, derived by multivariate logistic regression analysis. Error bars represent 95% CIs in all cases.
Considering all swabs together, carriage of CoNS and carriage of corynebacterial species were each inversely associated with carriage of S. aureus, while no significant effect was seen with streptococci (Table 2). These findings were reproduced on multivariate analysis (Fig. 5b). When the three time points were examined separately, it became clear that the interaction between S. aureus and the other bacterial groups was not constant with time. The putatively antagonistic effect of CoNS carriage was most marked at week 2 and had disappeared by week 24 (Table 2; Fig. 5b), and on species-specific analysis, it was found to be due entirely to S. epidermidis (representing 70% [441 of 633] of all CoNS isolates). The negative association between carriage of S. aureus and corynebacteria was only present at the 12-week time point and could not be related to a specific corynebacterial species. Thus, all associations with S. aureus carriage had disappeared by 24 weeks. Streptococci were not associated with S. aureus carriage at any stage. There were no significant associations (and by inference, bacterial competition) at any time point between carriage of CoNS, corynebacteria, and streptococci.
TABLE 2.
Antagonism between S. aureus and other bacterial groupsa
| Bacterial group | OR (95% CI) at wk:
|
Overall OR (95% CI) | ||
|---|---|---|---|---|
| 2 | 12 | 24 | ||
| Corynebacteria | 1.3 (0.55-2.8)b | 2.8 (1.4-5.6)c | 1.5 (0.65-3.4)b | 2.0 (1.3-3.0)d |
| CoNS | 8.6 (2.8-26.7)e | 2.1 (1.0-4.2)f | 0.78 (0.34-1.8)b | 1.7 (1.1-2.5)g |
| Streptococci | 0.78 (0.27-2.3)b | 2.3 (0.97-5.6)b | 0.57 (0.25-1.3)b | 1.3 (0.81-2.1)b |
OR (95% CI) and P values are given for the absence of S. aureus given colonization with each bacterial group. ORs were derived from univariate analyses, with conditional logistic regression used to calculate the overall effect, allowing for correlation between swabs taken from the same infant.
Result was not significant.
P = 0.003.
P = 0.002.
P < 0.0001.
P = 0.04.
P = 0.014.
S. aureus carriage at 24 weeks was predicted by S. aureus carriage at 2 weeks (OR, 2.4; 95% CI, 1.1 to 5.3; P = 0.033) and at 12 weeks (OR, 5.6; 95% CI, 2.4 to 12.8; P < 0.0001); this effect was not seen for carriage of other bacterial groups at any of the three time points. Considering S. aureus and S. epidermidis together, there was evidence for an antistaphylococcal effect which increased with time; the number of infants who were noncarriers for these two staphylococcal species increased from 12 (7.6%) at 2 weeks to 41 (26%) at 12 weeks and 68 (43%) at 24 weeks (P < 0.0001). This was independent of any bacterial interference from corynebacteria or streptococci.
DISCUSSION
This study has provided an opportunity to follow the evolution of nasal carriage of S. aureus during the first 6 months of life and to explore factors that influence this process. One of the advantages of studying carriage in this age group is that newborns are immunologically and microbiologically naive. Observing the development of bacterial colonization during the emergence of acquired immunity could provide important insights into how host factors and bacterial factors influence carriage.
The concordance in carriage between mother-infant pairs in this study indicates a strong influence from a shared environment and/or common host genetics. The study design does not allow us to differentiate between the two. However, the observation that most infants of noncarrier mothers were also noncarriers suggests that genetic susceptibility is a major factor, since infants are likely to be exposed to S. aureus carried by others on a regular basis. Previous studies that shed light on host factors in relation to S. aureus carriage relate largely to the immune response. Regulation of carriage via acquired immunity was strongly implied by a study in which a strain of S. aureus was established in the nares of healthy volunteers for at least 5 days (6). After spontaneous nasal elimination, duration of colonization with a second strain reflected that of the primary inoculation but carriage of the same strain after reinoculation was uniformly brief. Carriage rates have also been reported to be higher in groups of patients who are immunocompromised because of a variety of causes, including insulin-dependent diabetics, patients on continuous ambulatory peritoneal dialysis and hemodialysis, and in those infected with human immunodeficiency virus (11). Other soluble antibacterial factors secreted in the nose may also be involved in determining carriage, based on work by Cole et al., who reported that nasal secretions from 3 individuals who were S. aureus carriers lacked antimicrobial activity against S. aureus in vitro while fluid from noncarriers was bactericidal (4).
