Abstract
The nasopharyngeal carriage of Streptococcus pneumoniae is thought to pose a risk for invasive pneumococcal diseases, and the evaluation of carriage strains is thus often used to inform antibiotic treatment and vaccination strategies for these diseases. In this study, the age-specific prevalences, resistance to antibiotics, and serotype distributions of 1,340 carriage strains were analyzed and compared to 71 pneumococcal strains isolated from the cerebrospinal fluid of children under 5 years old with meningitis. Overall, the nasal carriage rate was 47%. One-fourth (26%) of the infants under 1 month of age and one-half (48%) of the infants under 12 months of age were colonized with S. pneumoniae. Rural children were colonized earlier than those from urban areas. Approximately one-fourth and one-half of the cases of pneumococcal meningitis occurred in the first 3 and 6 months of life, respectively. The respective rates of resistance for carriage and meningitis strains to penicillin (7 and 3%), cotrimoxazole (77 and 69%), and erythromycin (2 and 1%) were similar, whereas chloramphenicol resistance was lower among carriage strains (3%) than among meningitis strains (15.5%). The predominant serogroups of carriage and invasive isolates were variable and widely divergent. Thus, hypothetical 7-, 9-, and 11-valent vaccines, based on the predominant carriage strains of the present study, would cover only 23, 26, and 30%, respectively, of the serotypes causing meningitis. Further, currently available 7-, 9-, and 11-valent vaccines would protect against only 26, 43, and 48%, respectively, of these meningitis cases. In conclusion, while the surveillance of carriage strains for resistance to antibiotics appears useful in the design of empirical treatment guidelines for invasive pneumococcal disease, data on the serotypes of carriage strains have limited value in vaccine formulation strategies, particularly for meningitis cases.
Streptococcus pneumoniae is one of the leading causes of childhood pneumonia and meningitis. It accounts for 20 to 40% of the estimated annual global burden of 2.7 million childhood deaths from pneumonia in developing countries (11, 22, 25) and is the most common cause of pneumonia and the second leading cause of meningitis in children in Bangladesh (26, 31).
Data on the serotype composition and antibiotic resistance of invasive pneumococcal strains from the developing world are scarce. Previously, it has been shown that the serotype distribution of invasive S. pneumoniae in Bangladesh differs from the distribution in many other parts of the world, and the proposed conjugate vaccines developed on the basis of data from other, primarily Western, countries cover at most 50% of the invasive strains (29). High levels of resistance to cotrimoxazole and low levels of resistance to penicillin have also been found (28, 30); for Bangladesh, these findings bring into question the present World Health Organization recommendation that cotrimoxazole be used as a first-line empirical therapy for the treatment of pneumonia in the community. However, the data on pneumococcal serotype composition and drug resistance were based on small numbers of meningitis strains isolated from hospitalized patients and may not accurately reflect community-acquired infections in the whole country.
The incidence of pneumococcal disease is thought to be related to the prevalence of asymptomatic carriage (15, 21). Thus, carriage strains are used to monitor and predict drug resistance patterns and serotype prevalences for treatment and vaccine formulation, respectively (17, 19, 38). The present study was conducted to determine age-specific prevalences and to compare the antibiotic resistance and serotype patterns of carriage and invasive pneumococcal isolates among children in Bangladesh. The implications of these data for vaccine formulation and treatment strategies are discussed.
MATERIALS AND METHODS
Study population.
Healthy children under 5 years old (n = 2,839) from urban and rural areas of Bangladesh were enrolled for the isolation of nasal carriage strains of S. pneumoniae during 1999 and 2000. A total of 591 healthy children who attended the Outpatient Department of Dhaka Shishu Hospital (DSH) for immunization or elective surgical procedures were enrolled in the study and constituted the urban sample. The rural sample (n = 2,248) was selected from a village, Kaliganj, near Dhaka. Children who were on antibiotics or had a recent history of hospitalization were excluded from the study.
Isolation of carriage strains.
Anterior nasal swab specimens were collected with a cotton-tipped wooden swab and immediately plated onto sheep blood agar containing gentamicin (5 μg/ml) and incubated at 37°C with 5% CO2. Specimens collected at Kaliganj were also plated immediately and transported within 2 h, at ambient temperature, to the DSH microbiology laboratory. After 20 to 24 h of incubation, colonies typical for S. pneumoniae were selected and tested for sensitivity to optochin and bile solubility to confirm their identity.
Isolation of invasive strains.
