Abstract
Background
Palmitoylation is emerging as one of the most important posttranslational modifications of excitatory synaptic proteins in mammalian brain cells. As a reversible and regulatable modification sensitive to changing synaptic inputs, palmitoylation of ionotropic glutamate receptors contributes to not only the modulation of normal receptor and synaptic activities, but also the pathogenesis of various neuropsychiatric disorders. Here, we report that palmitoylation of the AMPA receptor is regulated by the psychostimulant, cocaine, and such regulation is involved in cocaine action.
Methods
We tested palmitoylation and surface expression of AMPA receptors in striatal neurons and psychomotor behavior in responses to cocaine in rats.
Results
All four AMPA receptor subunits (GluA1-4 or GluR1-4) are palmitoylated in the nucleus accumbens (NAc) of adult rats. Among them, GluA1 and GluA3 are preferentially upregulated in their palmitoylation levels by a systemic injection of cocaine. The upregulated GluA1 and 3 palmitoylation is a transient and reversible event. Consequently, it increases the susceptibility of surface-expressed GluA1 and 3 to internalization trafficking, leading to a temporal loss of surface receptor expression. Blockade of the regulated GluA1/3 palmitoylation with a palmitoylation inhibitor in the local NAc reverses the loss of surface GluA1/3. The inhibition of palmitoylation also concurrently sustains behavioral responsivity to cocaine.
Conclusions
Our data identify a novel drug-palmitoylation coupling in the center of limbic reward circuits. Through palmitoylating selective AMPA receptor subunits, cocaine activity-dependently regulates trafficking and subcellular localization of the receptor in NAc neurons and dynamically controls psychomotor sensitivity to the psychoactive drug in vivo.
Keywords: Glutamate, dopamine, synapse, nucleus accumbens, addiction, drug abuse
Glutamate is the major excitatory neurotransmitter in the mammalian brain. Its functions are mediated through the interaction with glutamate receptors. Ionotropic glutamate receptors are ligand-gated ion channels that process the fast excitatory synaptic transmission. These receptors are classified into several subtypes: AMPA, kainate, δ, and NMDA receptors. AMPA receptors (AMPARs) are composed of four subunits, GluA1-4 or GluR1-4 (1). Most AMPARs become fully functional upon heterotetrameric assembly of these subunits (2). Since AMPARs are the most commonly found receptor in many parts of the brain, they play critical roles in normal cellular and synaptic activities and in the pathogenesis of various neuropsychiatric and neurodegenerative diseases (3).
Early studies have demonstrated that posttranslational modifications, such as phosphorylation of AMPARs, actively regulate AMPARs and synaptic plasticity (4,5). Recent work identifies fatty acylation, such as palmitoylation, as another important type of posttranslational modification of the receptor (6). Thio-palmitoylation is the covalent attachment of a 16 carbon saturated fatty acid, palmitate (palmitic acid), to a cysteine residue most commonly via a thioester bond (7,8). This type of lipidation increases the lipophilicity or hydrophobicity of modified proteins, which often results in an altered affinity for the plasma membrane, leading to protein trafficking from one membrane system to another. Palmitoylation can also alter interactions of modified proteins with their binding partners, thereby regulating their subcellular distribution and function. Like phosphorylation and unlike other forms of lipidation, palmitoylation (the thioester bond) is labile and reversible. In the case of AMPARs, all four subunits (GluA1-4) are palmitoylated constitutively in transfected HEK 293T cells or cultured cortical neurons (9). Such palmitoylation is subject to the dynamic regulation by changing synaptic inputs. Given the fact that palmitate is the most abundant fatty acid in the brain, palmitoylation could be a common mechanism for regulating distribution and function of AMPARs.
Psychostimulants increase the synaptic availability of dopamine in the striatum (10,11). Similarly, stimulants modify glutamatergic transmission. The impact on glutamatergic transmission is deemed to mediate drug’s effects (12). AMPARs are densely expressed in medium spiny neurons of the striatum (13–15). These receptors are likely to be sensitive targets of psychostimulants in terms of their labile palmitoylation. Thus, in this study, we investigated whether AMPARs in the ventral striatum/nucleus accumbens (NAc) undergo basal palmitoylation and whether their palmitoylation is subject to the regulation by the psychostimulant cocaine and is involved in the drug action in vivo.
Materials and Methods
Animals
Adult male Wistar rats weighing 225–275 g (Charles River) were individually housed in a controlled environment at a constant temperature of 23ºC and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12-h/12-h light/dark cycle. Rats were allowed 6–7 days of habituation to the animal colony. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Systemic drug injection
Rats received an intraperitoneal (i.p.) injection of saline or cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO). The dose of cocaine was calculated as the salt. Doses of cocaine were chosen based on their effects on motor activity and posttranslational modification (phosphorylation) of GluA1 in the mouse striatum (16,17). Age-matched rats received an acute injection of saline (1 ml/kg) and served as controls.
