Abstract
Chronic stress is a vulnerability factor for a number of psychiatric disorders, including anxiety and affective disorders. Social defeat in rats has proven to be a useful paradigm to investigate the neural mechanisms underlying physiologic and behavioral adaptation to acute and chronic stress. Previous studies suggest that serotonergic systems may contribute to the physiologic and behavioral adaptation to chronic stress, including social defeat in rodent models. In order to test the hypothesis that repeated social defeat alters the emotional behavior and the excitability of brainstem serotonergic systems implicated in control of emotional behavior, we exposed adult male rats either to home cage control conditions, acute social defeat, or social defeat followed 24 h later by a second social defeat encounter. We then assessed behavioral responses during social defeat as well as the excitability of serotonergic neurons within the dorsal raphe nucleus using immunohistochemical staining of tryptophan hydroxylase, a marker of serotonergic neurons, and the protein product of the immediate-early gene, c-fos. Repeated social defeat resulted in a shift away from proactive emotional coping behaviors, such as rearing (explorative escape behavior), and toward reactive emotional coping behaviors such as freezing. Both acute and repeated defeat led to widespread increases in c-Fos expression in serotonergic neurons in the dorsal raphe nucleus. Changes in behavior following a second exposure to social defeat, relative to acute defeat, were associated with decreased c-Fos expression in serotonergic neurons within the dorsal and ventral parts of the mid-rostrocaudal dorsal raphe nucleus, regions that have been implicated in 1) serotonergic modulation of fear- and anxiety-related behavior and 2) defensive behavior in conspecific aggressive encounters, respectively. These data support the hypothesis that serotonergic systems play a role in physiologic and behavioral responses to both acute and repeated social defeat.
Keywords: dorsal raphe nucleus, serotonin, social defeat, stress, anxiety, fear
1. Introduction
Chronic stress has been identified as an important vulnerability factor for a number of psychiatric disorders, including anxiety disorders such as panic disorder and post-traumatic stress disorder, and affective disorders such as major depressive disorder. The mechanisms through which chronic stress increases vulnerability to anxiety and affective disorders are unclear.
Social defeat, which is the result of intraspecific confrontation between male rats, is an ethologically relevant paradigm that can be used to understand the physiologic and behavioral adaptations to repeated stress. Rats and Syrian hamsters that have experienced a single social defeat display changes in neuroendocrine, autonomic, and behavioral responses [1, 2], including increases in fear- and anxiety-like behaviors [3–5]. Rats exposed to repeated social defeat (i.e., 2 exposures to social defeat 24 h apart) also respond with increases in anxiety-like behaviors [6], but also hippocampal dendritic reorganization [7], decreased food intake and body weight gain [8] and altered sleep patterns [6]. Rats exposed to chronic social defeat, when compared to controls, (i.e., 4–7 daily exposures to social defeat) respond with long-lasting depressive-like behaviors [9], changes in defensive behaviors [10], and long-term impairment of autonomic circadian rhythms [10]. One mechanism through which repeated or chronic social defeat may elicit these physiologic and behavioral adaptations is through altered activity of brainstem neuromodulatory systems, such as serotonergic systems.
Previous studies support a role for serotonergic systems in physiologic and behavioral adaptations following social defeat. A single exposure to social defeat increases serotonergic neuronal activity, as evidenced by increases in expression of the protein product of the immediate-early gene, c-fos [11, 12], and increases in extracellular serotonin within the dorsal raphe nucleus (DR) [13]. Studies by Herbert and colleagues suggest that both acute and chronic social defeat equally increase c-Fos expression within the DR; however, it is unknown whether these increases are in serotonergic neurons or if they are specific to subregions of the DR [14, 15].
A single exposure to social defeat has been found to selectively activate serotonergic neurons in the dorsal part of the mid-rostrocaudal and caudal dorsal raphe nucleus (mid-rostrocaudal DRD and DRC) [11]. Serotonergic neurons in the DRD and DRC have been shown to be activated following exposure to a number of fear- and anxiety-related stimuli, including anxiogenic drugs such as the adenosine receptor antagonist caffeine, the serotonin 5-HT2A/2C receptor agonist m-chlorophenyl piperazine (mCPP), and the partial inverse agonist at the benzodiazepine allosteric site on the γ-aminobutyric acid A (GABAA) receptor, N-methyl-beta-carboline-3-carboxamide (FG-7142) [16], the anxiety-related neuropeptide urocortin 2 (Ucn 2) [17, 18], and inescapable stress [19]. The responses of topographically organized subpopulations of serotonergic neurons following repeated exposure to social defeat have not been tested.
In order to test the hypothesis that behavioral adaptations following exposure to social defeat are associated with changes in the excitability of topographically organized populations of serotonergic neurons, we exposed rats to either a single social defeat, or social defeat followed, 24 h later, by a second social defeat encounter. We then assessed, using immunohistochemical detection of the protein product of the immediate-early gene, c-fos, the functional excitability of serotonergic neurons within topographically organized subregions of the DR, the source of the majority of serotonergic projections to forebrain limbic structures regulating fear and anxiety states.
2. Experimental Procedures
2.1. Subjects
Male Long Evans rats (Harlan Laboratories, Indianapolis, IN, USA; 238–298 g, mean ± S.E.M., 271 ± 1.28 g) were housed in groups of 3 in cages (38 cm W × 48 cm L × 21 cm H; Techniplast cages, Techniplast, Kettering, UK) containing a thin layer of bedding (Cat. No. 7090; Teklad Sani-Chips; Harlan Laboratories). Rats were maintained on a 12 h light/12 h dark cycle (lights on at 0700 h) with free access to food (Cat. No. 8640; Teklad 22/5 Rodent diet, Harlan Laboratories) and tap water stored in 16 oz reduced-height water bottles (Cat. No. WB16RH; Alternative Designs, Siloam Springs, AR, USA) with screw lids (Cat. No. FSPCST2.5; AnCare Corp., Bellmore, NY, USA). Male Long Evans retired breeders (Harlan Laboratories, 411–598 g, mean ± S.E.M., 511 ± 18.2 g) were used as resident males for the social defeat. Residents were singly housed in transparent polycarbonate cages (26 cm W × 47.6 cm L × 20.3 cm H; Cat. No., RC88D-PC, Alternative Designs) and maintained as described above. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Colorado Institutional Animal Care and Use Committee (IACUC).