Invasive strains were isolated from the cerebrospinal fluid (CSF) of children under 5 years old with meningitis (n = 71) admitted to DSH during the same period of time. The inclusion of the meningitis cases was based on the presence in the CSF of ≥100 leukocytes with >50% polymorphs. CSF specimens were tested for antigens of five common organisms (Haemophilus influenzae, S. pneumoniae, Neisseria meningitidis, group B streptococcus, and Escherichia coli) with latex agglutination reagents (Murex, Dartford, Kent, United Kingdom) following the manufacturer's instructions.
Antimicrobial susceptibility testing.
S. pneumoniae strains were screened for resistance to penicillin, cotrimoxazole, chloramphenicol, and erythromycin by the disk diffusion method described by Jorgensen et al. (12). Resistant and intermediate strains, as determined by NCCLS guidelines (24), were subjected to the E-test for the determination of drug MICs (30). The E-tests were performed on Mueller-Hinton agar (Oxoid, Basingstoke, Hampshire, United Kingdom) supplemented with 5% defibrinated sheep blood. Inocula were prepared in Mueller-Hinton broth by direct suspension of pneumococcal colonies grown overnight on sheep blood agar to a density that matched a 0.5 McFarland opacity standard tube (12).
Based on the results, strains were interpreted as susceptible, intermediate, or completely nonsusceptible according to NCCLS-defined breakpoints. Only penicillin-nonsusceptible strains were tested for sensitivity to ampicillin and cephalosporins (24).
Serotyping.
Pneumococcal strains were serotyped by the capsular swelling procedure (the Quellung reaction) with type-specific antipneumococcal omni, pool, type or group, and factor sera (Statens Seruminstitute, Copenhagen, Denmark) (6, 29). ATCC strains 6314, 6301, and 10341 and Johns Hopkins University strains 9, 23, and 4 (kindly provided by Mark Steinhoff) were used as known control strains. Nontypeable S. pneumoniae strains were screened out with omni sera at the first step of serotyping. The serotyping of 10 randomly selected strains was repeated at the National Public Health Institute, Helsinki, Finland, and confirmed to be identical with our results.
Data analysis.
EpiInfo statistics program 6.02 (J. Dean, A. D. Coulombier, C. Smith, et al., Centers for Disease Control and Prevention, Atlanta, Ga.) was used for analyzing the data.
RESULTS
The overall nasal carriage rate of S. pneumoniae was 47% (1,340 isolates from 2,839 children). One-fourth of the infants less than 1 month of age and one-half of the infants less than 12 months of age were colonized (Table 1). Rural children were colonized earlier than those from urban areas. The prevalence of nasal colonization reached a plateau in rural children within 6 months but increased more gradually in urban children until the levels became equivalent with those of rural children in the fifth year of life (Table 1).
TABLE 1.
Prevalence of S. pneumoniae nasopharyngeal colonization among children by age and residence (rural and urban)
| Age (mo) | Rural
|
Urbana
|
Total
|
|||
|---|---|---|---|---|---|---|
| No. of children tested | No. of children positive (%) | No. of children tested | No. of children positive (%) | No. of children tested (%) | No. of children positive (%) | |