Analysis of palmitoylation with acyl-biotin exchange (ABE) method
Protein palmitoylation was analyzed using an ABE method (18–20). The detailed protocol was modified from Wan et al. (21) and Hayashi et al. (22) and was carried out on the affinity-purified palmitoyl proteins en masse from adult rat brain tissue. Main steps of a series of biochemical procedures are illustrated in Fig. S1. They include 1) extraction and denaturation of proteins from the NAc, followed by in vitro blockade of unmodified free cysteine thiols (-SH) with N-ethylmaleimide (NEM); 2) cleavage of palmitoylation thioester linkage with hydroxylamine (HA, NH2OH); 3) labeling newly-exposed cysteinyl thiols with a thiol-specific biotinylation reagent; 4) affinity purification of resulting biotinylated proteins with neutravidin-agarose; 5) elution of affinity-purified proteins with β-mercaptoethanol (β-ME); and 6) identification of palmitoylated proteins using Western blot with a panel of antibodies specific to individual proteins. Specifically, rats were anesthetized with Equithesin (9 ml/kg, i.p.) and decapitated at time points indicated. Brains were quickly removed and cut into coronal sections. The NAc was removed into a 1.5 ml microtube containing ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 2% SDS for denaturing proteins, and a protease inhibitor cocktail-Completetm, Roche) with 10 mM NEM (Pierce) for blocking free thiols,. The sample was homogenized by sonication. The homogenate was centrifuged at 760 g for 10 min at 4° C. Supernatant was subject to three sequential acetone precipitations to remove unbound free NEM. For each precipitation, 4 volumes of cold acetone were added and incubated for 60 min at −20ºC. Samples were centrifuged for 10 min at 14,000 g. Pellets were resuspended in RIPA buffer. After the final precipitation, pellets were dissolved in RIPA buffer and divided into two equal portions. One portion was incubated for 1 h at room temperature with 4 volumes of RIPA buffer containing 0.7 M HA (Sigma) for cleaving thioester bonds and 1 mM sulfhydryl-reactive HPDP-biotin (Pierce) for linking biotin to newly-exposed cysteinyl thiols. Another portion was incubated with RIPA buffer containing only HPDP-biotin to serve as controls. Three acetone precipitations were performed to remove HA and unreacted HPDP-biotin. After first precipitation, pellets were dissolved in RIPA buffer plus 0.2 mM HPDP-biotin and incubated for 1 h at room temperature. After last two precipitations, pellets were dissolved in RIPA buffer. Biotinylated proteins in samples were affinity-purified using neutravidin-conjugated beads (Thermo Fisher Scientific) for 1 h at 4ºC. To cleave the protein-biotin disulfide linkages and elute bound proteins from the beads, 1% (v/v) β-ME (Fisher) was added and incubated for 30 min at 37°C. Eluted proteins in the supernatant were resolved in Western blotting and analyzed with specific antibodies (23).
Surface receptor crosslinking assays
Surface expression of GluA1-4 was assayed using the membrane-impermeable crosslinking reagent bis(sulfosuccinimidyl)suberate (BS3) as described previously (24,25). Briefly, brains were removed following anesthesia and were cut into coronal sections (400 μm). The NAc was dissected and added to Eppendorf tubes containing ice-cold artificial cerebrospinal fluid (ACSF). BS3 (Pierce) was added to 2 mM and incubated for 30 min at 4°C. The reaction was terminated by quenching with 20 mM glycine (10 min, 4°C). The tissue was then washed four times in cold ACSF (10 min each), homogenized to obtain total protein homogenate, and analyzed directly by SDS-PAGE (4–12% Tris-glycine gels, Invitrogen).
Other procedures, including behavioral assessments, Western blot, primary striatal neuronal culture preparation, analysis of palmitoylation by metabolic labeling, surgeries for intra-NAc injection, assessments of cellular damage in striatal sections and statistics, are described in supplementary methods (see Supplement).
Results
Palmitoylation of AMPARs in the NAc
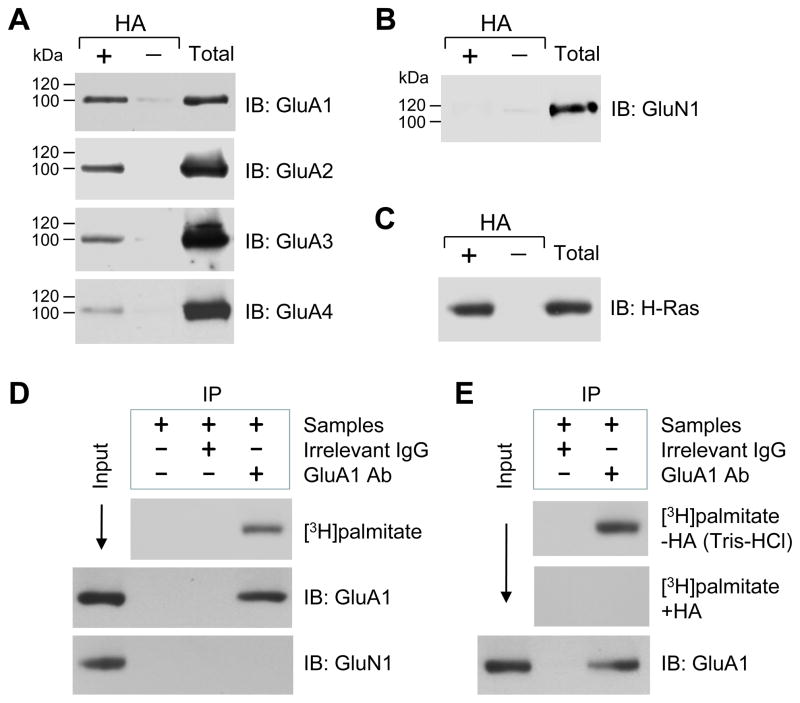
All four AMPAR subunits (GluA1-4) showed basal palmitoylation in transfected HEK 293T cells and cultured cortical neurons as detected by either [3H]palmitate metabolic labeling or the ABE method (9,20). It is however unclear whether GluA1-4 subunits undergo similar palmitoylation in the NAc of adult brains in vivo. To determine this, we performed ABE assays to assess the palmitoylation status of these subunits in normal rat NAc tissue. We found that all four endogenous subunits consistently displayed detectable palmitoylation (Fig. 1A). In contrast, the NMDA receptor GluN1 subunit did not (Fig. 1B), a result similar to previous observations in cultured cortical neurons (9,22). This result therefore served as a negative control. For a positive control, a well-known palmitoylated protein, H-Ras (26), was tested. It showed strong palmitoylation in our assays (Fig. 1C). These data demonstrate that AMPAR subunits (GluA1-4) are constitutively palmitoylated in NAc neurons of adult rats.
