Abstract
To clarify the relationship between reactive oxygen species (ROS) and cell death during ischemia-reperfusion (I/R), we studied cell death mechanisms in a cellular model of I/R. Oxidant stress during simulated ischemia was detected in the mitochondrial matrix using mito-roGFP, a ratiometric redox sensor, and by Mito-Sox Red oxidation. Reperfusion-induced death was attenuated by over-expression of Mn-superoxide dismutase (Mn-SOD) or mitochondrial phospholipid hydroperoxide glutathione peroxidase (mito-PHGPx), but not by catalase, mitochondria-targeted catalase, or Cu,Zn-SOD. Protection was also conferred by chemically distinct antioxidant compounds, and mito-roGFP oxidation was attenuated by NAC, or by scavenging of residual O2 during the ischemia (anoxic ischemia). Mitochondrial permeability transition pore (mPTP) oscillation/opening was monitored by real-time imaging of mitochondrial calcein fluorescence. Oxidant stress caused release of calcein to the cytosol during ischemia, a response that was inhibited by chemically diverse antioxidants, anoxia, or over-expression of Mn-SOD or mito-PHGPx. These findings suggest that mitochondrial oxidant stress causes oscillation of the mPTP prior to reperfusion. Cytochrome c release from mitochondria to the cytosol was not detected until after reperfusion, and was inhibited by anoxic ischemia or antioxidant administration during ischemia. Although DNA fragmentation was detected after I/R, no evidence of Bax activation was detected. Over-expression of the anti-apoptotic protein Bcl-XL in cardiomyocytes did not confer protection against I/R-induced cell death. Moreover, murine embryonic fibroblasts with genetic depletion of Bax and Bak, or over-expression of Bcl-XL, failed to show protection against I/R. These findings indicate that mitochondrial ROS during ischemia triggers mPTP activation, mitochondrial depolarization, and cell death during reperfusion through a Bax/Bak-independent cell death pathway. Therefore, mitochondrial apoptosis appears to represent a redundant death pathway in this model of simulated I/R.
INTRODUCTION
Tissue ischemia is characterized by severe hypoxia, acidosis, energy depletion and cell death. Although timely restoration of blood flow is currently the most effective means of minimizing ischemic injury, reperfusion of ischemic tissue triggers a paradoxical increase in cell death [6]. Excessive oxidant stress is well accepted as an important component of ischemia-reperfusion (I/R) injury [5,6]. ROS production begins early in ischemia, and is followed by a large burst of oxidant stress during the first few minutes of reperfusion [2,30,41,46]. Potential sources of ROS include mitochondria, NAD(P)H oxidases, nitric oxide (NO) synthase, and xanthine oxidase, and the critical targets of oxidant stress may include proteins, membrane lipids, and DNA [7,14,25,37]. Although many of the details regarding the sources and targets of oxidant stress during I/R are not known, a consensus regarding the importance of ROS in I/R injury has developed, based on studies showing that cells are protected during I/R by pretreatment with antioxidants or by over-expression of antioxidant enzymes [8,9,16,34].
Other reports have implicated mitochondrial apoptosis in the cell death triggered by I/R. This highly conserved process can be initiated by the activation of BH3-containing pro-death proteins in response to a variety of stimuli including hypoxia, nutrient deprivation, ROS and DNA damage [20]. Apoptotic triggers cause the Bcl-2 family members Bax and Bak to translocate to the mitochondria and trigger the release of cytochrome c to the cytosol [19]. This leads to the activation of caspases and subsequent cell death through an ATP-dependent pathway [42,49]. Consistent with that model, transgenic mice that over-express Bcl-2, a Bax/Bak suppressor, or that are deficient in the Bax protein, have been reported to exhibit smaller infarcts compared with wild-type mice subjected to I/R [23,26,43]. Other studies report that pharmacological inhibition of caspases is protective against I/R injury in vitro [36,40] although there is debate as to whether caspase inhibition can prevent cell death following cytochrome c release to the cytosol, due to the existence of redundant effector pathways of apoptosis that act downstream of the mitochondria.
Although oxidant stress and apoptosis have both been implicated in I/R-induced cell death, the relationship between these processes is not clearly established. One possibility is that ROS generation during I/R leads to the BH3-dependent activation of mitochondrial apoptosis, cytochrome c release, and caspase-mediated cell death. Alternatively, oxidant stress generated during I/R could trigger opening of the mitochondrial permeability transition pore, leading to cytochrome c release to the cytosol, bioenergetic failure, and cell death by a necrotic rather than an apoptotic pathway [11,12]. In the former case cytochrome c (cyt-c) release would mediate cell death, whereas in the latter case the release of cyt-c would represent only a marker of lethal cell damage. The present study examined the relationship between oxidant stress during I/R and mitochondria-mediated cell death, using a model of simulated I/R in which the cells were superfused with hypoxic, hypercarbic and acidotic medium lacking glucose. The data show that oxidant stress generated during simulated ischemia triggers oscillation of the mitochondrial permeability transition pore, and that this response precedes the irreversible opening of the pore and cyt-c release during reperfusion. Although cytochrome c release during reperfusion leads to caspase activation, interventions that inhibit BH3-dependent apoptosis do not protect against cell death in this model. These findings indicate that apoptosis becomes activated in simulated I/R, but it is not required for subsequent cell death.
METHODS
Cell culture and perfusion system
Embryonic chick cardiomyocytes were prepared as previously described and grown at a density of 1 × 105 on 25 mm glass cover slips or 6 well plates in a humidified incubator [47]. Experiments were performed on spontaneously contracting cells at 3–5 days after isolation. Primary mouse embryonic fibroblasts (MEFs) derived from wild type and Bax-Bak double knockout mice were immortalized with a plasmid containing SV40 genomic DNA. Immortalized wild-type MEFs were also used to develop a stable cell line over-expressing Bcl-XL. MEFs were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine (200 mmol/L) and antibiotics (pen/strep). Embryonic stem cells devoid of cytochrome c (cyt-c−/− ES cells) were cultured under conditions that compensate for their defect in mitochondrial respiration using media supplemented with uridine and pyruvate as previously described [31]. Wild-type embryonic stem cells containing cytochrome c were cultured in parallel with null (cyt-c−/−) ES cells and used as controls.
Simulated ischemia-reperfusion
Simulated I/R was achieved in a flow through chamber as previously described [47]. The chamber was comprised of two cover slips separated by a stainless steel spacer ring, with the cultured cells on the lower cover slip. The space between the cover slips was perfused with balanced salt solutions (BSS) at a flow rate of 0.5 ml/min. All solutions were equilibrated by bubbling vigorously with calibrated gases in a heated, water jacketed reservoir (Radnoti Glass Co.). The reservoir was connected to the flow-through chamber with stainless steel tubing to minimize entry of ambient oxygen. The chamber was mounted on a heated stage (37° C) on an inverted microscope (Nikon). During baseline conditions the cells were superfused for 20 min with BSS (NaCl 117 mmol/L, KCl 4 mmol/L, NaHCO3 18 mmol/L, MgSO4 0.76 mmol/L, NaH2PO4 1 mmol/L, CaCl2 1.21 mmol/L and glucose 5.6 mmol/L) bubbled with a normoxic gas mixture (5% CO2/21% O2/74% N2). The same solution was used during simulated reperfusion. Simulated ischemia was achieved by perfusing the chamber with ischemic media (NaCl 108.9 mmol/L, KCl 8 mmol/L, NaHCO3 21.4 mmol/L, MgSO4 0.76 mmol/L, NaH2PO4 1 mmol/L, CaCl2 1.21 mmol/L) devoid of glucose and bubbled with 20% CO2/0 % O2/80% N2 (pH 6.8). This resulted in an extracellular PO2 of approximately 7 mmHg within 10 min of hypoxia as determined by a porphyrin-based phosphorescence quenching assay [33].
