Abstract
Hereditary and sporadic neurodegenerative ataxias are movement disorders that affect the cerebellum. Robust and objective biomarkers are critical for treatment trials of ataxias. In addition, such biomarkers may help discriminate between ataxia subtypes because these diseases display substantial overlap in clinical presentation and conventional MRI. Profiles of 10–13 neurochemical concentrations obtained in vivo by high field proton magnetic resonance spectroscopy (1H MRS) can potentially provide ataxia-type specific biomarkers. We compared cerebellar and brainstem neurochemical profiles measured at 4 T from 26 patients with spinocerebellar ataxias (SCA1, N=9; SCA2, N=7; SCA6, N=5) or cerebellar multiple system atrophy (MSA-C, N=5) and 15 age-matched healthy controls. The Scale for the Assessment and Rating of Ataxia (SARA) was used to assess disease severity. The patterns of neurochemical alterations relative to controls differed between ataxia types. Myo-inositol levels in the vermis, myo-inositol, total N-acetylaspartate, total creatine, glutamate, glutamine in the cerebellar hemispheres and myo-inositol, total N-acetylaspartate, glutamate in the pons were significantly different between patient groups (Bonferroni corrected p<0.05). The best MRS predictors were selected by a tree classification procedure and lead to 89% accurate classification of all subjects while the SARA scores overlapped considerably between patient groups. Therefore, this study demonstrated multiple neurochemical alterations in SCAs and MSA-C relative to controls and the potential for these neurochemical levels to differentiate ataxia types. Studies with higher numbers of patients and other ataxias are warranted to further investigate the clinical utility of neurochemical levels as measured by high-field MRS as ataxia biomarkers.
Keywords: Neurochemical profile, Magnetic resonance spectroscopy, SCA, Multiple system atrophy, Ataxia
Introduction
Neurodegenerative ataxias are movement disorders characterized by progressive gait and limb ataxia, dysarthria and a variable pattern of other progressive neurological deficits [1]. Their primary pathological characteristic is cerebellar and, in many cases, brainstem neurodegeneration. A number of genetic forms exist, such as the autosomal dominant spinocerebellar ataxias (SCAs). SCA1, SCA2, SCA3, and SCA6 are the most common SCAs worldwide, making up about half of the SCA patient population [2]. The most common nonhereditary form is cerebellar multiple system atrophy (MSA-C) [3].
SCAs and MSA-C, as most neurodegenerative diseases, pose significant challenges for assessing whether therapies effect disease progression because of their slow progression and phenotypic variability. Hence, long clinical trials with large sample sizes are needed due to limitations of clinical outcome measures that are typically used in such trials [4]. On the other hand, biomarkers may provide useful surrogate outcome measures to gauge therapies after proper validation with clinical scales. Therefore, robust biomarkers that can eventually be used as surrogate markers in trials will have a high impact on development of neuroprotective therapies. Major advances were made in understanding disease mechanisms in neurodegenerative ataxias [2, 5], resulting in various treatment possibilities [6]. Therefore, the need for reliable ataxia biomarkers is greater than ever.
Neurodegenerative ataxias also pose challenges for diagnosis. The advent of genetic testing for SCAs had a great impact in classifying these disorders; however, running the full profile of available tests is expensive. Clinical evaluation can discriminate groups of SCAs, e.g., pure cerebellar disorders from ataxias associated with extracerebellar features; however, it cannot distinguish the many SCA genotypes [2]. Diagnosis of sporadic forms is primarily based on clinical evaluation and conventional MRIs that demonstrate cerebellar atrophy. Therefore, identifying robust and objective imaging markers that can differentiate between ataxias has been a long-term interest in the ataxia field [7–17]. Such biomarkers could guide genetic testing for hereditary ataxias, help with diagnosis of nonhereditary ataxias, and contribute to the understanding of differences in the disease processes between ataxias.
Distinguishing ataxia types by imaging has been challenging. Conventional T1-, T2-, and proton density-weighted MRI do not distinguish SCA subtypes and MSA-C [7, 12, 16]. The disease specificity of nonconventional imaging methods, such as voxel-based morphometry, diffusion tensor imaging (DTI), and proton MRS (1H MRS), has been investigated with variable success [7–15]. For example, volumetric analysis distinguished SCA2 and SCA3, but not SCA1 from either disease [10]. In a recent multicenter volumetric study, SCA1, SCA3, and SCA6 could be distinguished with a 81.7% reclassification probability using data from five brain regions, underlining the utility of multiparametric approaches [13]. Despite initial difficulties in distinguishing SCA1 and SCA2 based on DTI [12], a recent pilot study demonstrated potential to distinguish SCA1 from MSA-C and SCA2 by using multiple DTI measures [7].
Magnetic resonance spectroscopy enables quantification of endogenous concentrations of neurochemicals and has great potential to yield metabolic biomarkers that can report on neuronal dysfunction prior to irreversible structural damage and during progressive neurodegeneration. MRS studies at 1.5 T typically focus on three metabolites (N-acetylaspartate (NAA), creatine, choline) or their ratios [8, 11, 18]. A common observation in neurodegenerative diseases is a decrease in NAA levels or the NAA/creatine ratio [17], making the MRS findings nonspecific. For example, cerebellar and pontine NAA levels were lower than controls in both SCA1 and SCA2, with no difference between patient groups [9]. A higher cerebellar NAA/creatine ratio was found in SCA6 (N=3) than SCA2 (N=4); however, this difference may have been due to more severe disease in patients with SCA2 and not due to the genetic difference alone [8]. A recent study reported the ability to distinguish SCA2 from MSA-C based on the observation of lactate in the cerebellum in SCA2 [11].