Given the likely role of acquired immunity in carriage of S. aureus, it is possible that the decline in carriage rate after the 8th week of life in infants in this study reflects the development of an immune response to S. aureus and subsequent eradication of carriage in some individuals. Another possible explanation for the fall in carriage was that the number of mothers changing from breastfeeding to bottle feeding increased over the course of the study. This is an important potential confounder, given that breastfeeding was a risk factor for carriage. However, this did not appear to be the case, as the reduction in carriage rate with time appeared to be independent of breastfeeding. S. aureus bacterial factors examined in this study also failed to explain the fall in infant carriage. There was no evidence for strain switching at around the time that the decline in rate began or at any point thereafter. In addition, the ecological fitness of the strain (as measured by the frequency of a given ST in the bacterial group as a whole) did not appear to equate with the duration of carriage. However, it is still possible that infants became colonized with new bacterial species on leaving the home environment and that this influenced carriage through a process of bacterial interference.
Although acquired immunity could be responsible for the decline in carriage in infants over time, this does not explain the finding that around one-quarter of the infant group did not appear to carry S. aureus at any time point. It seems likely, therefore, that one or more additional host factors are involved. One possible explanation for the apparent inability to carry S. aureus is a lack of host receptors for bacterial adherence in the nares. This is supported by a study which found that adherence of S. aureus to desquamated epithelial cells from carriers was significantly greater than that for noncarriers (1), although the nature of putative epithelial cell receptors is unknown.
Environmental factors were also shown to influence carriage of S. aureus. One possible reason why breastfed infants had a greater chance of carriage is that S. aureus may colonize the nipples, an idea that is consistent with the fact that breast infections with S. aureus during lactation are relatively common. The presence of older siblings may increase the risk of carriage through an increased chance of being in regular and close contact with an S. aureus carrier; however, this association was weak and may have been due to chance.
The observation that bacterial strains were often the same in mother-infant pairs suggests that the mother is the usual source and that the strain acquired is therefore dictated by environmental factors. It is also possible that shared host genetics determine the strain carried by imposing restriction barriers to certain strains. This seems unlikely, however, since the carriage strain appeared to be established by the time of the first infant swab at 2 weeks of age, and the mechanism of restriction to given strains would probably rely on a developed immune response. Bacterial factors may, nonetheless, influence the strain carried once primary colonization is established through a process of colonization resistance, where a resident strain of S. aureus resists replacement by a second S. aureus strain (3, 12, 13, 21). This may explain why most individuals carried a single ST throughout the study period. Bacterial factors also had an effect on transmissibility, with strains with common STs being more frequent in both mother and infant than in one of the pair alone. It is interesting to speculate whether this is one of the factors on which bacterial ecological success relies.
Studies defining the relevance of bacterial interference between members of the flora residing in the nose on S. aureus carriage are few to date; Uehara et al. found the rate of methicillin-resistant S. aureus acquisition by newborn infants in a neonatal intensive care unit to be lower in those who were colonized in the mouth and nose with viridans group streptococci than in those who were not (25). The same investigator reported a low incidence of nasal S. aureus carriage in hospital staff with nose swabs positive for corynebacteria (26). A recent cross-sectional study by Lina et al. reported that S. aureus colonization was lower in individuals colonized by Corynebacterium spp. and/or S. epidermidis (14). This effect was dependent on the number of colonies present and was seen when >103 colonies of Corynebacterium spp. or >102 S. epidermidis organisms were present. Our data based on qualitative culture suggest that bacterial interference occurs early in infancy in the form of antagonism between S. aureus and other bacterial members of the normal flora, and the longitudinal design of the study allowed us to conclude that this is not sustained. The observation that early isolation of S. aureus predicted carriage at 24 weeks may reflect predetermination of S. aureus carriage at birth by host factors in a proportion of individuals. However, the antistaphylococcal effect which increased with age implies that acquired host factors, such as the development of an immune response, modulate the number of individuals ultimately carrying S. aureus.