Cell Viability
Cell death was assessed using propidium iodide (PI, 5 µmol/L) in the perfusate. This dye is excluded from live cells, but enters upon disruption of the plasma membrane. Fluorescence images were obtained using a 10× objective (excitation: 555 nm, emission: 605 nm) at baseline, at the end of ischemia, and at 1, 2 and 3 hrs into reperfusion. Following the 3 hr measurement, cells were permeabilized with digitonin (300 mmol/L) for 45 min. Cell death during the experiment was calculated as the percent of the post-digitonin value. In most experiments, cell death was assessed in 3 separate regions per cover slip using a computer-controlled motorized stage that returned to specific X-Y positions for repeated measurements. An LDH cytotoxicity assay (BioVision) was used to assess cell viability in response to staurosporine treatment and serum withdrawal. LDH is released into the extracellular space upon loss of plasma membrane integrity. The LDH assay was used according to the manufacturer’s specifications with minor modifications. Supernatants were collected after treatment with staurosporine (1 µmol/L in 1.5 ml of serum-free media) or prolonged serum withdrawal. Cells were incubated in Triton-X (1.5 ml of 2% in serum-free media) for 30 min. Lysates were collected and centrifuged at 11,000 RPM for 10 min. Samples were mixed with the reaction mixture and incubated for 30 min in a 96 well plate, at which point the absorbance was measured at 490 nm. Cytotoxicity was calculated as the absorbance in the supernatant divided by the sum of absorbance in the supernatant and lysates.
Mitochondrial Membrane Potential
ΔΨm was assessed with tetramethylrhodamine ethyl ester (TMRE) under non-quenching conditions as previously described [30]. Cells were loaded with TMRE (100 nmol/L) at 37° C for 30 min. The media was then replaced with a maintenance concentration of TMRE (25 nmol/L). Mitochondrial fluorescence intensity was assessed using excitation at 555 nm and emission at 605 nm. Images were obtained during baseline and at periodic intervals thereafter (10× objective). Intensity values were corrected for background. Stage positions were programmed using data acquisition software (Metamorph, Universal Imaging) allowing repeated images to be obtained from the same fields of cells throughout each experiment.
DNA Fragmentation assay
Cardiomyocytes (2.1 ×106) were disrupted using lysis buffer, and the DNA was extracted using phenol:chloroform after 2 hrs of proteinase K digestion at 50° C. Genomic DNA (1.5 µg per lane) was treated with 100 µg /ml of RNase A and run on a 2.0% agarose gel containing 0.06 mg/ml of ethidium bromide. Apoptotic DNA fragments consist of multimers of 180–200 base pairs and appear as a DNA ladder. DNA of non-apoptotic populations of cells has a high molecular weight and does not migrate far into the gel.
Immunofluorescence labeling of cytochrome c and Bax
To determine subcellular localization of cytochrome c, both treated and untreated cells were fixed in 3% paraformaldehyde with 0.02% glutaraldehyde in PBS for 15 min and subsequently permeabilized with ice-cold methanol. Cells were incubated in 0.5 mg/ml sodium borohydride (Sigma–Aldrich) solution, blocked for 30 min in PBS containing 1% goat serum, and incubated overnight at 4° C with 1:100 dilution of mouse anti-cytochrome c antibody (BD Bioscience) or mouse anti-Bax (BD Bioscience). Cells were then incubated with a fluorescent secondary antibody (Alexa Fluor 488-labeled anti-mouse IgG, (1:100)) for 1 hr at room temperature and visualized under confocal fluorescent microscopy using a 40× objective. Cells with a normal mitochondrial localization of cytochrome c exhibit a fluorescence pattern that is punctuate in appearance, while cells that have undergone redistribution of cytochrome c to the cytosol exhibit a more diffuse pattern of fluorescence. Conversely, cells with a normal cytosolic localization of Bax exhibit a fluorescence pattern that is diffuse in appearance while cells that have undergone Bax translocation to the mitochondria exhibit a punctuate pattern of fluorescence. Diffuse cells were enumerated independently by two blinded investigators who examined three separate visual fields each containing 50–60 cells. Values were expressed as percent of total cells. In separate experiments, cardiomyocytes were transduced for 48 hrs with an adenovirus expressing a Green Fluorescent Protein mutant (roGFP) targeted to the mitochondrial matrix using the targeting sequence from Cytochrome oxidase subunit IV. This targeting results in expression of the protein exclusively in the matrix compartment [48]. Cells were then subjected to I/R. They were then fixed, immunostained for cytochrome c or Bax, and were imaged using a laser scanning confocal microscope (Zeiss, 60X) to assess the extent of co-localization.
Reagents
Adenoviruses expressing catalase or mitochondria-targeted catalase were obtained from Drs. Bai and Cederbaum [3]. Adenoviruses expressing Cu,Zn-SOD (SOD I) or Mn-SOD (SOD II) were obtained from the Gene Transfer Vector Core Facility at the University of Iowa [50,51]. Viral doses were established in preliminary studies evaluating expression as a function of viral dosage (multiplicity of infection, MOI, data not shown). The effective concentrations of the chemical antioxidants were reported previously [29,30,41,44]. Embryonic stem cells with homozygous deletion of cytochrome c were obtained from ATCC. TMRE, Calcein-AM, and Alexa-Fluor secondary antibodies were obtained from Molecular Probes-Invitrogen. Digitonin was obtained from Aldrich. Other chemicals were obtained from Sigma.
Statistical Analysis
Data were analyzed by ANOVA, and are reported as mean values ± SEM. When a statistically significant effect was detected, individual differences were explored using a Newman-Keuls post hoc analysis. Statistical significance was determined at the 0.05 level.
RESULTS
Cellular response to simulated I/R
Cardiomyocytes on cover slips were subjected to simulated I/R in a flow-through chamber as previously reported [41]. Phase contrast images revealed evidence of cellular swelling, membrane irregularity, and increased PI uptake during reperfusion (Fig 1A). To clarify the role of oxidant stress in I/R, antioxidant enzymes targeted to specific intracellular compartments were expressed in cultured cardiomyocytes using adenoviral vectors, and examined in terms of their ability to protect against I/R-induced cell death. The adenoviral titers needed for optimal expression of catalase, catalase targeted to the mitochondrial matrix, Cu,Zn-SOD or Mn-SOD targeted to the mitochondrial matrix, were determined at 48 hr for each adenovirus by Western blotting (Fig. 1B). Cells transduced with these adenoviruses or an empty viral vector (Y5) were incubated for 48 hrs prior to simulated I/R. Paired control experiments were performed on the same day in order to minimize the effects of any differences due to cell age or isolation batch. Negligible cell death was present at baseline, and no significant increases in cell death were detected during ischemia prior to reperfusion (Fig. 1C). During 3 hrs of reperfusion, cell death increased significantly in cells expressing catalase, mitochondrial catalase or Cu,Zn-SOD, whereas cells over-expressing Mn-SOD showed dramatic protection (9±3%) compared to Y5 transduced controls (54±4%). These findings indicate that mitochondrial superoxide, which would have been scavenged by Mn-SOD, must have been a critical contributor to the I/R-induced cell death in the cardiomyocytes.
Figure 1.
Cell death in simulated ischemia-reperfusion. (A) Cardiomyocytes exposed to 1 hr of simulated ischemia followed by 3 hrs of reperfusion in a flow-through chamber. Phase images show increased cellular swelling, plasma membrane irregularity and PI uptake after I/R. (B) Western blot analysis of adenovirus-transduced cardiomyocytes showing over-expression of catalase, mitochondria-targeted catalase, Cu,Zn-SOD and Mn-SOD after 48 hrs. (C) Cardiomyocytes transduced with either Y5 (1000 MOI, n=10), cytosolic catalase (1000 MOI, n=6), mitochondria-targeted catalase (500 MOI, n=6), Cu,Zn-SOD (500 MOI, n=5) or Mn-SOD (20 MOI, n=5) for 48 hrs prior to simulated I/R (* p<.05 compared with controls).
Protection by antioxidant compounds
Addition of the antioxidant N-acetyl-L-cysteine (NAC), 2-mercaptoproprionyl glycine (2-MPG) or reduced glutathione peptide (GSH) to the perfusate throughout baseline and ischemia, but not during reperfusion, resulted in significant protection from I/R-induced cell death (Fig 2A). Addition of the O2 scavenger Oxyrase to the ischemia buffer lowers the O2 tension to anoxic levels, thereby preventing ROS generation during ischemia. As seen previously [41], Oxyrase administration during ischemia confers significant protection against later cell death during reperfusion (Fig. 2A). However, administration of NAC+2-MPG only during reperfusion did not result in significant protection compared with control cells. Mitochondrial potential (ΔΨm), assessed using TMRE fluorescence, decreased significantly during ischemia, and no recovery of TRME fluorescence was detected during reperfusion (Fig. 2B). Similar responses have been reported previously in this model [30].
Figure 2.