At higher fields, MRS enables quantification of 10–18 neurochemicals making up a “neurochemical profile” [19, 20]. Furthermore, metabolites are quantified with higher precision due to reduced measurement error, increasing the potential for disease-specific MRS findings. We have recently demonstrated altered neurochemical concentrations in the cerebellum and pons of patients with early-to-moderate SCA1 and correlations of a subset of these (NAA, myo-inositol, glutamate) with the scores on a validated ataxia rating scale [21]. Furthermore, the same neurochemicals displayed robust correlations with pathological changes and could be used longitudinally to monitor pre-symptomatic and progressive neurodegeneration in a mouse model of SCA1 [22]. Our goal in the current study was to assess neurochemical alterations in other neurodegenerative ataxias and the potential to use the neurochemical levels as ataxia-type specific biomarkers. Because the pathogenic pathways appear to differ between ataxia subtypes [2, 23] and different neurochemical patterns were demonstrated between mice with different ataxia-causing mutations [24], we hypothesized that the pattern of neurochemical profile alterations would differ between ataxia subtypes. To test this hypothesis, we measured cerebellar and brainstem neurochemical profiles of patients with SCA2, SCA6, and MSA-C by high field (4T) 1H MRS. These data combined with our prior data from patients with SCA1 and healthy controls [21] allowed us to test the disease specificity of MRS biomarkers. To examine if any potential differences in neurochemical alterations between disease groups can be explained by differences in disease severity, we also evaluated the patients with a validated ataxia rating scale [25].
Subjects and Methods
Subjects
Eight patients with SCA2 (age 51±6 (SD) years), five patients with SCA6 (53±8 years), and five patients with MSA-C (55±3 years; Table 1) enrolled in the study after giving written informed consent using procedures approved by the Institutional Review Board: Human Subjects Committee of University of Minnesota. Patients with SCA2 and SCA6 were diagnosed by genetic testing (Table 1). The diagnosis of probable MSA-C was established according to the criteria from the consensus statements on the diagnosis of MSA [3]. Exclusion criteria were identical to our prior study [21]. Patients underwent MR scanning and were evaluated with an ataxia rating scale [25] within 1 day of MR scanning. MRS data could not be obtained from one patient with SCA2 due to claustrophobia. Data for the nine patients with SCA1 and controls (N=15, age range matched to all patient groups) were taken from our prior study [21]. Subjects, including the patients with SCA1 and controls, were recruited from February 2005 to May 2009, and data acquired using identical methods for all groups.
Table 1.
Demographic and clinical characteristics of subjects from whom MRS data were acquired
| SCA1 (N=9) | SCA2 (N=7) | SCA6 (N=5) | MSA-C (N=5) | Controls (N=15) | P value | |
|---|---|---|---|---|---|---|
| Gender (female/male) | (4/5) | (2/5) | (3/2) | (3/2) | (8/7) | .721 |
| Age (years) | 54±6 | 51±7 | 53±8 | 55±3 | 52±8 | .779 |
| Age of onset (years) | 44±12 a | 29±7 b | 38±12 ab | 47±9 a | N/A | .026 |
| Disease duration (years) | 11±10 a | 22±11 b | 16±6 ab | 8±6 a | N/A | .064 |
| # of expanded CAG triplet repeats | 43±3 (range 40–19) | 38±1 (range 36–40) | 25±3 (range 21–27) | N/A | N/A | – |
| SARA score | 9±4 a | 10±4 ab | 16±1 bc | 21±11 c | 0.2±0.3d (N=10) | <.001 |
| % CSF, vermis | 33±10% a | 37±8% a | 51±3% b | 50±8% b | 12±5% c | <.001 |
| % CSF, cerebellar hemispheres | 7±6% a | 12±5% b | 4±2% ac | 10±4% ab | 2±1% c | <.001 |
| % CSF, pons | 4±3% a | 8±6% b | 4±2% a | 8±6% b | 3±1% a | .017 |
Values given as counts or as mean±SD, as appropriate. SCA1 and control data taken from [21]. Comparisons are between groups within each row. Means which share a letter are not significantly different; means with no letters in common are significantly different (p<0.05)
MR Protocol and Metabolite Quantification
The MR protocol at 4 T was identical to our prior study [21]. Briefly, 1H MR spectra from vermis (1×2.5×2.5 cm3), cerebellar hemisphere (1.7×1.7×1.7 cm3), and pons (1.6×1.6×1.6 cm3) were acquired with an ultra-short echo stimulated-echo acquisition mode (STEAM) sequence (echo time TE=5 ms, repetition time TR=4.5 s) and quantified using an automated deconvolution program (LCModel) [26] with the basis set described previously [21]. All metabolite concentrations were corrected for the amount of cerebrospinal fluid (CSF) in the volume-of-interest (VOI) [21]. The criteria to select the reliable concentrations were based on Cramér-Rao lower bounds (CRLB) and were identical to the prior study [21]. Based on these criteria, we report 13 statistically independent concentrations in the vermis, 11 in the cerebellar hemisphere, and 10 in the pons.
Ataxia Rating Scale
Subjects were assessed by the standardized Scale for the Assessment and Rating of Ataxia (SARA) [25], which yields a composite ataxia score in the range of 0 (no ataxia)—40 (most severe ataxia) and correlates with disease duration in SCA1, SCA2, and SCA6 [1].
