Abstract
Signals through the B cell antigen receptor (BCR) are necessary but not sufficient for cellular activation. Co-stimulatory signals must be provided through other immune recognition receptor systems, such as MHC class II/CD40 and the toll-like receptor (TLR) 9 that can only productively acquire their ligands in the processive environment of specialized late endosomes (MHC class II containing compartment or MIIC). It has long been appreciated that the BCR, by effectively capturing complex antigens and delivering them to late endosomes, is the link between activation events on the cell surface and those dependent on late endosomes. However, it has become increasingly apparent that the BCR also directs the translocation of MHC class II and TLR9 into the MIIC and that the endocytic flow of these receptors coincides with that of the BCR. This likely ensures close apposition of receptor complexes within the MIIC and the efficient transfer of ligands from the BCR to MHC class II and TLR9. This complex orchestration of receptor endocytic movement is dependent upon the quality of signals elicited through the BCR. Failure to activate specific signaling pathways, such as occurs in anergic B cells, prevents the entry of the BCR and TLR9 into the MIIC and abrogates TLR9 activation. Like anergy, this block in endocytic trafficking is rapidly reversible. These findings indicate that cellular responsiveness can be determined by mechanisms that control the subcellular location of important immune recognition receptors.
Introduction
For most peripheral immune responses, lymphocytes require two signals to become fully activated. The first is elicited by antigen recognition while the second can be provided by a number of receptors that assess the context in which antigen is recognized. For T cells, the second signal is usually provided through surface co-stimulatory receptors expressed on activated antigen presenting cells (APCs)(Greenwald et al., 2005). The integration of antigen receptor and co-stimulatory receptors, at the cell surface, determine cell fate. In contrast, B cells are dependent upon processes exclusive to late endosomes for most second signals required for cellular activation (Clark et al., 2004; Herlands et al., 2008; Krieg and Vollmer, 2007; Watts, 1997).
It has long been appreciated that the recruitment of T cell help requires antigens to be delivered to late endosomes (Ferrari et al., 1997; Qiu et al., 1994) which provide an environment where polypeptides can be processed and efficiently loaded onto MHC class II (Drake et al., 1999; Kleijmeer et al., 1997; Watts, 2001). Recently it has become apparent that the Toll-like receptors 7 and 9, which can effectively provide activation signals, are restricted to endocytic compartments. TLR activation plays a role in normal peripheral B cell responses, by detecting single stranded (ss) RNA (TLR7) and unmethylated CpG motifs within ssDNA (TLR9)(Krieg and Vollmer, 2007). They also are obligatory for many autoimmune humoral responses (Christensen et al., 2006; Leadbetter et al., 2002; Sadanaga et al., 2007).
In contrast to other APCs, non-specific mechanisms for the delivery of antigen to late endosomes, such as pinocytosis and phagocytosis, are not efficient in B cells (Pierce et al., 1988; Siemasko and Clark, 2001; Song et al., 1995). Rather, the MHC class II and TLR rich late endosomes of B cells are privileged environments preferentially targeted by immune receptors such as the BCR (Clark et al., 2004; Hess et al., 2000). The primacy of antigen receptors as portals for entry ensure the recruitment of second signals, and cellular activation, is dependent upon the nature of the antigenic complexes that are captured and delivered to late endosomes.
Herein, we will review our current understanding of how BCRs are selected for internalization and what are the molecular processes that direct the endocytosed BCR, and the TLRs, to late endosomes. Furthermore, we will review recent findings from our laboratory that receptor endocytic trafficking is abrogated in anergic cells. These latter data reveal a new level of regulation in which the subcellular location of important immune response receptors determine how B cells respond to immunogenic and tolerogenic ligands.
BCR Internalization: retention of signaling complexes on the cell surface
Recent in vivo studies have revealed several mechanisms by which antigen is provided to B lymphocytes (Batista and Harwood, 2009) including capture and presentation by macrophages (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2009), dendritic cells (Qi et al., 2006) and directly by diffusion through a conduit network composed of collagen bundles surrounded by follicular reticular cells (Gretz et al., 2000; Sixt et al., 2005). Non-cognate marginal zone B cells can also shuttle antigen to follicular dendritic cells for presentation to B cells (Batista and Harwood, 2009; Phan et al., 2009). Through most of these mechanisms, antigen is provided to B cells in membrane-bound arrays that induce receptor activation and clustering (Liu et al., 2010).
Receptor clustering elicits a series of signaling events that greatly accelerate the internalization of the BCR and its delivery to late endosomes. These events can be organized into an afferent signaling pathway, which activates the endocytic machinery and an efferent limb that allows primed endocytic machinery to discriminate between surface BCR complexes destined for internalization and those that will assemble a signalsome at the cell surface (A in Figure 1). The afferent signaling pathway emanating from the BCR (Figure 2) involves proximal activation of one or more Src-family tyrosine kinases (SFTKs) and is associated with the phosphorylation of clathrin heavy chain and actin polymerization (Guagliardi et al., 1990; Salisbury et al., 1980; Stoddart et al., 2002; Stoddart et al., 2005). Internalization also requires the B lymphocyte adaptor protein LAB which is inductively phosphorylated and then recruits Grb2-dynamin and Vav (Malhotra et al., 2009a; Malhotra et al., 2009b). Alternatively, BLNK may serve to link Grb2 to BCR-mediated kinase activation {Kabak, 2002#2870}. Downstream of Vav, Rac1/2 is required for BCR endocytosis.