Figure 1. Palmitoylation of native AMPAR subunits GluA1-4 in rat NAc neurons in vivo and in vitro.
(A) Palmitoylation of GluA1-4 subunits in the NAc. (B) Palmitoylation of NMDA receptor GluN1 subunits in the NAc. (C) Palmitoylation of H-Ras in the NAc. Palmitoylation was examined in homogenates from the normal rat NAc tissue using the ABE assay (see Materials and Methods). Proteins that were purified through the ABE method, both in the presence (+) and absence ( ) of HA, were analyzed by immunoblot (IB) using the indicated antibodies. HA-dependent detection of a given protein indicates its palmitoylation. As a control, total proteins from homogenates (before ABE purification) were also examined at the same blot. (D) Palmitoylation of GluA1 as detected in cultured rat NAc neurons by [3H]palmitate metabolic labeling. (E) HA-dependent palmitoylation of GluA1 in cultured NAc neurons. GluA1 proteins were purified via immunoprecipitation (IP), followed by film detection of incorporation of [3H]palmitate into GluA1 and immunoblot confirmation of immunoprecipitated GluA1.
To confirm the palmitoylation of AMPAR subunits in NAc neurons, we performed metabolic labeling with [3H]palmitate in cultured rat NAc neurons. Because of the conserved amino acid sequence surrounding the palmitoylation sites of all four AMPAR subunits (9), we selected GluA1 palmitoylation as a representative of these subunits in this study. As shown in Fig. 1D, GluA1 was metabolically labeled with [3H]palmitate, establishing an existence of GluA1 palmitoylation. To determine if [3H]palmitate was linked to GluA1 via HA-sensitive thioester bonds (27), we treated GluA1 samples immunoprecipitated from [3H]palmitate-labeled cultures with HA or a control buffer (Tris-HCl). We found that HA eliminated the [3H]palmitate labeling of GluA1, while Tris-HCl did not (Fig. 1E). Thus, the labeling was due to palmitoylation at cysteine residues via thioester bonds.
Cocaine increases palmitoylation of GluA1 and GluA3 in NAc neurons
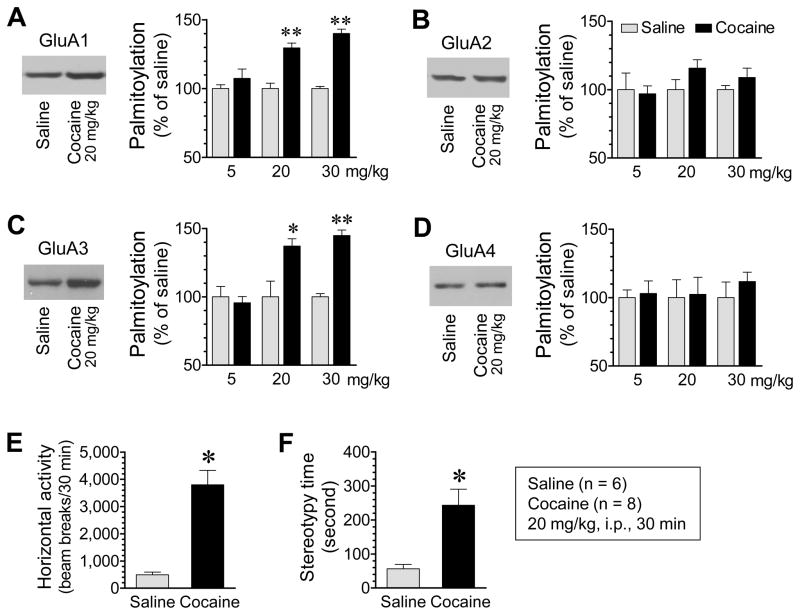
Palmitoylation of many receptor, signaling, and scaffold proteins at excitatory synapses is regulated by changing synaptic inputs (9,22,28,29). To determine whether cocaine alters palmitoylation of GluA1-4 in the NAc, we subjected rats to a single dose of cocaine (5, 20 or 30 mg/kg, i.p.). We sacrificed animals 30 min after drug injection to assess changes in GluA1-4 palmitoylation in the NAc. Cocaine at the two higher doses significantly increased palmitoylation of GluA1, while cocaine at a lower dose did not (Fig. 2A). Palmitoylation of GluA3 was also elevated in cocaine-treated rats relative to saline-treated rats (Fig. 2C). Noticeably, in contrast to GluA1 and GluA3, GluA2 and GluA4 showed insignificant changes in their palmitoylation levels (GluA2, Fig. 2B; GluA4, Fig. 2D). These results identify the AMPAR as a sensitive target of cocaine in the in vivo NAc. Their basal levels of palmitoylation are subject to the upregulation by cocaine. Since total amounts of proteins of all four subunits remained unchanged in cocaine-treated rats compared to saline-treated rats (Fig. S2), the increase in GluA1 and 3 palmitoylation should result from an increased portion of palmitoylated GluA1 and 3 proteins in their total abundance, but not from an overall addition of proteins. Similar results were observed in the dorsal striatum (Fig. S3). Behavioral stimulation by cocaine was confirmed by enhanced locomotion (Fig. 2E) and stereotypy (Fig. 2F).
Figure 2. Effects of cocaine on palmitoylation of AMPAR subunits GluA1-4 in the rat NAc.
(A–D) Effects of cocaine at different doses on palmitoylation of GluA1 (A), GluA2 (B), GluA3 (C), and GluA4 (D) in the NAc. Representative immunoblots from rats receiving 20 mg/kg of cocaine are shown left to the quantified data. Note that cocaine at the two higher doses increased palmitoylation of GluA1 and GluA3, but not GluA2 and GluA4. (E and F) Effects of cocaine on motor activities in terms of horizontal locomotor activity (E) and stereotypy time (F). Rats were given a single dose of cocaine or saline and sacrificed 30 min after injection (A–D). Motor responses to cocaine or saline were continuously measured for 30 min (E and F). Data are presented as means ± SEM (n = 4–8 per group). *P < .05 and **P < .01 versus saline (Student t test).