Statistical Analysis
Demographic data were compared between groups with ANOVA and chi-square. Groups were compared with respect to each metabolite using a mixed-effects linear model with fixed-effects terms for brain region, group, and their interaction, but no adjustment for confounders; patients were a random effect and correlation between regions within patients was estimated without assuming any structure for the covariance matrix. These models accommodate missing values; therefore, no imputation was used. Unadjusted means and standard errors from these models are reported. ANOVA overall F tests to compare the four ataxia groups with respect to each metabolite were adjusted for multiple comparisons within each brain region by the Bonferroni method: for six possible pairwise comparisons between four ataxia groups, family-wise error rate is <0.05 when nominal p<0.05/6=.0083 [27]. A binary classification tree selected the metabolites that best distinguished the groups. Mixed-effects models were fitted with Proc Mixed in SAS version 9.2 (SAS Institute, Cary NC, 2008). Classification trees were computed using the “tree” package in R (R Foundation for Statistical Computing, Vienna, Austria, 2009. URL http://www.R-project.org) and by inspection of the data.
Results
Patient Characteristics
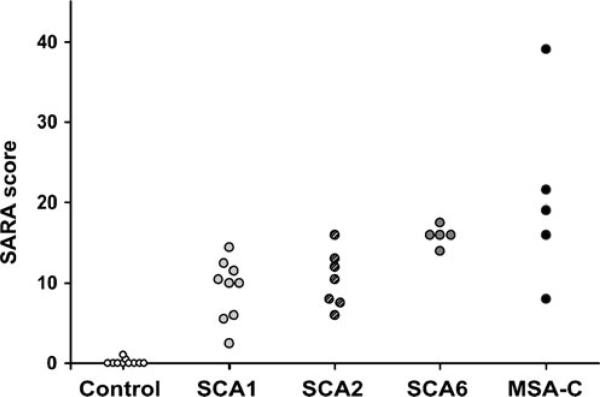
Subject groups (including the SCA1 and controls from the prior study [21]) did not differ in age and gender (Table 1). Subjects with SCA1 and MSA-C presented with a later age-of-onset and had shorter disease duration than those with SCA2 and SCA6. SARA scores of patient groups overlapped considerably (Fig. 1). Patients with SCA1 had lower SARA scores than those with SCA6 and MSA-C and patients with SCA2 had lower SARA scores than those with MSA-C (Table 1). Cerebellar atrophy in ataxia groups was apparent by higher % CSF contribution to the vermis and hemisphere VOI than in controls, while SCA2 and MSA-C groups also showed higher % CSF in pons than controls (Table 1). Vermian atrophy was more severe in patients with SCA6 and MSA-C than those with SCA1 and SCA2 and hemisphere atrophy more severe in patients with SCA2 and MSA-C than those with SCA1 and SCA6 (Table 1).
Fig. 1.
SARA scores of all subjects
Neurochemical Alterations in SCA2, SCA6, and MSA-C
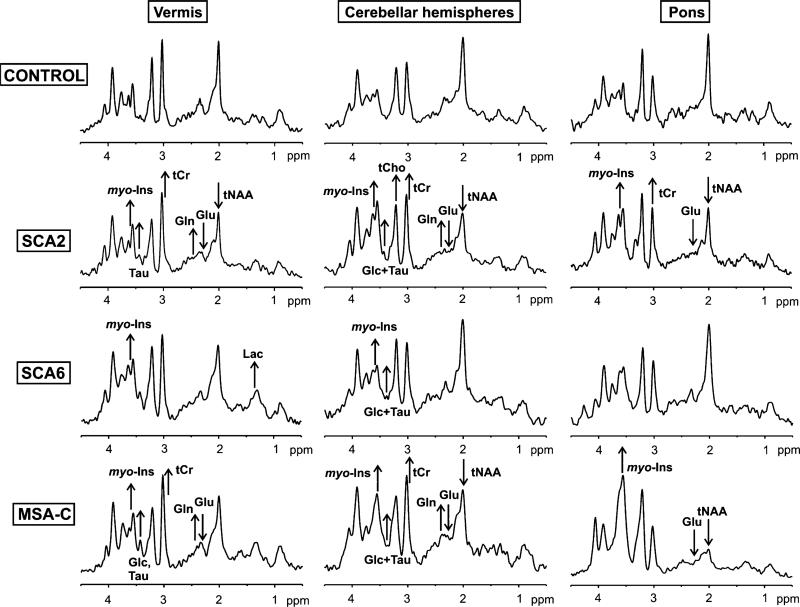
Artifact free MR spectra with a high signal-to-noise ratio (SNR) were acquired from all three VOIs (Fig. 2) in all subject groups (Fig. 3). As a result of the high SNR, differences in the spectral patterns between ataxia groups and between ataxia groups and controls were visible in individual spectra. Note for example the substantially lower NAA and higher myo-inositol in the pons spectrum in MSA-C relative to others.
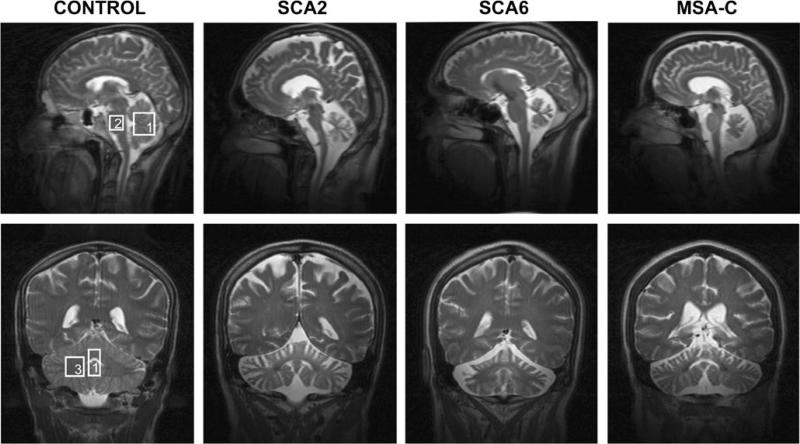
Fig. 2.