Figure 1. Decision points along the endocytic pathway to the MIIC.
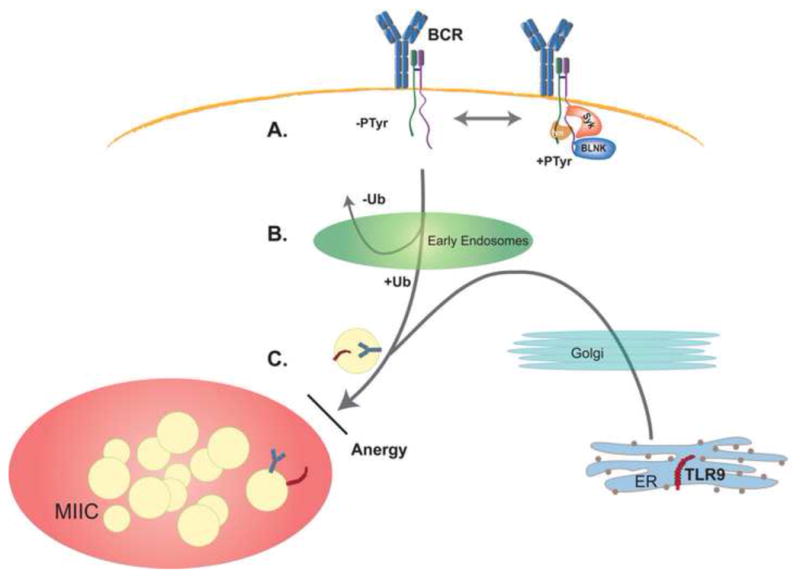
(A) Following recognition of antigen, only a small subfraction of surface BCR complexes are tyrosine phosphorylated (PTyr) and these assemble a signalsome at the cell surface. The majority of BCR complexes remain unphosphorylated and therefore are targeted for internalization through tyrosine-based internalization motifs within the cytosolic tails of Igα and Igβ. (B) The second decision point occurs in early endosomes. Receptors that are ubiquitinylated (Ub) are sorted towards late endosomes while those that are not ubiquitinylated recycle back to the cell surface. (C) Analysis of anergic B cells has revealed a third decision point along the endocytic pathway, at entry into the MIIC. BCR signals also control the transit of TLR9 and the block induced in anergy affects entry of both the BCR and TLR9 into the MIIC. These and other observations indicate that the BCR and TLR9 share a common final pathway into the MIIC and that, in wild-type cells, they enter together.
Figure 2. Selection of BCR complexes for internalization.
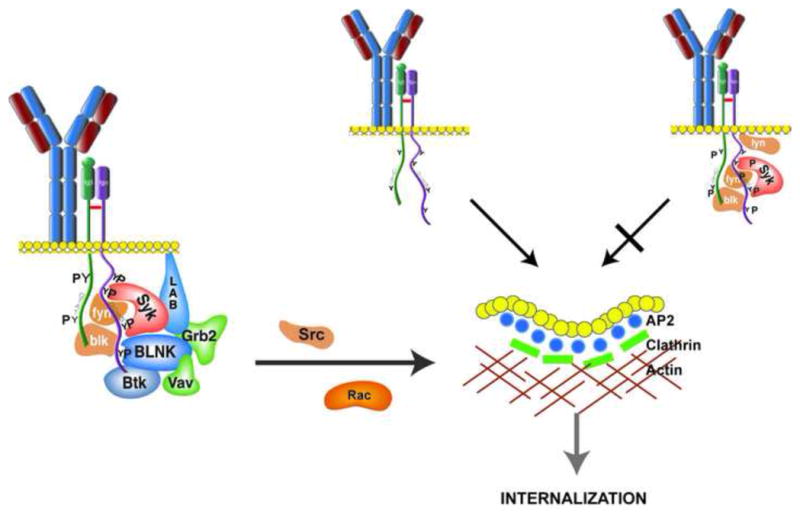
Clustering of the BCR induces the assembly of a signalsome at the receptor containing Syk, BLNK and BLNK-associated molecules. The complex, in conjunction with the adaptor LAB activates signaling cascades that include the molecules Vav, Rac and Src-family kinases (such as fyn and blk). These signaling pathways activate endocytic machinery (the adaptor AP2, clathrin and actin) such that they can select cargo (receptors) for endocytosis and entry into the endocytic pathway. In our model, BCR complexes are selected for inclusion in clathrin-coated pits if they have AP2 docking sites available (YXXL). If these motifs are blocked because they are phosphoryated, or they are obscured by recruited signaling molecules, the receptor is retained on the cell surface. Through this mechanism, BCR complexes involved in signal propagation are retained on the plasma membrane and are not targeted for degradation.