| 0-1 | 34 | 10 (29) | 16 | 3 (19) | 50 | 13 (26) |
| 2-5 | 129 | 64 (50) | 171 | 45 (26)** | 300 | 109 (36) |
| 6-11 | 180 | 89 (49) | 119 | 36 (30)** | 299 | 125 (42) |
| 12-23 | 343 | 189 (55) | 105 | 35 (33)** | 448 | 224 (50) |
| 24-35 | 405 | 212 (52) | 71 | 29 (41) | 476 | 241 (51) |
| 36-48 | 654 | 308 (47) | 67 | 25 (37) | 721 | 333 (46) |
| ≥48 | 503 | 236 (47) | 42 | 20 (48) | 545 | 256 (47) |
| Total | 2,248 | 1,108 (49) | 591 | 193 (33)** | 2,839 | 1,301 (46) |
**, comparison of results of urban and rural populations (P < 0.001).
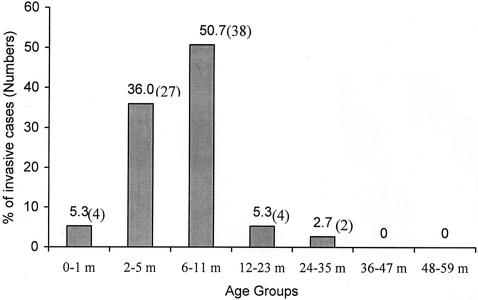
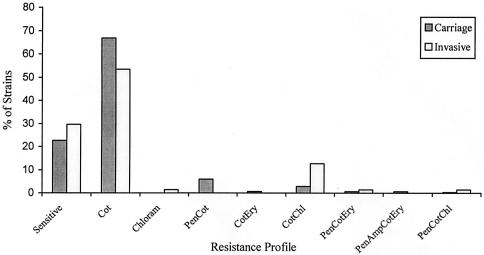
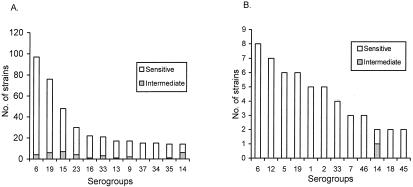
The age distribution of pneumococcal meningitis cases (culture and/or latex agglutination positive) showed that 5% of the cases occurred during the first month of life, and approximately one-fourth of the cases occurred in the first 3 months and one-half occurred within the first 6 months; nearly all cases (96%; 72 of 75) occurred within the first year of life (Fig. 1). Approximately equal proportions of carriage (22.6%; 303 of 1340) and meningitis (29.6%; 21 of 71) strains were susceptible to all antibiotics tested (P = 0.20) (Fig. 2). Similarly, the rates of resistance to more than one antibiotic did not differ significantly (P = 0.33) between carriage (15.5%; 142 of 1340) and meningitis (10.6%; 11 of 71) strains (Fig. 2). Among the penicillin nonsusceptible carriage (7.0%; 94 of 1340) and meningitis (2.9%; 2 of 71) strains (P = 0.26), all were intermediate except one in the carriage group for which the MIC was 8.0 μg/ml. An association of penicillin nonsusceptibility with serotype 14 was observed in both carriage and meningitis strains (Fig. 3). The rates of resistance in carriage and meningitis strains to cotrimoxazole (77.0%, or 1,037 of 1,340, and 69.0%, or 49 of 71, respectively; P = 0.10) and erythromycin (1.6%, or 22 of 1,340, and 1.4%, or 1 of 71; P = 0.70) were also similar (Fig. 2); only nonsusceptibility to chloramphenicol was significantly lower in carriage than in meningitis strains (3.2%, or 44 of 1,340, and 15.5%, or 11 of 71; P < 0.001). Chloramphenicol resistance among the meningitis isolates was mainly observed in serogroups 12 (44%; 4 of 9) and 46 (33%; 3 of 9). Among the carriage isolates, the susceptibilities of rural and urban strains were as follows, respectively: to penicillin, 93.8 and 87.9% (P = 0.19); to chloramphenicol, 96.8 and 96.1% (P = 0.35); to cotrimoxazole, 23.1 and 19.8% (P = 0. 68); and to erythromycin, 99.2 and 93.7% (P < 0.001).
FIG. 1.
Age distribution of invasive S. pneumoniae cases (n = 75). m, month(s).
FIG. 2.
Resistance profile of carriage (n = 1,340) and invasive (n = 71) S. pneumoniae strains. Cot, cotrimoxazole; Chl(oram), chloramphenicol; Pen, penicillin; Ery, erythromycin; Amp, ampicillin.
FIG. 3.
Comparative serogroup distributions of carriage (A) and invasive (B) S. pneumoniae strains and their relation to penicillin resistance (MIC, ≥0.12 μg).
Serotype distribution was diverse and widely divergent among nasal carriage and meningitis strains (Fig. 3). Serogroup 6 was the most prevalent in both strains, and serogroup 19 ranked second and third in carriage- and meningitis-causing strains, respectively. However, there are several predominant serogroups among the carriage strains which were never encountered in meningitis cases, such as serogroups 16, 13, 37, and 34. On the other hand, several predominant serogroups (12, 5, 1, and 2) found in cases of meningitis (ranking 2, 3, 5, and 6, respectively) were rarely isolated as carriage strains (ranking 20, 33, 34, and 35, respectively). Three different vaccine serotypes or groups (5, 23F, and 9V) were rarely (1.5%) isolated from meningitis cases, which resulted in diminished coverage of the meningitis cases by the established 7-, 9-, and 11-valent vaccines (Table 2).
TABLE 2.