T2-weighted images acquired in the mid-sagittal (top row) and coronal planes (bottom row) in a control subject, a patient with SCA2, a patient with SCA6 and a patient with MSA-C. Boxes indicate the voxel locations (1 cerebellar vermis; 2 pons, 3 cerebellar hemisphere)
Fig. 3.
MR spectral differences in patients with SCA2, SCA6, and MSA-C relative to controls. Localized proton MR spectra (STEAM, TE=5 ms, TR=4.5 s, 128 transients) obtained from the vermis, cerebellar hemisphere, and pons are shown. All spectra were processed identically and weighted with the same Gaussian function prior to Fourier transformation. Visible metabolite alterations are shown with arrows that indicate the direction of change. tNAA total N-acetylaspartate, Glu glutamate, Gln glutamine, myo-Ins myo-inositol, tCr total creatine, tCho total choline, Lac lactate, Glc glucose, Tau taurine
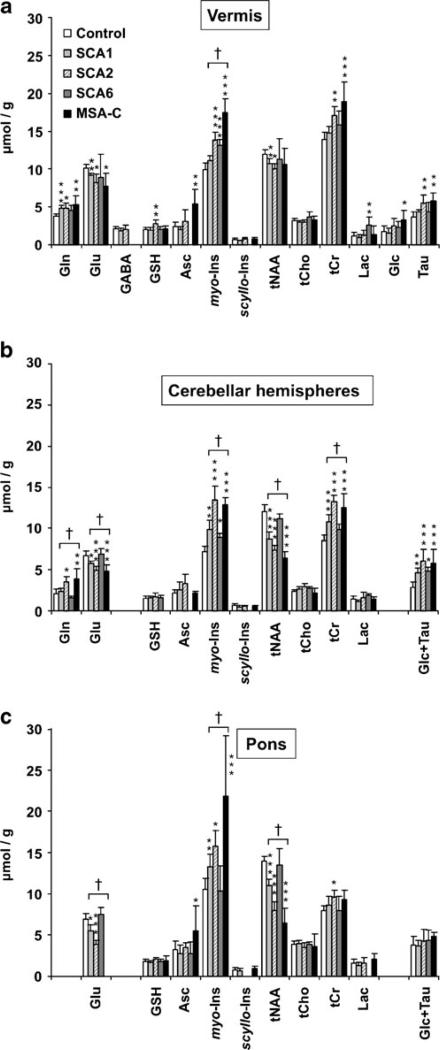
Neurochemical profiles of SCA2 and MSA-C were similar (Fig. 4): total NAA (tNAA, NAA + N-acetylaspartyl-glutamate) and glutamate were lower than controls and total creatine (tCr, creatine + phosphocreatine), glutamine and myo-inositol higher than controls. Some of these alterations were shared by SCA1. However, the extent of some alterations differed between the diseases, such as myo-inositol and tCr in the vermis and myo-inositol in the pons. Also, glutathione was only high in SCA2 and ascorbate only high in MSA-C although these are weakly represented metabolites; hence, these findings need to be investigated further. SCA6 displayed fewer neurochemical alterations from controls and was distinguished from other ataxia groups by higher lactate levels in the vermis and lack of involvement of the pons.
Fig. 4.
Neurochemical profiles of SCA1, SCA2, SCA6, and MSA-C. Mean cerebellar (a, b) and brainstem (c) neurochemical concentrations ± 2 × S.E. (95% C.I.) are shown. Only metabolites quantified with CRLB ≤50% in most patients in a group are plotted. Statistically significant differences between the patients and controls are shown with *p<0.05, **p<0.01, ***p<0.001. Metabolites significantly different between ataxia groups (Bonferroni corrected p<0.05) are marked with †. Gln glutamine, Glu glutamate, GABA γ-aminobutyric acid, GSH glutathione, Asc ascorbate, myo-Ins myo-inositol, scyllo-Ins scyllo-inositol, tNAA NAA + N-acetylaspartylglutamate, tCho glycerophosphocholine + phosphocholine, tCr creatine + phosphocreatine, Lac lactate, Glc glucose, Tau taurine
Overall comparisons of ataxia groups found differences in myo-inositol in the vermis; myo-inositol, tNAA, tCr, glutamate, glutamine in the hemispheres; and myo-inositol, tNAA, glutamate in the pons (Fig. 4).
Classification of SCA1, SCA2, SCA6, and MSA-C by MRS
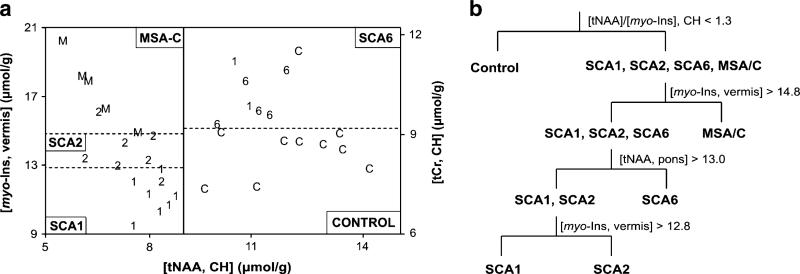
Two tree classification procedures were developed to separate the groups based on MRS markers. Only subjects with complete datasets from the three brain regions were included in this analysis, thus excluding five control subjects for whom data were available from only one or two VOI. Three metabolites measured from two brain regions resulted in 31/36 (86%) correct classifications (Fig. 5a). The second tree included among predictors the tNAA/myo-inositol ratio in the cerebellar hemispheres because this ratio had differentiated patients with SCA1 from controls with 100% specificity and sensitivity in our prior study [21]. This second tree improved the classification to 32/36 (89%) correctly identified (Fig. 5b). Restricting the first tree to SCA2, SCA6, MSA-C, and controls gave 22/24 (92%) correct classifications (Fig. 5a).