In conclusion, determining the individual roles of host, environmental, and bacterial factors in the process of S. aureus carriage is complex. The findings of this study suggest that host factors play a key role in this process. Environmental factors also influence carriage status, whereas bacterial factors appear to be involved in defining the strain carried rather than the actual carriage status. Bacterial interference, while present early after birth, did not appear to determine S. aureus carriage by the age of 6 months. We propose that host factors are of overriding importance and that studies of host genetic susceptibility to carriage may help to elucidate the nature of these.
Acknowledgments
We thank Lesley Daniels, Marion Archer, and Linda Diggle, who performed the nasal swabs. We are very grateful to the mothers and infants who participated in this study.
This project was supported by Wellcome Trust Grants awarded to S.P., D.C., and N.D.
REFERENCES
- 1.Aly, R., H. I. Shinefield, W. G. Strauss, and H. I. Maibach. 1977. Bacterial adherence to nasal mucosal cells. Infect. Immun. 17:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boelaert, J. R., H. W. Van Landuyt, C. A. Godard, R. F. Daneels, M. L. Schurgers, E. G. Matthys, Y. A. De Baere, D. W. Gheyle, B. Z. Gordts, and L. A. Herwaldt. 1993. Nasal mupirocin ointment decreases the incidence of Staphylococcus aureus bacteraemias in haemodialysis patients. Nephrol. Dial. Transplant. 8:235-239. [PubMed] [Google Scholar]
- 3.Boris, M., H. R. Shinefield, P. Romano, D. P. McCarthy, and A. L. Florman. 1968. Bacterial interference. Protection against recurrent intrafamilial staphylococcal disease. Am. J. Dis. Child. 115:521-529. [DOI] [PubMed] [Google Scholar]
- 4.Cole, A. M., P. Dewan, and T. Ganz. 1999. Innate antimicrobial activity of nasal secretions. Infect. Immun. 67:3267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Silva, G. D., A. Justice, A. R. Wilkinson, J. Buttery, M. Herbert, N. P. Day, and S. J. Peacock. 2001. Genetic population structure of coagulase-negative staphylococci associated with carriage and disease in preterm infants. Clin. Infect. Dis. 33:1520-1528. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenkranz, N. J. 1966. Nasal rejection of experimentally inoculated Staphylococcus aureus: evidence for an immune reaction in man. J. Immunol. 96:509-517. [PubMed] [Google Scholar]
- 7.Emori, T. G., and R. P. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu, L., A. Umeda, S. Kondo, and K. Amako. 1995. Typing of Staphylococcus aureus colonising human nasal carriers by pulsed-field gel electrophoresis. J. Med. Microbiol. 42:127-132. [DOI] [PubMed] [Google Scholar]
- 10.Kluytmans, J. 1998. Reduction of surgical site infections in major surgery by elimination of nasal carriage of Staphylococcus aureus. J. Hosp. Infect. 40(Suppl. B):S25-S29. [DOI] [PubMed] [Google Scholar]
- 11.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Light, I. J., J. M. Sutherland, and J. E. Schott. 1965. Control of a staphylococcal outbreak in a nursery: use of bacterial interference. JAMA 193:699-704. [DOI] [PubMed] [Google Scholar]
- 13.Light, I. J., L. Walton, J. M. Sutherland, H. R. Shinefield, and V. Brackvogel. 1967. Use of bacterial interference to control a staphylococcal nursery outbreak: deliberate colonization of all infants with a 502A strain of Staphylococcus aureus. Am. J. Dis. Child. 113:291-300. [DOI] [PubMed] [Google Scholar]