Coverage of invasive cases by hypothetical and established pneumococcal vaccine formulations
| Vaccine formulation type and coverage (vaccine serotypes)a | Coverage of invasive cases (%) |
|---|---|
| Hypothetical | |
| 7-valent (6, 19F, 16F, 13, 34, 37, 14) | 22.3 |
| 9-valent (6, 19F, 16F, 13, 34, 37, 14, 15C, 23F) | 25.7 |
| 11-valent (6, 19F, 16F, 13, 34, 37, 14, 15C, 23F, 15B, 33F) | 29.5 |
| Established | |
| 7-valent (4, 14, 18C, 19F, 23F, 6B, 9V) | 26.1 |
| 9-valent (1, 4, 5, 14, 18C, 19F, 23F, 6B, 9V) | 43.1 |
| 11-valent (1, 3, 4, 5, 7F, 14, 18C, 19F, 23F, 6B, 9V) | 47.7 |
Hypothetical vaccines are based on carriage strains. Vaccine serotype 6 is 6A plus 6B.
DISCUSSION
This study is based on the largest number of pneumococcal strains from any single country in Asia and the Middle East, with 1,340 carriage and 71 invasive strains causing meningitis isolated during the same period of time at a single institution. The prevalence of nasal carriage of pneumococci among children in Bangladesh (47%) was similar to the carriage rate reported in India (43%) (16), Indonesia (48%) (35), and other developing countries (41), but the prevalence was much greater than that in countries of higher socioeconomic levels, such as Singapore (9%) (16) and Sweden (12%) (1). Likewise, the early acquisition of pneumococcal carriage in the first month of life contrasts with the later colonization reported in Finland (9% in the second month of life and the steepest increase in months 15 to 24) (18) and other developed countries (8, 9). The acquisition of nasal carriage has been found to be closely associated with the degree of intrafamilial contact with another carrier, particularly an older sibling (18). Perhaps the more common presence in rural compared to urban Bangladeshi households of an extended family network caring for a young infant under the same roof contributed to the higher rate in rural children than in urban children of pneumococcal carriage.
Similar to the high prevalence rate of S. pneumoniae carriage in early infancy, meningitis was also found predominantly in the postneonatal period of infancy, which appears to corroborate observations that the risk of pneumococcal invasive diseases is high among children with high rates of carriage (5). We found, however, that the serotype distributions of carriage and meningitis strains were widely divergent, suggesting that other factors, in addition to colonization, are associated with the risk for invasive disease. Perhaps pneumococcal carriage is a more dynamic process than previously thought. The early development of meningitis cases emphasizes the need for an effective vaccine in this young age group. Since about one-fourth of the cases occurred in the first 3 months of life and about one-half occurred in the first 6 months, maternal immunization may be a promising strategy until a conjugate vaccine with a good immune response in early infancy is available. However, studies of maternal immunization are still preliminary, and vaccine efficacy beyond 8 weeks of age is yet to be demonstrated (23).
Drug resistance patterns were similar in both carriage and meningitis strains, except for chloramphenicol; resistance to this drug was significantly higher (P < 0.001) among the meningitis strains than among the carriage strains. It appears that resistance to chloramphenicol among meningitis-causing pneumococci in Bangladeshi children is increasing over time, as it has now reached a level of 15.5% compared to 2.8% in 1997 (30). The increase in chloramphenicol resistance may reflect widespread use of this drug in the hospital and communities. However, the rate of resistance should be interpreted with caution since patients with treatment failure due to antibiotic resistance may preferentially present to DSH and may not reflect the true picture of the country (32). Nevertheless, this trend should be followed as it may herald increased chloramphenicol resistance and treatment failure in the future if widespread use of the antibiotic persists. Chloramphenicol-resistant strains belonged primarily to serogroups 12 (44%) and 46 (33%), which were predominant among the meningitis strains but rarely encountered among carriage strains. The association of serogroups 12 and 46 with chloramphenicol resistance needs to be followed up with larger numbers of strains isolated over a longer period of time.
The high prevalence (74%) of in vitro cotrimoxazole resistance observed in our patients was consistent with findings from other countries in this region (10, 16) and also with a previous study with invasive pneumococcal strains (30). High cotrimoxazole resistance among pneumococci has, in fact, reflected a national trend toward increasing resistance to the drug over time, possibly due to widespread use by community health workers (2). Although it has been recommended that use of cotrimoxazole be discontinued in areas with a high prevalence of nonsusceptible strains (14), debate continues about the association between in vitro resistance and clinical responses to cotrimoxazole therapy for pneumonia (33, 37), as direct evidence in favor of or against such an association is lacking. In this study, qualitative resistance by the disk diffusion method was quantitated further by the E-test, and a high cotrimoxazole MIC was found for most of the strains (the MIC at which 50% of the strains were inhibited was >32 μg/ml, the highest range of E-strips). Several reports of the high prevalence of cotrimoxazole resistance from Bangladesh and other countries in the region (10, 16, 30) and the corroborating evidence of high cotrimoxazole MICs for approximately half of the invasive pneumococci in this study are of great concern for public health specialists and policy makers (33). At this point, it is very important to clarify the clinical impact of the treatment of pneumonia with cotrimoxazole to decide (i) whether cotrimoxazole use should be discouraged in populations with a high percentage of cotrimoxazole resistance or (ii) whether the nonsusceptibility of S. pneumoniae to this antimicrobial, in terms of the cutoff point, has to be redefined.