Once activated, the endocytic machinery must be targeted to the fraction of receptors fated for internalization (Figure 2). How is this accomplished? It had been assumed that active signaling complexes are efficiently or even preferentially targeted for degradation and that this would serve to directly down modulate BCR signaling. However, this is not the case. The BCR motifs that are recognized by the endocytic machinery are the same ones that, when phosphorylated, initiate BCR-dependent signaling cascades (Gazumyan et al., 2006; Hou et al., 2006)(A in Figure 1 and Figure 2). In the non-phosphorylated state, the tyrosines of the immunoreceptor tyrosine-based activation motifs (ITAMs) in the BCR subcomponents Igα/Igβ and the Igα non-ITAM motif direct internalization of the receptor. Available data indicate that all of the conserved tyrosine-based motifs in both Igα and Igβ additively contribute to BCR internalization (Gazumyan et al., 2006; Hou et al., 2006). The exact mechanism by which this occurs is not clear. However, the ITAMs are predicted to recruit the adaptor AP2 (Traub, 2004) which has been demonstrated to couple receptors to internalization through clathrin-coated pits. Recognition by AP2 requires an intact phenyl group that can be provided by either a tyrosine or phenylalanine. Nonconservative mutations or phosphorylation of the AP2 motif tyrosines on the receptor ablates recognition by AP2. Such a mechanism is consistent with observations that mutation of Igα ITAM and non-ITAM tyrosines to alanine impedes internalization and that following ligation, phosphorylated BCR complexes are selectively retained on the cell surface (Hou et al., 2006).
These findings indicate that BCR internalization attenuates signaling by limiting the number of BCR complexes available for phosphorylation rather than targeting already phosphorylated complexes for degradation. Mathematical modeling predicts that selective internalization of non-phosphorylated BCRs, as opposed to non-selective internalization of all surface BCR complexes, would both increase the sensitivity of the receptor to low avidity ligands and limit the maximal response that can be elicited through the BCR (Hou et al., 2006). Selective internalization could also provide a mechanism of quality control, as hypophosphorylated BCR complexes would also be removed from the cell surface thereby increasing the efficiency of signal initiation.
If only non-phosphorylated BCR complexes are internalized, how are polyvalent ligands, those best at inducing BCR phosphorylation, delivered to late endosomes? There are several possibilities. Even with saturating ligation, only a small fraction of BCR complexes (<10%) are inducibly phosphorylated within two minutes (Hou et al., 2006). This fraction is then rapidly dephosphorylated such that only a few percent of BCR complexes remain phosphorylated ten minutes after receptor clustering. Therefore, at most, BCR phosphorylation might delay the internalization of a small fraction of antigen-bound receptors. While this would not limit the capture of prevalent antigens it might impede the capture of rare epitopes. Alternatively, it has been demonstrated that in anergic B cells, and in B cell lines, receptor ligation induces the dissociation of the antigen recognition subunit, membrane bound immunoglobulin (mIg), from the signaling subunits Igα/Igβ (termed receptor destabilization), allowing each to be internalized through different mechanisms (Kim et al., 2005; Vilen et al., 1999). While receptor destabilization has been demonstrated in biochemical and immunoelectron micrographic studies, it has not been observed in separate fluorescent resonance energy-transfer (FRET) studies (Tolar et al., 2005). However, this latter technique might not be sensitive to small subpopulations of receptors undergoing destabilization.
Targeting Late Endosomes: Mechanisms of BCR Ubiquitinylation and Endocytic Trafficking
Once the ligated BCR is internalized, it rapidly sorts through early endosomes to late endosomes. In APCs, late endosomes are specialized for antigen processing. They are rich in MHC class II and contain machineries for processing peptides and MHC loading (MIIC) (Watts, 1997; Watts, 2001). Ultrastructurally, typical MIIC vesicles consist of a limiting membrane studded with lysosomal associated membrane protein-1 (Lamp-1) and a lumen containing intraluminal multivesicular bodies (IMBs) (Ferrari et al., 1997; Siemasko and Clark, 2001; West et al., 1994). The IMBs are derived from transport vesicles that have gained access to the MIIC compartment (Railborg et al., 2003; Riberdy et al., 1994).
Work from several laboratories has provided a general model for how endocytosed receptor complexes are sorted through early endosomes and delivered into late endosomes (Railborg and Stenmark, 2009). Central to the model is the monoubiquitinylation of receptors (Hicke and Dunn, 2003; Stahl and Barbieri, 2002; Umebayashi, 2003) and the recognition of these ubiquitin modifications by a protein complex containing Hrs, Eps15 and STAM (collectively termed the endosomal complex required for transport or ESCRT-0)(Railborg and Stenmark, 2009; Slagsvold et al., 2006). ESCRT-0 engaged receptors are retained within the endosomal pathway while unbound receptors recycle to the cell surface. Retained receptors then bind Tsg 101, a subunit of ESCRT-I. Successive recruitment of the multimeric complexes ESCRT-II and ESCRT-III target receptors to late endosomes. These receptors are then sorted into intraluminal multivesicular bodies where they are degraded (Rodahl et al., 2009). Ubiquitinylation can also facilitate receptor internalization (Belouzard and Rouille, 2006). However, there are examples in which receptor ubiquitinylation has no role in receptor internalization (Duan et al., 2003; Huang et al., 2006).
This general model of receptor endocytic trafficking can be applied to the BCR (B in Figure 1). Within the BCR, Igβ ubiquitinylation is required for sorting through early endosomes and for trafficking into late endosomes (Zhang et al., 2007). Igα can become ubiquitinylated but is detectable only well after receptor ligation when most endocytosed complexes have been delivered to the MIIC. Mutation of Igβ cytosolic lysines to arginines, which ablates ubiquitinylation, yields a receptor that enters the recycling early endosomal pool upon internalization [(Zhang et al., 2007) and unpublished observations] and does not target to late endosomes. Igβ ubiquitinylation does not facilitate BCR internalization indicating that the mechanisms mediating BCR internalization are different from those directing endocytic trafficking.
