Abstract
Background
Agonist replacement treatment is a promising strategy to manage cannabis-use disorders. The aim of this study was to assess the combined effects of the synthetic cannabinoid agonist nabilone and Δ9-tetrahydrocannabinol (Δ9-THC) using drug-discrimination procedures, which are sensitive to drug interactions. Testing the concurrent administration of nabilone and Δ9-THC was also conducted to provide initial safety and tolerability data, which is important because cannabis users will likely lapse during treatment.
Methods
Six cannabis users learned to discriminate 30 mg oral Δ9-THC from placebo and then received nabilone (0, 1 and 3 mg) and Δ9-THC (0, 5, 15 and 30 mg), alone and in combination. Subjects completed the Multiple-Choice Procedure to assess drug reinforcement, and self-report, task performance and physiological measures were collected.
Results
Δ9-THC and nabilone alone shared discriminative-stimulus effects with the training dose of Δ9-THC, increased crossover point on the Multiple-Choice Procedure, produced overlapping subject ratings and decreased skin temperature. Nabilone alone also elevated heart rate. In combination, nabilone shifted the discriminative-stimulus effects of Δ9-THC leftward/upward and enhanced Δ9-THC effects on the other outcome measures.
Conclusions
These results replicate a previous study demonstrating that nabilone shares agonist effects with the active constituent of cannabis in cannabis users, and contribute further by indicating that nabilone would likely be safe and well tolerated when combined with cannabis. These data support the conduct of future studies to determine if nabilone treatment would produce cross-tolerance to the abuse-related effects of cannabis and reduce cannabis use.
Keywords: agonist replacement, drug-discrimination, marijuana, multiple-choice procedure, subjective effects, time reproduction, repeated acquisition task, digit-symbol-substitution task, cardiovascular, temperature
1. Introduction
Cannabis remains the most commonly used illicit drug in the United States and worldwide (Leggett, United Nations Office on Drugs and Crime, 2006). Data from the 2008 National Survey on Drug Use and Health (NSDUH; SAMHSA, 2009) indicate that 10% of the U.S. population aged 12 and over (25.8 million persons) have used cannabis in the past year. For comparison, the number of people who have used all other illicit drugs combined is slightly less (8%). Moreover, approximately a quarter (4.2 million persons) of past-year cannabis users meet criteria for cannabis-use disorders (DSM IV-TR; American Psychiatric Association, 2000), with the total number of users meeting criteria for cannabis-use disorders being at least twice that of any other illicit drug (SAMHSA, 2009). Results from the Treatment Episode Data Set for 2007 (SAMHSA, 2009) indicated that admissions to publicly funded treatment programs for primary cannabis related-disorders (16% of admissions for all substances) is comparable to cocaine (13%) and opiates (19%). Similarly, rates of lapse and relapse for cannabis use are comparable to other illicit drugs, and in one report were as high as 71% in 82 individuals who attained 2 weeks of initial abstinence (Moore and Budney, 2003). These epidemiological and clinical data underscore the need for effective therapies for cannabis-use disorders. Until recently, however, there has been relatively limited research that has focused on the development of pharmacological adjuncts to treatment.
Agonist replacement treatment is a particularly effective treatment for opioids, nicotine and stimulant substance-use disorders (e.g., Fant et al., 2009; Grabowski et al., 2004; Ling et al., 1994). The rationale for agonist pharmacotherapies is that they could reduce drug seeking and drug use through several mechanisms, including the reduction of other agonist effects through cross-tolerance, satiety and the prevention of withdrawal. Repeated, daily administration of Δ9-tetrahydrocannabinol (Δ9-THC; a cannabinoid agonist and the primary active constituent of cannabis) produces these effects when combined with cannabis. For example, Jones and colleagues (1981) reported that cannabis-induced subject ratings of Intoxicated, elevated heart rate, and decreased peripheral temperature and intraocular pressure were attenuated during Δ9-THC maintenance. Hart and colleagues (2002) examined cannabis self-administration using a choice procedure during Δ9-THC maintenance. Oral Δ9-THC did not significantly reduce cannabis self-administration, but did attenuate some of the “positive” subjective effects (i.e., Good Drug Effect and High). In addition, repeated Δ9-THC administration reduces the signs and symptoms of spontaneous cannabis withdrawal under controlled laboratory conditions (Haney et al., 2004; Jones et al., 1981; Budney et al., 2007). In agreement with laboratory data suggesting that Δ9-THC produces effects that overlap with other efficacious agonist replacement medications, two recent case reports suggested that treatment with oral Δ9-THC (with concomitant medications) was effective for abstinence induction in cannabis-dependent individuals (Levin and Kleber, 2008).
Although results with Δ9-THC are promising, cannabinoid agonist medications other than Δ9-THC are needed as treatment options. Because abstinence from smoked cannabis is most efficiently verified by toxicology testing for Δ9-THC or its primary metabolite 11-nor-9-carboxy-Δ9-THC, urine samples from individuals maintained on Δ9-THC would also test positive, making the objective determination of cannabis abstinence difficult. Although there are certain metabolites that can be detected after the administration of smoked cannabis but not oral Δ9-THC (e.g., Δ9-tetrahydrocannabivarin; ElSohly et al., 2001), a recent study demonstrated that other metabolites might not be reliable indicators of recent cannabis use because their concentrations vary across strains of cannabis (Levin et al., 2010). Despite this major limitation, there are no published clinical studies that have evaluated the potential therapeutic use of cannabinoid agonists other than Δ9-THC for cannabis-use disorders.