Time course of cocaine-regulated palmitoylation of AMPARs
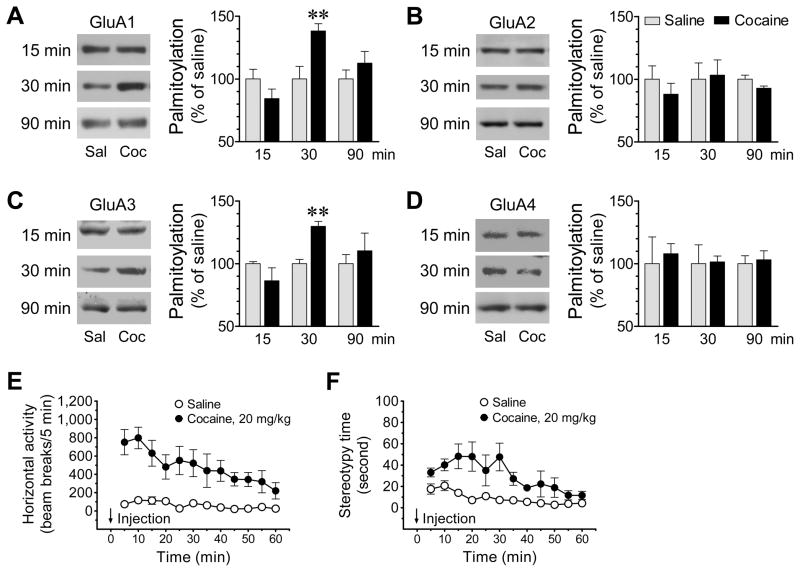
To determine the temporal property of the cocaine-regulated AMPAR palmitoylation, we subjected rats to acute cocaine (20 mg/kg). We then sacrificed animals at different time points (15, 30, and 90 min after drug injection) for assessments of changes in palmitoylation of GluA1-4 in the NAc. Cocaine showed no significant effect on GluA1 palmitoylation in the NAc at 15 min after injection (84.4 ± 7.6% of saline, P > 0.05) (Fig. 3A). The drug increased GluA1 palmitoylation at 30 min (138.3 ± 5.7% of saline, P < 0.01). This effect dissipated at 90 min as GluA1 palmitoylation returned to a level insignificantly different from saline (112.6 ± 9.4% of saline, P > 0.05). GluA2 was not significantly modified in palmitoylation at 15 min (88.1 ± 8.7% of saline, P > 0.05) (Fig. 3B). Similar results were observed at 30 min (103.3 ± 12.1% of saline, P > 0.05) and 90 min (92.8 ± 1.8% of saline, P > 0.05) (Fig. 3B). GluA3, like GluA1, was sensitive to cocaine in a time-dependent fashion (Fig. 3C). Its palmitoylation was unaffected at 15 min (86.4 ± 10.3% of saline, P < 0.05). An increase was observed at 30 min (129.8 ± 3.9% of saline, P < 0.01), which returned to the normal level at 90 min (110.3 ± 14.1% of saline, P > 0.05). Finally, GluA4 palmitoylation was resistant to cocaine as its palmitoylation was not altered throughout the time points tested (15 min: 107.9 ± 8.1% of saline, P > 0.05; 30 min: 101.4 ± 4.7% of saline, P > 0.05; and 90 min: 103.1 ± 7.2% of saline, P > 0.05) (Fig. 3D). There was no change in total GluA1-4 expression at all time points (Fig. S4). These data establish the selectivity of GluA1/3 and the insensitivity of GluA2/4 to cocaine in their palmitoylation status at all three time points surveyed. They further characterize the cocaine-regulated GluA1/3 palmitoylation as a transient and reversible event. As compared to the motor stimulation, this time-dependent modification of palmitoylation was developed after the occurrence of typical behavioral responses to cocaine (Fig. 3E and 3F).
Figure 3. Time-dependent effects of cocaine on palmitoylation of AMPAR subunits GluA1-4 in the rat NAc.
(A–D) Time-dependent effects of cocaine on palmitoylation of GluA1 (A), GluA2 (B), GluA3 (C), and GluA4 (D) in the NAc. Representative immunoblots are shown left to the quantified data. Note that cocaine time-dependently increased palmitoylation of GluA1 and GluA3, but not GluA2 and GluA4. (E and F) Temporal effects of cocaine on motor activities in terms of horizontal locomotor activity (E) and stereotypy time (F). Rats were given a single dose of cocaine (20 mg/kg, i.p.) or saline and sacrificed at different time points (15, 30, or 90 min after drug injection) for ABE assays of changes in GluA1-4 palmitoylation (A–D). For rats sacrificed at 90 min, their motor responses to cocaine or saline in terms of horizontal locomotor activity (E) and stereotypy time (F) were continuously measured for 60 min. Data are presented as means ± SEM (n = 5–7 per group). **P < .01 versus saline (Student t test).