In resting B cells, Igβ is constitutively ubiquitinylated and this does not change with receptor ligation. Therefore, receptor clustering, and the initiation of tyrosine kinase- mediated signal cascades, are not necessary for tagging the receptor with ubiquitin. This is consistent with earlier studies demonstrating that, once internalized, both ligated and unligated BCR complexes target late endosomes (Brown et al., 1999; Song et al., 1995). In normal B cells expressing wild-type BCR complexes, we have not observed significant recycling, or early endosomal retention, of clustered, endocytosed BCR complexes (unpublished observations). This suggests that the majority of surface BCRs are tagged with ubiquitin. One of the E3 ligases involved in Igβ ubiqutinylation is the HECT family member Itch (Zhang et al., 2007). It is not clear how Itch is recruited to the BCR or even if it directly ubiquitinylates Igβ. However, mutation of the Igβ ITAM tyrosines to alanines in vivo is associated with decreased Igβ ubiquitinylation of the resting receptor (Gazumyan et al., 2006). This observation suggests a role for the Igβ ITAM in coupling the BCR to Itch.
The unphosphorylated TCR is not ubiquitinylated in thymocytes. Ubiquitinylation requires receptor phosphorylation and the recruitment of the E3 ligase c-Cbl (Dragone et al., 2009; Myers et al., 2006). C-Cbl has also been implicated in controlling BCR surface expression and in mediating the ubiquitinylation and degradation of internalized receptor complexes (Dragone et al., 2009; Dragone et al., 2006). This function is dependent upon co-expression of the SFTK-associated adaptor protein (SLAP). However, we could not detect c-Cbl recruitment to the ligated BCR in peripheral naïve B cells (manuscript in preparation). Therefore, it is unclear how c-Cbl and SLAP are contributing to BCR degradation in peripheral B cells. While these data indicate that both the BCR and TCR are ubiquitinylated, and this is required for normal endocytic transit, the mechanisms by which each receptor is tagged with ubiquitin are different.
Coordination of BCR endocytic trafficking with MHC class II and TLR9
Stimulation of the BCR regulates and coordinates the endocytic transit of other immune receptors destined for the MIIC. The most well described example is newly synthesized MHC class II that, in resting cells, primarily resides outside the MIIC. Stimulation of the BCR induces the rapid targeting of MHC class II into the MIIC (Lankar et al., 2002; Siemasko et al., 1998). The activation of specific BCR-dependent signaling pathways is required for the trafficking of MHC class II and the biogenesis of IMBs (Lankar et al., 2002; Le Roux et al., 2007; Siemasko et al., 1998; Siemasko et al., 2002). However, it is also likely that normal IMB biogenesis requires the physical transit of the BCR into the MIIC. This latter point is suggested by studies of chimeric receptors containing only the cytosolic tail of Igα, and not Igβ, that can activate signaling pathways but arrest in early endosomes (Siemasko et al., 1999). In these cells, intracellular MHC class II co-localizes with the endocytosed Igα receptor and is not detectable in late endosomes (unpublished observations).
In addition to the BCR and BCR-dependent signaling pathways, cathespin S and invariant chain (Ii) are required to form IMBs (Boes et al., 2005) implicating MHC class II endocytic transit and/or processing in IMB biogenesis. A physical requirement for both MHC class II and the BCR to form IMBs would ensure that the MIIC intraluminal vesicles containing endocytosed BCR complexes were also decorated with MHC class II. Such close juxtaposition of receptors would enhance the loading of peptides derived from BCR-captured antigens onto MHC class II.
The BCR also controls and coordinates the transit of TLR9 into the MIIC (Chaturvedi et al., 2008; O’Neill et al., 2009). In resting cells, TLR9 is resident outside late endosomes, presumably in endoplasmic reticulum (Latz et al., 2004). Significant surface expression cannot be detected (Trinchieri and Sher, 2006). Upon BCR ligation, and the initiation of BCR-dependent signaling cascades, TLR9 transits into the MIIC. Like MHC class II, BCR and TLR9 transit are concurrent. Furthermore, as described below, analysis of anergic B cells indicates that the BCR and TLR9 enter the MIIC together, on common transport vesicles (O’Neill et al., 2009). Like MHC class II, segregation of TLR9 into the same IMBs as ligand-bearing BCRs should facilitate the productive transfer of ligand to TLR9. This juxtaposition of BCR and TLR9 occurs in the permissive environment of late endosomes where TLR9 is cleaved to its active form by acid-dependent cathepsins (Barton and Kagan, 2009; Park et al., 2008).
It is not clear if similar or different BCR-dependent signaling pathways control the endocytic transit of MHC class II and TLR9. Proximally, B cell linker protein (BLNK or SLP65)(Ishiai et al., 1999) and the downstream activation of one or more protein kinase Cs (PKCs) have been implicated in MHC class II endocytic trafficking (Siemasko et al., 1999; Siemasko et al., 2002). For TLR9 trafficking, MAP kinase (MAPK), c-Jun N-terminal kinase (JNK)(O’Neill et al., 2009) and phospholipase D (Chaturvedi et al., 2008) activation have been demonstrated to be important. However, there has been no direct comparison of the signaling requirements for targeting MHC class II and TLR9 to the MIIC. There are several possible downstream effectors that could have a role in controlling the entry of the BCR, MHC class II and TLR9 into late endosomes. The Rab family of GTPases are possible targets as they are important for the internalization and endocytic targeting of a variety of receptors and they can be regulated by the MAPKs (Cavalli et al., 2001; Zwang and Yarden, 2006). Other possibilities include the adaptor protein Cbl-interacting protein of 85 kDa (CIN85) and/or its homolog the CD2 adaptor protein (CD2AP). CIN85 has mostly been studied in the context of EGF receptor trafficking where it co-localizes with late endosomes (Zhang et al., 2009) and is important for receptor degradation (Haglund et al., 2002). CD2AP plays a prominent role in TCR function (Lee et al., 2003).