Nabilone (Cesamet®) is a synthetic cannabinoid used to treat nausea associated with cancer chemotherapy. Nabilone is a potent cannabinoid agonist, having an affinity of 2.2 nM for human CB1 receptors and 1.8 nM for human CB2 receptors (Gareau et al., 1996). With respect to intrinsic receptor efficacy, a recent study indicated that nabilone was more effective at stimulating [35S]GTPγS binding in mouse whole brain tissue compared to Δ9-THC, but less effective than the synthetic cannabinoid CP55940 (Baillie et al., 2010). Laboratory studies that have compared nabilone to Δ9-THC in animals have demonstrated similar behavioral and physiological responses (Stark and Dews, 1980a,b), and nabilone occasions drug-appropriate responding in rats trained to discriminate Δ9-THC (Browne and Weissman, 1981). In humans, nabilone also engendered drug-appropriate responding in subjects who learned to discriminate Δ9-THC and produced a profile of subject-rated effects that was similar to cannabis and Δ9-THC (Lile et al., 2010). Importantly, nabilone is not cross-reactive in urine assays used to detect recent cannabis use (Fraser and Meatherall, 1989). Together, these data indicate that nabilone is a promising synthetic cannabinoid agonist for evaluation as a potential replacement medication.
In the present study, the separate and combined effects of nabilone and Δ9-THC were assessed in cannabis users who had learned to discriminate Δ9-THC. Drug discrimination procedures were chosen because the data from these procedures are concordant with the central actions of a drug at the receptor level, and therefore are a sensitive means to evaluate drug interactions (Holtzman and Locke, 1988). In addition, these procedures provided initial safety and tolerability data for the combination of nabilone and Δ9-THC, which is important because cannabis dependent individuals are likely to lapse and use cannabis during treatment. The findings from the drug discrimination task were supplemented by testing the reinforcing, subject-rated, performance, cardiovascular and thermoregulatory effects of nabilone and Δ9-THC combinations.
2. Methods
2.1 Subjects
Adult men and women with a history of cannabis use were recruited from the local community. Potential subjects completed demographic, drug-use history and medical history questionnaires, as well as medical screens. Individuals with current or past histories of Axis I or DSM IV psychiatric disorder, including substance dependence disorders (except nicotine), were excluded from participating. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document.
Six subjects (3 white males, 1 black male, 2 white females) completed the experiment. They ranged in age from 19 to 29 years (median = 24 years), in education from 11 to 16 years (median = 15), and in weight from 52 to 145 kg (median = 72 kg). All subjects reported cannabis use (range of 1–7 days/week; mean = 5.3). Subjects reported consuming 0 to 12 standard alcohol-containing beverages per week (mean = 2.8). Three subjects reported using 1–3 tobacco cigarettes per day. Other lifetime non-medical drug use was reported, but the frequency of use was low and no use was reported in the month prior to screening.
2.2 General Procedures
Subjects were enrolled as outpatients at the University of Kentucky General Clinical Research Center. They completed two drug-free practice sessions to become familiarized with the procedures prior to completing between 23 and 26 (mean = 24.7) experimental sessions. Study participation lasted over a period of 7–14 weeks (mean = 10).
Subjects were informed that they would receive placebo, Δ9-THC and nabilone and that these drugs could be given alone or in combination, but were blind to the dose and order of administration. They were asked to abstain from illicit drugs other than cannabis for the duration of the experiment, and any drug use on the day of experimental sessions to avoid potentially unsafe drug interactions. They were also asked to avoid any over-the-counter medication, with the exception of non-steroidal anti-inflammatory analgesics. In addition, subjects were asked to refrain from food or caffeine intake for 4 hours prior to each experimental session, or alcohol for 12 hours prior to and following each experimental session. Subjects who smoked tobacco cigarettes were also asked to abstain from smoking the morning of each session, but were allowed to smoke a single tobacco cigarette upon arrival to the laboratory to avoid testing under conditions of nicotine withdrawal. They were not allowed to smoke again until the session had ended.
Experimental sessions were conducted at a fixed time, Monday through Friday; subjects participated in 1-5 sessions per week. Individual subjects were tested separately in a standard hospital room. At the beginning of each session, breath (Alcolyzer, AK Solutions USA, Palisades Park, NJ) and urine tests to assess drug use (Integrated E-Z Split Cut, Acon Laboratories, San Diego, CA) and possible pregnancy (hCG Assay, Rapid Detect, Inc., Poteau, OK) were conducted. Urine samples were negative for substances other than cannabis metabolites (i.e., 11-nor-9-carboxy-Δ9-THC) and hCG throughout the study. Subjects then completed a modified version of the U.S. Department of Transportation Drug Evaluation and Classification Screening (walk and turn, timed one-leg balance or Romberg balance, time interval reproduction and the finger-to-nose tests; Toland and Green, 1991) and were observed by the research staff for signs of cannabis intoxication (e.g., bloodshot, glassy eyes); no cannabis intoxication was detected during intake throughout the study. Subjects then consumed a low-fat snack approximately 20 minutes prior to drug administration.
2.3 Drug-Discrimination Procedure
Well-established drug-discrimination procedures (e.g., Lile et al., 2009) were used to teach subjects to discriminate between a “Drug X” condition (i.e., 30 mg Δ9-THC) and a “Not Drug X” condition (i.e., placebo).
2.3.1 Sampling Phase
During two sampling sessions, subjects ingested four capsules that contained a total of 30 mg Δ9-THC. The capsules were identified by a letter code (e.g., Drug X; a unique letter code used for each subject); subjects were not informed that the capsules contained Δ9-THC.
