Abstract
A rapidly growing mycobacterium was isolated five times from blood cultures from a 6-year-old female patient with relapsed pre-B-cell acute lymphocytic leukemia. All five isolates had identical nucleotide sequences for the first 500 bp of the 16S rRNA gene, indicative of a single species. High-performance liquid chromatography analysis of mycolic acids indicated that the species was similar to Mycobacterium smegmatis. Sequence analysis of the 16S rRNA gene (1,455 bp) for one isolate demonstrated that the species was closely related to Mycobacterium diernhoferi. Based on the phenotypic features and phylogenetic analysis, it was concluded that the isolates represented a novel rapidly growing Mycobacterium species. The name “Mycobacterium hackensackense” is proposed for this unique strain, 147-0552T, which was deposited in the American Type Culture Collection as ATCC BAA-823T.
By definition, rapidly growing mycobacteria (RGM) demonstrate visible growth on culture media within 7 days. The species of RGM capable of causing human infections are primarily of the Mycobacterium fortuitum group, the M. chelonae/M. abscessus group, and the M. smegmatis group. Some of the diseases caused by RGM include posttraumatic and postsurgical wound infections, bone and joint infections, catheter-related infections, postinjection abscesses, disseminated cutaneous disease, pulmonary disease, central nervous system disease, and cervical lymphadenitis. The most common mycobacterial pathogen associated with catheter infections is M. fortuitum. However, M. chelonae, M. abscessus, M. immunogenum, the M. smegmatis group, and M. mucogenicum have also been associated with catheter-related infections (4).
Traditionally, clinical laboratory identification of RGM involved selected biochemical tests, pigmentation, and colony morphology. However, it has been shown that these methods lack sensitivity and can be influenced by phenotypic variability (13). The development of high-performance liquid chromatography (HPLC) analysis of cell wall-bound, species-specific mycolic acids (5) and nucleic acid-based technologies provided fast and accurate identification tests for Mycobacterium species. Additionally, 16S rRNA gene sequence analysis has been used to describe the phylogenetic relationships among mycobacterial species (12). In this report, a novel rapidly growing Mycobacterium species involved in a catheter-related mycobacteriosis of a young female patient with relapsed pre-B-cell acute lymphocytic leukemia is described.
MATERIALS AND METHODS
Case report.
In May 2002, a 6-year-old female with relapsed pre-B-cell acute lymphocytic leukemia and a history of multiple previous infections was admitted at Hackensack University Medical Center for evaluation with a fever of 39.5°C. She was initially diagnosed in November 1997 and received chemotherapy (Pediatric Oncology Group [POG] protocol 9606), which was finished in July 2000. Chemotherapy (POG protocol 9411) was resumed for a bone marrow relapse, diagnosed in June 2001. Since then, the patient has had multiple infections, including a Bacillus species port infection, hepatosplenic candidiasis, urinary tract infections with Enterococcus and Pseudomonas spp., and central venous catheter infections with Staphylococcus aureus and a Corynebacterium sp. with the current catheter, which was in place 6 months prior to admission. Medications on admission included trimethoprim-sulfamethoxazole for Pneumocystis carinii prophylaxis, amphotericin B liposome and rifampin for long-term treatment of hepatosplenic candidiasis, and penicillin prophylaxis for a splenectomy performed 9 months prior to admission to confirm the diagnosis of hepatosplenic candidiasis. Temperature on admission was 40.5°C, and no source of infection was identified on physical examination. The catheter site was clean, dry, and intact. No new infiltrates or effusions were seen on the chest radiograph at admission. White blood cell count was 17,500/mm3 with 11% bands, 79% segmented neutrophils, 5% lymphocytes, 4% monocytes, and 1% eosinophils. Hemoglobin was 11.9 g/dl, hematocrit was 35.3%, and platelets were 243,000/mm3. Empirical vancomycin (40 mg/kg of body weight/day) and meropenem (60 mg/kg/day) therapy was started intravenously. The patient was afebrile on the first day of hospitalization, and on the second day gram-positive coccobacilli were identified in blood cultures taken at admission from the Broviac catheter white and red lumens. Gram-positive coccobacilli were also identified in the peripheral blood cultures. Subsequently, on the fourth day the organisms were identified as rapidly growing acid-fast bacilli, and intravenous amikacin (15 mg/kg/day) and oral clarithromycin (15 mg/kg/day) were started. Vancomycin was discontinued. Three sets of catheter blood cultures and two peripheral blood cultures collected on follow-up 5 days later were sterile. The central venous catheter was removed on the seventh day, and peripheral intravenous access was maintained for three additional days. A new central venous catheter was surgically implanted on the tenth day, and amikacin was discontinued after 7 days. The patient was discharged to complete 4 weeks of clarithromycin and meropenem therapy. She was well on a follow-up exam conducted 10 months later.
Blood culture.
Blood culture was performed at the microbiology laboratory of Hackensack University Medical Center using a BacT/Alert automated blood culture instrument and pediatric FAN blood culture bottles (bioMerieux, Durham, N.C.). Isolator blood culture tubes (Wampole Laboratory, Cranbury, N.J.) were also used.