Entry into the MIIC by the BCR, TLR9 and MHC class II is a highly coordinated process that has specific signaling requirements and likely involves the physical delivery of one or more of these receptors. It is also a process critical for coupling BCR recognition to the recruitment of additional signaling pathways, ultimately downstream of MHC class II/CD40 and endocytic TLRs, necessary for full B cell activation. Therefore, processes that disrupt the signaling pathways necessary for normal receptor endocytic trafficking would be expected to abrogate B cell activation. Indeed, this is what happens in anergic B cells.
BCR endocytic trafficking in B cell tolerance
Receptor editing and clonal deletion irreversibly eliminate high affinity autoreactive B cell clones from the naïve repertoire (Erikson et al., 1991; Gay et al., 1993; Goodnow et al., 1989; Goodnow et al., 2010; Nemazee and Buerki, 1989; Tiegs et al., 1993). However, these mechanisms of tolerance inefficiently eliminate clones with relatively low affinity for self-antigen. Up to 20% of B cells that survive central tolerance, and that are selected into the peripheral B cell pool, express self-reactive immunoglobulins (Yurasov et al., 2005). However, this large and potentially dangerous pool of lymphocytes does not contribute to normal immune responses. In murine models of B cell tolerance, most of these peripheral B cell populations are intrinsically unresponsive to antigen, or anergic (Andrews and Wilson, 2010; Benschop et al., 2001; Borrero and Clarke, 2002; Nguyen et al., 1997). While anergy has been most convincingly demonstrated in immunoglobulin transgenic mice, populations of cells with features of anergy have been identified in both unmanipulated mice (Merrell et al., 2006) and in humans (Duty et al., 2009; Isnardi et al., 2010). Therefore, anergy appears to be a common and important mechanism of B cell tolerance.
In contrast to deletion and receptor editing, anergy is a reversible state that must be reinforced by chronic antigen exposure and signaling through the BCR (Cambier et al., 2007; Cornall et al., 1995; Cyster and Goodnow, 1995; Goodnow et al., 1991). This has been most convincingly demonstrated in the Ars/A1 transgenic model in which Ars-Tyr hapten effectively competes with single-stranded DNA for BCR binding and rapidly reverses anergy (Gauld et al., 2005). These data suggest that one way that B cells discriminate self from non-self is by the chronicity of antigenic exposure.
Immunogenic and tolerogenic stimuli induce different proximal BCR signaling responses (Jun and Goodnow, 2003). Immunogenicity is associated with the coordinated activation of a network of signaling pathways, including those leading to rapid intracellular calcium elevation as well as extracellular signal related kinase (ERK) and JNK activation (Skaggs and Clark, 2004). Most anergic B cells manifest aberrant BCR-induced calcium responses and attenuated JNK activation (Cambier et al., 2007; Healy et al., 1997). In contrast, it has been difficult to identify a characteristic transcriptional profile for anergic B cells (Cambier et al., 2007; Merrell et al., 2006). The apparent lack of correlation between transcriptional events and B cell anergy is not surprising given the rapidity with which anergy can be reversed.
If the proximal signaling aberrancies of anergy are not inducing tolergenic transcriptional programs, how are they attenuating cellular activation? We propose that a major function of BCR anergic signaling is to prevent the subsequent solicitation of the second signals needed for cellular activation (C in Figure 1). In anergic, ssDNA-specific B cells this is accomplished by excluding both the BCR and TLR9 from the MIIC (O’Neill et al., 2009). Without access to the processive environment of the MIIC DNA ligands captured by the BCR cannot be productively transferred to TLR9 (Park et al., 2008) (Barton and Kagan, 2009). This is particularly important for preventing the secretion of anti-DNA antibodies, which requires TLR9 (Christensen et al., 2006) and the downstream activation of MyD88 (Sadanaga et al., 2007).
The failure of both the BCR and TLR9 to enter the MIIC is a consequence of aberrant proximal BCR signaling in anergic cells. Pharmacologically complementing BCR signaling rapidly restores BCR and TLR9 endocytic trafficking within 30 minutes as does reversing BCR anergic signaling by competing away self-ligand (O’Neill et al., 2009). The kinetics of regulation implicates mechanisms of control that do not depend upon changes in transcription or protein translation. Such mechanisms are in keeping with the observed reversibility of anergy.
The block in receptor trafficking in anergic B cells appears to affect both the BCR and TLR9 receptors at the same level, along a common route into the MIIC. This is apparent from confocal images of anergic B cells in which the BCR and TLR9 co-localize in small vesicles lying outside Lamp-1+ late endosomes. For the BCR, the endocytic block is after early endocytic sorting, consistent with regulation at the level of entry into late endosomes (O’Neill et al., 2009). However, it should be noted that these conclusions are based on fixed cell images so it is possible that instead of inducing a static block in receptor trafficking, anergy changes the balance or flow of receptors through the endocytic pathway. Also, it is not known if receptors that cannot enter the MIIC adopt an alternative endocytic fate (e.g., trafficking to terminal lysosomes). Resolution of these questions will require additional live cell imaging studies.