Penicillin nonsusceptibility was noted to be low in both carriage (7.1%) and meningitis (2.9%) strains, and all strains except one were intermediately susceptible. The rates of nonsusceptibility to penicillin and the penicillin MICs were comparable to those reported from China, India, and the Philippines (16) and contrast with the high prevalence of nonsusceptibility reported in the United States (40) and several other Asian (17) and European countries (20). The strong association of serotype 14 with penicillin nonsusceptibility was also similar to previous findings (30) and to reports from Slovakia (27) and the United States (36, 38).
In contrast to the trend of nonsusceptibility to cotrimoxazole and chloramphenicol in Bangladesh, the frequency of penicillin susceptibility among the S. pneumoniae isolates has not changed in the past decade (28, 30). Overall, the low prevalence of nonsusceptible strains and the similarity of antibiograms between carriage and meningitis strains indicate that penicillin or ampicillin remains a reasonable empirical drug of choice for community-acquired pneumonia in Bangladesh in children under 5 years old. Overall, the surveillance of carriage strains to formulate strategies for the treatment of pneumococcal invasive diseases, particularly meningitis, appears useful and rational. Further, the susceptibilities of rural and urban carriage isolates were similar, and there was no significant difference except for erythromycin, for which higher susceptibility was observed in the urban isolates. However, nonsusceptibilty to erythromycin is still very rare (1.6%), and it is thus difficult to draw a conclusion. The similarity between rural and urban strains with respect to their susceptibilities to antibiotics may be due to the proximity of the rural study area to Dhaka city.
The serotype distribution of the carriage strains of this study was similar to that of another study conducted at a similar time in a different rural population of Bangladesh (M. M. Zakaria, K. M. M. Rahman, S. Granat, M. Das, T. Kaijalainen, E. Herva, L. Piirainen, J. Ollgren, and P. H. Makela, Abstr. 3rd Int. Symp. Pneumococci Pneumococcal Dis., abstr. 49B, 2002) as well as to the distribution reported from other countries in this region (16). Overall, the distribution of the serotypes of carriage strains is diverse and significantly more diverse than the distribution of meningitis strains, as reported elsewhere (3). Thus, to cover 50% of the carriage and meningitis cases, as many as 14 and 8 serotypes, respectively, would be needed for vaccines. In contrast, national pneumococcal surveillance in the United States and the United Kingdom showed that only three and two serotypes, respectively, accounted for about 50% of the invasive cases (3, 40). Pneumococcal vaccine formulation for Bangladesh is further complicated by the fact that remarkable changes in serotype prevalences have occurred in recent years (29).
Extrapolating from the present data, hypothetical 7-, 9-, and 11-valent vaccines based on the predominant nasal carriage strains in children under 5 years old would cover only 23, 26, and 30%, respectively, of the meningitis cases (Table 2). The low coverage of the meningitis cases with the carriage strains was due to several predominant carriage strains (i.e., serotypes 16, 13, 37, and 24) which were rarely isolated from CSF. This result may be due to the fact that serotypes or groups carried for a long period would be recovered more frequently from nasal swabs than those carried only transiently, and invasive disease is more likely to occur soon after the acquisition and, thus, is less well associated with the period of colonization (3).
None of the available vaccines can provide good coverage for pneumococcal meningitis strains in Bangladesh, as established 7-, 9-, and 11-valent vaccines cover only 26, 43, and 48% of meningitis cases, respectively, considering the cross-protection of 6A and 6B. The coverage of meningitis cases by the established formulation of vaccines is low due to the wide diversity of the invasive isolates and to the very low coverage by some of the vaccine types or groups. For example, among the 7-, 9-, and 11-valent formulations, each of three different serotypes or groups (i.e., 5, 23F, and 9V) covered only 1.5% of meningitis cases. The low coverage of invasive cases with the established vaccine packages is similar to previous findings with a larger number of invasive strains (29) than used in this study. This finding emphasizes the need for additional, nationwide, population-based studies before recommendations can be made on the proper vaccine to protect the children of Bangladesh against pneumococcal invasive disease(s). The coverage of invasive cases with the 23-valent lipopolysaccharide vaccine was calculated to be 68%, although coverage increases if serogroups are considered instead of serotypes. We limited our consideration of cross-protection to serotypes 6A and 6B, however, as the cross-reactivity of only these two types is proven (39).