2.3.2 Control Phase
The control phase was conducted to determine whether subjects could discriminate 30 mg Δ9-THC from placebo. During this phase, subjects ingested capsules under double-blind conditions. The order of drug administration was random except that all subjects received each training condition, 30 mg Δ9-THC and placebo, at least twice every four sessions. The criterion for having acquired the discrimination was ≥ 80% correct responding on the drug-discrimination task during the final 5-h assessment for four consecutive sessions. If a subject did not meet the control criteria within 12 sessions, they were dismissed from the study.
2.3.3 Test Phase
Drug doses administered during the test phase included placebo, Δ9-THC (5, 15 and 30 mg) and nabilone (1 and 3 mg), alone and in combination. The acute recommended dosing range in adults for Δ9-THC is 2.5–10 mg and 1–2 mg for nabilone (PDR, 2000). Each drug dose and dose combination was administered once. The order of drug administration was random during this phase except that an active drug dose was never administered on more than three consecutive sessions, and the highest dose of Δ9-THC (30 mg) and nabilone (3 mg) were not administered together before a lower dose combination was tested.
Control sessions (i.e., 30 mg Δ9-THC or placebo) were also included in the test phase to monitor drug-discrimination performance, and comprised approximately 28% of sessions during the test phase. If a subject responded incorrectly on a control session, additional control sessions were scheduled until the subject accurately identified both of the training conditions once each across consecutive sessions.
2.4 Outcome measures
Drug discrimination was the primary outcome measure, supplemented by self-report questionnaires, performance tasks, physiological assessments, and a measure of drug reinforcement. Data were collected in fixed order, immediately prior to drug administration, and 1, 2, 3, 4 and 5 h after drug administration, with the following exceptions. The drug-discrimination task was completed only 3, 4 and 5 h after drug administration because of the slow onset (3–4 h) of the effects of Δ9-THC observed in our previous studies (Lile et al., 2009, 2010). A Multiple-Choice Procedure was completed at the end of the 5-h assessment. Except for physiological assessments, data were collected on an Apple Macintosh computer (Apple Computer, Inc., Cupertino, CA).
2.4.1 Drug-Discrimination Task
Two circles labeled Drug X and Not Drug X and associated counters were displayed on a computer screen. Button presses increased the counter for a particular circle according to a fixed-interval 1-sec schedule for 60 s. At the end of each session, following presentation of the final task battery, subjects were informed whether it was a control session, (i.e., Drug X or Not Drug) or a test session. During control sessions, points accumulated on the correct option were exchangeable for money at a rate of $0.23/point. Thus, subjects were able to earn up to approximately $40.00/session on this task. During test sessions, when drugs and/or doses other than the control conditions were administered, subjects earned the average from all previous sessions in which control conditions were tested. These monetary contingencies prompted subjects to acquire points on the counters based on the presence (or absence) of the training drug cue at the time of task performance during both control and test sessions. The dependent variable for this task was the percent responding on the drug-appropriate option at the 5-h time point.
2.4.2 Multiple-Choice Procedure
This task provides a contingency-based assessment of the monetary value of each dose condition and is an efficient and valid method to assess drug reinforcement in humans (Griffiths et al., 1993, 1996). Subjects made a series of nine discrete choices between a drug dose and ascending amounts of money available as a gift card to a local grocery store. The dollar value of this gift card increased across the choices ($0.10, 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00 and 32.00). Subjects randomly selected one of their previous choices at the end of the penultimate experimental session. This choice (drug or gift card) was then presented to the subject during a final session. The dependent measure on the Multiple-Choice Procedure was the maximum dollar value at which subjects chose drug over money (i.e., “crossover point”).
2.4.3 Visual Analog Scale (VAS) Subject-Rated Drug-Effect Questionnaire
Subjects rated 20 items (I feel: any drug effect, a bad drug effect, dizzy, forgetful, a good drug effect, high, hungry, nauseated, restless, a rush, shaky or jittery, stimulated, stoned, suspicious, thirsty; I am having difficulty concentrating; I am seeing or hearing unusual things; I like the drug effect; I would pay for the drug; I would take this drug again) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely”.
2.4.4 Performance Tasks
These tasks were chosen because prior research has found that following tasks are sensitive to the impairing effects of oral Δ9-THC (Hart et al., 2005; Kamien et al., 1994) or smoked cannabis (e.g., Heishman et al., 1989; Kelly et al., 1990, 1993; Wilson et al., 1994).
2.4.5 Digit-Symbol-Substitution Test (DSST)
A modified version of the computerized DSST was used (Griffiths et al., 1993, 1996). Briefly, subjects used a numeric keypad to enter the geometric pattern associated with one of nine patterns identified on a given trial for 90 s. The dependent measures were the number of patterns the subject entered correctly (i.e., trials correct) and the total number of patterns entered (i.e., trials completed).
2.4.6 Repeated Acquisition of Response Sequences Task (RA task)
Subjects pressed 4 keys (1, 3, 7 and 9) on a numeric keypad to learn a new, randomly-determined 10-response sequence (a “chain”) for 180 s. When a correct key in the sequence was pressed, a “position” counter on the screen increased by 1. When the tenth and final key in the sequence was pressed, a “points” counter increased by one, and the position counter reset. A 60-s performance version of this task was also included, in which the 10-response sequence remained the same across trials. The primary dependent measures for this task were the number of chains completed, the number of errors committed and the percentage of correct responses.
2.4.7 Time Reproduction Task
Four time periods, 3, 30, 60 and 180s were presented. Subjects responded to start a timer, and held down the response key until they believed that the interval had elapsed.
2.4.8 Physiological Indices
2.4.8.1 Heart Rate and Blood Pressure
Heart rate and blood pressure were recorded using an automated monitor (DINAMAP, Johnson and Johnson, Alexandria, TX).