To induce and/or maintain the anergic state, signaling through other intrinsic immune receptors, such as the TLRs and CD40, must be attenuated. However, anergy is phenotypically and undoubtedly mechanistically heterogeneous (Cambier et al., 2007). For example, in anergic B cells expressing a high affinity BCR specific for HEL, there is not a substantial block in BCR endocytic transit (Blery et al., 2006; O’Neill et al., 2009). Rather, in these cells constitutive ERK activation induces a global inhibition in intrinsic TLR mediated activation (Rui et al., 2003). In contrast, constitutive ERK activation is not a feature of anergic anti-ssDNA B cells (O’Neill et al., 2009). However, these cells are also hyporesponsive to the TLR4 ligand lipopolysaccharide (Nguyen et al., 1997), even though this receptor should be available on the cell surface and therefore not regulated by endocytic transit (Kagan et al., 2008). Therefore, in different anergic cell populations, and probably within single anergic cells, multiple mechanisms ensure that cells do not become spuriously activated through intrinsic immune response receptors.
Conclusions and Perspectives
The findings discussed above illustrate that anergy cannot be adequately characterized by the biochemical or transcriptional state of the cell. In addition, cell biological processes, and the spatial relationships of immune recognition receptors, are important features of the anergic state. Such mechanisms of regulation, which can be rapidly reversed if the need arises, are consistent with current concepts that anergy must be constantly reinforced by the presence of self-antigen.
These mechanisms of receptor sequestration not only reveal something novel about anergy, they reveal how basic cell biological processes can be regulated in lymphocytes to determine peripheral responsiveness. The mechanisms that mediate endocytic vesicular trafficking have been studied in great detail and in a variety of models. However, they have been viewed as largely constitutive processes that ensure cellular homeostasis. The above observations indicate that they are also potential points of regulation that can be modulated to determine how a cell responses to environmental cues. From the perspectives of both the cell biologist and the immunologist, it would be revealing to understand how BCR signaling controlled the kinetics of immune receptor endocytic trafficking as well as docking and entry into the MIIC.
If anergy is potentially reversible, why are these autoreactive and potentially pathogenic cells allowed to persistent in the periphery? In addition to being a mechanism to sequester autoreactivity, anergic B cells could play active roles in some immune responses. Anergic cells could directly suppress autoreactivity, through unknown mechanisms, and/or they could give rise to regulatory B cells (Andrews and Wilson, 2010). Alternatively, it could be that the broader autoimmune “naïve” or starting repertoire potentially afforded by anergic cells might enable productive immune responses against some infections. This possibility is suggested by observations that polyreactivity with self-antigens is a feature of many broadly neutralizing HIV antibodies (Haynes et al., 2005; Mouquet et al., 2010). Therefore, preserving a degree of autoreactivity may enable some protective immune responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews SF, Wilson PC. The anergic B cell. Blood. 2010;115:4976–4978. doi: 10.1182/blood-2010-03-276352. [DOI] [PubMed] [Google Scholar]
- Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- Belouzard S, Rouille Y. Ubiquitylation of leptin receptor OB-Ra regulates its clathrin mediated endocytosis. EMBO J. 2006;25:932–942. doi: 10.1038/sj.emboj.7600989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- Blery M, Tze LE, Miosge LA, Jun JE, Goodnow CC. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-kappa B in B cell clonal anergy. J Exp Med. 2006;203:1773–1783. doi: 10.1084/jem.20060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes M, van der Wel N, Peperzak V, Kim Y-m, Peters PJ, Ploegh H. In vivo control of endosomal architecture by class II-associated invariant chain and cathepsin S. Eur J Immunol. 2005;35:2552–2562. doi: 10.1002/eji.200526323. [DOI] [PubMed] [Google Scholar]
- Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- Brown BK, Li C, Cheng PC, Song W. Trafficking of the Iga/Igb heterodimer with membrane Ig and bound antigen to the major histocompatibility complex class II peptide-loading compartment. J Biol Chem. 1999;274:11439–11446. doi: 10.1074/jbc.274.16.11439. [DOI] [PubMed] [Google Scholar]
- Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nature Rev. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–71. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Grunberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Clark MR, Massenburg D, Siemasko K, Hou P, Zhang M. B-cell antigen receptor signaling requirements for targeting antigen to the MHC class II presentation pathway. Cur Opin Immunol. 2004;16:382–387. doi: 10.1016/j.coi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Cornall RJ, Goodnow CC, Cyster JG. The regulation of self-reactive B cells. Curr Opin Immunol. 1995;7:804–811. doi: 10.1016/0952-7915(95)80052-2. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Dragone L, Shaw LA, Myers MD, Weiss A. SLAP, a regulator of immunoreceptor ubiquitinylation, signaling, and trafficking. Immunol Rev. 2009;232:218–228. doi: 10.1111/j.1600-065X.2009.00827.x. [DOI] [PubMed] [Google Scholar]
- Dragone LL, Myers MD, White C, Sosinowski T, Weiss A. Src-like adaptor protein regulates B cell development and function. J Immunol. 2006;176:335–345. doi: 10.4049/jimmunol.176.1.335. [DOI] [PubMed] [Google Scholar]
- Drake JR, Lewis TA, Condon KB, Mitchell RN, Webster P. Involvement of MIIC-like late endosomes in B cell receptor-mediated antigen processing in murine B cells. J Immunol. 1999;162:1150–1155. [PubMed] [Google Scholar]
- Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Lakku RA, Ghosh AK, Fernandes N, Shou P, Mullane-Robinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitinylation is required for lysosomal sorting of EGF receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–28960. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias MD, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson P. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–4. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Knight AM, Watts C, Pieters J. Distinct intracellular compartments involved in invariant chain degradation and antigenic peptide loading of major histocompatibility complex (MHC) class II molecules. J Cell Biol. 1997;139:1433–1446. doi: 10.1083/jcb.139.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–7. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- Gay D, Saunders T, Camper S, Weigert M. Receptor editing: An approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazumyan A, Reichlin A, Nussenzweig MC. Igbeta tyrosine residues contribute to the control of B cell receptor signaling by regulating receptor internalization. J Exp Med. 2006;203:1785–1794. doi: 10.1084/jem.20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–6. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–91. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Ann Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–40. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardi LE, Koppelman B, Blum JS, Marks MS, Cresswell P, Brodsky FM. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990;343:133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Haglund K, Shimokawa N, Szymkiewicz I, Dikic I. Cbl-directed monoubiquitination of CIN85 is involved in regulation of ligand-induced degradation of EGF receptors. Proc Natl Acad Sci. 2002;99:12191–12196. doi: 10.1073/pnas.192462299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson JF, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two boradly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–28. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- Herlands RA, Christensen S, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess MW, Schwendinger MG, Eskelinen EL, Pfaller K, Pavelka M, Dierich MP, Prodinger WM. Tracing uptake of C3dg-conjugated antigen into B cells via complement receptor 2 (CR2, CD21) Blood. 2000;95:2617–2623. [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Ann Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hou P, Araujo E, Zhao T, Massenburg D, Veselits M, Doyle C, Dinner AR, Clark MR. B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biology. 2006:e200. doi: 10.1371/journal.pbio.0040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi SP, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitinylation with the kinase domain. Mol Cell. 2006;17:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan AC, Kurosaki T. BLNK required for coupling Syk to PLCg2 and Rac1-JNK in B Cells. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E. Complement receptor 2/CD21-human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JE, Goodnow CC. Scaffolding of antigen receptors for immunogenic versus tolergenic signaling. Nat Immunol. 2003;4:1057–1064. doi: 10.1038/ni1001. [DOI] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–4. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:343–5. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Cramer L, Mueller H, Wilson B, Vilen BJ. Independent trafficking of Ig-alpha/Ig-beta and micro-heavy chain is facilitated by dissociation of the B cell antigen receptor complex. J Immunol. 2005;175:147–154. doi: 10.4049/jimmunol.175.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijmeer MJ, Morkowski S, Griffith JM, Rudensky AY, Gueze HJ. Major histocompatibility complex class II compartments in human and nouse B lymphoblasts represent conventional endocytic compartments. J Cell Bio. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251–69. doi: 10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Lankar D, Vincent-Schneider H, Briken V, Yokozeki T, Raposo G, Bonnerot C. Dynamics of major histocompatibility complex class II compartments during B cell receptor-mediated cell activation. J Exp Med. 2002;195:461–72. doi: 10.1084/jem.20011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- Le Roux D, Lankar D, Yuseff MI, Vascottto F, Yokozeki T, Faure-Andre G, Mougneau E, Glaichenhaus N, Manoury B, Bonnerot C, Lennon-Dumenil AM. Syk-dependent actin dynamics regulate endocytic trafficking and processing of antigens internalized through the B-cell receptor. Mol Biol Cell. 2007;18:3451–3462. doi: 10.1091/mbc.E06-12-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik M, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:595–598. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen PM, Dustin ML, Chakraborty AK, Shaw A. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Liu W, Sohn HW, Tolar P, Pierce SK. It’s all about change: the antigen-driven initiation of B-cell receptor signaling. Cold Spring Harb Perspect Biol. 2010;2:a002295. doi: 10.1101/cshperspect.a002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Kovats S, Zhang W, Coggeshall KM. B cell antigen receptor endocytosis and antigen presentation to T cells require Vav and dynamin. J Biol Chem. 2009a;284:24088–24097. doi: 10.1074/jbc.M109.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Kovats S, Zhang W, Coggeshall KM. Vav and Rac activation in B cell antigen receptor endocytosis involves Vav recruitment to the adapter protein LAB. J Biol Chem. 2009b;284:36202–36212. doi: 10.1074/jbc.M109.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–62. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov M, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AD, Hope T, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010 doi: 10.1038/nature09385. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MD, Sosinowski T, Dragone LL, White C, Band H, Gu H, Weiss A. Src-like adaptor protein regulates TCR expression on thymocytes by linking the ubiquitin ligase c-Cbl to the TCR complex. Nat Immunol. 2006;7:57–66. doi: 10.1038/ni1291. [DOI] [PubMed] [Google Scholar]