2.2. Social defeat procedure
Following two days of acclimation, rats were exposed to either no defeat (home cage control, HCC) on days 1 and 2, a social defeat encounter on day 1 (acute defeat, AD) or social defeat encounters, separated by 24 h, on both day 1 and day 2 (repeated defeat, RD). Social defeat occurred during the light phase (~450 lux) at 0800 hr, one hour following lights on. It is common for social defeat experiments to be conducted during either the light phase [11, 20, 21] or the dark phase on a reversed light/dark cycle, usually under a dim red light [14, 22–25]. Social defeat encounters lasted for 20 min and consisted of both a pre-defeat phase (10 min) and defeat phase (10 min). The social defeat encounters occurred in the resident male’s home cage. During the pre-defeat phase the resident and intruder were separated by a transparent 0.3 cm-wide Plexiglas® partition with 9 (0.3 cm diameter) holes drilled 5 cm apart in it so that physical contact was prevented, but visual, auditory and olfactory cues remained. For the defeat phase, the partition was removed allowing the rats to freely interact. The behavior of the intruder and resident was recorded with a digital video camera (Sony Handycam DCR-HC52 and DCR-HC35E, Sony Corporation of America, New York, NY, USA) mounted on a tripod and later quantified “offline” using Noldus, The Observer (Version 5, Noldus Information Technology, Wageningen, The Netherlands) by an experimenter blind to treatment group. Home cage control rats consisted of two groups (home cage control 1 and home cage control 2), which were time matched for perfusion with fixative in preparation for immunohistochemical procedures (see below) with AD rats on day 1 and RD rats on day 2, respectively. No differences in cell counts were observed between home cage control 1 and home cage control 2 groups (see results below), therefore both groups were merged into one group called home cage control (HCC). Home cage control rats were transferred to an adjacent room 1 hr prior to the social defeat period (3 hrs prior to transcardial perfusion), weighed, and returned to their home cages where they remained during the social defeat period for time-matched rats exposed to social defeat.
2.3. Behavioral analysis
The specific behaviors that were scored during both the pre-defeat and defeat phases were based on previous work by Gardner et al. 2005 [11] (Table 1). During the defeat phase the style of behavioral coping was further divided into reactive coping, proactive coping and neutral behaviors (Table 1). Reactive coping includes sniffing bedding, freezing, full submission, sideways submission passive genital sniff and genital sniff. Proactive coping includes rearing, defensive burying, aggression, escape, upright defensive behavior and social interaction. Finally, neutral behaviors consisted of locomotion, self-grooming and inactivity.
Table 1.
Definitions of behavioral categories and individual behaviors quantified during the pre-defeat and defeat phases of social defeat.
| Pre-defeat behaviors | Defeat behaviors | Definition |
|---|---|---|
| Reactive coping | Behaviors with vigilance, anxiety-related, fear-related, or risk assessment components as well as submissive behaviors | |
| Sniffing bedding | Sniffing bedding | Sniffing the substrate without locomotion |
| Freezing | Freezing | Intruder is crouching with his back arched, occasionally exhibiting piloerection, and is motionless except for movement associated with respiration and scanning of the environment with the head |
| Full submission | The intruder lies on its back with its full belly exposed to the resident | |
| Sideways submission | The intruder crouches below the resident and turns to expose part of its belly | |
| Passive genital sniff | Being sniffed by the resident | |
| Genital sniff | Sniffing the resident’s genitals | |
| Proactive coping | Confrontational behaviors and behaviors with exploration or escape components | |
| Rearing* | Rearing* | Bipedal posture |
| Defensive burying | Defensive burying | Shoveling bedding towards the resident |
| Aggression | Biting, kicking, boxing, wrestling and fighting the resident | |
| Escape | Fleeing from the resident | |
| Upright defensive behavior | Rearing while facing the resident | |
| Social interaction | Grooming and sniffing the resident outside the ano-genital region, nosing and crawling over/under the resident | |
| Neutral | ||
| Locomotion | Locomotion | Walking around the cage |
| Self-grooming | Self-grooming | Licking or scratching coat |
| Inactivity | Inactivity | Lying or sitting motionless |
(E.g. Explorative escape as defined by De Boer and Koolhaas, 2003).
2.4. Tissue preparation
Two hours following the onset of social defeat, rats were deeply anesthetized with sodium pentobarbital (Fatal-Plus, MWI Veterinary Supply, Meridian, ID, USA; 200 mg/kg, intraperitoneal (i.p.)) and transcardially perfused with ice-cold 0.05 M phosphate-buffered saline (PBS, pH 7.4) followed by ice-cold 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB) containing 1.5% sucrose. The brains were dissected out and post-fixed overnight in the same fixative, followed by two 12 h washes in 0.1 M PB and then switched to 0.1 M PB containing 30% sucrose for 2–3 days until saturated. Brains were blocked into forebrain and hindbrain sections by placing the brain into a rat brain matrix (RBM-4000C, ASI Instruments, Warren, MI, USA) and bisecting the brain, in the coronal plane, with a razorblade directly caudal to the mammillary bodies (approximately −5.60 mm from bregma). Next, the forebrain and hindbrain sections were flash-frozen with isopentane (cooled between −30 and −40 °C with dry ice) and stored at −80 °C until sectioning. Coronal tissue slices (30 μm) were prepared using a precision cryostat (Leica CM1900) and stored as six alternate sets of sections in 24-well tissue culture plates containing cryoprotectant (30% ethylene glycol, 20% glycerol, 0.05 M PB, pH 7.4) at −20 °C until further immunohistochemical staining.
2.5. Immunohistochemistry
Double-immunohistochemical staining for c-Fos and tryptophan hydroxylase (TPH) is described below. One set of sections, representing every sixth section throughout the DR from each rat was removed from cryoprotectant and washed twice in 0.05 M PBS for 15 min. Endogenous peroxidase activity was then neutralized in 0.05 M PBS containing 1% H202 (15 min), then sections were rinsed twice with 0.05 M PBS (15 min each time), rinsed with 0.05 M PBS containing 0.3% Triton X-100 (15 min) and subsequently incubated overnight at room temperature (RT) in rabbit anti-c-Fos 1° polyclonal antibody (Cat. No. PC3 8; Lot No. D00080180; Calbiochem (EMD Chemicals), Gibbstown, NJ, USA) diluted to 1:3000 with 0.05 M PBS containing 0.1% Triton X-100 and 0.01% sodium azide. The next day, the tissue was rinsed twice with 0.05 M PBS (15 min each time), then incubated for 90 min in biotinylated donkey anti-rabbit 2° antibody (Cat. No. 711-065-152; Lot No. 86689; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) diluted to 1:500 with 0.05 M PBS, washed twice with 0.05 M PBS (15 min each time), incubated for 90 min in an avidin-biotin-peroxidase complex (Elite ABC reagent, Cat. No. PK-6100; Vector Laboratories, Burlingame, CA, USA) diluted to 1:200 in 0.05 M PBS, washed twice with 0.05 M PBS (15 min) and finally reacted in a peroxidase substrate (Cat. No. SK4700; Vector SG chromogen kit; Vector Laboratories, diluted as recommended by the vendor). Following the reaction, the tissue was washed twice in 0.05 M PBS (15 min each time), then placed in 0.05 M PBS containing 1% H202 (15 min) followed by two additional 0.05 M PBS washes (15 min each time). Next, the tissue was incubated overnight at RT in sheep anti-TPH 1° antibody (Cat. No. T8575; L ot No. 047K1223; Sigma-Aldrich, St. Louis, MO, USA) diluted to 1:12,000 in 0.05 M PBS containing 0.1% Triton X-100 and 0.01% sodium azide. The next day, tissue was rinsed twice with 0.05 M PBS (15 min each time) and then incubated for 90 min in biotinylated rabbit anti-sheep 2° polyclonal antibo dy (Cat No. PK-6106; Vector Elite kit; Vector Laboratories) diluted to 1:200 with 0.05 M PBS. Following incubation in 2° antibody, tissue was rinse d twice in 0.05 M PBS (15 min each time), then incubated for 90 min in the avidin-biotin-peroxidase complex reagent diluted to 1:200 in 0.05 M PBS, rinsed twice in 0.05 M PBS (15 min each time), reacted with 0.01% 3-3′-diaminobenzidine tetrahydrochloride (DAB; Cat. No. D9015, Sigma-Aldrich) in 0.05 M PBS containing 0.005% H202, rinsed twice in 0.05 M PBS (15 min) and finally stored at 4 °C in 0.1 M PB containing 0.01% sodium azide. Tissue was then floated onto glass slides; after the tissue was air dried cover slips were mounted using Entellen mounting medium (Electron Microscopy Science, Hatfield, PA, USA) and cell counts were conducted as specified below.