Oxidant stress and mitochondrial depolarization in simulated ischemia-reperfusion. (A) N-acetyl-L-cysteine (NAC, 500 µmol/L) or 2-MPG (2-mercaptopropionyl glycine, 400 µmol/L) added prior to and during simulated ischemia decreased cell death after 3 hrs of reperfusion. Similar protection was conferred by anoxic ischemia, achieved by adding Oxyrase to the ischemic media, or by addition of reduced glutathione (GSH, 100 µmol/L) throughout simulated I/R. The combined use of NAC and 2-MPG, beginning 10 min prior to and throughout reperfusion, did not significantly decrease cell death during I/R (Controls, n=9; NAC, 2-MPG, Oxyrase, n=4; GSH, n=3; NAC/2-MPG during reperfusion n=4; * p<.05). (B) TMRE fluorescence in cardiomyocytes, to assess changes in mitochondrial potential. Fluorescence intensities were monitored in control cells maintained under normoxia and in cells challenged with simulated I/R. A significant decrease in TMRE fluorescence was detected during 20–40 min of simulated ischemia. Values are expressed as percent of baseline intensity (Controls, n=6; I/R, n=4; * p<.05). White bars indicate controls; dark bars indicate I/R.
Assessment of mitochondrial oxidant stress during ischemia
To assess oxidant stress in the mitochondrial matrix during ischemia, cells were transduced with an adenovirus expressing mito-roGFP, a ratiometric redox-sensitive sensor [15]. This protein was targeted to the mitochondrial matrix by appending the mitochondrial targeting sequence from cytochrome oxidase subunit IV. The specificity of expression was confirmed by co-localization studies comparing the distribution of mito-roGFP fluorescence and cytochrome c immunostaining, by confocal microscopy (Fig. 3A). Cardiomyocytes expressing mito-roGFP exhibited partial oxidation of the sensor under baseline conditions (Fig. 3B). During simulated ischemia, a significant increase in oxidation was detected, which was abrogated by continuous treatment with NAC, or by anoxic ischemia. Mock ischemia, wherein cells were superfused with ischemic buffer equilibrated with 20% CO2 and 21% O2 (no hypoxia), was included to control for possible effects arising from the low pH conditions during ischemia. No significant change in the mito-roGFP oxidation state was detected during mock ischemia, confirming that the changes observed during simulated ischemia were not an artifact arising from low pH conditions. The percent oxidation of the mito-roGFP protein during ischemia was determined by calibrating the sensor at the end of the ischemia challenge. This was achieved by perfusing the cells with media containing dithiothreitol (1 mM), followed by t-butyl hydroperoxide (1 mM) in order to fully reduce and fully oxidize the protein, respectively (Fig. 3C). To confirm the generation of oxidant stress in the mitochondrial matrix during simulated ischemia, cells were loaded with Mito-Sox Red, a cationic probe that distributes to the mitochondrial matrix by virtue of the transmembrane potential (Fig. 3D). Oxidation of this probe was detected during simulated ischemia, but that response was significantly attenuated by NAC administration or by Mn-SOD over-expression (Fig. 3E). Collectively, these findings reveal that simulated ischemia is associated with a significant increase in oxidant stress within the mitochondrial matrix.
Figure 3.
Detection of oxidant stress in the mitochondrial matrix during simulated ischemia. (A) Isolated cardiomyocytes were transduced with an adenovirus to express mito-roGFP in the mitochondrial matrix. Cytochrome c immunostaining and merged images demonstrate the expected co-localization within the mitochondria. (B) Ratiometric measurements made using the mito-roGFP sensor showed a significant increase in the oxidation state within the mitochondrial matrix during simulated ischemia. This oxidation response was attenuated during “mock ischemia” (20%CO2, 21%O2, 59% N2), anoxic ischemia (PO2 <0.5 mmHg), or ischemia in the presence of NAC (500µmol/L) (Mock ischemia, anoxic ischemia, ischemia + NAC, n=3; Controls, n=4; * p<.05). (C) Representative tracing of ratiometric measurements show the dynamics of the mito-roGFP sensor in cardiomyocytes during simulated ischemia, followed by calibration of the sensor using dithiothreitol (DTT, 1 mmol/L) followed by t-butyl hydroperoxide (TBH 1mmol/L). (D) Mito-Sox Red staining of the mitochondrial matrix in cultured cardiomyocytes. (E). Increases in Mito-Sox Red fluorescence relative to baseline values were evident within 20 min of simulated ischemia. No significant increase was detected in control cells maintained under baseline conditions for 20 min (Control) or in cells exposed to ischemia in the presence of NAC (Ischemia + NAC). Over-expression of Mn-SOD, induced using an adenoviral vector, significantly attenuated the rise in Mito-Sox fluorescence observed during simulated ischemia (Ischemia + NAC) (Control, n=6; ischemia, n=8; ischemia + NAC, n=4; ischemia + Mn-SOD, n=3; * p<.05).
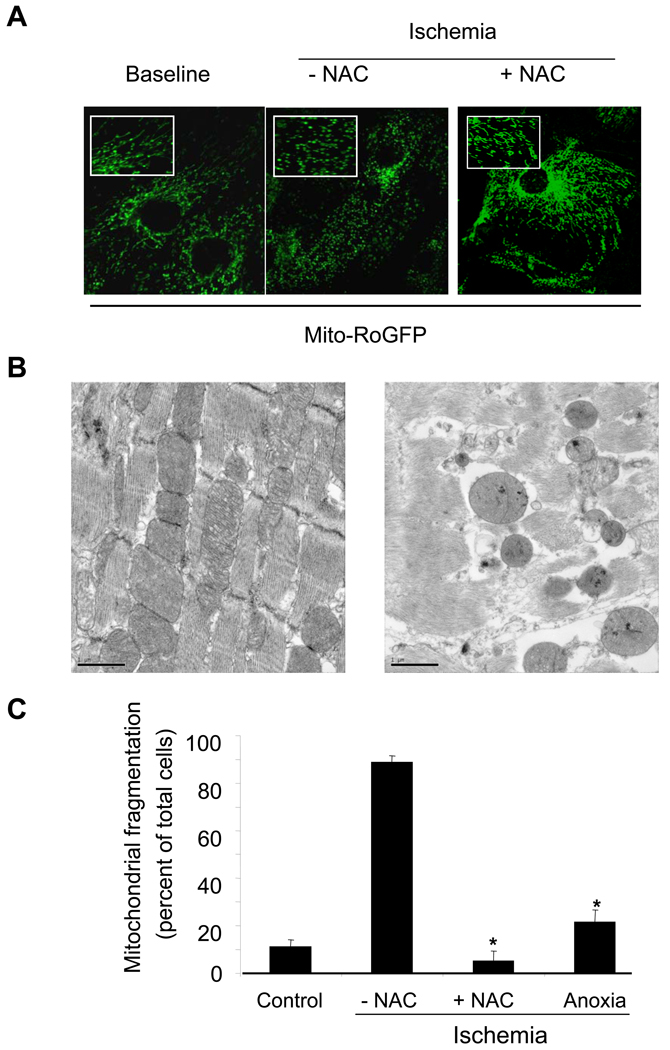
Alterations in mitochondrial morphology induced by ischemic oxidant stress
Cells expressing mito-roGFP exhibited a reticular fluorescence pattern that was concentrated in the perinuclear region. During ischemia this pattern changed, as the fluorescence became more punctate in nature (Fig. 4A). Adult mouse ventricular muscle (400 µm slice) was harvested, rapidly chilled to prevent ischemic damage, fixed, and examined by transmission electron microscopy. Another slice was subjected to simulated ischemia in the flow-though chamber (30 min), followed by rapid cooling and fixation to permit analysis of the ultrastructural changes associated with ischemia in the absence of reperfusion. In the normal ventricular slice, cardiac muscle fibers in both longitudinal and cross sectional orientations exhibited normal ultrastructure (Fig. 4B, left). Bundles of myofibrils arranged along the length of each muscle fiber were interspersed with numerous mitochondria. Ventricular slices subjected to ischemia without reperfusion showed morphological changes in mitochondria that included rounding and blebbing of the mitochondrial outer membrane (Fig. 4B, right). Mitochondria were disorganized and dispersed, in comparison to the tightly packed organization in control slices. To determine the role of ischemic oxidant stress in the alteration of mitochondrial morphology, the fraction of cells exhibiting fragmentation in the fluorescent labeling studies (Fig. 4A) was quantified (Fig. 4C). Administration of NAC during ischemia, or scavenging of residual O2 during ischemia with Oxyrase (anoxic ischemia), significantly attenuated the fragmented mitochondrial appearance. These findings implicate ischemic oxidant stress as the cause of these changes in mitochondrial morphology.