Cocaine causes redistribution of GluA1 and GluA3 in NAc neurons in vivo
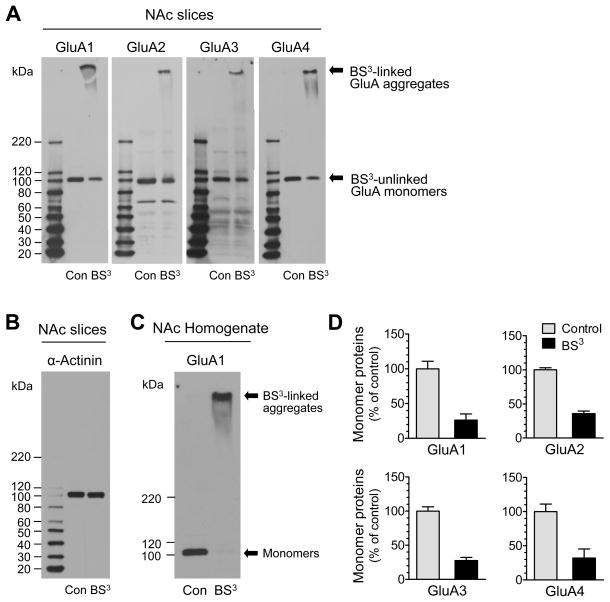
Palmitoylation increases the lipophilicity of modified proteins, which often alters the affinity of proteins for plasma membranes. Palmitoylation can also alter the interaction rate of surface membrane-bound proteins with their submembranous scaffold proteins. These alterations alone or together can regulate protein trafficking from one membrane system to another and redefine subcellular distribution of synaptic proteins in response to altered synaptic activity (6). To this end, we examined whether cocaine-stimulated palmitoylation of AMPAR subunits occurs in parallel with redistribution of these subunits. We used a recently developed biochemical crosslinking method to isolate native receptors from specific surface and intracellular pools in intact neurons in vivo (24,25). In this crosslinking assay, we treated viable rat NAc slices with BS3. As a membrane-impermeable reagent, BS3 selectively crosslinks surface membrane-bound receptors to form high-molecular weight aggregates. On gel electrophoresis, these aggregates can be readily separated from unlinked intracellular receptors bound on light membranes of the intracellular endoplasmic reticulum, Golgi, and vesicular structures. In BS3-treated normal rat NAc slices, we observed a high molecular weight band (BS3-linked surface proteins) for each subunit tested (Fig. 4A). We also observed a low monomeric molecular weight band (BS3-unlinked intracellular proteins) for each subunit. This band was proportionally less in optical density as compared to a single monomeric molecular weight band seen in non-BS3-treated control slices, which contains both surface and intracellular subunits (Fig. 4A). The surface selectivity of BS3 was confirmed by the lack of crosslinking effect of BS3 on α-actinin, an intracellular protein (Fig. 4B). In addition, adding BS3 into NAc homogenates completely eliminated the monomeric band of GluA1 (Fig. 4C), GluA2, GluA3 or GluA4. In normal NAc neurons, BS3 assays revealed similar estimates of the percentages of four subunits in surface and intracellular pools. Typically, a larger portion of AMPAR subunits is expressed in the surface pool. A smaller portion is expressed in the intracellular pool as indicated by much lower levels of intracellular monomer subunits in BS3-treated slices relative to control slices (Fig. 4D).
Figure 4. Surface and intracellular expression of AMPARs in the rat NAc.
(A) Representative GluA1-4 immunoblots from rat viable NAc slices treated with BS3 or vehicle control (Con). (B) A representative α-actinin immunoblot from rat viable NAc slices treated with BS3 or vehicle control. (C) An immunoblot of GluA1 from NAc homogenates treated with BS3 or vehicle control. (D) Quantification of monomer subunit protein expression (BS3-unlinked intracellular subunits) in normal NAc neurons. Data are presented as means ± SEM (n = 3–6 per group).
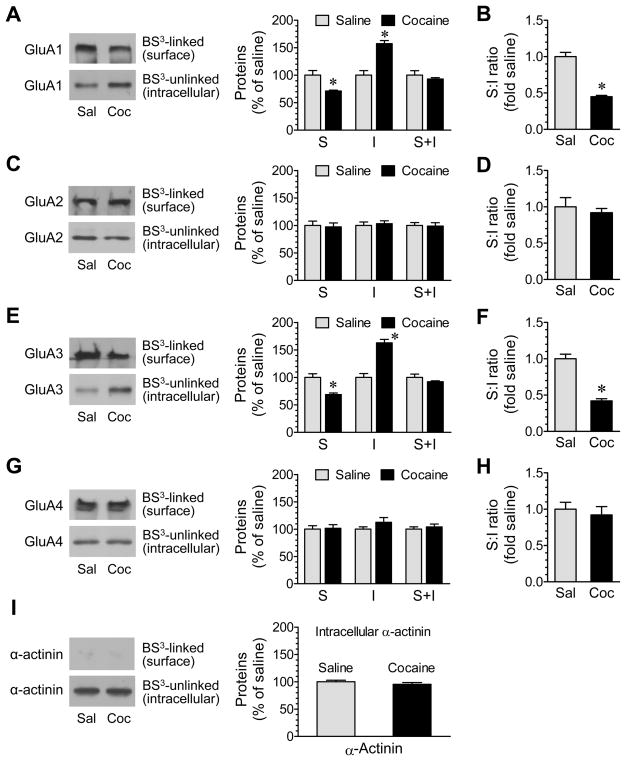
We next examined whether cocaine regulates surface and/or intracellular AMPAR expression. Cocaine (20 mg/kg, i.p., 30 min) induced a decrease in GluA1 protein abundance in the surface pool of NAc (71.1 ± 1.7% of saline, P < 0.05; Fig. 5A). In contrast, cocaine increased GluA1 proteins in the intracellular pool (157.4 ± 5.5% of saline, P < 0.05; Fig. 5A). The total amount of GluA1 (surface + intracellular proteins) remained unchanged (Fig. 5A). Due to concurrent changes in both surface and intracellular pools, the surface:intracellular ratio of GluA1 expression was markedly reduced (Fig. 5B). No significant change was found in GluA2 protein levels in either the surface or the intracellular pool (Fig. 5C) or in the surface:intracellular ratio (Fig. 5D). Similar to GluA1, GluA3 expression in surface and intracellular pools was reduced (68.8 ± 2.8% of saline, P < 0.05) and elevated (163.0 ± 6.2% of saline, P < 0.05), respectively (Fig. 5E). The surface:intracellular ratio of GluA3 was therefore lower in cocaine-treated rats than saline-treated rats (Fig. 5F). There was no significant change in GluA4 expression in the two pools (Fig. 5G) and in the surface:intracellular ratio (Fig. 5H). Expression of α-actinin showed no change (Fig. 5I). These data indicate that cocaine can trigger a rapid redistribution of GluA1 and GluA3 subunits, which is characterized by a loss of surface receptors and a simultaneous accumulation of intracellular receptors, probably due to an accelerated internalization trafficking.