2.6. Cell counts
The anatomical regions of the DR were identified using a stereotaxic rat brain atlas [26] and an atlas illustrating the distribution of TPH immunostaining throughout the rat DR [27]. Four rostrocaudal levels (−7.46 mm, −8.00 mm, −8.18 mm and −8.54 mm bregma; Figure 1) of the DR along with the corresponding subdivisions were selected for analysis. The subdivisions analyzed included: the dorsal raphe nucleus, dorsal part (DRD) and dorsal raphe nucleus, ventral part (DRV) at −7.46 mm bregma; the DRD, DRV and dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG) at −8.00 mm bregma; the DRD, DRV, DRVL/VLPAG and dorsal raphe nucleus, interfascicular part (DRI) at −8.18 mm bregma; and the DRI and dorsal raphe nucleus, caudal part (DRC) at −8.54 mm bregma. Cell counts included the numbers of c-Fos-immunoreactive (c-Fos-ir) serotonergic neurons (c-Fos-ir/TPH-ir neurons; distinguished by a dark blue/black-stained nucleus located entirely within a light brown-stained cytoplasm), the numbers of c-Fos-ir non-serotonergic cells (c-Fos-ir/TPH-immunonegative cells; distinguished by a dark blue/black stained nucleus) and total numbers of TPH-ir neurons (c-Fos-ir/TPH-ir neurons and c-Fos-immunonegative/TPH-ir neurons; identified by a light brown-stained cytoplasm). An experimenter blind to treatment group conducted the cell counts using bright-field microscopy with a 10× objective lens; c-Fos-ir/TPH-ir neurons were confirmed with a 40× objective lens.
Figure 1.
Photomicrographs illustrating the rostrocaudal levels and subdivisions of the dorsal raphe nucleus sampled for analysis of immunohistochemical staining. Dashed lines delineate each subdivision. Numbers in the lower left of each panel indicate the distance from bregma, based on a standard stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). (A) −7.46 mm bregma, (B) −8.00 mm bregma, (C) −8.18 mm bregma, and (D) −8.54 mm bregma. Abbreviations: Aq, cerebral aqueduct; bv, blood vessel; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus. Scale bar, 250 μm.
2.7.1. Statistical analysis of behavior
The behavioral data were separated into pre-defeat and defeat phases, and the frequency and duration of each behavior in acute and repeated defeat groups were compared using independent Student’s t-tests (PASW Statistics 17.0.2 for Macintosh, SPSS Inc., Chicago, IL, USA). Additionally, the behaviors during the defeat phase were grouped into behaviorally-related categories, including reactive coping behaviors, proactive coping behaviors and neutral behaviors, that were analyzed separately using independent Student’s t-tests.
Prior to statistical analysis, outliers were identified by Grubb’s test [28]; outliers were excluded from further analysis and were not included in the tables and graphical representation of the data. For the pre-defeat phase data, 1 out of 161 data points for frequency were excluded (0.6% of total data) and 5 out of 161 data points for duration were excluded (3.1% of total data); for the defeat phase data, 13 out of 345 data points for frequency were excluded (3.8% of total data) and 13 out of 345 data points for duration were excluded (3.8% of total data).
2.7.2. Statistical analysis of cell counts for home cage groups
Prior to the analysis of cell counts, a two-factor analysis of variance (ANOVA) with repeated measures (PASW statistics), using day (two levels: home cage control 1 and home cage control 2) as the between-subjects factor and region (11 levels) as the within-subjects factor, was used to determine if home cage control 1 and home cage control 2 rats differed in the numbers of c-Fos-ir/TPH-ir neurons, c-Fos-ir/TPH-immunonegative cells and the total number of serotonergic neurons sampled. A Greenhouse-Geisser correction epsilon (ε) was used to correct for potential violation of the sphericity assumption.
Outliers were identified using the Grubb’s test [28] and excluded from further analysis. Any missing values were replaced using the Peterson method in order to run the repeated measures ANOVA [29], but these values were not included in the post hoc analyses or the graphical representation of the data. The Grubb’s test analysis identified 9 outliers out of 308 data points (2.9%) for c-Fos-ir/TPH-ir cell counts, 10 outliers out of 308 data points (3.2%) for c-Fos-ir/TPH-immunonegative cell counts and 2 outliers out of 308 data points (0.6%) for the total number of serotonergic neurons sampled.
2.7.3. Statistical analysis of treatment effects for cell counts
Cell counts were analyzed using separate two-factor analysis of variance (ANOVA) with repeated measures (PASW statistics) on the numbers of 1) c-Fos-ir/TPH-ir neurons, 2) c-Fos-ir/TPH-immunonegative cells and 3) the total number of serotonergic neurons with treatment (three levels: acute social defeat, repeated social defeat and home cage control) as a between-subjects factor and region (11 levels) as a within-subjects factor. A Greenhouse-Geisser correction epsilon (ε) was used to correct for potential violation of the sphericity assumption. When appropriate, post hoc analyses using Fisher’s Protected LSD tests (PASW statistics) were conducted.
Outliers were identified using the Grubb’s test [28] and excluded from further analysis. Any missing values were replaced using the Peterson method in order to run the repeated measures ANOVA [29], but these values were not included in the post hoc analyses or the graphical representation of the data. The Grubb’s test analysis identified 11 outliers out of 539 data points (2.0%) for c-Fos-ir/TPH-ir cell counts, 9 outliers out of 539 data points (1.7%) for c-Fos-ir/TPH-immunonegative cell counts and 2 outliers out of 539 data points (0.4%) for the total number of serotonergic neurons sampled.
2.7.4 Statistical analysis of correlations
Both the frequency and duration of individual behaviors that were different between acute and repeated social defeat subjects were correlated with numbers of c-Fos-ir/TPH-ir neurons in specific subregions of the DR, where treatment effects were observed, using the Pearson Product Moment correlation test.