Figure 4.
Mitochondrial fragmentation in cardiomyocytes during simulated ischemia. (A) Cardiomyocytes were transduced with mito-roGFP and visualized under fluorescent microscopy (excitation: 484nm, emission: 525 nm) to detect real time changes in mitochondrial morphology. Cells perfused under baseline conditions or subjected to simulated ischemia in the presence of N-acetylcysteine (NAC, 500µmol/L) retained a normal reticular mitochondrial morphology. Cells exposed to ischemia in the absence of NAC demonstrated a significantly fragmented mitochondrial morphology. (B) Representative electron micrographs of mouse ventricular tissue from 400 µm slices maintained under baseline conditions (left) or after 30 min simulated ischemia without reperfusion (right). Bars are 1 µm in length. (C) Cardiomyocytes expressing mito-roGFP were subjected to baseline conditions or simulated ischemia with or without NAC or anoxia treatment. Images were analyzed by 2 independent observers in order to quantify the number of cells with fragmented mitochondria, as a percent of the total cells per high power field (50–70 cells from 3 distinct regions per cover slip). NAC and anoxic ischemia using Oxyrase both significantly reduced the percentage of cells with fragmented mitochondria. (n=3 each; * p<.05 compared with Ischemia – NAC).
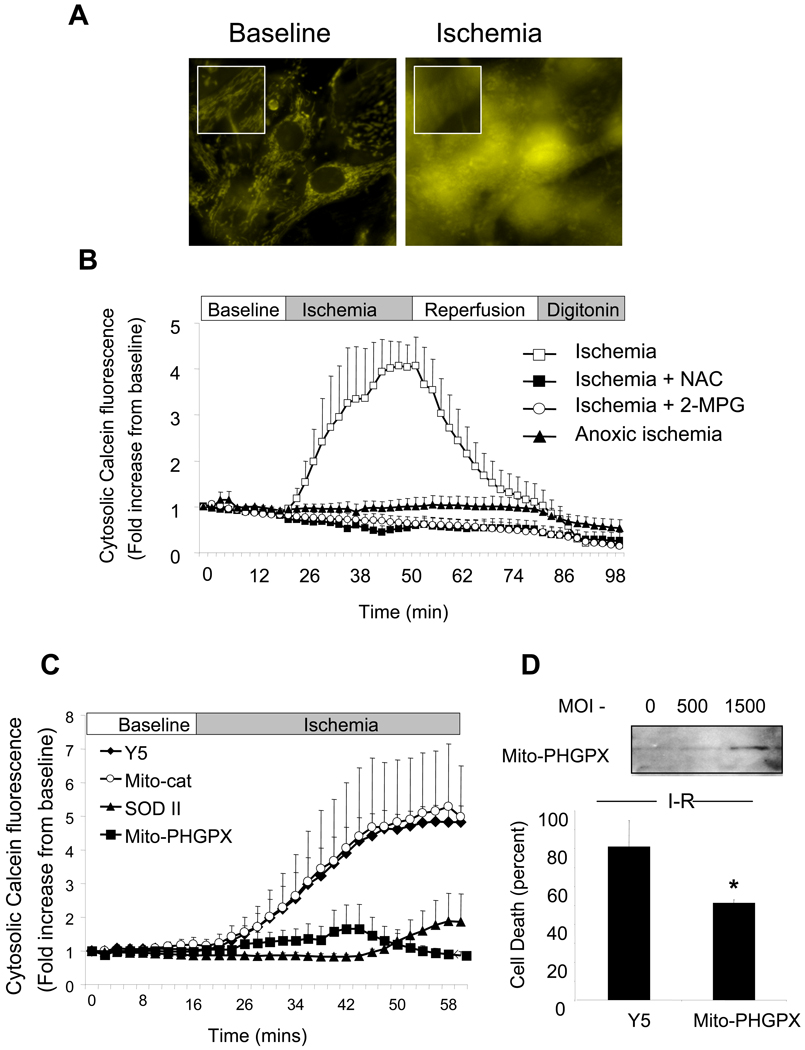
Role of ischemic oxidant stress in regulating the mitochondrial permeability transition pore
Opening of the mitochondrial permeability transition pore (mPTP) at reperfusion has been implicated in causing cyt-c redistribution to the cytosol during I/R [4,11,27]. To determine whether activation of the pore is responsible for the depolarization during ischemia, mitochondria were loaded with calcein-AM as previously described [38]. After quenching the cytosolic fluorescence with cobalt chloride, a pattern of mitochondrial fluorescence was observed in the cells (Fig. 5A), that remained stable during baseline. During ischemia, the mitochondrial fluorescence pattern shifted to a diffuse cytosolic distribution, which was associated with a significant increase in cellular fluorescence intensity (Fig. 5A,B), indicating escape of calcein from the mitochondrial matrix to the cytosol. Addition of NAC or 2-MPG during baseline and ischemia abolished this response, as did anoxic ischemia achieved using Oxyrase to scavenge O2 from the ischemia buffer. These findings implicate ischemic oxidant stress in the activation of the mPTP during simulated ischemia.
Figure 5.
Monitoring the opening of the mitochondrial permeability transition pore (mPTP) during simulated ischemia using mitochondrial calcein fluorescence. (A) Cardiomyocytes were loaded with calcein dye (1 mmol/L) in the presence of cobalt chloride (CoCl2 1 mmol/L) for 30 min and then studied in the flow-through chamber. During baseline perfusion a mitochondrial pattern of calcein fluorescence was observed. Within 16 min after beginning simulated ischemia, a rapid and profound release of calcein to the cytosol was observed. (B) A significant increase in calcein fluorescence intensity was detected during simulated ischemia. This increase was significantly attenuated by NAC (500 µmol/L) or 2-MPG (400 µmol/L) administration prior to and during ischemia. Anoxic ischemia using Oxyrase also prevented calcein release (2-MPG, n=3; controls, NAC, anoxic ischemia n=4). (C) Adenoviral over-expression of mitochondrial phospholipid hydroperoxide glutathione peroxidase (mito-PHGPx, 1500 MOI) or manganese superoxide dismutase (Mn-SOD, 100 MOI) conferred significant protection against calcein escape from mitochondria during ischemia. Mitochondrial-targeted catalase (Mito-cat, 500 MOI) did not attenuate the rise in cytosolic calcein fluorescence (Controls, mito-PHGPx, n=5; Mn-SOD, n=6, Mito-cat, n=4). (D) Over-expression of mito-PHGPx also conferred significant protection against cell death after 1 hr of ischemia and 3 hrs of reperfusion, compared with cells transduced with Y5 empty virus (n=5; *p<0.05).
To further explore the role of mitochondrial oxidant stress in the activation of the mPTP, cardiomyocytes were transduced with adenoviruses to express mitochondria-targeted catalase, Mn-SOD, or mitochondrial phospholipid hydroperoxide glutathione peroxidase (mito-PHGPx). The increase in cytosolic calcein fluorescence during ischemia was abrogated by mito-PHGPx and by Mn-SOD expression, but not by mito-catalase or by infection with the empty Y5 virus (Fig. 5C). Expression of mito-PHGPx was confirmed by Western blotting. Also, mito-PHGPx over-expression conferred protection against cell death in this model (Fig. 5D).