Fig. 5.
Classification of SCA1, SCA2, SCA6, and MSA-C by MRS. a Metabolite concentrations for individual subjects are shown in this two-part figure. Total NAA concentration in the cerebellar hemispheres (CH) distinguished patients with SCA1 (1), SCA2 (2), and MSA-C (M) from controls (C) and patients with SCA6 (6), who were further classified by the myo-inositol (myo-Ins) levels in the vermis and total creatine levels in the CH, respectively. b Tree classification procedure that includes the [tNAA]/[myo-Ins] ratio in CH. The MRS predictors and the threshold levels used (no units for the [tNAA]/[myo-Ins] ratio, μmol/g for concentrations) are shown at each step, e. g. subjects who had a [tNAA]/[myo-Ins] ratio in the CH higher than 1.3 were classified as controls. At each step of this analysis, one patient was misclassified
Discussion
Here, we report multiple neurochemical alterations in SCA2, SCA6, and MSA-C relative to age-matched controls. Thanks to the increased sensitivity and resolution at high field, some of these neurochemical differences were visible in individual spectra (Fig. 3). Together with our earlier data that demonstrated robust correlations between neurochemical levels and ataxia scores in SCA1 [21] and the excellent test–retest reproducibility of neurochemical levels measured at high field [22], these data indicate MRS biomarkers as potential surrogate markers in future treatment trials of neurodegenerative ataxias. In addition, we observed different patterns of neurochemical alterations in different SCAs and MSA-C and were able to accurately classify ataxia subtypes based on these. Myo-inositol, tNAA, tCr, glutamate, and glutamine levels were significantly different between patient groups, with the highest number of differences in the cerebellar hemispheres. Of these neurochemicals, myo-inositol, tNAA, tCr, and the tNAA/myo-inositol ratio were the best MRS predictors that distinguished the subject groups.
Due to the pilot nature of this study, raw p values for the comparisons of the patient groups vs. controls are shown in Fig. 4; therefore, differences with p<0.05 and some with p<0.01 should be considered as trends to be investigated further in future studies. However, at least half of the differences (those with p<0.001 in Fig. 4) would remain significant after correction for multiple comparisons of 10–13 metabolites in each brain region for each patient group vs. controls. On the other hand, the standard Bonferroni correction for multiple comparisons was applied within each brain region to reveal those metabolites that most robustly distinguished the groups. This approach identified multiple neurochemicals (marked with † in Fig. 4) which were significantly different between patient groups despite the small sample size, likely as a result of low measurement errors at 4 T. To select the best MRS predictors for the ataxia subtypes, a simple tree approach was chosen in these pilot data although more sophisticated approaches that take into account patterns of neurochemical alterations are possible.
Low cerebellar and pontine NAA levels and NAA/Cr ratios in SCA2 and MSA-C relative to controls were reported previously [9, 11, 28] and indicate neuronal dysfunction/loss in these brain regions [29]. In addition to this well-established biomarker, we detected differences in myo-inositol, tCr (both putative markers of gliosis [30, 31]), glutamate, glutamine (potentially reflecting disruptions in glutamatergic neurotransmission [21]), and glutathione and ascorbate (potentially indicating oxidative stress) in patients with SCA2 and MSA-C relative to controls (Fig. 4). At variance with reports by Boesch et al. [8, 11], we did not observe high lactate levels in SCA2, and the reason for this discrepancy may be the different MRS methods utilized. Namely, these authors detected higher lactate levels in long echo MRS images (MRSI), which may be due to a longer T2 relaxation time of lactate than controls. They reported the ability to distinguish SCA2 from MSA-C based on the observation of the lactate peak in SCA2, while high vermian myo-inositol levels distinguished MSA-C from all three SCAs in our study (Fig. 5). On the other hand, we confirmed these authors’ finding of higher cerebellar NAA/Cr levels in SCA6 than SCA2, which they detected both at short and long echo MRSI data, and demonstrated that this difference is not due to a difference in clinical severity as the SARA scores of patients with SCA2 and SCA6 in our study were not different (Table 1). Another prior MRS study compared cerebellar and pontine neurochemical levels of two patients with SCA6 to controls and reported lower NAA levels in the cerebellar hemisphere in SCA6 [32]. While the trend for lower NAA in cerebellar hemispheres did not reach significance in the current study (Fig. 4b), we observed several other neurochemical differences in patients with SCA6 relative to controls. Although fewer than in SCA2 and MSA-C, these included higher myo-inositol in the vermis and hemispheres indicating gliotic activity and higher lactate in the vermis indicating defects in oxidative metabolism (Fig. 4). Glucose + taurine was also higher in SCA6 than controls in the cerebellar hemispheres; however, it is unclear which of the two metabolites, hence which cellular/biochemical process, lead to this difference. Note that some of these differences, such as lower tNAA and higher myo-inositol, were also reported in other neurodegenerative diseases [33, 34] likely because these are markers of neuronal dysfunction and gliosis, processes common to all neurodegenerative diseases. Therefore, these are not markers specific to ataxias. What does confer specificity to these MRS predictors is the extent to which they are altered in each brain region and ataxia subtype studied.