3. Results
3.1. Pre-defeat behavior
Rats exposed to social defeat 24 h prior to testing, relative to naive rats, responded with altered duration and frequency of a number of behaviors during the pre-defeat phase (Table 2). Analysis of the duration of pre-defeat behaviors revealed that repeated defeat subjects, compared to acute defeat subjects, responded with a greater duration of freezing (p = 0.028). Repeated defeat subjects displayed significantly less rearing (p = 0.009) and locomotion (p = 0.010). Additionally, rats exposed to repeated defeat tended to respond with a greater duration of inactivity, although this comparison only approached statistical significance (p = 0.056). Results from analysis of the frequency of pre-defeat behaviors paralleled the results from analysis of the duration of pre-defeat behaviors. Rats exposed to repeated defeat, compared to rats exposed to acute defeat, demonstrated more freezing (p = 0.018) and inactivity (p = 0.009), but less rearing (p = 0.035) and locomotion (p = 0.007). No additional differences were found for either the duration or frequency of any other pre-defeat behaviors.
Table 2.
Duration and frequency of the intruders’ behavior during the pre-defeat phase of social defeat.
| Behavior | Duration | Frequency | ||
|---|---|---|---|---|
| Acute | Repeated | Acute | Repeated | |
| Sniffing bedding | 68.7±9.6 | 62.9±8.0 | 21.2±2.7 | 19.2±2.0 |
| Freezing | 0.3±0.3 | 36.9±11.7* | 0.2±0.1 | 3.2±1.4* |
| Rearing | 173.1±14.8 | 97.6±23.2** | 34.3±2.8 | 22.5±4.7* |
| Defensive burying | 6.6±2.0 | 2.0±1.1 | 2.8±0.8 | 1.0±0.5 |
| Locomotion | 73.0±14.3 | 25.2±4.3* | 29.8±3.4 | 15.4±3.1** |
| Self-grooming | 70.5±8.8 | 70.9±9.0 | 6.0±0.6 | 7.3±1.2 |
| Inactivity | 12.0±3.7 | 38.6±9.6 | 5±1.4 | 12.7±2.5** |
P<0.05;
P<0.01. Values are presented as mean±S.E.M.
3.2. Defeat behavior
Rats exposed to social defeat 24 h prior to testing, relative to naive rats, responded with altered duration and frequency of a number of behaviors during the defeat phase (Table 3). Analysis of the duration of behaviors during the defeat phase revealed that repeated defeat, when compared with acute defeat, resulted in increased freezing (p = 0.035) and reductions in rearing (p = 0.039), social interaction (p = 0.024) and locomotion (p < 0.001). The analysis of the frequency of defeat behaviors was largely consistent with the analysis of the duration of defeat behaviors. Repeated defeat rats, compared to acute defeat rats, respond with a higher frequency of freezing (p = 0.012) and inactivity (p = 0.008). In contrast, repeated defeat rats displayed less frequent genital sniffing (p = 0.048), rearing (p = 0.035), social interaction (p = 0.011) and locomotion (p = 0.001).
Table 3.
Duration and frequency of the intruders’ behavior during the defeat phase of social defeat.
| Behavior | Duration | Frequency | ||
|---|---|---|---|---|
| Acute | Repeated | Acute | Repeated | |
| Reactive coping | ||||
| Sniffing bedding | 5.8±2.9 | 6.7±5.3 | 1.9±0.9 | 2.0±1.3 |
| Freezing | 42.6±16.1 | 115.7±30.7* | 2.5±0.7 | 7.9±2.1* |
| Full submission | 10.2±2.8 | 12.1±2.8 | 2.5±0.8 | 1.7±0.2 |
| Sideways submission | 2.8±1.4 | 5.3±1.6 | 0.9±0.3 | 1.7±0.5 |
| Passive genital sniff | 25.0±6.7 | 32.5±7.3 | 7.6±1.6 | 7.4±1.1 |
| Genital sniff | 2.1±0.9 | 0.0±0.0 | 1.1±0.5 | 0.0±0.0* |
| Proactive coping | ||||
| Rearing | 43.8±10.4 | 16.3±4.8* | 11.4±2.9 | 3.2±0.9* |
| Defensive burying | 0.2±0.1 | 0.0±0.0 | 0.2±0.1 | 0.0±0.0 |
| Aggression | 0.3±0.2 | 0.2±0.2 | 0.3±0.1 | 0.1±0.1 |
| Escape | 0.7±0.3 | 1.8±1.2 | 0.7±0.3 | 2.6±1.2 |
| Upright defensive behavior | 153.4±37.9 | 182.3±53.7 | 13.9±2.2 | 9.8±1.6 |
| Social interaction | 13.7±3.0 | 4.2±2.1* | 4.6±1.0 | 1.3±0.5* |
| Neutral | ||||
| Locomotion | 30.5±5.3 | 4.5±1.0*** | 13.9±2.2 | 2.9±0.8** |
| Self-grooming | 20.0±6.7 | 12.7±5.6 | 2.5±0.9 | 1.4±0.6 |
| Inactivity | 34.2±12.7 | 55.2±17.1 | 3.1±0.9 | 10.7±2.7** |
P<0.05;
P<0.01;
P<0.001. Values are presented as mean±S.E.M.
Rats exposed to social defeat 24 h prior to testing, relative to naive rats, responded with altered duration and frequency of reactive and proactive behavioral strategies during the defeat phase (Figure 2) Rats exposed to repeated defeat responded with a greater duration of reactive coping behavior relative to rats exposed to a single defeat (p = 0.026; Figure 2A). In addition, the comparison of the ratio of the duration of reactive versus proactive coping behavior approached statistical significance (p = 0.054; Figure 2D). Rats exposed to repeated defeat responded with a lower frequency of proactive behaviors (p = 0.018; Figure 2F) and a greater ratio of the frequency of reactive versus proactive behaviors relative to rats exposed to a single defeat (p = 0.005; Figure 2H). No statistically significant differences were observed in either the duration (Figure 2C) or frequency (Figure 2H) of neutral behaviors.
Figure 2.
Graphs illustrating the duration and frequency of reactive, proactive, and neutral behaviors during the defeat phase of social defeat in rats exposed to either acute or repeated social defeat. Behavioral classifications include (A) duration and (E) frequency of reactive coping behaviors, (B) duration and (F) frequency of proactive coping behaviors and (C) duration and (G) frequency of neutral behaviors. Graphs also illustrate the ratio of (D) duration and (H) frequency of reactive coping behaviors versus the duration of proactive coping behaviors. *p < 0.05; **p < 0.01 compared with acute defeat group, independent Student’s t-tests. Results are presented as the mean + S.E.M. (n = 13 for acute defeat; n = 10 for repeated defeat).