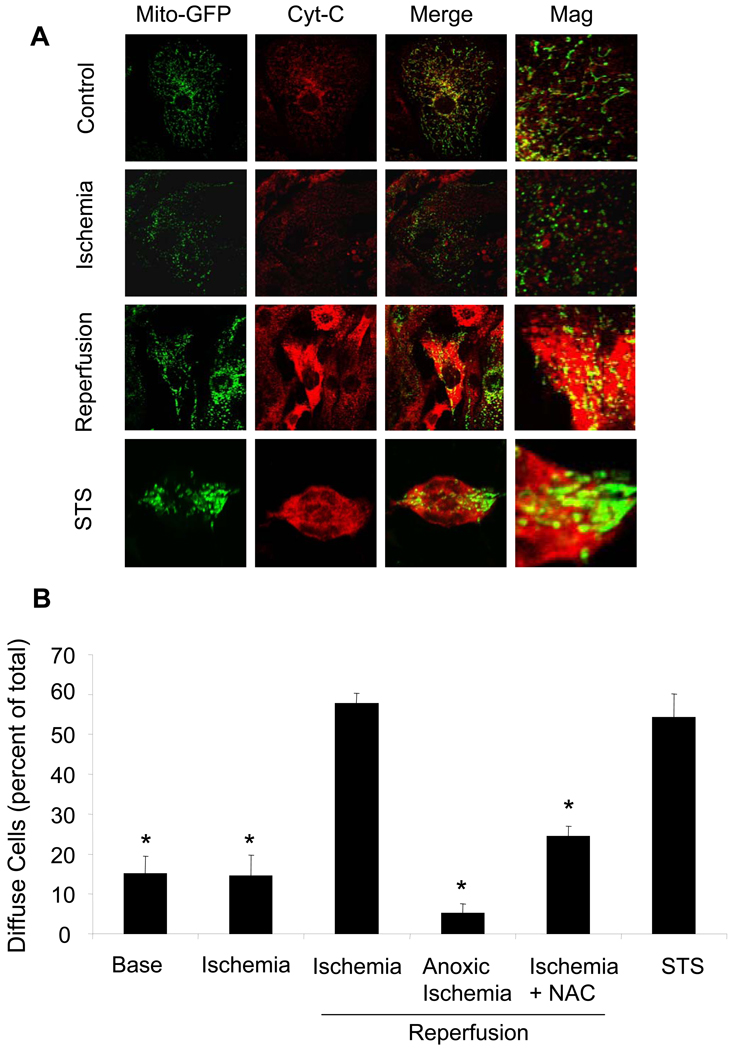
Relationship between ischemia and reperfusion in terms of cytochrome c release during I/R
The release of cytochrome c from the intermembrane space to the cytosol represents a threshold event in the activation of mitochondrial apoptosis. Cardiomyocytes immunostained for cytochrome c under baseline conditions demonstrated a punctate pattern of fluorescence in confocal images, which exhibited significant co-localization with mitochondria-targeted roGFP, consistent with mitochondrial localization (Fig. 6A). During simulated ischemia, no significant increase in cells exhibiting diffuse cyt-c staining was detected (Fig. 6B). However, a significant increase in the number of cells exhibiting a diffuse pattern of fluorescence was detected during reperfusion, which was mirrored in the cells treated with the apoptosis-inducing agent staurosporine. Treatment of cardiomyocytes with NAC during baseline and ischemia, or with Oxyrase to scavenge O2 during ischemia, resulted in a significant decrease in the extent of cyt-c redistribution during reperfusion. These findings indicate that, despite release of calcein during the ischemia phase, cyt-c redistribution does not occur until after reperfusion. Moreover, ischemic oxidant stress is required for both the release of calcein during ischemia, and the release of cyt-c during reperfusion in this model.
Figure 6.
Release of cytochrome c during simulated I/R. (A). Cardiomyocytes were transduced with mito-roGFP for 48 hrs to identify the mitochondria. Cells were fixed at baseline, after 45 minutes of ischemia, or after 2 hrs of reperfusion. Cells were then immunostained for cytochrome c to assess the degree of co-localization with the mito-roGFP sensor. Significant redistribution of cytochrome c to the cytosol was noted after 2 hrs of reperfusion, while only trace levels of redistribution were detected during ischemia (Mag. 60X). (B) Cells showing redistribution of cytochrome c were enumerated and expressed as a percent of the total cell population. Staurosporine (STS) caused a significant increase in the percentage of cells with cytochrome c release. A similar increase was seen in cells subjected to simulated I/R. Cytochrome c release was attenuated in cells treated with NAC prior to and during ischemia, and in cells challenged with anoxic ischemia using Oxyrase. No significant difference at baseline or after (base, ischemia, ischemia/reperfusion, STS, n=4; NAC, anoxic ischemia, n=3; * p<0.05).
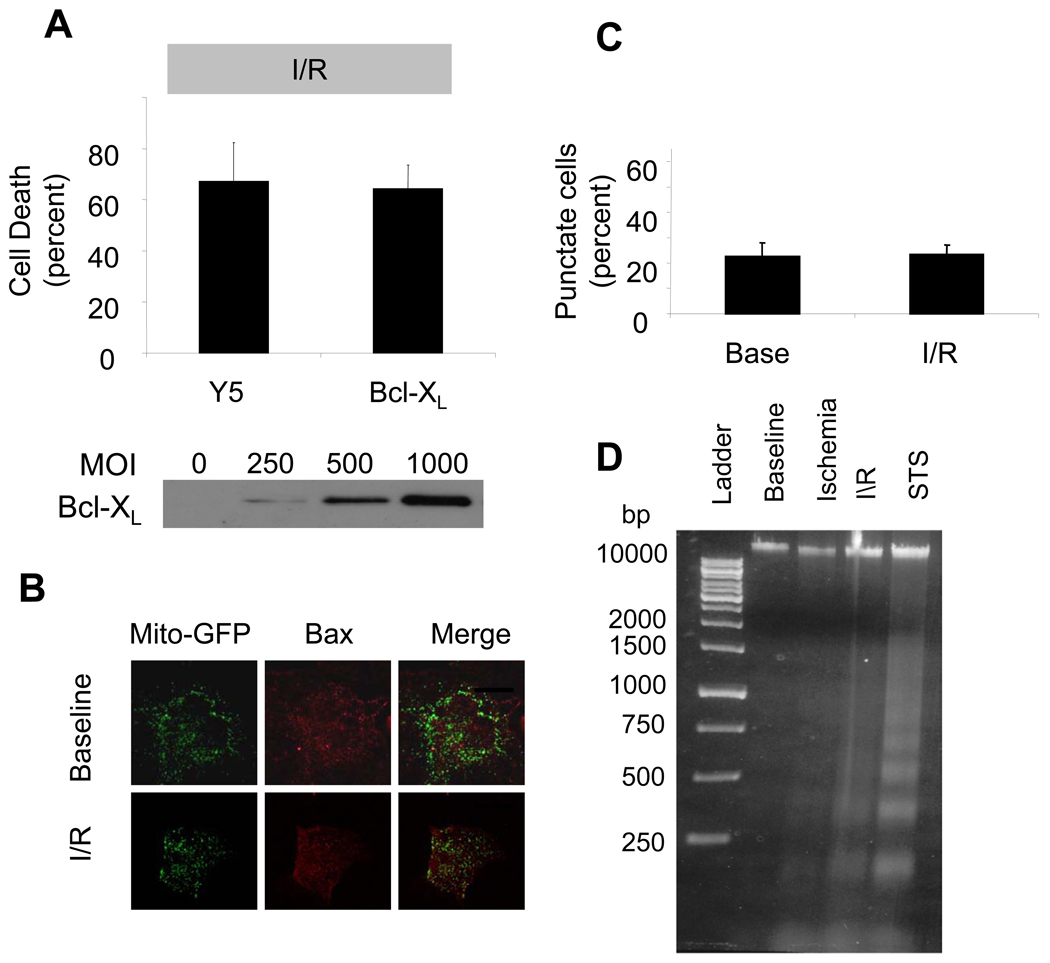
Role of mitochondrial apoptosis in simulated I/R
To clarify the requirement for apoptosis in the response to I/R, we examined the role of BH domain-containing proteins in the regulation of cell death. Cardiomyocytes were transduced with an adenovirus to induce over-expression of the antiapoptotic protein Bcl-XL. While Bcl-XL over-expression conferred significant protection against cell death induced by staurosporine (data not shown), it did not confer protection against simulated I/R (Fig. 7A). Simulated I/R was not associated with a translocation of Bax from the cytosol to the mitochondria, as evidenced by a lack of co-localization of mitochondria-targeted roGFP and activated Bax (Fig. 7B). Similarly, there was no increase in the percentage of total cells exhibiting a punctuate pattern of Bax immunostaining in I/R (Fig. 7C), indicating an absence of Bax oligomerization. However, DNA harvested from cardiomyocytes subjected to I/R did show evidence of laddering (Fig. 7D), indicating that caspase activation had occurred. These results indicate that the release of cyt-c during simulated I/R is not inhibited by anti-apoptotic proteins, and is not associated with Bax activation and translocation to the mitochondria.
Figure 7.