In conclusion, while surveillance of the resistance to antibiotics of carriage strains appears useful in the design of empirical treatment guidelines for pneumococcal meningitis, data about the serotypes of carriage strains have little or no value in vaccine formulation for the prevention of pneumococcal meningitis. The anticipated levels of protection of currently available vaccines against all pneumococcal diseases cannot be determined based on the data; however, previous reports have failed to show differences in serotype distribution among isolates from cases of meningitis and pneumonia (30). The distribution of diverse serotypes of both carriage and invasive strains in Bangladesh and the Asia region (5) and poor coverage of invasive cases in Bangladesh and India with established conjugate vaccines indicate the need for a species-wide protein-based vaccine to protect against invasive pneumococcal diseases in this region. Alternatively, given the early age of both colonization and invasive disease, the option of maternal immunization should be considered, at least until a vaccine effective in early infancy is identified. However, transplacental antibody persists for only about 8 weeks, when pneumococcal disease prevalence is low (23, 34).
This study has the limitation that it is not population based and has a small number of meningitis cases from the same region of the country, which may not be representative of the country as a whole. Considering the limitations, we are now in the process of starting a nationwide multicenter study on the surveillance of invasive pneumococcal diseases.
Acknowledgments
The quality control for pneumococcal serotyping was kindly done by E. Herva at the National Public Health Institute, Helsinki, Finland. We gratefully acknowledge the technical assistance rendered by Maksuda Islam.
The study was funded by the Child Health Foundation (Columbia, Md.), the Ministry of Health of Japan, and the Bangladesh Medical Research Council.
REFERENCES
- 1.Aniansson G., B. Alm, B. Anderson, P. Larsson, O. Nylen, H. Peterson, P. Rigner, M. Svanborg, and C. Svanborg. 1992. Nasopharyngeal colonization during the first year of life. J. Infect. Dis. 165(Suppl. 1):S38-S42. [DOI] [PubMed] [Google Scholar]
- 2.Bangladesh Ministry of Health and Family Welfare. 1993. CARI (Care for Acute Respiratory Infection) project: management of the young child with an acute respiratory infection. Directorate General of Health Services, Government of Bangladesh, Dhaka, Bangladesh.
- 3.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 4.Butler, J. C., R. F. Breiman, H. B. Lipman, J. Hofman, and R. R. Facklam. 1995. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171:885-889. [DOI] [PubMed] [Google Scholar]
- 5.Coles, C. L., R. Kanungo, L. Rahamathullah, R. D. Thulasiraj, J. Katz, M. Santosham, and J. M. Tielsch. 2001. Pneumococcal nasopharyngeal colonization in young South Indian infants. Pediatr. Infect. Dis. J. 20:289-295. [DOI] [PubMed] [Google Scholar]
- 6.Facklam R. R, and J. A. Washington II. 1991. Streptococcus and related catalase-negative gram-positive cocci, p. 238-257. In A. Balows, W. J. Hausler, Jr., K. Hermann, H. D. Isenberg, and H. J. Shadomy (ed.), Manual of clinical microbiology, 5th ed. American Society for Microbiology, Washington, D.C.