3.3. Immunohistochemistry
3.3.1. Cell counts in home cage control rats
Statistical analysis using two-factor repeated measures ANOVA revealed that there were no differences between home cage control 1 and home cage control 2 rats on the numbers of c-Fos-ir/TPH-ir neurons (day × region interaction, F(10, 260) = 1.30, p = 0.273, ε = 0.44; day main effect, F(1, 26) = 1.13, p = 0.297, ε = 0.44; region main effect, F(10, 260) = 13.58, p < 0.001, ε = 0.44; data not shown), c-Fos-ir/TPH-immunonegative cells (day × region interaction effect, F(10, 260) = 0.94 p = 0.410, ε = 0.24; day main effect, F(1, 26) = 0.47, p = 0.500, ε = 0.24; region main effect, F(10, 260) = 70.26, p < 0.001, ε = 0.24; data not shown) or TPH-ir neurons (day × region interaction effect, F(10, 260) = 0.38 p = 0.819, ε = 0.39; day main effect, F(1, 26) < 0.001, p = 0.983, ε = 0.49; region main effect, F(10, 260) = 46.78, p < 0.001, ε = 0.39; data not shown). Consequently, cell counts from the two control groups were combined for analysis of treatment effects.
3.3.2. c-Fos-ir/TPH-ir neurons
Acute and repeated defeat differentially increased c-Fos expression in DR serotonergic neurons. Statistical analysis using multifactor ANOVA with repeated measures revealed that social defeat altered c-Fos expression within serotonergic neurons (i.e., altered the numbers of c-Fos-ir/TPH-ir neurons) in the DR (social defeat × region interaction effect, F(20, 460) = 4.89, p < 0.001, ε = 0.49; treatment main effect, F(2, 46) = 28.10, p < 0.001, ε = 0.49; region main effect, F(10, 460) = 27.08, p < 0.001, ε = 0.49; Figures 3 and 4). Post hoc Fisher’s Protected LSD tests revealed that both acute and repeated social defeat, compared to home cage control conditions, increased c-Fos-ir/TPH-ir staining in several subdivisions of the DR. These included the DRD and DRV at −7.46 mm bregma, the DRD at −8.00 mm bregma, the DRD, DRV and DRVL/VLPAG at −8.18 mm bregma and the DRC at −8.54 mm bregma. The comparison of c-Fos-ir/TPH-ir staining within the DRVL/VLPAG at −8.00 mm bregma approached statistical significance for rats exposed to either acute (p = 0.073) or repeated (p = 0.051) defeat (Figure 3). In addition, subjects exposed to acute social defeat displayed increased c-Fos-ir/TPH-ir staining in the DRV at −8.00 mm bregma and the DRI at −8.54 mm bregma; subjects exposed to repeated social defeat exhibited increased c-Fos-ir/TPH-ir staining in the DRI at −8.18 mm bregma while staining in the DRI at −8.54 mm bregma approached statistical significance (p = 0.062). When comparing acute to repeated social defeat subjects, repeated social defeat rats showed decreased c-Fos-ir/TPH-ir staining within the DRD (p = 0.018) and DRV (p = 0.015) at −8.00 mm bregma; this pattern of c-Fos-ir/TPH-ir staining approached statistical significance within the DRD (p = 0.061) and DRV (p = 0.066) at −8.18 mm bregma, suggesting a consistent pattern of responses in the mid-rostrocaudal DRD and DRV. Finally, repeated social defeat subjects, when compared with acute defeat, exhibited increased c-Fos-ir/TPH-ir staining in the DRI (p = 0.004) at −8.18 mm bregma.
Figure 3.
Graphs illustrating the effects of acute defeat, repeated defeat or home cage control conditions on serotonergic neurons in the dorsal raphe nucleus. Graphs illustrate the total numbers of tryptophan hydroxylase (TPH)-positive neurons (open bars) and the numbers of c-Fos-positive/TPH-positive neurons (filled bars). *p < 0.05; **p < 0.01; ***p < 0.001 compared with home cage controls; ap < 0.05 versus acute defeat group; bp < 0.01 versus acute defeat group, Fisher’s Protected Least Significant Difference (LSD) tests. Bar graphs represent the means + S.E.M. (n = 28 for home cage control; n = 11 for acute defeat; n = 10 for repeated defeat). For abbreviations, see Figure 1 legend.
Figure 4.
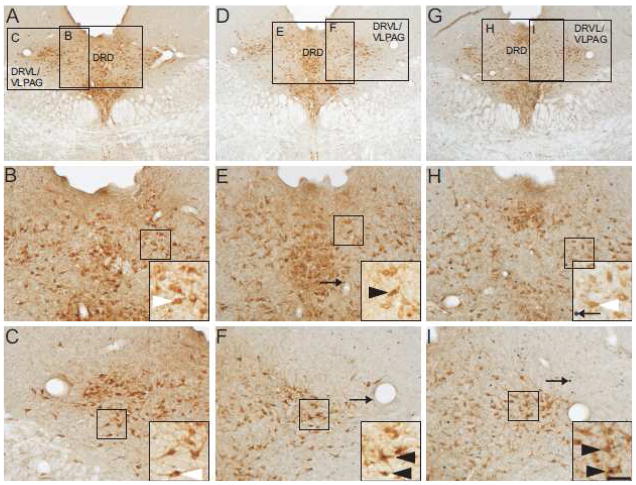
Photomicrographs illustrate tryptophan hydroxylase/c-Fos immunostaining in the mid-rostrocaudal dorsal raphe nucleus (−8.00 mm bregma) in representative rats from each treatment group. Photomicrographs illustrate immunostaining in rats exposed to (A–C) home cage control conditions, (D–F) acute social defeat and (G–I) repeated social defeat. Black boxes in A, D and G indicate regions displayed at higher magnification in B, C, E, F, H and I. Black boxes in B, C, E, F, H and I indicate regions shown at higher magnification within insets located in the lower-right hand corner of these respective panels. Black arrows indicate c-Fos-immunoreactive non-serotonergic cells (blue/black nuclear staining); white arrowheads indicate TPH-immunoreactive/c-Fos-immunonegative neurons (brown/orange cytoplasmic staining); black arrowheads represent c-Fos-immunoreactive/TPH-immunoreactive neurons (brown/orange cytoplasmic staining with blue/black nuclear staining). Abbreviations: DRD, dorsal raphe nucleus, dorsal part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray. Scale bar in panel I applies as follows: 250 μm for panels A, D, G; 100 μm for panels B, C, E, F, H, I; 50 μm for insets.
3.3.3. c-Fos-ir/TPH-immunonegative cells
Acute and repeated defeat increased c-Fos expression in non-serotonergic cells within the DR. A separate two-factor ANOVA with repeated measures showed that social defeat altered the expression of c-Fos within non-serotonergic cells (i.e., c-Fos-ir/TPH-immunonegative cells) in subregions of the DR (social defeat × region interaction effect, F(20, 460) = 4.90, p < 0.001, ε = 0.23; treatment main effect, F(2, 46) = 16.62, p < 0.001, ε = 0.23; region main effect, F(10, 460) = 121.01, p < 0.001, ε = 0.23; Figures 4 and 5). Further analysis using post hoc Fisher’s Protected LSD tests revealed that rats exposed to either acute or repeated social defeat, compared to rats exposed to home cage control conditions, exhibited increased numbers of c-Fos-ir/TPH-immunonegative cells in all regions studied, excluding the DRI at −8.18 mm bregma where only rats exposed to repeated social defeat responded with increased c-Fos expression.
Figure 5.