2.4.8.2 Temperature
An infrared thermographic scanner (Derma-Temp, Exergen Corporation, Watertown, MA) was used to measure skin temperature on the tip of the index finger. A standard thermometer was used to measure oral temperature.
2.5 Drug Administration
During each experimental session, subjects ingested four opaque green size 00 capsules. Doses of Δ9-THC and nabilone were prepared by encapsulating commercially available capsules of Marinol® (Δ9-THC in sesame oil, Solvay Pharmaceuticals, Marietta, GA) and/or Cesamet® (Valeant Pharmaceuticals North America, Aliso Viejo, CA). Cornstarch was used to fill the remainder of all capsules. Placebo capsules contained only cornstarch.
2.6 Data Analyses
Drug-discrimination data were analyzed using two-factor, repeated-measure analysis of variance (ANOVA; JMP, SAS Institute Inc., Cary, NC) with Δ9-THC and nabilone as the factors. For the 30 mg Δ9-THC and placebo conditions, data were averaged across the sessions in which these conditions were presented during the test phase. Raw data from the self-reported drug-effect questionnaires, performance tasks and physiological measures were analyzed for each drug as the peak-effect (i.e., the mean of the maximum or minimum value observed for each subject 1–5 hr after drug administration) using two-factor, repeated-measure ANOVA. Peak-effects analysis was used rather than Area-Under-the-Curve or using three-factor ANOVA to facilitate data presentation and interpretation, both in the Results section and in the Figures. Crossover point data from the Multiple-Choice Procedure were first subjected to a square-root transformation because of violations in the assumptions of ANOVA (i.e., monetary increments across successive choices range from $0.15 to $16.00). For all measures, effects were considered significant for p ≤ 0.05. If a main effect of nabilone, Δ9-THC or their interaction attained statistical significance, contrast statements were used to compare active drug doses to placebo, and to compare each dose of Δ9-THC alone compared to that dose of Δ9-THC administered in combination with nabilone.
3. Results
3.1 Drug-discrimination task
All subjects met the discrimination criterion, which took an average of 5.3 sessions (range = 4–9). During the final four sessions of the control phase, subjects reported an average of 0.0 (SEM = 0.0) percent Δ9-THC-appropriate responding on the drug-discrimination task during placebo sessions and 100.0 (SEM = 0.0) percent drug-appropriate responding during sessions when the training dose of Δ9-THC (i.e., 30 mg) was administered.
During the test phase, placebo and the training dose of Δ9-THC occasioned an average of 10.8 (SEM = 4.9) and 100 (SEM = 0.0) percent Δ9-THC-appropriate responding, respectively. In general, discriminative control of behavior was maintained throughout the test phase. Four of the six subjects correctly identified placebo, and all subjects correctly identified 30 mg Δ9-THC, every time a training condition was presented during the test phase. Two subjects inaccurately identified the placebo condition during the test phase on a single occasion, but correctly identified each of the training conditions when each was administered during the next two consecutive sessions.
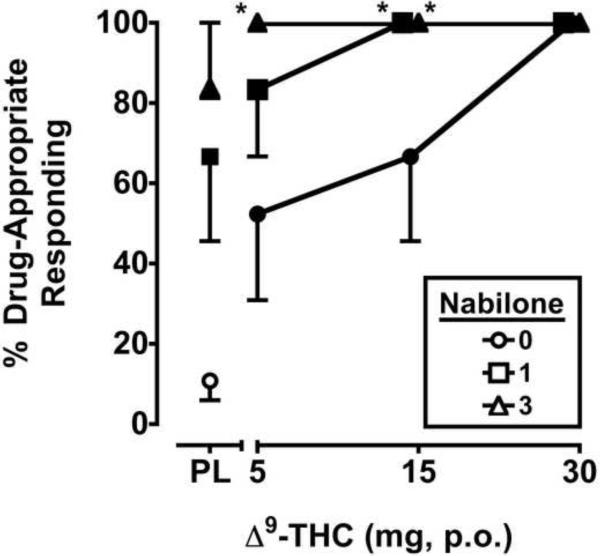
The two-factor, repeated-measure ANOVA revealed significant main effects of Δ9-THC (F3,15 = 12.9, p ≤ 0.001) and nabilone (F2,10 = 9.3, p ≤ 0.01) for percentage of Δ9-THC-appropriate responding. Δ9-THC dose-dependently increased drug-appropriate responding on the drug-discrimination task. Nabilone alone also dose-dependently increased drug-appropriate responding, and significantly shifted the discriminative-stimulus effects of the 5 and 15 mg doses of Δ9-THC leftward/upward (Figure 1).
Figure 1.
Separate and combined effects of Δ9-THC and nabilone on Δ9-THC-appropriate responding on the drug-discrimination task. Filled symbols indicate values that are significantly different from placebo. Asterisks indicate combinations of Δ9-THC and nabilone that are significantly different from that dose of Δ9-THC alone. The x-axis represents the Δ9-THC dose in mg; PL denotes placebo. Data points show means of 6 subjects. Uni-directional brackets indicate 1 SEM.