The neurochemical differences between ataxias were likely not due to differences in disease severity because the SARA scores of patients overlapped considerably (Fig. 1). For example, while the SARA scores of the SCA6 group were not different from the SCA2 and MSA-C groups (Table 1), these patients showed the fewest neurochemical alterations. Furthermore, the SARA scores of patients with SCA6 were higher than those with SCA1 while patients with SCA1 had more neurochemical alterations than those with SCA6. Distinguishing patients with SCA1 and SCA2 with MRI and MRS has been particularly challenging in prior studies [9, 10, 12]. Patients in these groups had almost identical SARA scores (Fig. 1) while the extent of neurochemical alterations was almost always higher in patients with SCA2 and vermian myo-inositol levels most clearly distinguished the two groups. It could be argued that the longer disease duration of patients with SCA2 than those with SCA1 could have resulted in the more severe neurochemical alterations in SCA2. On the other hand, patients with MSA-C had the shortest disease duration and the most severe alterations and patients with SCA6 intermediate disease duration and the fewest alterations. Therefore, although neurochemical alterations are expected to correlate with clinical severity (as we have shown in SCA1 [21]) and disease duration within each disease group, differences in neurochemical alterations between different ataxias cannot be simply explained by clinical severity or disease duration.
The question then remains what causes these differences in neurochemical profiles. One possibility is that they reflect differences in the extent of atrophy between the groups. The % CSF contribution is a measure of atrophy within the MRS VOI. All concentrations were corrected for this contribution and hence represent metabolite concentrations in the remaining tissue. Therefore, neurochemical alterations may only reflect atrophic changes if the extent of overall atrophy is also a measure of damage in the remaining cells. In the vermis, patients with SCA6 and MSA-C had more atrophy than those with SCA1 and SCA2 (Table 1), while patients with SCA6 had the fewest neurochemical alterations, indicating the functional integrity of the remaining tissue. Similarly, the extent of pontine atrophy was not different between SCA1 and SCA6 and between SCA2 and MSA-C while pontine neurochemical alterations differed between these groups. Therefore the neurochemical alterations also cannot be explained by atrophy alone. On the other hand, altered contributions of gray (GM) and white matter (WM) to VOI in patients relative to controls may explain some of the observed differences in the neurochemical levels since metabolite levels are different in GM and WM and our data were not corrected for fractional contributions of GM vs. WM in the VOI. Of the three VOI, the cerebellar hemispheres is the most likely one to be affected by altered GM/WM contributions because this VOI primarily contains WM and atrophy may increase its GM content in patients (while in the other two VOI, atrophy primarily increases the CSF content of the VOI). The potential effects of altered GM/WM contributions to the cerebellar hemisphere VOI can be assessed by comparing metabolite levels in this VOI to those in the vermis, which represent metabolite levels in cerebellar GM. Thus, higher levels of some metabolites in cerebellar hemispheres of patients relative to controls can in part be explained by their higher levels in GM (vermis) than WM (cerebellar hemispheres) and a higher GM contribution to the cerebellar hemisphere VOI in patients than controls. Myo-inositol, tCr, and glutamine are three such metabolites. However, when the extent of the increase of these metabolite levels in the cerebellar hemispheres of patients is considered, it becomes clear that the GM/WM fraction alone cannot explain these differences. For example, the almost doubling of cerebellar hemisphere myo-inositol levels (from ~7 μmol/g in controls to ~13 μmol/g in SCA2 and MSA-C) cannot be primarily due to higher GM content in the VOI since myo-inositol levels in GM (vermis) are only ~10 μmol/g. Similarly, to explain the ~50% higher tCr levels in cerebellar hemispheres of patients with SCA2 and MSA-C relative to controls by an increased GM content, the entire cerebellar hemisphere VOI should be replaced by GM (with ~14 μmol/g tCr content), which was clearly not the case. The reductions in tNAA could also not be explained by altered GM/WM ratios in patients since we did not observe a difference in tNAA levels between normal vermis and hemispheres. A reduction of glutamate levels in cerebellar hemispheres could clearly not be due to a higher GM content in the hemisphere VOI since glutamate levels are higher in GM (vermis) than WM (cerebellar hemispheres). In summary, the neurochemical alterations we observed in patient groups relative to controls could not be simply explained by altered GM/WM contributions to selected VOI either.
There are pathological differences between different SCAs [35–37] which may cause the different neurochemical alterations. The degree of Purkinje cell atrophy, the pathological hallmark in these diseases, is comparable in SCA1, SCA2, and SCA6 [35, 36] and, therefore, does not explain the neurochemical profile differences in the vermis. Dentate nucleus (which is included in the cerebellar hemisphere VOI) is severely affected in SCA1 but relatively spared in SCA2 [36, 37]; hence, the more extensive alterations in the hemispheres in SCA2 than SCA1 does not reflect dentate involvement. On the other hand, while dentate neurons are also spared in SCA6, significant gliosis is observed in this region [38], which may be reflected by the increased myo-inositol in the presence of unchanged NAA levels (Fig. 4). Furthermore, the lack of involvement of pons in SCA6, which is considered a pure cerebellar ataxia, led to its separation from SCA1 and SCA2 (Fig. 5b). Interestingly, some pontine involvement was indicated by the MRS data of two patients with SCA6, consistent with brainstem involvement in 25% of patients [1] and the slight pontine atrophy recently detected in SCA6 by volumetry [13]. Another pathologic feature that differs between SCA1, SCA2, and SCA6 is the degree of synaptic loss [35]. Namely, cerebellar and brainstem synaptic loss is most severe in SCA2, followed by SCA1 and SCA6, where no synaptic loss was observed in the two cases studied [39]. The same ranking was observed in the severity of neurochemical alterations between SCA2, SCA1, and SCA6, raising the intriguing possibility that neurochemical differences may in part reflect synaptic density. After all, both MRS and synaptic density are more functional, rather than structural, measures. Lastly, MSA-C stood out by the very high myo-inositol levels in the vermis and pons, consistent with a prominent involvement of glial cells in the pathology. Interestingly, glial cytoplasmic inclusions (GCI) are a hallmark of MSA pathology and appear closely associated with the neurodegenerative process [40]. Whether the high myo-inositol levels are in any way linked to the GCI pathology in MSA remains to be determined.