Figure 5. Effects of cocaine on surface and intracellular expression of AMPAR subunits in rat NAc neurons.
(A–H) Effects of saline (Sal) or cocaine (Coc) on surface (S) and intracellular (I) expression of GluA1 (A), GluA2 (C), GluA3 (E), and GluA4 (G) and on the S:I ratio of GluA1 (B), GluA2 (D), GluA3 (F), and GluA4 (H) in NAc neurons. (I) Effects of saline and cocaine on expression of α-actinin. Rats were given a single dose of cocaine (20 mg/kg, i.p.) or saline. They were sacrificed 30 min after drug injection for BS3 crosslinking assays of changes in surface versus intracellular expression of GluA1-4 in the NAc. Representative immunoblots are shown to the left of the quantified date (A, C, E, G, and I). Data are presented as means ± SEM (n = 6 per group). *P < .05 versus saline (Student t test).
Palmitoylation links to internalization trafficking of AMPARs
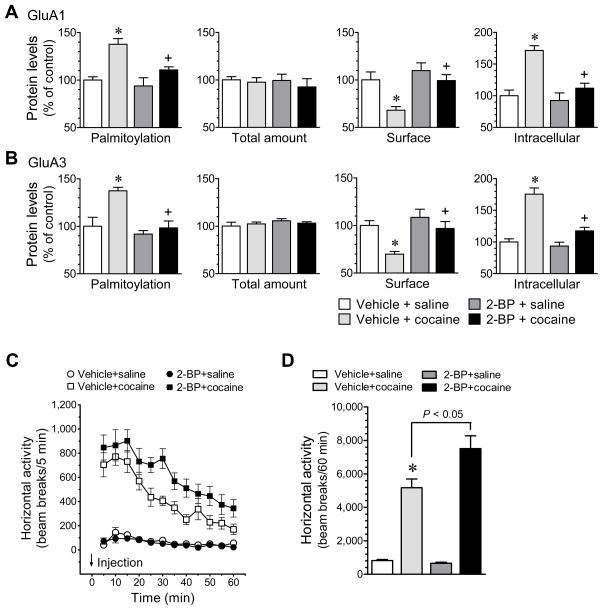
Palmitoylation is an enzymatically catalyzed event and is catalyzed by a family of membrane-bound enzymes known as palmitoyl acyltransferases (PATs). All PATs contain an Asp-His-His-Cys (DHHC) Cys-rich domain which is conserved in PATs from yeast to mammals and is recognized as the molecular signature of PATs (8). At least 23 mammalian DHHC-containing PATs (DHHC1-23) have been identified. These PATs noticeably have the confined substrate selectivity (8). For glutamate receptors, a Golgi apparatus-specific protein with a DHHC zinc finger domain (GODZ, also known as DHHC3) has the PAT activity toward constitutive AMPAR palmitoylation in cultured cortical neurons and heterologous cells (9,30). In addition to the Golgi-resident DHHC3, a dendritically localized DHHC2 activity-dependently translocates to the postsynaptic density (PSD) to induce a rapid palmitoylation of PSD-95 in cultured hippocampal neurons (29). To determine roles of PATs in mediating cocaine-stimulated AMPAR palmitoylation and evaluate the causal linkage between altered palmitoylation and redistribution of the receptor, we examined whether 2-BP, a nonmetabolizable palmitate and a general inhibitor of protein palmitoylation (31), affects cocaine-regulated palmitoylation and surface/intracellular expression of GluA1/3. In chronically cannulated and freely-moving rats, microinjection of 2-BP into the NAc (0.3 nmol/0.5 μl, 1 h prior to cocaine) abolished the increase in GluA1 palmitoylation in the NAc induced by an i.p. injection of cocaine (20 mg/kg, 30 min) (Fig. 6A). This effect was accompanied by reversal of the cocaine-stimulated changes in surface and intracellular expression of GluA1 (Fig. 6A). Similar results were observed from assays for GluA3 (Fig. 6B). These results indicate that cocaine stimulates PATs to induce GluA1/3 palmitoylation. In addition, as compared to vehicle injection, 2-BP induced no difference in the number of apoptotic cells as detected by fragment-end labeling and the number of pyknotic cells in NAc slices, indicating that 2-BP caused no toxicity to NAc neurons.
Figure 6. Effects of 2-BP on cocaine-stimulated palmitoylation and redistribution of GluA1/3 in rat NAc neurons and on behavioral responses to cocaine.
(A and B) Effects of 2-BP on cocaine-stimulated palmitoylation and redistribution of GluA1 (A) and GluA3 (B) in the NAc. Data were analyzed with one-way ANOVA: palmitoylation, surface and intracellular GluA1 for F(3,20) = 9.9, F(3,20) = 6.8 and F(3,20) = 15.2, respectively; P < .05, and GluA3 for F(3,20) = 10.2, F(3,20) = 7.1 and F(3,20) = 29.1, respectively; P < .05. (C) Intra-NAc injection of 2-BP augmented the cocaine-stimulated horizontal activity. Data were analyzed with two-way ANOVA [drug F(3,28) = 31.7, P < .05; time F(11,308) = 16.8, P < .05; interaction F(33,308) = 3.8, P < .05]. (D) A graph of the data from (C) obtained during 60 min after i.p. injection of saline or cocaine. Vehicle or 2-BP (0.3 nmol/0.5 μl) was injected into bilateral NAc 1 h prior to an i.p. injection of saline or cocaine (20 mg/kg, i.p.). Data are presented as means ± SEM. *P < .05 versus vehicle + saline. +P < .05 versus vehicle + cocaine.