3.2 Multiple-Choice Procedure
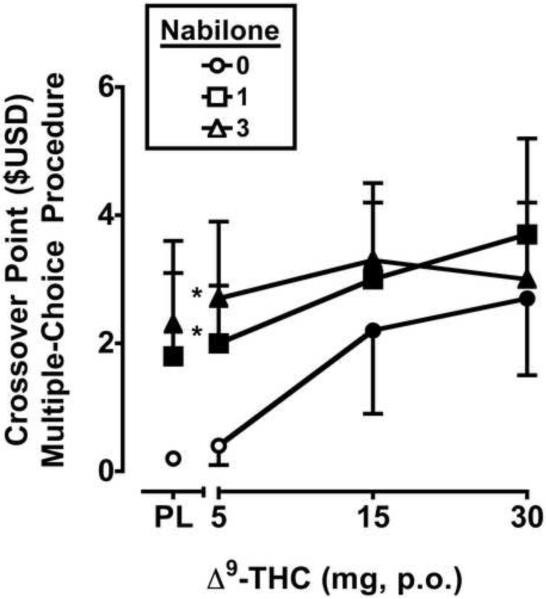
Significant main effects were observed for Δ9-THC (F3,15 = 12.9, p ≤ 0.001) and nabilone (F2,10 = 9.3, p ≤ 0.01) on the crossover point. Δ9-THC and nabilone dose-dependently increased crossover points, suggesting that both drugs functioned as reinforcers. Nabilone significantly increased the reinforcing effects of the 5 mg dose of Δ9-THC (Figure 2).
Figure 2.
Crossover Point in $USD for Δ9-THC and nabilone, alone and in combination, on a Multiple-Choice Procedure. All other details are as in Figure 1.
3.3 Subject Ratings
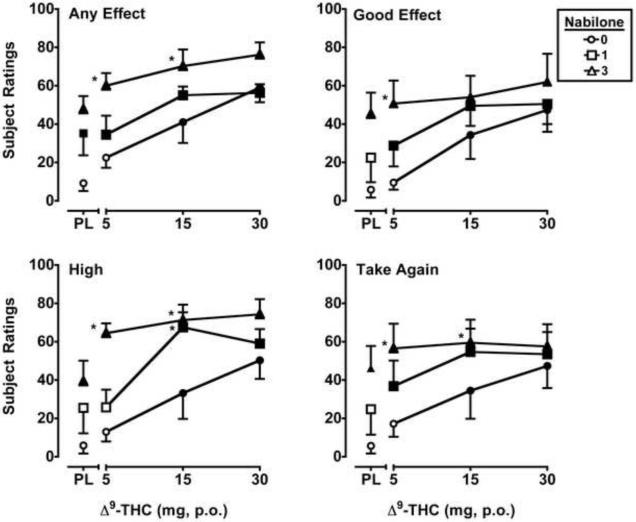
Δ9-THC (F's3,15 = 6.1–15.3, p's ≤ 0.01) and nabilone (F's2,10 = 7.6–25.6, p's ≤ 0.01) dose-dependently increased ratings for seven VAS items: Any Effect*, Good Effects*, High*, Take Again*, Like Drug, Pay For and Thirsty. The data from VAS items marked with an asterisk* are presented in Figure 3. As illustrated in those graphs, both drugs increased ratings on these items alone, and the effects of the 5 mg or 5 and 10 mg doses of Δ9-THC were significantly enhanced by nabilone in a dose-dependent manner. In addition, Δ9-THC increased ratings of Stoned (F3,15 = 6.7, p ≤ 0.01; trend for nabilone, p = 0.06) and Stimulated (F3,15 = 3.4, p ≤ 0.05).
Figure 3.
Peak (maximum value) Visual Analog Scale ratings for Δ9-THC and nabilone, alone and in combination, on the drug-effect questionnaire items Any Effect, Good Effects, High and Take Again. All other details are as in Figure 1.
3.4 Performance
Δ9-THC and nabilone did not impact performance on the DSST and produced relatively small and inconsistent impairment on the RA and Time Reproduction tasks.
Δ9-THC had no effect during either the acquisition or performance components of the RA task. Nabilone had no effect during the acquisition component, but increased the number of errors (F2,10 = 5.4, p ≤ 0.05) and decreased rate (F2,10 = 5.0, p ≤ 0.05) and accuracy (F2,10 = 5.1, p ≤ 0.05) during the performance component. Following placebo administration, subjects committed an average of 7.7 errors (±1.3), which was increased to 13.2 ±3.3 and 11.3 ±2.0 errors after administration of 1 and 3 mg of nabilone, respectively. Decreases in rate and accuracy were observed only when nabilone was combined with 30 mg Δ9-THC (reductions of approximately 20%).
A main effect of nabilone was observed on the Time Reproduction task for the 30-s interval (F2,10 = 11.6, p ≤ 0.01), but planned comparisons revealed significant differences from placebo only when nabilone was combined with Δ9-THC. Compared to placebo, the combination of nabilone and Δ9-THC resulted in an under-reproduction of the 30-s time interval of approximately 6 s for multiple dose combinations. Δ9-THC alone did not affect reproduction of the 30-s interval and neither Δ9-THC or nabilone impacted reproduction of the 3, 60 or 180-s time intervals.
3.5 Heart Rate, Blood Pressure and Temperature
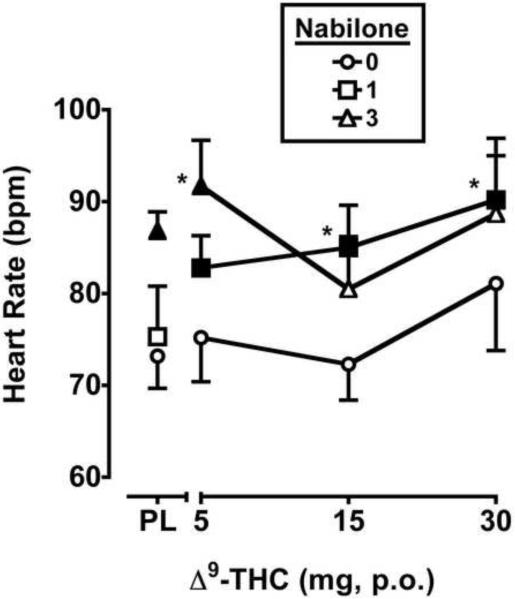
Nabilone, but not Δ9-THC, significantly increased heart rate (F2,10 = 20.5, p ≤ 0.001) when administered alone. Compared to placebo, the 3 mg dose of nabilone alone elevated peak heart rate (Figure 4). In addition, the combination of the 3 mg dose of nabilone with the 5 mg dose of Δ9-THC significantly increased heart rate above placebo levels, and the combination of the 1 mg dose of nabilone raised heart rate above placebo levels at all doses of Δ9-THC. Blood pressure was not impacted by Δ9-THC or nabilone.