The main limitation of our study is the small sample size in each patient group. Thus, a higher number of patients are needed to investigate the sensitivity of the MRS biomarkers to disease severity by examining the correlation of neurochemical levels with SARA scores in each ataxia subtype. In addition, the utility of neurochemical levels as surrogate markers in treatment trials of neurodegenerative ataxias needs to be examined in longitudinal studies. Finally, the ability of the MRS predictors identified here to distinguish different ataxia subtypes needs to be tested in different patient groups using blind analyses.
Conclusion
In summary, the different neurochemical profiles observed here in different SCAs and MSA-C are not simply explained by differences in disease severity, duration, GM/WM composition of the VOI or atrophy and likely provide unique information about the complex pathology in each disease. Studies with higher numbers of patients and other ataxias are warranted to fully demonstrate the utility of high field MRS to differentiate between ataxia subtypes and to monitor progression in each disease and will be possible with the increasing availability of 3 T scanners. While the final diagnosis in hereditary ataxias will always rely on genetic testing, MRS, together with other imaging modalities, can narrow down the options for genotypes to be tested in the absence of sufficient family history. We expect that a combination of imaging techniques together with clinical evaluation will have the best chance for predicting genotype in these disorders and for reliably monitoring disease progression as well as treatment response.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke grant R21 NS056172 (GÖ) and Jay D. Schlueter Ataxia Research Fund. The 4 T TEM coil was built with a grant (3761-9236-07) from the Minnesota Medical Foundation (GÖ). The Center for MR Research is supported by National Center for Research Resources (NCRR) biotechnology research resource grant P41RR008079 and Neuroscience Center Core Blueprint Award P30 NS057091. The General Clinical Research Center is supported by NCRR grant M01RR00400. We thank the staff of the Center for MR Research for maintaining and supporting the NMR system.
Footnotes
Conflict of Interest The authors have no conflicts of interest to disclose.
Contributor Information
Gülin Öz, Center for MR Research, Department of Radiology, Medical School, University of Minnesota, 2021 6th St. S.E., Minneapolis, MN 55455, USA.
Isabelle Iltis, Center for MR Research, Department of Radiology, Medical School, University of Minnesota, 2021 6th St. S.E., Minneapolis, MN 55455, USA.
Diane Hutter, Center for MR Research, Department of Radiology, Medical School, University of Minnesota, 2021 6th St. S.E., Minneapolis, MN 55455, USA.
William Thomas, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Khalaf O. Bushara, University of Minnesota Ataxia Center, Department of Neurology, University of Minnesota, Minneapolis, MN, USA
Christopher M. Gomez, Department of Neurology, University of Chicago, Chicago, IL, USA
References
- 1.Schmitz-Hübsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, et al. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology. 2008;71(13):982–9. doi: 10.1212/01.wnl.0000325057.33666.72. [DOI] [PubMed] [Google Scholar]
- 2.Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3(5):291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- 3.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller SG, Schuff N, Weiner MW. Evaluation of treatment effects in Alzheimer's and other neurodegenerative diseases by MRI and MRS. NMR Biomed. 2006;19(6):655–68. doi: 10.1002/nbm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoghbi HY, Orr HT. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1. J Biol Chem. 2009;284(12):7425–9. doi: 10.1074/jbc.R800041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Prospero NA, Fischbeck KH. Therapeutics development for triplet repeat expansion diseases. Nat Rev Genet. 2005;6(10):756–65. doi: 10.1038/nrg1690. [DOI] [PubMed] [Google Scholar]
- 7.Prakash N, Hageman N, Hua X, Toga AW, Perlman SL, Salamon N. Patterns of fractional anisotropy changes in white matter of cerebellar peduncles distinguish spinocerebellar ataxia-1 from multiple system atrophy and other ataxia syndromes. Neuroimage. 2009;47(Suppl 2):T72–81. doi: 10.1016/j.neuroimage.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Boesch SM, Schocke M, Burk K, Hollosi P, Fornai F, Aichner FT, et al. Proton magnetic resonance spectroscopic imaging reveals differences in spinocerebellar ataxia types 2 and 6. J Magn Reson Imaging. 2001;13(4):553–9. doi: 10.1002/jmri.1078. [DOI] [PubMed] [Google Scholar]
- 9.Guerrini L, Lolli F, Ginestroni A, Belli G, Nave RD, Tessa C, et al. Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study. Brain. 2004;127(Pt 8):1785–95. doi: 10.1093/brain/awh201. [DOI] [PubMed] [Google Scholar]
- 10.Klockgether T, Skalej M, Wedekind D, Luft AR, Welte D, Schulz JB, et al. Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain. 1998;121(Pt 9):1687–93. doi: 10.1093/brain/121.9.1687. [DOI] [PubMed] [Google Scholar]
- 11.Boesch SM, Wolf C, Seppi K, Felber S, Wenning GK, Schocke M. Differentiation of SCA2 from MSA-C using proton magnetic resonance spectroscopic imaging. J Magn Reson Imaging. 2007;25(3):564–9. doi: 10.1002/jmri.20846. [DOI] [PubMed] [Google Scholar]