Mitochondrial apoptosis in staurosporine-treated and I/R-challenged cardiomyocytes. (A) Bcl-XL over-expression with an adenovirus (1000 MOI) for 36 hrs in cardiomyocytes was not protective against simulated I/R compared with cells transduced with empty virus (Y5, 1000 MOI)(n=4). (B) Cardiomyocytes transduced with an adenovirus to express mito-roGFP for 48 hrs, in order to identify mitochondria. Cell were then subjected to simulated I/R and immunostained for Bax, in order to assess oligomerization as evidenced by co-localization with the mitochondrial fluorescence, by confocal microscopy. I/R did not induce significant Bax activation, as seen by the lack of co-localization in this representative image (Mag. 60X). (C) No significant increase in the number of cells exhibiting a mitochondrial localization of Bax after simulated I/R was detected, indicating that I/R was not a significant stimulus for the activation of mitochondrial apoptosis (n=4). (D) Representative gel showing the presence of DNA fragmentation after treatment with I/R (lanes: 1- Ladder, 2- Baseline, 3- Ischemia, 4-Ischemia+Reperfusion (I/R), 5-Staurosporine (STS)).
Role of Bax/Bak and cytochrome c release in I/R- induced cell death
To further explore the requirement for apoptotic cell death in the setting of I/R, immortalized mouse embryonic fibroblast cells (MEFs) with genetic deletion of the proapoptotic proteins Bax and Bak (Bax−/−/Bak−/− double knockout cells) were compared with immortalized MEFs from isogenic wild type animals. Wild type immortalized MEFs, immortalized Bax/Bak double knockout MEFs, and immortalized MEFs with stable over-expression of Bcl-XL were grown on glass cover slips and subjected to serum withdrawal (48 hrs). Cell death in response to serum withdrawal was significantly attenuated in Bax/Bak-deficient cells, and by over-expression of Bcl-XL (Fig. 8A). By contrast, neither Bax/Bak deletion nor Bcl-XL over-expression conferred significant protection against simulated I/R.
Figure 8.
Cell death in wild-type MEFs, Bax/Bak double knockout (KO) MEFs, Bcl-XL over-expressing MEFs, wild-type ES cells and cytochrome c deficient ES cells. (A) Bcl-XL over-expression and genetic deletion of Bax/Bak conferred protection against growth factor withdrawal for 72 hrs as assessed by LDH cytotoxicity assays. However, in cells subjected to 30 min of simulated ischemia followed by 3 hrs reperfusion there was no significant difference in cell death between groups as determined by PI uptake (n=5; * p<.05 compared with controls). (B) Murine embryonic stem (ES) cells lacking cytochrome c (cyt-c−/−) were not significantly protected against 60 min of simulated ischemia followed by 3 hrs of reperfusion compared with wild-type ES cells as shown by analysis of PI uptake (n=4).
As cytochrome c is released to the cytosol during simulated reperfusion (Fig. 6), we sought to clarify the significance of this event in I/R-induced cell death. Murine embryonic cells (ES cells) devoid of cytochrome c (cyt-c −/−) were obtained from ATCC. Previous studies demonstrated that these cells are resistant to various apoptotic stimuli including UV irradiation, staurosporine, and serum withdrawal [31]. Cytochrome c-deficient ES cells and murine wild type (WT ES cells) were grown on cover slips and subjected to simulated I/R. However, deletion of cytochrome c did not confer protection against I/R (Fig. 8B). These findings indicate that, although apoptosis is activated during simulated I/R as evidenced by cytochrome c release and DNA laddering, cell death does not require cytochrome c release in this model.
Temporal relationship between PI uptake and cytochrome c release
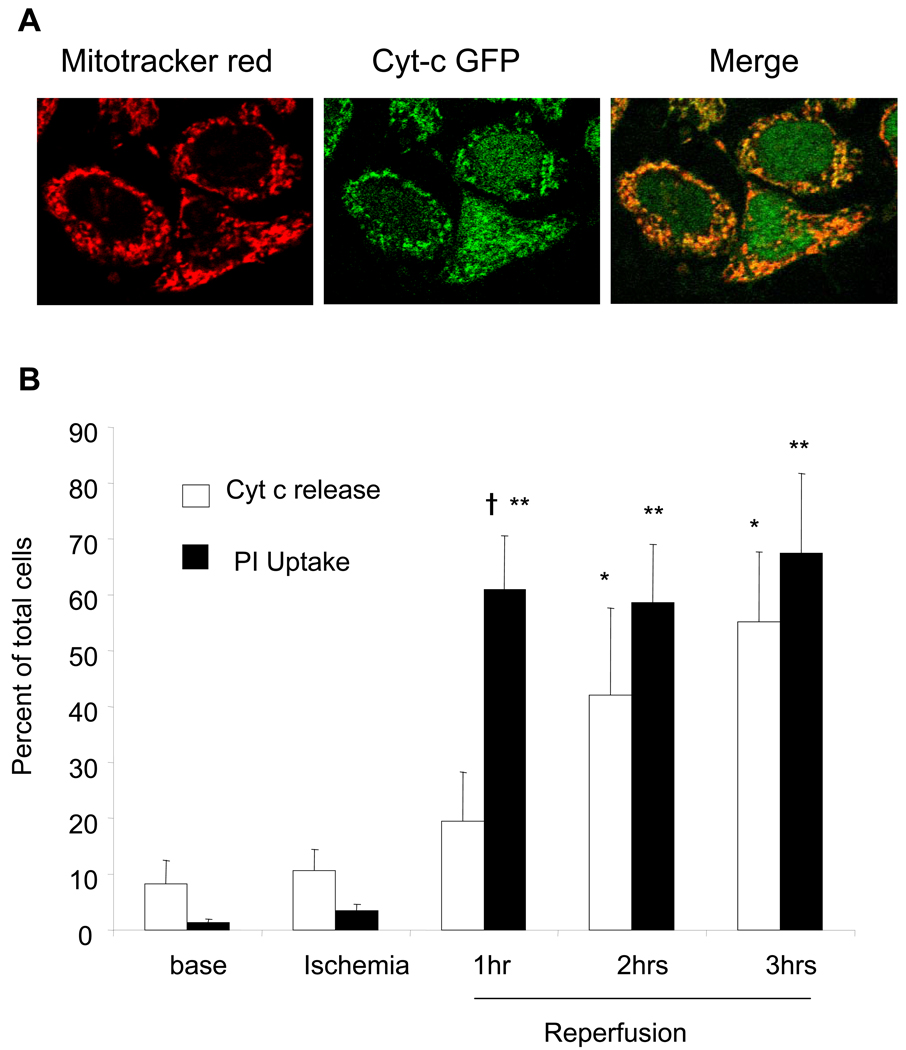
HeLa cells stably expressing a fusion protein consisting of cyt-c and GFP [18] were used to assess the temporal relationship between cytochrome c release to the cytosol and propidium uptake, during simulated I/R. Co-localization studies using Mitotracker Red and GFP fluorescence demonstrated significant co-localization under confocal microscopy (Fig. 9A). During ischemia, there was minimal release of cyt-c and minimal uptake of PI in these cells (Fig. 9B). Within 1 hr after reperfusion, significant PI uptake was observed, indicative of plasma membrane disruption. By contrast, a progressive increase in cyt-c release was observed during 3 hr of reperfusion. These findings suggest that cyt-c release is not the primary mechanism driving cell death in this model.
Figure 9.
Cytochrome c release and PI uptake during simulated I/R in HeLa cells with stable expression of cytochrome c-GFP. (A) Cytochrome c-GFP- expressing HeLa cells were loaded with Mitotracker Red (100 nM) for 30 min to label mitochondria. Merged confocal image shows co-localization of the cytochrome c-GFP fluorescence and Mitotracker Red (Mag. 60X). (B) HeLa cells challenged with simulated I/R. Live cell images were obtained during baseline, ischemia, and during reperfusion. Rapid and extensive dissipation of punctuate cyt-c-GFP fluorescence was observed within 2 hrs of reperfusion. In separate experiments, propidium iodide was used to detect the extent of cell death in the HeLa cells during simulated I/R. Significantly greater cell death within 1 hr of reperfusion was observed using PI uptake compared with cellular cytochrome c redistribution. The percentage of cells demonstrating cytochrome c release approximated the percentage of cells exhibiting PI uptake by 2 hrs of reperfusion. (Cyt-c redistribution and PI uptake, n=4; *p<0.05 compared to cyt-c release at baseline; **p<0.05 compared to PI uptake at baseline; † p<0.05, PI uptake compared to percent of cells with diffuse cyt-c staining).