- 7.Felmingham D., R. R. Renert, Y. Hirakata, and A. Rodloff. 2002. Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and compatative in vitro activity of the ketolide, telithromycin. J. Antimicrob. Chemother. 50(Suppl. S1):25-37. [DOI] [PubMed] [Google Scholar]
- 8.Gray, B. M., G. M. Converse, and H. C. J. Dillon. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 9.Gratten, M., H. Gratten, A. Poli, E. Carrad, M. Raymer, and G. Koki. 1986. Colonization of Haemophilus influenzae and Streptococcus pneumoniae in the upper respiratory tract of neonates in Papua New Guinea: primary acquisition, duration of carriage in mothers. Biol. Neonate 50:114-120. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, and K. T. Luh. 1999. Extremely high incidence of macrolide and trimethoprim-sulphamethoxazole resistance among clinical isolates of Streptococcus pneumoniae in Taiwan. J. Clin. Microbiol. 37:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine. 1986. New vaccine development establishing priorities, vol. 2. Disease importance in developing countries. National Academies Press, Washington, D.C. [PubMed]
- 12.Jorgensen, J. H., A. W. Howell, and L. A. Maher. 1991. Quantitative antimicrobial susceptibility testing of Haemophilus influenzae and Streptococcus pneumoniae by using the E-test. J. Clin. Microbiol. 29:109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klugman, K. P., and H. J. Koornhof. 1988. Drug resistance pattern and serogroups or serotypes of pneumococcal isolates from cerebrospinal fluid or blood, 1979-1986. J. Infect. Dis. 158:956-964. [DOI] [PubMed] [Google Scholar]
- 14.Klugman, K. P. 1990. Pneumococcal resistance to antibiotics. Clin. Microbiol. Rev. 3:171-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leach, A. J., J. B. Boswell, V. Asche, and T. G. Nienhuys. 1994. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants. Pediatr. Infect. Dis. J. 13:983-989. [DOI] [PubMed] [Google Scholar]
- 16.Lee, N. M., J. H. Song, S. Kim, et al. 2001. Carriage of antibiotic resistant pneumococci among Asian children: a multinational surveillance by the Asian network for surveillance of resistant pathogens (ANSORP). Clin. Infect. Dis. 32:1463-1469. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann, D., M. Gratten, and J. Montgomery. 1997. Susceptibility of pneumococcal carriage isolates to penicillin provides a conservative estimate of susceptibility of invasive pneumococci. Pediatr. Infect. Dis. J. 16:297-305. [DOI] [PubMed] [Google Scholar]
- 18.Leino, T., K. Auranen, J. Jokinen, M. Leinonen, P. Tervonen, and A. K. Takala. 2001. Pneumococcal carriage in children during their first two years: important role of family exposure. Pediatr. Infect. Dis. J. 20:1022-1027. [DOI] [PubMed] [Google Scholar]
- 19.Mastro, T. D., N. K. Nomani, Z. Ishak, A. Ghafoor, N. F. Shaukat, E. Esko, M. Leinonen, J. Henrichsen, R. F. Breiman, B. Schwartz, et al. 1993. Use of nasopharyngeal isolates of Streptococcus pneumoniae and Haemophilus influenzae from children in Pakistan for surveillance for antimicrobial resistance. Pediatr. Infect. Dis. J. 12:824-830. [DOI] [PubMed] [Google Scholar]
- 20.Maugein, J., D. Guillemot, M. J. Dupont, T. Fosse, G. Laurans, M. Roussel-Delvallez, J. Thierry, M. Vergnaud, M. Weber, and B. Poirier. 2003. Clinical and microbiological epidemiology of Streptococcus pneumoniae bacteremia in eight French countries. Clin. Microbiol. Infect. 9:280-288. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery, J. M., D. Lehmann, T. Smith, A. Michael, B. Joseph, T. Lupiwa, C. Coakley, V. Spooner, B. Best, I. D. Riley, et al. 1990. Bacterial colonization of the upper respiratory tract infections in highland children of Papua New Guinea. Rev. Infect. Dis. 12 (Suppl. 8):S1006-S1016. [DOI] [PubMed] [Google Scholar]
- 22.Mulholland, K. 1999. Magnitude of the problem of childhood pneumonia. Lancet 354:590-592. [DOI] [PubMed] [Google Scholar]