Figure 4.
Peak (maximum value) heart rate for Δ9-THC and nabilone, alone and in combination. All other details are as in Figure 1.
An interaction of nabilone and Δ9-THC was found for index finger temperature (F6,30 = 2.8, p ≤ 0.05). Following placebo administration, peak index finger temperature was 29.6 C. Nabilone alone significantly decreased index finger temperature at both doses by approximately 2 degrees C. Likewise, the 15 and 30 mg doses of Δ9-THC also reduced temperature by approximately 2 degrees C. In addition, the combination of 3 mg nabilone with 5 mg Δ9-THC significantly decreased skin temperature compared to that dose of Δ9-THC alone.
4. Discussion
The aim of this study was to assess the separate and combined effects of the synthetic cannabinoid agonist nabilone and Δ9-THC using drug-discrimination procedures. Δ9-THC functioned as a discriminative stimulus and dose-dependently occasioned drug-appropriate responding. Both nabilone doses occasioned Δ9-THC-appropriate responding, consistent with its actions as a non-selective CB1 and CB2 agonist like Δ9-THC. These results are in agreement with a previous study in which a wider range of nabilone doses (1, 2, 3 and 5 mg) dose-dependently substituted for the Δ9-THC discriminative stimulus (Lile et al., 2010). When combined with Δ9-THC, nabilone increased drug-appropriate responding, resulting in full substitution of the two lowest doses of Δ9-THC. This leftward/upward shift in the dose-effect curve is consistent with what would be predicted following the acute administration of two agonists sharing a common mechanism of action.
Relatively few studies in humans have evaluated the effects of a putative agonist pharmacotherapy on the discriminative-stimulus effects of an abused drug. In one recent study, sustained release d-amphetamine was administered as a pretreatment in subjects who had learned to discriminate methamphetamine (Vansickel et al., unpublished data). d-Amphetamine enhanced the discriminative-stimulus effects of all but the training dose of methamphetamine. Similarly, another study demonstrated the ability of pretreatment with an oral dose of cocaine to increase drug-appropriate responding in subjects who had learned to discriminate intravenous cocaine (Johanson et al., 2007). In addition, application of a transdermal nicotine patch was found to enhance the discriminative-stimulus effects of the training dose of intranasal nicotine in male subjects, and a low dose of intranasal nicotine in women, although the highest doses were attenuated by patch application in the female subjects (Perkins et al., 2001). The present study is in agreement with the previous agonist pretreatment studies, and extends those findings to cannabinoid agonists.
Although the effects of an abused drug would likely be enhanced following acute pretreatment with another agonist, as demonstrated in the present study, human laboratory data indicate that following maintenance dosing with a putative agonist pharmacotherapy, the effects of the abused drug are diminished. For instance, the application of a transdermal nicotine patch for one week significantly decreased the effects of intravenous nicotine in cigarette-smoking stimulant users (Sobel et al., 2004). Similarly, methadone and levo-alpha-acetylmethadol maintenance reduced the effects of heroin and hydromorphone, respectively (Donny et al., 2005; Houtsmuller et al., 1998). A recent study showed that the subject-rated effects of intranasal methamphetamine were attenuated during d-amphetamine maintenance (Rush et al., 2010), consistent with other studies in which the response to acute cocaine administration was evaluated during maintenance on an agonist pharmacotherapy (Collins et al., 2006; Rush et al., 2009; Winhusen et al., 2006). Studies in which cannabis-using subjects were maintained on Δ9-THC agree with these results (Jones et al., 1981; Hart et al., 2002).
In the present study, nabilone significantly increased crossover point on a Multiple-Choice Procedure, suggesting that it functioned as a reinforcer. Nabilone also increased ratings on “positive” items from the self-reported drug-effect questionnaire. Although these results indicate that nabilone might have the potential for abuse in cannabis users, its slow onset, and low availability and high cost compared to cannabis appear to limit its diversion and misuse (Ware et al., 2010). The availability of nabilone to cannabis users as a treatment could increase the likelihood of non-therapeutic use, however. When Δ9-THC and nabilone were combined, their reinforcing and positive subject-rated effects were enhanced, suggesting that the effects of cannabis might be enhanced by administration of nabilone if a lapse in abstinence were to occur. However, a reduction in the effects of cannabis as a result of cross-tolerance appears to be a more likely outcome during nabilone maintenance. Worth noting is that there appears to be a ceiling on the combined effects of nabilone and Δ9-THC on self-reported items and the Multiple-Choice Procedure, further supporting the safety and tolerability of concurrent use of nabilone with cannabis.
Notable impairing effects of nabilone and nabilone-Δ9-THC combinations were limited to the performance component of the Repeated Acquisition Task. One possible explanation for the task-specific performance deficits is that response rates on this task were higher than on any other performance task, and high response rates may be more sensitive to disruption by drug administration (Kelleher and Morse, 1964). That nabilone had minimal impact on other performance measures is consistent with a previous study in cannabis users (Lile et al., 2010) and prior research in non-cannabis users who received repeated doses of nabilone (Frank et al., 2008; Kurzthaler et al., 2005). In contrast, a recent study tested doses of 1–3 mg in healthy subjects and found impairment on measures of attention, psychomotor speed and working and episodic memory (Wesnes et al., 2009). However, these discrepancies across studies can be associated with the development of tolerance to the performance-impairing effects of nabilone following multiple dose administrations (Frank et al., 2008; Kurzthaler et al., 2005), and cross-tolerance from regular use of cannabis (Lile et al., 2010; present findings).