DISCUSSION
Oxidant stress and mitochondrial apoptosis have both been implicated in the cell death observed during I/R, but the relationship between these contributing factors is not clear. A substantial body of evidence indicates that ROS production occurs during I/R [46,52], and that chemical antioxidants confer protection against I/R-induced cell death [30]. However, apoptosis also has been reported to be activated by I/R, and to be important for mediating I/R-induced cell death [23,43]. It is also conceivable that oxidant stress during I/R mediates cell death by activating a programmed cell death pathway [40]. We previously reported that oxidant stress generated during simulated ischemia is required for cell death during reperfusion [41]. The present study sought to examine how oxidant stress generated during ischemia contributes to cell death, and the relationship between ischemic oxidant stress and the activation of mitochondrial apoptosis.
Ischemia causes oxidant stress in the mitochondrial matrix during simulated I/R
We utilized mito-roGFP, a ratiometric redox sensor, to detect protein thiol oxidation during ischemia in the mitochondrial matrix. During simulated ischemia, oxidation of this sensor increased from less than 50% to more than 90%, indicating that mitochondrial ROS production increases paradoxically during ischemia, before experiencing the ROS burst that occurs upon reperfusion. Ischemic oxidant production was abrogated by chemical antioxidants, and by scavenging all of the residual O2 from the ischemia media to prevent ROS generation, indicating that ROS were involved. Mitochondrial ROS production was also detected using Mito-SOX, a superoxide-sensitive cationic dye that accumulates in mitochondria. Oxidation of Mito-SOX was attenuated by antioxidants and by Mn-SOD over-expression, indicating that superoxide is the initial oxidant generated in that compartment. A paradoxical increase in ROS production during ischemia was originally predicted by Misra and Fridovich, who postulated that the increase in reduction state of the mitochondrial electron carriers during hypoxia would promote ROS generation despite a decrease in the availability of O2 levels [35]. We previously detected such oxidant stress in the cytosol of cardiomyocytes in our simulated I/R model [41]. The present finding extends that work by showing that oxidant stress increases in the matrix as well, suggesting that ischemic oxidants originate from the electron transport chain. It seems unlikely that each of the chemically diverse antioxidants was able to scavenge superoxide directly. More likely, some of these agents may have acted by protecting the redox status of proteins that were the targets of superoxide attack.
Ischemic oxidant stress is critical for cell death in simulated I/R
ROS production during ischemia is required for cell death during reperfusion. This conclusion is based on the observation that chemical antioxidants given during the ischemia were protective, as was anoxic ischemia achieved by O2 scavenging. These findings are consistent with our previous work [41].
Ischemic oxidant stress causes a change in mitochondrial morphology, from a reticular to a punctate or fragmented appearance. In association with this morphological change, mitochondria release calcein from the matrix to the cytosol, a response that signals the formation of a large conductance pore in the mitochondrial membranes. The most likely source of this leak is the mPTP, which has previously been implicated in the depolarization of mitochondria during reperfusion after ischemia [10,13]. Antioxidants and anoxic ischemia prevented the calcein leak, as did over-expression of mito-PHGPx. This protein is targeted to the mitochondrial membranes and is responsible for scavenging lipid hydroperoxides [17]. Over-expression of mito-PHGPx conferred protection against I/R-induced cell death, and it abrogated the ischemia-induced calcein leakage. These results suggest that mitochondrial oxidant stress is responsible for triggering the mPTP opening, which is responsible for the mitochondrial depolarization and cell death. A previous report also noted that mito-PHGPx over-expression was protective in I/R [24].
Protection against calcein leak and cell death was also conferred by Mn-SOD, which suggests that superoxide production in the mitochondrial matrix is critical for regulating cell death in this model. This finding is consistent with studies showing that protection from hepatic and cardiac I/R injury in vivo is conferred by over-expression of Mn-SOD in mice [8]. Loss of this critical antioxidant in mice causes a severe dilated cardiomyopathy and neonatal lethality [28,32], underscoring the potential toxicity of intra-mitochondrial superoxide. Recently, Ago et al. reported that NOX4 may be expressed in mitochondria, and that over-expression of NOX4 can lead to cell death in aging and hypertrophic hearts [1]. While the electron transport chain has been regarded as the apparent source of superoxide in ischemia, those findings raise the question of whether NOX4 contributes importantly to the generation of superoxide during ischemia. In either case, superoxide appears to be critical for the cell damage, as catalase expression in either the cytosol or in the mitochondrial matrix failed to provide protection, which indicates that H2O2 is a less important factor in this model. Although there is little doubt that excessive H2O2 can potentially injure a cell, our findings do suggest that superoxide in the mitochondrial matrix may act on different targets than does H2O2, with important consequences for the rapid initiation of cell death pathways. Over-expression of Cu,Zn-SOD provided no significant protection in this model. Hydrogen peroxide generated in one intracellular compartment can diffuse through membranes to access other compartments. By contrast, superoxide generated in I/R cannot easily cross membranes, which explains why Mn-SOD was protective in the I/R model whereas Cu,Zn-SOD was not.
Previous studies have suggested that mPTP opening only occurs after reperfusion in intact heart, as the low pH during the ischemic insult inhibits pore activation [11,21,22]. Although our cellular model of simulated ischemia includes hypercarbic acidosis, this did not prevent activation of the pore during the ischemia. Despite this difference, important similarities are seen between this model and the intact heart, including the ability to undergo preconditioning protection [45], involvement of ROS in the cell death [30], and the development of cell death after reperfusion [47]. Further studies are needed to determine whether more severe acidosis would delay the onset of mPTP opening until after the start of reperfusion.
Relationship between mPTP activation and cytochrome c release
Previous studies of I/R have shown that mPTP opening at reperfusion is associated with cytochrome c release to the cytosol [11,12]. Our findings confirm that negligible cyt-c release occurs before reperfusion, yet our results consistently show that calcein release occurs in parallel with mitochondrial depolarization during ischemia, in an ROS-dependent manner. One possibility is that mPTP activation during ischemia represents oscillation or flickering of the pore, whereas irreversible opening occurs at reperfusion. Our findings are consistent with the mPTP oscillations reported by Di Lisa and colleagues, who concluded that cell death was not triggered by these short mPTP openings [39]. The only difference between their findings and ours is that we observed release of both calcein and TMRE during this oscillation, whereas they observed release of calcein but not TMRM. In either case, our findings suggest that the ROS-mediated oscillation of the mPTP during ischemia is an important determinant of later reperfusion-induced cell death. We agree with their conclusion that mPTP oscillation by itself is not lethal for the cell, based on our observation that cyt-c release and cell death do not increase significantly until reperfusion, even when the ischemic insult is extensively prolonged.
Role of mitochondrial apoptosis in cell death following I/R
Simulated I/R caused a redistribution of cyt-c from the intermembrane space to the cytosol. This, plus the associated DNA laddering, suggests that caspases were activated by that event. However, over-expression of Bcl-XL in cardiomyocytes and MEFs failed to induce protection, and there was no evidence of Bax oligomerization in cardiomyocytes during I/R. Furthermore, MEFs derived from Bax/Bak double knockout embryos were not protected against I/R, but did show protection against serum withdrawal, a potent stimulus for activating mitochondrial apoptosis. These findings indicate that activation of BH-domain proteins is not required for I/R-induced cell death.
Activation of redundant death pathways mediated by mPTP oscillation
Although cytochrome c release occurs in response to I/R, our data suggest that this is a marker rather than a mediator of cell death. First, genetic depletion of cytochrome c was not protective against cell death in this model. Second, when comparing the kinetics of PI uptake and the release of cyt-c, it is apparent that PI uptake occurs within the first hour after reperfusion whereas cytochrome c release develops later. This suggests that cytochrome c release is not required for the loss in plasma membrane integrity. Rather, events early in reperfusion, most likely the ROS burst, appear to be responsible for triggering both the loss of plasma membrane integrity and the later release of cytochrome c. Once the plasma membrane integrity is lost, a cell becomes committed to death regardless of whether or not cytochrome c is later released. Our data suggest that low levels of oxidant stress during ischemia trigger oscillation of the mPTP, which causes cell death during reperfusion, possibly by initiating a lethal ROS burst. Although the specific mechanism is not fully understood, we speculate that mPTP activation during ischemia may prime the cell for undergoing a burst in oxidant stress at reperfusion by releasing NAD(P)H from the matrix to the cytosol. Di Lisa and colleagues demonstrated the feasibility of such a process by showing that mitochondrial NAD+ release to the cytosol occurs in response to mPTP activation [13]. A burst of ROS generation at reperfusion, resulting from the oxidation of mitochondria-derived NADH in the cytosol, could then trigger plasma membrane disruption and also cause irreversible mPTP opening and cytochrome c release. In that respect, oxidant stress during ischemia would represent a causative factor in the lethal ROS burst during reperfusion, which would undermine cell survival by activating multiple redundant death pathways. A fuller test of this hypothesis is warranted.