- 12.Mandelli ML, De Simone T, Minati L, Bruzzone MG, Mariotti C, Fancellu R, et al. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. AJNR Am J Neuroradiol. 2007;28(10):1996–2000. doi: 10.3174/ajnr.A0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49(1):158–68. doi: 10.1016/j.neuroimage.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Lukas C, Schöls L, Bellenberg B, Rub U, Przuntek H, Schmid G, et al. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett. 2006;408(3):230–5. doi: 10.1016/j.neulet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Della Nave R, Ginestroni A, Tessa C, Salvatore E, De Grandis D, Plasmati R, et al. Brain white matter damage in SCA1 and SCA2. An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. Neuroimage. 2008;43(1):10–9. doi: 10.1016/j.neuroimage.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Döhlinger S, Hauser TK, Borkert J, Luft AR, Schulz JB. Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum. 2008;7(2):204–14. doi: 10.1007/s12311-008-0025-0. [DOI] [PubMed] [Google Scholar]
- 17.Viau M, Boulanger Y. Characterization of ataxias with magnetic resonance imaging and spectroscopy. Parkinsonism Relat Disord. 2004;10(6):335–51. doi: 10.1016/j.parkreldis.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Fukatsu H, Katsuno M, Sugiura M, Hamada K, Okada Y, et al. Multiple regional 1H-MR spectroscopy in multiple system atrophy: NAA/Cr reduction in pontine base as a valuable diagnostic marker. J Neurol Neurosurg Psychiatry. 2004;75(1):103–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeuffer J, Tkáč I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J Magn Reson. 1999;141(1):104–20. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 20.Tkáč I, Öz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62(4):868–79. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Öz G, Hutter D, Tkáč I, Clark HB, Gross MD, Jiang H, et al. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010;25(9):1253–61. doi: 10.1002/mds.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Öz G, Nelson CD, Koski DM, Henry PG, Marjańska M, Deelchand DK, et al. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010;30(10):3831–8. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matilla-Duenas A, Sanchez I, Corral-Juan M, Davalos A, Alvarez R, Latorre P. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum. 2010;9(2):148–66. doi: 10.1007/s12311-009-0144-2. [DOI] [PubMed] [Google Scholar]
- 24.Lalonde R, Strazielle C. Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain Res. 2007;1140:51–74. doi: 10.1016/j.brainres.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 26.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg Y, Tamhane AC. Multiple comparison procedures. Wiley; New York: 1987. [Google Scholar]
- 28.Terakawa H, Abe K, Watanabe Y, Nakamura M, Fujita N, Hirabuki N, et al. Proton magnetic resonance spectroscopy (1H MRS) in patients with sporadic cerebellar degeneration. J Neuroimaging. 1999;9(2):72–7. doi: 10.1111/jon19999272. [DOI] [PubMed] [Google Scholar]
- 29.Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20(4–5):271–6. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- 30.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3–5):289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 31.Guerrini L, Belli G, Mazzoni L, Foresti S, Ginestroni A, Della Nave R, et al. Impact of cerebrospinal fluid contamination on brain metabolites evaluation with 1H-MR spectroscopy: a single voxel study of the cerebellar vermis in patients with degenerative ataxias. J Magn Reson Imaging. 2009;30(1):11–7. doi: 10.1002/jmri.21804. [DOI] [PubMed] [Google Scholar]
- 32.Viau M, Marchand L, Bard C, Boulanger Y. 1H magnetic resonance spectroscopy of autosomal ataxias. Brain Res. 2005;1049(2):191–202. doi: 10.1016/j.brainres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Kantarci K, Knopman DS, Dickson DW, Parisi JE, Whitwell JL, Weigand SD, et al. Alzheimer disease: postmortem neuropatho-logic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248(1):210–20. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firbank MJ, Harrison RM, O'Brien JT. A comprehensive review of proton magnetic resonance spectroscopy studies in dementia and Parkinson's disease. Dement Geriatr Cogn Disord. 2002;14(2):64–76. doi: 10.1159/000064927. [DOI] [PubMed] [Google Scholar]
- 35.Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4(1):62–73. doi: 10.1080/14734220510007950. [DOI] [PubMed] [Google Scholar]
- 36.Robitaille Y, Lopes-Cendes I, Becher M, Rouleau G, Clark AW. The neuropathology of CAG repeat diseases: review and update of genetic and molecular features. Brain Pathol. 1997;7(3):901–26. doi: 10.1111/j.1750-3639.1997.tb00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwabuchi K, Tsuchiya K, Uchihara T, Yagishita S. Autosomal dominant spinocerebellar degenerations. Clinical, pathological, and genetic correlations. Rev Neurol (Paris) 1999;155(4):255–70. [PubMed] [Google Scholar]
- 38.Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, et al. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 1997;42(6):933–50. doi: 10.1002/ana.410420616. [DOI] [PubMed] [Google Scholar]
- 39.Koeppen AH, Dickson AC, Lamarche JB, Robitaille Y. Synapses in the hereditary ataxias. J Neuropathol Exp Neurol. 1999;58(7):748–64. doi: 10.1097/00005072-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127(Pt 12):2657–71. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]