Nabilone significantly increased heart rate, consistent with previous research (Glass et al., 1980; Lemberger et al., 1982; Lile et al., 2010; Mendelson and Mello, 1984). Heart rate was elevated at the 3 mg dose of nabilone alone and when combined with certain doses of Δ9-THC, although worth noting is that the response to the drug combination was not dose dependent. The lack of dose dependency is similar to what was observed in our previous study, in which all doses of nabilone tested, but not Δ9-THC, significantly increased heart rate. The relative effectiveness of nabilone to elevate heart rate compared to Δ9-THC could be a function of the greater intrinsic efficacy of nabilone (Baillie et al., 2010). In 4 of the 6 subjects increases in heart rate following the addition of nabilone to Δ9-THC were not large enough to meet criteria for tachycardia (>100 bpm) or to be of significant clinical concern for an acute drug effect, although two subjects did experience heart rates above 100 bpm following several dose combinations, with a maximum response of 111 and 113 bpm. In these subjects, there were no additional symptoms (e.g., blurry vision, sweating) indicative of a more serious cardiovascular problem. That significant increases in heart rate were observed following concurrent administration of nabilone and Δ9-THC suggest that similar increases could occur following administration of cannabis during nabilone maintenance, highlighting the need for caution and close monitoring of cardiovascular signs in future studies.
Nabilone has several characteristics that make it an attractive candidate agonist replacement medication, most notably the lack of cross-reactivity in toxicology assays to detect recent cannabis use. In addition, the present results replicate our previous study (Lile et al., 2010) by demonstrating that nabilone shares many of the agonist effects of Δ9-THC. These results contribute further by demonstrating that the combination of nabilone and Δ9-THC is safe and well tolerated, suggesting that cannabis use during nabilone maintenance would not pose a serious risk. Future studies using maintenance dosing with nabilone should be conducted to confirm the safety and tolerability of cannabis administration during nabilone treatment and the ability of nabilone maintenance to engender cross-tolerance to the abuse-related effects of cannabis that foster its continued use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Fourth edition, Text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baillie GL, Ross RA, Pertwee RG. The ability of nabilone to interact with cannabinoid CB1 and CB2 receptors. 20th annual symposium of the International Cannabinoid Research Society; Lund, Sweden. 2010. [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J. Clin. Pharmacol. 1981;21:S227–234. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral Δ9-THC suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend. 2006;82:158–167. doi: 10.1016/j.drugalcdep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100:1496–1509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, deWit H, Wachtel SR, Feng S, Murphy TP. Δ9-tetrahydrocannabivarin as a marker for the ingestion of marijuana versus Marinol: results of a clinical study. J. Anal. Toxicol. 2001;25:565–571. doi: 10.1093/jat/25.7.565. [DOI] [PubMed] [Google Scholar]
- Fant RV, Owen LL, Henningfield JE. Nicotine replacement therapy. Prim. Care. 2009;26:633–52. doi: 10.1016/s0095-4543(05)70121-4. [DOI] [PubMed] [Google Scholar]
- Frank B, Serpell MG, Hughes J, Matthews JN, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ. 2008;336:199–201. doi: 10.1136/bmj.39429.619653.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AD, Meatherall R. Lack of interference by nabilone in the EMIT d.a.u. cannabinoid assay, Abbott TDx cannabinoid assay, and a sensitive TLC assay for Δ9-THC-carboxylic acid. J. Anal. Toxicol. 1989;13:240. doi: 10.1093/jat/13.4.240. [DOI] [PubMed] [Google Scholar]
- Gareau Y, Dufresne C, Gallant M, Rochette C, Sawyer N, Slipetz DM, Tremblay N, Weech PK, Metters KM, Labelle M. Structure activity relationships of tetrahydrocannabinol analogues on human cannabinoid receptors. Bioorg. Med. Chem. Lett. 1996;6:189–194. [Google Scholar]
- Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW. A single dose study of nabilone, a synthetic cannabinoid. Psychopharmacology. 1980;71:137–42. doi: 10.1007/BF00434401. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp. Clin. Psychopharmacol. 1996;4:97–106. [Google Scholar]
- Griffiths RR, Troisi JR, II, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav. Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181:237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman WM, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports and performance. Pharmacol. Biochem. Behav. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Locke KW. Neural mechanisms of drug stimuli: experimental approaches. Psychopharmacol. Ser. 1988;4:138–153. [PubMed] [Google Scholar]
- Houtsmuller EJ, Walsh SL, Schuh KJ, Johnson RE, Stitzer ML, Bigelow GE. Dose-response analysis of opioid cross-tolerance and withdrawal suppression during LAAM maintenance. J. Pharmacol. Exp. Ther. 1998;285:387–396. [PubMed] [Google Scholar]