2-BP increases behavioral sensitivity to cocaine
To determine whether inhibition of palmitoylation has any impact on behavioral responsivity to the drug, we tested locomotor responses to cocaine in rats pretreated with a local injection of 2-BP into the NAc, a drug treatment which was shown to block the cocaine-stimulated GluA1/3 palmitoylation and subsequent internalization (see above). We subjected chronically-cannulated and freely moving rats to microinjections of 2-BP into bilateral NAc (0.3 nmol/0.5 μl/side). We then injected cocaine to these rats (20 mg/kg, i.p., 1 h after 2-BP) and monitored their motor responses to the drug. We found that cocaine induced a greater and more sustained motor response in rats pretreated with 2-BP relative to rats pretreated with a vehicle control (Fig. 6C and 6D). Rats pretreated with 2-BP alone exhibited a minimal change in spontaneous motor activity (Fig. 6). These data demonstrate that the local inhibition of activity-dependent protein palmitoylation in the NAc with 2-BP can augment and sustain behavioral responses to cocaine.
Discussion
PATs catalyze the site-specific palmitoylation. While all PATs clearly contain a DHHC Cys-rich domain, it is unclear if there exists a consensus palmitoylation motif surrounding palmitoyl cysteines (8). Nevertheless, as more palmitoylated proteins are discovered, a pattern of palmitoylation motif seems to have emerged. In a preferred algorithm, basic and hydrophobic residues and additional cysteines seem to appear as more favorable and common residues around palmitoyl cysteines (32,33). In the case of AMPARs, four subunits are palmitoylated on two conserved cysteine residues: one within C-terminal regions (GluA1-Cys811; GluA2-Cys836; GluA3-Cys841; and GluA4-Cys817) and another within the transmembrane domain (TMD) 2 (GluA1-Cys585; GluA2-Cys610; GluA3-Cys615; and GluA4-Cys611) (9). These sites are surrounded by basic and hydrophobic residues. They are adjacent to transmembrane domains, consistent with a pattern of palmitoylation for transmembrane proteins, i.e., palmitoylation at intracellular cysteines near transmembrane domains (7,34,35). The Golgi-resident PAT DHHC3 promotes the palmitoylation on the TMD 2 site, while the exact subtype of PATs that palmitoylates the C-terminal site still needs to be identified (9). In this study, we used the ABE method to detect overall, but not site-specific, palmitoylation of AMPARs. A detection of palmitoyl- and site-specific palmitoylation in the adult brain in vivo will have to rely on palmitoyl- and site-specific antibodies to be developed.
Glutamate receptor palmitoylation is much less studied in vivo than in vitro. We thus initiated this study to portray glutamate receptor palmitoylation in vivo. Several important in vivo characteristics were established. First, among various types of posttranslational modifications, palmitoylation like phosphorylation is unique because it is regulated activity-dependently by extracellular signals and it is a reversible process. This is evidently the case for AMPAR palmitoylation in NAc neurons in response to cocaine. Similar to this, Kang et al. (20) found that kainic acid, a drug enhancing neuronal excitability leading to seizure-like behavior (36), increased GluA1 palmitoylation in brains. Second, with regard to the underlying mechanism, palmitoylation cycle is regulated by the balance of PATs and palmitoyl thioesterases (PTEs) which depalmitoylate substrates. Thus, stimulating PATs and/or inhibiting PTEs could increase GluA1 and GluA3 palmitoylation. Given that the cocaine effect was sensitive to the PAT inhibitor 2-BP, elevated PAT activity is believed to contribute to the increase in GluA1/3 palmitoylation. At the receptor level, dopamine and/or glutamate receptors likely mediate cocaine action, a topic being investigated. Finally, the selective effect of cocaine on GluA1/3 is noteworthy. This is consistent with the finding that kainic acid increased GluA1, but not GluA2, palmitoylation (20). It appears that, in the complex in vivo brain, cocaine selectively affects the distinct AMPAR subtypes, either GluA1 homomers or GluA1/3 heteromers, likely in subsets of excitatory synapses via a mechanism involving the integrated influence of the drug on multiple neurotransmitter systems.
Palmitoylation is one of protein modifications that can translocate modified proteins from one membrane microdomain to another in an activity-sensitive fashion. Thus, it is interesting to explore whether altered AMPAR palmitoylation leads to changes in their subcellular localization. Our data indicate that inducible palmitoylation in response to cocaine indeed regulates localization of the receptor. The cocaine-stimulated palmitoylation of GluA1 and GluA3 was accompanied by a loss of surface GluA1/3 and an addition of intracellular GluA1/3. This pattern of redistribution was reversed when the induced palmitoylation was blocked by 2-BP. Precise mechanism(s) linking the palmitoylation to the redistribution are unclear. However, induced palmitoylation is believed to increase protein lipophilicity or regulate protein-protein interactions. Through either or both, palmitoylation can rapidly redistribute proteins in a dramatic or subtle way. According to Hayashi et al. (9), agonist-induced acute internalization of AMPARs is regulated by palmitoylation. By disrupting the interaction of receptors with 4.1N, a synapse-enriched cytoskeletal protein stabilizing surface AMPAR expression (37), palmitoylation on the C-terminal site reduced the affinity of the receptor for 4.1N and accelerated the activity-dependent receptor internalization in cultured cortical neurons. Thus, cocaine may reduce surface receptors via palmitoylation- and activity-sensitive internalization.
A single dose of cocaine can increase extracellular glutamate in the NAc (see below). This may reduce surface AMPAR expression to homeostatically control neuronal excitability and motor responses to cocaine. As such, limiting the palmitoylation-dependent endocytosis of AMPARs may have a behavioral consequence. In fact, the palmitoylation inhibitor that inhibited the cocaine-stimulated GluA1/3 palmitoylation in the local NAc sustained behavioral responses to cocaine. Thus, inducible and reversible palmitoylation seems to serve as a dynamic regulator controlling surface AMPAR abundance and psychomotor responsivity to cocaine.