- 14.Lina, G., F. Boutite, A. Tristan, M. Bes, J. Etienne, and F. Vandenesch. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl. Environ. Microbiol. 69:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 16.Luzar, M. A., G. A. Coles, B. Faller, A. Slingeneyer, G. D. Dah, C. Briat, C. Wone, Y. Knefati, M. Kessler, and F. Peluso. 1990. Staphylococcus aureus nasal carriage and infection in patients on continuous ambulatory peritoneal dialysis. N. Engl. J. Med. 322:505-509. [DOI] [PubMed] [Google Scholar]
- 17.Moellering, R. C., Jr. 1998. Problems with antimicrobial resistance in gram-positive cocci. Clin. Infect. Dis. 26:1177-1178. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen, M. H., C. A. Kauffman, R. P. Goodman, C. Squier, R. D. Arbeit, N. Singh, M. M. Wagener, and V. L. Yu. 1999. Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Ann. Intern. Med. 130:221-225. [DOI] [PubMed] [Google Scholar]
- 19.Peacock, S. J., G. D. de Silva, A. Justice, A. Cowland, C. E. Moore, C. G. Winearls, and N. P. Day. 2002. Comparison of multilocus sequence typing and pulsed-field gel electrophoresis as tools for typing Staphylococcus aureus isolates in a microepidemiological setting. J. Clin. Microbiol. 40:3764-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Fontan, M., T. Garcia-Falcon, M. Rosales, A. Rodriguez-Carmona, M. Adeva, I. Rodriguez-Lozano, and J. Moncalian. 1993. Treatment of Staphylococcus aureus nasal carriers in continuous ambulatory peritoneal dialysis with mupirocin: long-term results. Am. J. Kidney Dis. 22:708-712. [DOI] [PubMed] [Google Scholar]
- 21.Shinefield, H. R., J. C. Ribble, M. Boris, and H. F. Eichenwald. 1963. Bacterial interference: its effect on nursery-acquired infection with Staphylococcus aureus. I. Preliminary observations on artificial colonization of newborns. Am. J. Dis. Child. 105:646-654. [PubMed] [Google Scholar]
- 22.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg, J. P., C. C. Clark, and B. O. Hackman. 1996. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin. Infect. Dis. 23:255-259. [DOI] [PubMed] [Google Scholar]
- 24.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uehara, Y., K. Kikuchi, T. Nakamura, H. Nakama, K. Agematsu, Y. Kawakami, N. Maruchi, and K. Totsuka. 2001. Inhibition of methicillin-resistant Staphylococcus aureus colonization of oral cavities in newborns by viridans group streptococci. Clin. Infect. Dis. 32:1399-1407. [DOI] [PubMed] [Google Scholar]
- 26.Uehara, Y., H. Nakama, K. Agematsu, M. Uchida, Y. Kawakami, A. S. Abdul Fattah, and N. Maruchi. 2000. Bacterial interference among nasal inhabitants: eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J. Hosp. Infect. 44:127-133. [DOI] [PubMed] [Google Scholar]
- 27.Van Belkum, A., N. H. Riewarts Eriksen, M. Sijmons, W. Van Leeuwen, M. Van den Bergh, J. Kluytmans, F. Espersen, and H. Verbrugh. 1997. Coagulase and protein A polymorphisms do not contribute to persistence of nasal colonisation by Staphylococcus aureus. J. Med. Microbiol. 46:222-232. [DOI] [PubMed] [Google Scholar]
- 28.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 29.Weinke, T., R. Schiller, F. J. Fehrenbach, and H. D. Pohle. 1992. Association between Staphylococcus aureus nasopharyngeal colonization and septicemia in patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 11:985-989. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein, H. J. 1959. The relationship between the nasal staphylococcal carrier state and the incidence of post-operative complications. N. Engl. J. Med. 260:1303-1308. [DOI] [PubMed] [Google Scholar]
- 31.Yu, V. L., A. Goetz, M. Wagener, P. B. Smith, J. D. Rihs, J. Hanchett, and J. J. Zuravleff. 1986. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N. Engl. J. Med. 315:91-96. [DOI] [PubMed] [Google Scholar]