- Johanson CE, Lundahl LH, Schubiner H. Effects of oral cocaine on intravenous cocaine discrimination in humans. Exp. Clin. Psychopharmacol. 2007;15:219–227. doi: 10.1037/1064-1297.15.3.219. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J. Clin. Pharmacol. 1981;21:S143–152. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Higgins ST, Hughes JR. The effects of delta 9-tetrahydrocannabinol on repeated acquisition and performance of response sequences and on self-reports in humans. Behav. Pharmacol. 1994;5:71–78. doi: 10.1097/00008877-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol and diazepam. J. Anal. Toxicol. 1993;17:264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Fischman MW. Multidimensional behavioral effects of marijuana. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1990;14:885–902. doi: 10.1016/0278-5846(90)90075-r. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb. Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Kurzthaler I, Bodner T, Kemmler G, Entner T, Wissel J, Berger T, Fleischhacker WW. The effect of nabilone on neuropsychological functions related to driving ability: an extended case series. Hum. Psychopharmacol. 2005;20:291–293. doi: 10.1002/hup.688. [DOI] [PubMed] [Google Scholar]
- Leggett T. United Nations Office on Drugs and Crime. A review of the world cannabis situation. Bull. Narc. 2006;58:1–155. [PubMed] [Google Scholar]
- Lemberger L, Rubin A, Wolen R, DeSante K, Rowe H, Forney R, Pence P. Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat. Rev. 1982;9:17–23. doi: 10.1016/s0305-7372(82)80031-5. [DOI] [PubMed] [Google Scholar]
- Levin FR, Kleber HD. Use of dronabinol for cannabis dependence: two case reports and review. Am. J. Addict. 2008;17:161–164. doi: 10.1080/10550490701861177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Xie S, Murray KA. Δ9-tetrahydrocannabivarin testing may not have the sensitivity to detect marijuana use among individuals ingesting dronabinol. Drug Alcohol Depend. 2010;1:65–68. doi: 10.1016/j.drugalcdep.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Substitution profile of the cannabinoid agonist nabilone in humans discriminating Δ9-THC. Clin. Neuropharmacol. 2010;33:235–242. doi: 10.1097/WNF.0b013e3181e77428. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Δ9-tetrahydrocannabinol, triazolam, hydromorphone and methylphenidate in humans discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203:241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. The reinforcing, self-reported performance and physiological effects of Δ9-THC, triazolam, hydromorphone, and methylphenidate in cannabis users. Behav. Pharmacol. 2010;21:29–38. doi: 10.1097/FBP.0b013e32833470d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Rawson RA, Compton MA. Substitution pharmacotherapies for opioid addiction: from methadone to LAAM and buprenorphine. J. Psychoactive Drugs. 1994;26:119–128. doi: 10.1080/02791072.1994.10472259. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Reinforcing properties of oral delta 9-tetrahydrocannabinol, smoked marijuana and nabilone: influence of previous marijuana use. Psychopharmacology. 1984;83:351–356. doi: 10.1007/BF00428544. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. J. Subst. Abuse Treat. 2003;25:85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Lipton MA, Timmons MC, Wall ME, Brine DR, Davis KH. Pharmacology of orally administered Δ9-THC. Clin. Pharmacol. Ther. 1973;14:48–55. doi: 10.1002/cpt197314148. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Meeker J, White W, Wilson A. The discriminative stimulus and reinforcing effects of nicotine in humans following nicotine pretreatment. Behav. Pharmacol. 2001;12:35–44. doi: 10.1097/00008877-200102000-00004. Epub ahead of print. Accessed from http://www.springerlink.com/content/66p66432xtttt187/fulltext.pdf. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Lile JA, Glaser PE, Hays LR. Subjective and physiological effects of acute intranasal methamphetamine during d-amphetamine maintenance. Psychopharmacology. 2010 doi: 10.1007/s00213-010-2067-5. in press. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR. Cocaine effects during d-amphetamine maintenance: a human laboratory analysis of safety, tolerability and efficacy. Drug Alcohol Depend. 2010;99:261–271. doi: 10.1016/j.drugalcdep.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel BF, Sigmon SC, Griffiths RR. Transdermal nicotine maintenance attenuates the subjective and reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigaratte-smoking stimulant abusers. Neuropsychopharmacology. 2004;29:991–1003. doi: 10.1038/sj.npp.1300415. [DOI] [PubMed] [Google Scholar]
- Stark P, Dews PB. Cannabinoids I. Behavioral effects. J. Pharmacol. Exp. Ther. 1980;214:124–130. [PubMed] [Google Scholar]
- Stark P, Dews PB. Cannabinoids II. Cardiovascular effects. J. Pharmacol. Exp. Ther. 1980;214:131–138. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies . Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2009. (NSDUH Series H-36). DHHS Publication No. SMA 09-4434. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies . Treatment Episode Data Set (TEDS). Highlights 2007. National Admissions to Substance Abuse Treatment Services. Rockville, MD: 2009. (DASIS Series: S-45). DHHS Publication No. SMA 09-4360. [Google Scholar]
- Toland SL, Green W. DRE field testing of drug impaired drivers. In: Watts V, editor. The Effects of Drugs on Human Performance and Behavior: Drugs and Driving/Drugs in the Workplace. American Academy of Forensic Sciences; 1991. [Google Scholar]
- Ware MA, St Arnaud-Trempe E. The abuse potential of the synthetic cannabinoid nabilone. Addiction. 2010;105:494–503. doi: 10.1111/j.1360-0443.2009.02776.x. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Annas P, Edgar C, Deeprose C, Karlsten R, Philipp A, Kalliomaki J, Segerdähl M. Nabilone produces marked impairments to cognitive function and changes in subjective state in healthy volunteers. J. Psychopharmacol. 2009;24:1659–1669. doi: 10.1177/0269881109105900. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Matthew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry Res. 1994;51:115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Singal BM, Harrer J, Apparaju S, Mezinskis J, Desai P, Elkashef A, Chiang CN, Horn P. Methylphenidate and cocaine: a placebo-controlled drug interaction study. Pharmacol. Biochem. Behav. 2006;85:29–38. doi: 10.1016/j.pbb.2006.06.023. [DOI] [PubMed] [Google Scholar]