Graphs illustrating the effects of acute defeat, repeated defeat or home cage control conditions on c-Fos expression in non-serotonergic cells in the dorsal raphe nucleus. Graphs illustrate the numbers of c-Fos-positive/tryptophan hydroxylase (TPH)-immunonegative cells. *p < 0.05; **p < 0.01; ***p < 0.001 compared with home cage controls, Fisher’s Protected Least Significant Difference (LSD) tests. Bar graphs represent the means + S.E.M. (n = 28 for home cage control; n = 11 for acute defeat; n = 10 for repeated defeat). For abbreviations, see Figure 1 legend.
3.3.4. Total numbers of TPH-ir neurons
Acute and repeated defeat had no effect on the numbers of serotonergic neurons within the DR. A separate two-factor ANOVA with repeated measures showed that social defeat did not alter the total numbers of serotonergic neurons (i.e., the sum of c-Fos-immunonegative/TPH-ir neurons and c-Fos-ir/TPH-ir neurons) within subregions of the DR (social defeat × region interaction effect, F(20, 460) = 1.43, p = 0.103, ε = 0.48; treatment main effect, F(2, 46) = 0.012, p = 0.988, ε = 0.48; region main effect, F(10, 460) = 75.69, p < 0.001, ε = 0.48; Figures 3 and 4).
3.4. Correlations
Correlation analysis was conducted for the duration of freezing behavior and subregions of the DR that displayed statistically significant increases in the numbers of c-Fos-ir/TPH-ir neurons and that are known to be involved in regulating behavioral coping strategies (i.e., DRD and DRVL/VLPAG). A Pearson’s Product Moment Correlation test showed that the numbers of c-Fos-ir/TPH-ir neurons within the DRVL/VLPAG at −8.00 mm bregma were positively correlated with the duration of freezing behavior (r2 = 0.361, P =0.018, Figure 6). No behaviors during the pre-defeat phase were significantly correlated with numbers of c-Fos-ir/TPH-ir neurons in these regions.
Figure 6.
Graph illustrating the correlation between the number of c-Fos-immunoreactive serotonergic neurons within the dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG; −8.00 mm bregma) and the duration of freezing behavior during the defeat phase of social defeat. Open circles, acute defeat subjects; closed circles, repeated defeat subjects.
4. Discussion
Repeated defeat, relative to acute defeat, resulted in a shift away from a proactive emotional coping style during the defeat phase of the test and toward a reactive emotional coping style. Rats exposed to either acute defeat or repeated defeat responded with increased c-Fos expression in serotonergic neurons in multiple subdivisions of the DR, relative to rats exposed to home cage control conditions. In most cases, increases in c-Fos expression in serotonergic neurons were similar in rats exposed to acute or repeated defeat. However, rats exposed to repeated defeat responded with less c-Fos expression in serotonergic neurons, relative to rats exposed to acute defeat, within the dorsal (DRD) and ventral (DRV) parts of the mid-rostrocaudal DR.
Rats exposed to acute and repeated social defeat displayed different behavioral coping strategies. Rats exposed to repeated defeat responded with a decrease in proactive emotional coping behavior (e.g. decreased duration and frequency of rearing and social interaction, and decreased frequency of proactive coping) and an increase in reactive emotional coping behavior (e.g. increased duration and frequency of freezing, and increased duration of reactive coping) relative to rats exposed to a single defeat. Overall, rats exposed to a second social defeat encounter, relative to rats exposed to a single social defeat, responded with an increase in the ratio of the frequency of reactive to proactive emotional coping behaviors. Behavior during the pre-defeat phase paralleled these findings. During the pre-defeat phase, rats exposed to repeated social defeat, compared to acute social defeat, responded with a reduction in the duration and frequency of rearing and locomotion, and with an increase in the duration and frequency of freezing. A reactive emotional coping strategy or development of a subordinate status has been proposed to be a more adaptive, flexible behavioral strategy [30] during periods of unstable social structure that may serve to avoid danger, limit injury and conserve energy [31]. Previous studies have suggested that serotonergic systems may play a role in the inhibition of proactive coping responses including aggression [32, 33], escape behaviors [34, 35], and in the facilitation of passive-submissive behaviors [12, 22, 36], including fear- and anxiety-like behaviors [36].
Rats exposed to acute defeat responded with increased c-Fos expression in serotonergic neurons in multiple subdivisions of the DR, relative to rats exposed to home cage control conditions. These findings are consistent with other studies showing that social defeat increases 5-HT neurotransmission within the DR [13, 14, 23]. Widespread activation of DR serotonergic systems has been described with other stressors including restraint stress [37] and inescapable tail shock [37]. Thus, serotonergic responses to acute social defeat resemble those observed following exposure to relatively intense uncontrollable stressors. This widespread activation of serotonergic systems may be due to activation of multiple excitatory afferents to the DR, including those arising from the central nucleus of the amygdala, bed nucleus of the stria terminalis, lateral habenula, locus coeruleus, and lateral parabrachial nucleus [38–41]. Indeed, these structures that provide excitatory input to the DR are also activated by uncontrollable stressors [15, 24, 42–44]. Our own previous studies found more anatomically restricted increases in c-Fos expression following exposure to social defeat [11] compared to those in our study. However, rats in the control group in the previous study by Gardner and colleagues [11] were exposed to a novel cage and moved to a novel environment for the duration of the social defeat period for time-matched rats exposed to social defeat, whereas rats in the control group in our study were removed from their cage 1 hr prior to social defeat, moved to a novel environment to be weighed, and then returned to their home cage environment where they remained during the social defeat period for time-matched rats exposed to social defeat. Consistent with these differences in experimental design, the levels of c-Fos expression in the control group from the study by Gardner and colleagues [11] were considerably greater than in our study.
Also, a recent study by Hinwood et al., 2010 [45] reported that repeated social defeat, when compared to a home cage control condition (like the one used in the current study), increased ΔFosB expression in the infralimbic and prelimbic regions of the medial prefrontal cortex and both the core and shell of the nucleus accumbens; however, when comparing repeated social defeat subjects to a sham-stress control group (i.e. a control animal that is exposed to the resident’s cage in the absence of the resident) the increase in ΔFosB expression was limited to only the infralimbic region of the prefrontal cortex. Studies investigating the effects of social defeat stress on immediate-early gene expression often differ in the type of home cage controls utilized and either use a home cage control condition [20, 46], exposure to a novel cage [11, 14, 22, 23], or exposure to the resident’s cage in the absence of the resident [24, 25, 45], but, as the aforementioned studies illustrate, the type of control group can be critical for interpreting the results. Future studies should include a sham-stress control group to identify the specific contributions of psychosocial stress per se (as opposed to those of handling, novelty, olfactory stimuli, etc.) on the activation of DR serotonergic systems. The current study, however, is consistent with prior studies showing increased c-Fos expression in the DR following social defeat [14, 15, 24, 25], although these previous studies did not distinguish between serotonergic and non-serotonergic neurons in the DR.