- 23.Munoz, F. M., J. A. Englund, C. C. Cheesman, M. L. Maccato, P. M. Pinell, M. H. Hahm, E. O. Mason, C. A. Kozinetz, R. A. Thompson, and W. P. Glezen. 2001. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine 20:826-837. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2002. Performance of standards for antimicrobial susceptibility testing, twelfth informational supplement, vol. 22, no. 1. M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Obaro, S. K., M. A. Monteil, and D. C. Henderson. 1996. The pneumococcal problem. Br. Med. J. 312:1521-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman, M. F., F. Huq, and D. A. Sack. 1990. Acute lower respiratory infection in hospitalized patients with diarrhea in Dhaka, Bangladesh. Rev Infect. Dis. 12:S889-S906. [DOI] [PubMed] [Google Scholar]
- 27.Reichler, M. R., J. Rakovsky, A. Sobotova, M. Slacikova, B. Hlavacova, B. Hill, L. Drajcikova, P. Tarina, R. R. Facklam, and R. R. Breiman. 1995. Multiple antimicrobial resistance of pneumococci in children with otitis media, bacteremia, and meningitis in Slovakia. J. Infect. Dis. 171:1491-1496. [DOI] [PubMed] [Google Scholar]
- 28.Saha, S. K., W. A. Khan, M. S. Hoq, A. F. M. Selim, and M. S. Akbar. 1992. Penicillin resistant pneumococci in Bangladeshi children. Lancet 337:734-735. [DOI] [PubMed] [Google Scholar]
- 29.Saha, S. K., N. Rikitomi, and D. Biswas. 1997. Serotype of Streptococcus pneumoniae causing invasive childhood infections in Bangladesh, 1992 to 1995. J. Clin. Microbiol. 35:785-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saha, S. K., N. Rikitomi, M. Ruhulamin, H. Masaki, M. Hanif, M. Islam, K. Watanabe, K. Ahmed, K. Matsumoto, R. B. Sack, and T. Nagatake. 1999. Antimicrobial resistance and serotype distribution of Streptococcus pneumoniae strains causing childhood infections in Bangladesh, 1993 to 1997. J. Clin. Microbiol. 37:798-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha, S. K., N. Rikitomi, M. Ruhulamin, K. Watanabe, K. Ahmed, D. Biswas, M. Hanif, W. H. Khan, M. Islam, K. Matsumoto, and T. Nagatake. 1997. The increasing burden of disease in Bangladeshi children due to Haemophilus influenzae type meningitis. Ann. Trop. Paediatr. 17:5-8. [DOI] [PubMed] [Google Scholar]
- 32.Saha, S. K., S. Saha, M. Ruhulamin, M. Hanif, and M. Islam. 1997. Decreasing trend of multiresistant Salmonella typhi in Bangladesh. J. Antimicrob. Chemother. 39:554-556. [DOI] [PubMed] [Google Scholar]
- 33.Sazawal, S., and R. E. Black. Effect of pneumonia case management on mortality in neonates, infants and preschool children: a meta-analysis of community based trials. Lancet Infect. Dis., in press. [DOI] [PubMed]
- 34.Shahid, N. S., M. C. Steinhoff, S. S. Hoque, T. Begum, C. Thompson, and G. R. Siber. 1995. Serum, breast milk and infant antibody after maternal immunization with pneumococcal vaccine. Lancet 346:1252-1257. [DOI] [PubMed] [Google Scholar]
- 35.Soewignjo, S., D. B. Gessner, A. Sutanto, M. Steinhoff, M. Prijanto, C. Nelson, A. Widjaya, and S. Arjoso. 2001. Streptococcus pneumoniae nasopharyngeal carriage prevalence patterns among children on Lombok Island, Indonesia. Clin. Infect. Dis. 32:1039-1043. [DOI] [PubMed] [Google Scholar]
- 36.Spika, J. S., R. R. Facklam, B. D. Plikaytis, and M. J. Oxtboy. 1991. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979-1987. J. Infect. Dis. 163:1273-1278. [DOI] [PubMed] [Google Scholar]
- 37.Straus, W. L., S. A. Qazi, Z. Kundi, N. K. Nomani, and B. Schwartz. 1998. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus amoxycillin for pneumonia among children in Pakistan: randomized controlled trial. Lancet 352:270-274. [DOI] [PubMed] [Google Scholar]
- 38.Ussery, X. T., B. D. Gessner, H. Lipman, J. A. Elliott, M. J. Crain, P. C. Tien, A. J. Parkinson, M. Davidson, R. R. Facklam, and R. F. Breiman. 1996. Risk factors for nasopharyngeal carriage of resistant Streptococcus pneumoniae and detection of a multiply resistant clone among the children living in the Yukon-Kuskokwim Delta region of Alaska. Pediatr. Infect. Dis. J. 15:986-992. [DOI] [PubMed] [Google Scholar]
- 39.Vakevainen, M., C. Eklund, J. Eskola, and H. Kayhty. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J. Infect. Dis. 184:789-793. [DOI] [PubMed] [Google Scholar]
- 40.Whitney, C. G., M. M. Farley, J. Hadler, et al. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]
- 41.Yomo, A., V. R. Subramanyam, R. Fudzulani, H. Kamaga, S. M. Graham, R. L. Broadhead, L. E. Cuevas, and C. A. Hart. 1997. Carriage of penicillin-resistant pneumococci in Malawian children. Ann. Trop. Paediatr. 17:239-243. [DOI] [PubMed] [Google Scholar]