- Nemazee DA, Buerki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Mandik L, Bui A, Kavaler J, Norvell A, Monroe JG, Roark JH, Erikson J. Characterization of anti-single-stranded DNA B cells in a non-autoimmune background. J Immunol. 1997;159:2633–44. [PubMed] [Google Scholar]
- O’Neill SK, Veselits ML, Zhang M, Labno C, Cao Y, Finnegan A, Uccelini M, Alegre ML, Cambier JC, Clark MR. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic B cells. Proc Nat Acad Sci, USA. 2009;106:6262–6267. doi: 10.1073/pnas.0812922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;12:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:786–93. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SK, Morris JF, Grusby MJ, Kawmaya, Van Buskirk A, Srnivasan M, Grump B, Smolenski LA. Antigen-presenting function of B-lymphocytes. Immunological Rev. 1988;106:149–180. doi: 10.1111/j.1600-065x.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AYC, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Xu X, Wandinger-Ness A, Dalke DP, Pierce SK. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994;125:595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railborg C, Rusten TE, Stenmark H. Protein sorting into mutivesicular endosomes. Curr Op Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- Railborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- Riberdy JM, Avva RR, Geuze HJ, Cresswell P. Transport and intracellular distribution of MHC class II molecules associated with invariant chain in normal and antigen processing mutant cell lines. J Cell Biol. 1994;125:1215–1237. doi: 10.1083/jcb.125.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodahl LM, Stuffers S, Lobert VH, Stenmark H. The role of ESCRT proteins in attenuation of cell signaling. Biochem Soc Trans. 2009;37:137–142. doi: 10.1042/BST0370137. [DOI] [PubMed] [Google Scholar]
- Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, Sugiyama N, Niiro H, Harada M. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–28. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- Salisbury JL, Condeelis SJ, Satir P. Role of coated vesicles, micorfilaments and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J Cell Biol. 1980;87:132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemasko K, Clark MR. The control and facilitation of MHC class II antigen processing by the BCR. Curr Opin Immunol. 2001;13:32–6. doi: 10.1016/s0952-7915(00)00178-3. [DOI] [PubMed] [Google Scholar]
- Siemasko K, Eisfelder BJ, Stebbins C, Kabak S, Sant AJ, Song W, Clark MR. Ig-alpha and Ig-beta are required for efficient trafficking to late endosomes and to enhance antigen presentation. J Immunol. 1999;162:6518–6525. [PubMed] [Google Scholar]
- Siemasko K, Eisfelder BJ, Williamson E, Kabak S, Clark MR. Cutting edge: signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes. J Immunol. 1998;160:5203–8. [PubMed] [Google Scholar]
- Siemasko K, Skaggs BJ, Kabak S, Williamson E, Brown BK, Song W, Clark MR. Receptor-facilitated antigen presentation requires the recruitment of B cell linker protein to Iga. J Immunol. 2002;168:2127–2138. doi: 10.4049/jimmunol.168.5.2127. [DOI] [PubMed] [Google Scholar]
- Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Skaggs BJ, Clark MR. Proximal B cell receptor signaling pathways. Signal Transduction. 2004;5–6:173–194. [Google Scholar]
- Slagsvold T, Pattni K, Malerod L, Stgenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Song W, Cho H, Cheng P, Pierce SK. Entry of B cell antigen receptor and antigen into class II peptide-loading compartment is independent of receptor cross-linking. J Immunol. 1995;155:4255–4263. [PubMed] [Google Scholar]
- Stahl PD, Barbieri AM. Multivesicular bodies and multivesicular endosomes: The “ins and outs” of endosomal trafficking. Science’s SKTE. 2002 doi: 10.1126/stke.2002.141.pe32. http://www.skte.org/cgi/content/full/sigtrans;2002/141/pe32. [DOI] [PubMed]
- Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–462. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- Stoddart A, Jackson AP, Brodsky FM. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol Biol Cell. 2005;16:2339–2348. doi: 10.1091/mbc.E05-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar P, Sohn HW, Pierce SK. The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol. 2005;6:1168–1176. doi: 10.1038/ni1262. [DOI] [PubMed] [Google Scholar]
- Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2004;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defense. Nat Rev Immunol. 2006;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Umebayashi K. The roles of ubiquitin and lipids in protein sorting along the endocytic pathway. Cell Struct Function. 2003;28:443–453. doi: 10.1247/csf.28.443. [DOI] [PubMed] [Google Scholar]
- Vilen BJ, Nakamura T, Cambier JC. Antigen-Stimulated Dissociation of BCR mlg from Ig-a/Ig-b: Implications for Receptor Desensitizations. Immunity. 1999;10:239–248. doi: 10.1016/s1074-7613(00)80024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Ann Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- Watts C. Antigen processing in the endocytic compartment. Cur Opin Immunol. 2001;13:26–31. doi: 10.1016/s0952-7915(00)00177-1. [DOI] [PubMed] [Google Scholar]
- West MA, Lucocq JM, Watts C. Antigen processing and class II MHC peptide-loading in human B-lymphoblastoid cells. Nature. 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zheng XX, Yang X, Liao K. CIN85 associates with endosomal membrane and binds phosphatidic acid. Cell Res. 2009;19:733–746. doi: 10.1038/cr.2009.51. [DOI] [PubMed] [Google Scholar]
- Zhang M, Veselits M, O’Neill S, Hou P, Reddi AL, Berlin I, Ikeda M, Nash PD, Longnecker R, Band H, Clark MR. Ubiquitinylation of Ig-beta dictates the endocytic fate of the B cell antigen receptor. J Immunol. 2007;179:4435–43. doi: 10.4049/jimmunol.179.7.4435. [DOI] [PubMed] [Google Scholar]
- Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]