ACKNOWLEDGMENTS
The authors thank Bhumika Sharma for general technical assistance, Danijela Dokic for assistance with immunofluorescence studies, and Juan Li for the isolation of the cardiomyocytes. We also thank Dr. Douglas R. Green for providing the HeLa cell line with stable expression of cytochrome c-GFP, and Drs. J.X. Bai and A.I. Cederbaum for providing adenoviruses expressing catalase and mitochondrial catalase. This work was supported by HL35440, HL32646, HL66315, HL079650 and the American Heart Association, Midwest Affiliates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- 3.Bai JX, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J. Biol. Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 4.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J. Clin. Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox. Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J. Mol. Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZY, Oberley TD, Ho YS, Chua CC, Siu B, Hamdy RC, Epstein CJ, Chua BHL. Overexpression of CuZnSOD in coronary vascular cells attenuates myocardial ischemia/reperfusion injury. Free Radic. Biol. Med. 2000;29:589–596. doi: 10.1016/s0891-5849(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Lisa F. Mitochondrial contribution in the progression of cardiac ischemic injury. IUBMB. Life. 2001;52:255–261. doi: 10.1080/15216540152846073. [DOI] [PubMed] [Google Scholar]
- 11.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc. Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J. Biol. Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 13.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J. Biol. Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 14.Dixon IM, Kaneko M, Hata T, Panagia V, Dhalla NS. Alterations in cardiac membrane Ca2+ transport during oxidative stress. Mol. Cell Biochem. 1990;99:125–133. doi: 10.1007/BF00230342. [DOI] [PubMed] [Google Scholar]
- 15.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 16.Fischer UM, Cox CS, Jr, Allen SJ, Stewart RH, Mehlhorn U, Laine GA. The antioxidant N-acetylcysteine preserves myocardial function and diminishes oxidative stress after cardioplegic arrest. J. Thorac. Cardiovasc. Surg. 2003;126:1483–1488. doi: 10.1016/s0022-5223(03)00792-x. [DOI] [PubMed] [Google Scholar]
- 17.Godeas C, Sandri G, Panfili E. Distribution of phospholipid hydroperoxide glutathione peroxidase (PHGPx) in rat testis mitochondria. Biochim. Biophys. Acta. 1994;1191:147–150. doi: 10.1016/0005-2736(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 19.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J. Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 21.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim. Biophys. Acta Bio. -Energetics. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 23.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am. J. Physiol Heart Circ. Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 24.Hollander JM, Lin KM, Scott BT, Dillmann WH. Overexpression of PHGPx and HSP60/10 protects against ischemia/reoxygenation injury. Free Radic. Biol. Med. 2003;35:742–751. doi: 10.1016/s0891-5849(03)00400-3. [DOI] [PubMed] [Google Scholar]
- 25.Hurd TR, Filipovska A, Costa NJ, Dahm CC, Murphy MP. Disulphide formation on mitochondrial protein thiols. Biochem. Soc. Trans. 2005;33:1390–1393. doi: 10.1042/BST0331390. [DOI] [PubMed] [Google Scholar]
- 26.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ. Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 28.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebuffe G, Schumacker PT, Shao ZH, Anderson T, Iwase H, Vanden Hoek TL. ROS and NO trigger early preconditioning: relationship to mitochondrial KATP channel. Am. J. Physiol Heart Circ. Physiol. 2003;284:H299–H308. doi: 10.1152/ajpheart.00706.2002. [DOI] [PubMed] [Google Scholar]
- 30.Levraut J, Iwase H, Shao ZH, Vanden Hoek TL, Schumacker PT. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am. J. Physiol Heart Circ. Physiol. 2003;284:H549–H558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 31.Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Williams RS. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 33.Lo LW, Koch CJ, Wilson DF. Calibration of oxygen-dependent quenching of the phosphorescence of Pd-meso-tetra (4-carboxyphenyl) porphine: a phosphor with general application for measuring oxygen concentration in biological systems. Analyt. Biochem. 1996;236:153–160. doi: 10.1006/abio.1996.0144. [DOI] [PubMed] [Google Scholar]
- 34.McDonald MC, Zacharowski K, Bowes J, Cuzzocrea S, Thiemermann C. Tempol reduces infarct size in rodent models of regional myocardial ischemia and reperfusion. Free Radic. Biol. Med. 1999;27:493–503. doi: 10.1016/s0891-5849(99)00100-8. [DOI] [PubMed] [Google Scholar]
- 35.Misra HP, Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J. Biol. Chem. 1972;247:188–192. [PubMed] [Google Scholar]
- 36.Mueller TH, Kienle K, Beham A, Geissler EK, Jauch KW, Rentsch M. Caspase 3 inhibition improves survival and reduces early graft injury after ischemia and reperfusion in rat liver transplantation. Transplantation. 2004;78:1267–1273. doi: 10.1097/01.tp.0000141095.06273.10. [DOI] [PubMed] [Google Scholar]
- 37.Ostadal P, Elmoselhi AB, Zdobnicka I, Lukas A, Elimban V, Dhalla NS. Role of oxidative stress in ischemia-reperfusion-induced changes in Na+,K(+)-ATPase isoform expression in rat heart. Antioxid. Redox. Signal. 2004;6:914–923. doi: 10.1089/ars.2004.6.914. [DOI] [PubMed] [Google Scholar]
- 38.Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J. Biol. Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- 40.Qin YM, Hoek TLV, Wojcik K, Anderson T, Li CQ, Shao ZH, Becker LB, Hamann KJ. Caspase-dependent cytochrome c release and cell death in chick cardiomyocytes after simulated ischemia-reperfusion. Am. J. Physiol Heart Circ. Physiol. 2004;286:H2280–H2286. doi: 10.1152/ajpheart.01063.2003. [DOI] [PubMed] [Google Scholar]
- 41.Robin E, Guzy RD, Loor G, Iwase H, Waypa GB, Marks JD, Vanden Hoek TL, Schumacker PT. Oxidant stress during simulated ischemia primes cardiomyocytes for cell death during reperfusion. J. Biol. Chem. 2007;282:19133–19143. doi: 10.1074/jbc.M701917200. [DOI] [PubMed] [Google Scholar]
- 42.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Nakae S, Terry PD, Mokhtari GK, Gunawan F, Balsam LB, Kaneda H, Kofidis T, Tsao PS, Robbins RC. Cardiomyocyte-specific Bcl-2 overexpression attenuates ischemia-reperfusion injury, immune response during acute rejection, and graft coronary artery disease. Blood. 2004;104:3789–3796. doi: 10.1182/blood-2004-02-0666. [DOI] [PubMed] [Google Scholar]
- 44.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 45.Vanden Hoek TL, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ. Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- 46.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J. Mol. Cell. Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 47.Vanden Hoek TL, Shao Z, Li C, Zak R, Schumacker PT, Becker LB. Reperfusion injury in cardiac myocytes after simulated ischemia. Am. J. Physiol. 1996;270:H1334–H1341. doi: 10.1152/ajpheart.1996.270.4.H1334. [DOI] [PubMed] [Google Scholar]
- 48.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia Triggers Subcellular Compartmental Redox Signaling in Vascular Smooth Muscle Cells. Circ. Res. 2009;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei MC, Zong WX, Cheng EHY, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacCregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwacka RM, Dudus L, Epperly MW, Greenberger JS, Engelhardt JF. Redox gene therapy protects human IB-3 lung epithelial cells against ionizing radiation-induced apoptosis. Hum. Gene Ther. 1998;9:1381–1386. doi: 10.1089/hum.1998.9.9-1381. [DOI] [PubMed] [Google Scholar]
- 51.Zwacka RM, Zhou W, Zhang Y, Darby CJ, Dudus L, Halldorson J, Oberley L, Engelhardt JF. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-kappaB activation. Nat. Med. 1998;4:698–704. doi: 10.1038/nm0698-698. [DOI] [PubMed] [Google Scholar]
- 52.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]