In the majority of subdivisions analyzed, there were no differences between responses of DR serotonergic neurons in rats exposed to acute defeat or repeated defeat. However, in the mid-rostrocaudal DR, including both the dorsal (DRD) and ventral (DRV) parts, c-Fos expression in serotonergic neurons was lower in rats exposed to repeated defeat, compared to rats exposed to acute defeat. These regions of the DR receive a unique set of afferents and give rise to topographically organized projections to forebrain targets. The mid-rostrocaudal DRD gives rise to extensive projections to subcortical limbic sites involved in fear and anxiety, whereas the mid-rostrocaudal DRV gives rise to sensorimotor and motor structures, including extensive cortical projections (for reviews, see [47, 48]). Together, these structures may modulate fear and anxiety states and sensorimotor and motor function.
The mechanisms underlying the anatomically selective adaptations to repeated defeat in the mid-rostrocaudal DRD and DRV are not clear. However, functional anatomical studies suggest that the mid-rostrocaudal DRD and/or DRV are selectively activated by a number of anxiety- and stress-related stimuli [11, 17, 18, 36, 49, 50]. Interestingly, maternal separation, an adverse early life experience that results in a prolonged increase in anxiety state and a shift toward a more reactive emotional behavioral strategy during social defeat results in a pronounced increase in tph2 mRNA expression throughout the mid-rostrocaudal DRV [51]. Although previous studies have not shown selective activation of the mid-rostrocaudal DRV by social defeat, activation of the lateral orbital cortex, which gives rise to a dense projection to the mid-rostrocaudal DRV, has been associated with negative reward anticipation, losing outcome, and evaluation of wrong choices [52, 53]. It would be interesting to investigate whether a more chronic social defeat paradigm would amplify the site-specific effects of repeated social defeat on c-Fos expression within the mid-rostrocaudal DRD and DRV, as well as the functional consequences.
There are a number of mechanisms that could account for the decrease in c-Fos expression within serotonergic neurons in the mid-rostrocaudal DRD and DRV seen in animals exposed to repeated social defeat, compared to acute social defeat. It is possible that a selective increase in 5-HT1A receptor autoinhibition of serotonergic neurons in the mid-rostrocaudal DRD and DRV could explain a decrease in activation of this region during a second exposure to social defeat. Social defeat increases 5-HT1A mRNA expression throughout the DR [23][43], which could decrease activity in the DRD and DRV, but this is unlikely since these mRNA expression changes are not selective to subregions of the DR. It is also possible that excitatory input to the mid-rostrocaudal DRD and DRV is diminished, that serotonergic autoinhibition is increased, or that non-serotonergic inhibitory input is increased, during a second exposure to social defeat. Alternatively, the reduction in c-Fos expression within the mid-rostrocaudal DRD and DRV observed in repeated social defeat subjects could be due to autoinhibiton of gene expression via transrepression of the c-fos promotor by a c-Fos/c-Jun heterodimer complex [54, 55]. Future experiments should investigate whether the reductions in c-Fos expression in serotonergic neurons observed in subjects exposed to repeated social defeat are due to altered afferent input to the DR or autoinhibitory mechanisms.
The amount of c-Fos expression in the mid-rostrocaudal DRVL/VLPAG was positively correlated with the duration of freezing behavior. This is consistent with a number of studies demonstrating that the VLPAG plays an important role in regulating a type of freezing behavior related to defensive responses and conditioned fear [56, 57]; indeed, lesions of the VLPAG prevent freezing associated with conditioned fear [58, 59]. In our study, a greater level of activation of the DRVL/VLPAG region (as indicated by increased c-Fos expression in serotonergic neurons) was correlated with increased freezing behavior. As neurons in the DRVL/VLPAG region are known to project to the dorsal periaqueductal gray [60], this effect may be due to serotonergic inhibition of escape behaviors, resulting in a shift toward a more reactive emotional coping style. Numerous studies have shown that activation of 5-HT1A and 5-HT2A receptors within the dorsal periaqueductal gray inhibits escape behavior [61–64]. In contrast, c-Fos expression in non-serotonergic neurons within the DRVL/VLPAG region was not correlated with freezing behavior, suggesting that the effects of DRVL/VLPAG stimulation on freezing may be mediated by serotonergic mechanisms. Consistent with this hypothesis, intra-DR injections of corticotropin-releasing factor (CRF) increase freezing behavior that is temporally correlated with increased 5-HT release in the central amygdaloid nucleus [65], which, like the dorsal periaqueductal gray, is innervated by serotonergic neurons in the DRVL/VLPAG [66].
Acute and repeated defeat resulted in increased c-Fos expression in non-serotonergic neurons throughout all subregions of the DR relative to home cage control conditions. The DR contains a variety of neurotransmitters, including aspartate, dopamine, GABA, glutamate, glycine, nitric oxide, norepinephrine, and the peptide transmitters calbindin, calretinin, cholecystokinin, corticotropin-releasing factor, leu- and met-enkephalin, galanin, neuropeptide Y, neurotensin, somatostatin, substance P and vasoactive intestinal polypeptide [47, 67]. Social defeat is known to produce changes in other neurotransmitter systems such as dopamine [68] and a variety of neuropeptides [69], however, to our knowledge, the effects of social defeat on other neurotransmitters specifically within the DR has yet to be investigated. There were no differences in the level of c-Fos activation in non-serotonergic neurons in rats exposed to acute and repeated defeat. This is consistent with previous studies that did not distinguish between serotonergic and non-serotonergic neurons reporting that there were no differences in the patterns of c-Fos expression within the DR following exposure to either acute or repeated (chronic) social defeat [14, 15, 25]. Identifying other cell types that are activated following social defeat should be an important direction for future research.
In summary, rats exposed to repeated social defeat, when compared with rats exposed to acute social defeat, responded with an increase in reactive coping behavior that was associated with a decrease in c-Fos expression within serotonergic neurons in the mid-rostrocaudal DRD and DRV. Furthermore, c-Fos expression within serotonergic neurons in the mid-rostrocaudal DRVL/VLPAG was positively correlated with the duration of freezing. These data support the hypothesis that the DR serotonergic neurons have a functional topographical organization and that novel therapeutic strategies for stress-related neuropsychiatric disorders could target topographically organized subpopulations of serotonergic neurons.