A single high dose of cocaine increased extracellular glutamate in the NA (38,39). A lower dose of cocaine challenge was sufficient to increase glutamate in the NA of cocaine-sensitized rats (40–42). In addition to presynaptic glutamate release, surface expression of postsynaptic AMPARs in the NAc was elevated after withdrawal from repeated or self administration of cocaine (43,44). The elevated surface AMPAR expression returned to normal levels (43) and synaptic depression was induced (45,46) in the NAc in response to a single cocaine re-exposure (challenge) after extended withdrawal. The withdrawal-induced elevation of surface AMPARs seems to mediate the incubation of cocaine craving (44), although elevating AMPAR expression and function in the NAc via viral overexpression of AMPARs was found to attenuate cocaine sensitization and seeking and facilitate cocaine extinction (47,48). These data underscore the complexity of AMPARs in adaptations to cocaine and in regulating drug effects. Our study adds a new layer of AMPAR regulation by cocaine. Future studies will explore whether this palmitoylation-dependent regulation undergoes plastic changes in response to chronic drug administration and contributes to enduring AMPAR plasticity and cocaine addiction.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW) and a grant from Saint Luke’s Hospital Foundation.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 2.Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 4.Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]
- 5.Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- 6.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 7.El-Husseini Ael-D, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- 8.Planey SL, Zacharias DA. Palmitoyl acyltransferases, their substrates, and novel assays to connect them. Mol Mem Biol. 2009;26:14–31. doi: 10.1080/09687680802646703. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- 11.Church WH, Justice JB, Jr, Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur J Pharmacol. 1987;139:345–348. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- 12.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 13.Bernard V, Gardiol A, Faucheux B, Bloch B, Agid Y, Hirsch EC. Expression of glutamate receptors in the human and rat basal ganglia: effect of the dopaminergic denervation on AMPA receptor gene expression in the striatopallidal complex in Parkinson's disease and rat with 6-OHDA lesion. J Comp Neurol. 1996;368:553–568. doi: 10.1002/(SICI)1096-9861(19960513)368:4<553::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Kondo M, Okabe S, Sumino R, Okado H. A high GluR1:GluR2 expression ratio is correlated with expression of Ca2+-binding proteins in rat forebrain neurons. Eur J Neurosci. 2000;12:2812–2822. doi: 10.1046/j.1460-9568.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu HJ, Chen LW, Yung KK, Chan YS. Differential expression of AMPA receptor subunits in substance P receptor-containing neurons of the caudate-putamen of rats. Neurosci Res. 2004;49:281–288. doi: 10.1016/j.neures.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, Zhou HF, Xu M, Wang JQ. Activity–dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drisdel RC, Alexander JK, Sayeed A, Green WN. Assays of protein palmitoylation. Methods. 2006;40:127–134. doi: 10.1016/j.ymeth.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Roth AF, Wan J, Sun AO, Kuchar JA, Green WN, Phinney BS, Yates JRI, Davis NG. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, Green WN, Yates III, JR, Davis NG, El-Husseini A. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nat Protoc. 2007;2:1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LacKamp A, Zhang GC, Mao LM, Fibuch EE, Wang JQ. Loss of surface N-methyl-D-aspartate receptor proteins in mouse cortical neurones during anaesthesia induced by chloral hydrate in vivo. Br J Anaesth. 2009;102:515–522. doi: 10.1093/bja/aep009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baekkeskov S, Kanaani J. Palmitoylation cycles and regulation of protein function. Mol Membr Biol. 2009;26:42–54. doi: 10.1080/09687680802680108. [DOI] [PubMed] [Google Scholar]
- 27.Bizzozero OA. Chemical analysis of acylation sites and species. Methods Enzymol. 1995;250:361–379. doi: 10.1016/0076-6879(95)50085-5. [DOI] [PubMed] [Google Scholar]
- 28.El-Husseini Ael-D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 29.Noritake J, Fukata Y, Iwanaga T, Naoki H, Tsutsumi R, Matsuda N, Tani H, Iwanari H, Mochizuki Y, Kodama T, Matsuura Y, Bredt D, Hamakubo T, Fukata M. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J Cell Biol. 2009;186:147–160. doi: 10.1083/jcb.200903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uemura T, Mori H, Mishina M. Isolation and characterization of Golgi apparatus-specific GODZ with the DHHC zinc finger domain. Biochem Biophys Res Commun. 2002;296:492–496. doi: 10.1016/s0006-291x(02)00900-2. [DOI] [PubMed] [Google Scholar]
- 31.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 32.Xue Y, Chen H, Jin C, Sun Z, Yao X. NBA-Palm: prediction of palmitoylation site implemented in Naive Bayes algorithm. BMC Bioinformatics. 2006;7:458. doi: 10.1186/1471-2105-7-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng Des Sel. 2008;21:639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 35.Gauthier-Campbell C, Bredt DS, Murphy TH, El-Husseini AD. Regulation of dendritic branching and filopodia formation in hippocampal neurons by specific acylated protein motifs. Mol Biol Cell. 2004;15:2205–2217. doi: 10.1091/mbc.E03-07-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fannjiang Y, Kim CH, Huganir RL, Zou S, Lindsten T, Thompson CB, Mito T, Traystman RJ, Larsen T, Griffin DE, Mandir AS, Dawson TM, Dike S, Sappington AL, Kerr DA, Jonas EA, Kaczmarek LK, Hardwick JM. BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Dev Cell. 2003;4:575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 37.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-liked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- 39.Reid MS, Hsu K, Jr, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- 42.Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 43.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 46.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behavior. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 48.Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Euro J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.