Acknowledgments
M.J. Valentine and D.M. Sarchet were supported by Bioscience Undergraduate Research Skills and Training (BURST) fellowships; M. J. Valentine was supported by an Undergraduate Research Opportunities Program (UROP)/Howard Hughes Medical Institute (HHMI) Individual Grant funded by the Biological Sciences Initiative (BSI) through a grant from the Howard Hughes Medical Institute (HHMI). This work was supported by NIH award number R01MH086539 (to CAL). The funding sources had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Raab A, Dantzer R, Michaud B, Mormede P, Taghzouti K, Simon H, Le MM. Behavioural, physiological and immunological consequences of social status and aggression in chronically coexisting resident-intruder dyads of male rats. Physiol Behav. 1986;36:223–228. doi: 10.1016/0031-9384(86)90007-7. [DOI] [PubMed] [Google Scholar]
- 2.Herbert J. Neuroendocrine responses to social stress. Baillieres Clin Endocrinol Metab. 1987;1:467–490. doi: 10.1016/s0950-351x(87)80072-1. [DOI] [PubMed] [Google Scholar]
- 3.Razzoli M, Roncari E, Guidi A, Carboni L, Arban R, Gerrard P, Bacchi F. Conditioning properties of social subordination in rats: behavioral and biochemical correlates of anxiety. Horm Behav. 2006;50:245–251. doi: 10.1016/j.yhbeh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinn AM, Gronli J, Fiske E, Kuipers S, Ursin R, Murison R, Portas CM. A double exposure to social defeat induces sub-chronic effects on sleep and open field behaviour in rats. Physiol Behav. 2008;95:553–561. doi: 10.1016/j.physbeh.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Kole MH, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Meerlo P, Overkamp GJ, Daan S, Van Den Hoofdakker RH, Koolhaas JM. Changes in Behaviour and Body Weight Following a Single or Double Social Defeat in Rats. Stress. 1996;1:21–32. doi: 10.3109/10253899609001093. [DOI] [PubMed] [Google Scholar]
- 9.Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology (Berl) 2010;211:69–77. doi: 10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- 10.Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- 11.Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–1038. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- 15.Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- 16.Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 18.Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF(2)) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 19.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 20.Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- 21.Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 22.Chung KK, Martinez M, Herbert J. Central serotonin depletion modulates the behavioural, endocrine and physiological responses to repeated social stress and subsequent c-fos expression in the brains of male rats. Neuroscience. 1999;92:613–625. doi: 10.1016/s0306-4522(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 23.Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 27.Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomical and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- 28.Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 29.Petersen RG. Design and Analysis of Experiments. New York: Marcel Dekker, Inc; 1985. [Google Scholar]
- 30.Koolhaas JM, Korte SM, De Boer SF, van d V, Van Reenen CG, Hopster H, De JI, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 31.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Miczek KA, Mos J, Olivier B. Brain 5-HT and inhibition of aggressive behavior in animals: 5-HIAA and receptor subtypes. Psychopharmacol Bull. 1989;25:399–403. [PubMed] [Google Scholar]
- 33.Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- 34.Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Graeff FG, Zangrossi H., Jr The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent Nerv Syst Agents Med Chem. 2010;10:207–217. doi: 10.2174/1871524911006030207. [DOI] [PubMed] [Google Scholar]
- 36.Lowry CA, Hale MW. Serotonin and the neurobiology of anxious states. In: Mûller CP, Jacobs BL, editors. The Behavioural Neurobiology of Serotonin. Amsterdam: Elsevier; 2010. pp. 379–396. [Google Scholar]
- 37.Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav Brain Res. 2005;162:299–306. doi: 10.1016/j.bbr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 39.Baraban JM, Aghajanian GK. Noradrenergic innervation of serotonergic neurons in the dorsal raphe: demonstration by electron microscopic autoradiography. Brain Res. 1981;204:1–11. doi: 10.1016/0006-8993(81)90646-6. [DOI] [PubMed] [Google Scholar]
- 40.Kalen P, Strecker RE, Rosengren E, Bjorklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- 41.Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- 42.Jasnow AM, Cooper MA, Huhman KL. N-methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004;123:625–634. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 44.Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha(1b) adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinwood M, Tynan RJ, Day TA, Walker FR. Repeated Social Defeat Selectively Increases {Delta}FosB Expression and Histone H3 Acetylation in the Infralimbic Medial Prefrontal Cortex. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq080. [DOI] [PubMed] [Google Scholar]
- 46.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal BL, Jacobs BL, Nutt DL, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. 1. Basel: Birkhauser; 2008. pp. 25–68. [Google Scholar]
- 48.Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- 49.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 50.Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008 doi: 10.1196/annals.1410.004. (in press) [DOI] [PubMed] [Google Scholar]
- 51.Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA. Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009;163:991–1001. doi: 10.1016/j.neuroscience.2009.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Sassone-Corsi P, Sisson JC, Verma IM. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- 55.Lucibello FC, Lowag C, Neuberg M, Muller R. trans-repression of the mouse c-fos promoter: a novel mechanism of Fos-mediated trans-regulation. Cell. 1989;59:999–1007. doi: 10.1016/0092-8674(89)90756-3. [DOI] [PubMed] [Google Scholar]
- 56.Vianna DM, Graeff FG, Landeira-Fernandez J, Brandao ML. Lesion of the ventral periaqueductal gray reduces conditioned fear but does not change freezing induced by stimulation of the dorsal periaqueductal gray. Learn Mem. 2001;8:164–169. doi: 10.1101/lm.36101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav Brain Res. 2008;188:1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 58.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 60.Stezhka VV, Lovick TA. Projections from dorsal raphe nucleus to the periaqueductal grey matter: studies in slices of rat midbrain maintained in vitro. Neurosci Lett. 1997;230:57–60. doi: 10.1016/s0304-3940(97)00464-3. [DOI] [PubMed] [Google Scholar]
- 61.de Bortoli V, Nogueira RL, Zangrossi H., Jr Alprazolam potentiates the antiaversive effect induced by the activation of 5-HT(1A) and 5-HT (2A) receptors in the rat dorsal periaqueductal gray. Psychopharmacology (Berl) 2008;198:341–349. doi: 10.1007/s00213-008-1134-7. [DOI] [PubMed] [Google Scholar]
- 62.Jacob CA, Cabral AH, Almeida LP, Magierek V, Ramos PL, Zanoveli JM, Landeira-Fernandez J, Zangrossi H, Nogueira RL. Chronic imipramine enhances 5-HT(1A) and 5-HT(2) receptors-mediated inhibition of panic-like behavior in the rat dorsal periaqueductal gray. Pharmacol Biochem Behav. 2002;72:761–766. doi: 10.1016/s0091-3057(01)00785-7. [DOI] [PubMed] [Google Scholar]
- 63.Beckett S, Marsden CA. The effect of central and systemic injection of the 5-HT1A receptor agonist 8-OHDPAT and the 5-HT1A receptor antagonist WAY100635 on periaqueductal grey-induced defence behaviour. J Psychopharmacol. 1997;11:35–40. doi: 10.1177/026988119701100111. [DOI] [PubMed] [Google Scholar]
- 64.Beckett SR, Lawrence AJ, Marsden CA, Marshall PW. Attenuation of chemically induced defence response by 5-HT1 receptor agonists administered into the periaqueductal gray. Psychopharmacology (Berl) 1992;108:110–114. doi: 10.1007/BF02245294. [DOI] [PubMed] [Google Scholar]
- 65.Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience. 2006;140:1067–1077. doi: 10.1016/j.neuroscience.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 67.Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus--from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Nikulina EM, rrillaga-Romany I, Miczek KA, Hammer RP., Jr Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of mu-opioid receptor mRNA and FosB/DeltaFosB immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panksepp J, Burgdorf J, Beinfeld MC, Kroes RA, Moskal JR. Brain regional neuropeptide changes resulting from social defeat. Behav Neurosci. 2007;121:1364–1371. doi: 10.1037/0735-7044.121.6.1364. [DOI] [PubMed] [Google Scholar]