Morphological and biochemical characteristics.
The ability of the isolate to grow at different temperatures (24, 37, and 45°C) on blood agar and Löwenstein-Jensen (L/J) slants was tested. The isolate was observed for pigment formation on days 3, 7, and 14 of incubation at 24 and 37°C. Growth rate and colony morphology on 5% sheep blood and L/J slant agar (BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.) incubated at 37°C were determined. Testing for nitrate reduction, catalase, Tween hydrolysis, tellurite reduction, 5% NaCl tolerance, iron uptake, arylsulfatase activity at 3 days, growth on MacConkey agar without crystal violet, and urease was performed as previously described (7).
Antimicrobial susceptibility testing.
Broth microdilution susceptibility testing was performed on isolate 147-0552T according to the method of NCCLS M24-T2 for testing rapidly growing mycobacteria (10). Commercial Sensititre plates (Trek Diagnostics, Westlake, Ohio) containing test antibiotics were used. Isolate 147-0552T was tested for susceptibility against twofold dilutions of clarithromycin (0.03 to 64 μg/ml), imipenem (1 to 64 μg/ml), cefoxitin (2 to 256 μg/ml), amikacin (1 to 128 μg/ml), sulfamethoxazole (1 to 64 μg/ml), doxycycline (0.25 to 128 μg/ml), tobramycin (1 to 16 μg/ml), and ciprofloxacin (0.12 to 16 μg/ml).
Mycolic acid analysis.
Representative mycolic acid HPLC pattern standards of M. smegmatis ATCC 19420T and M. diernhoferi ATCC 19340T were compared with mycolic acid HPLC patterns for the study isolate, 147-0552T. Cell wall-bound mycolic acids were extracted and analyzed by the standard reverse-phase HPLC method using UV detection as recently reviewed (6). The relative positions of the mycolic acid peaks were located by comparison to a high-molecular-weight reference internal standard, a propriety compound (Corixa Corporation), which emerged from the column after the mycolic acids (5).
Sequencing of the 16S rRNA gene.
DNA was extracted from cells suspended in Tris-EDTA buffer with siliconized zirconium beads by using a FastPrep cell disrupter, model FP120 (Qbiogene, Inc.), set at 6.0 m/s for 45 s. The aqueous layer containing the DNA portion was isolated by the addition of chloroform and collected by centrifugation. DNA oligonucleotides GAGAGTTTGATCCTGGCTCAG and AAGGAGGTGATCCAGCCGCA were used to amplify the 16S ribosomal DNA (rDNA) fragments between positions 8 and 1542 (Escherichia coli numbering). The 16S rDNA amplicon was sequenced with a BigDye terminator kit (PE Applied Biosystems) and analyzed with a 373A DNA sequencer (Applied Biosystems). DNA segments from overlapping strands were used to determine a consensus sequence with the Navigator software (Applied Biosystems). A total of 1,455 nucleotides in the consensus sequence were compare to sequence data in the National Institutes of Health GenBank database to determine similarity. The evolutionary relationship of the clinical isolate to the other mycobacteria was determined by online analysis at the Michigan State University Center for Microbial Ecology, Ribosomal Database Project II site (http://rdp.cme.msu.edu/html/index.html) (9), with version 3.5c of the Phylogeny Inference Package (J. Felsenstein, PHYLIP: Phylogeny Inference Package, Department of Genetics, University of Washington, Seattle, 1993). The phylogenetic tree was generated with the NEIGHBOR program.
Nucleotide sequence accession number.
The GenBank accession number for the 16S rRNA gene sequence of strain 147-0552 T (ATCC BAA-823T) is AY266138.
RESULTS
Five of six blood cultures (three from central venous catheter and two from peripheral blood) were positive in 2 to 4 days. A Gram stain revealed small gram-positive coccobacilli. Aliquots of the positive blood culture were subcultured on 5% blood and chocolate agars (BBL) and incubated at 37°C in 5% CO2. Smooth, nonpigmented colonies were visible after 3 days of incubation. The coccobacilli stained partially acid-alcohol fast, and colonies remained nonpigmented after prolonged incubation.
Conventional biochemical tests showed that the study isolates were negative for 5% NaCl tolerance and positive for SQ catalase, indicating they were different from either M. diernhoferi or M. smegmatis (Table 1). Susceptibility testing indicated that the isolates were susceptible to clarithromycin and imipenem, intermediate to amikacin and cefoxitin, and resistant to other antibiotics. MIC results were as follows: clarithromycin, 0.25 μg/ml; imipenem, 4 μg/ml; cefoxitin, 32 μg/ml; amikacin, 32 μg/ml; sulfamethoxazole, >64 μg/ml; doxycycline, >128 μg/ml; tobramycin, 16 μg/ml, ciprofloxacin, 16 μg/ml.
TABLE 1.
Biochemical comparison of species closely related to the study isolate
| Test | Resultd for:
|
||
|---|---|---|---|
| M. smegmatis sensu strictoa | M. diernhoferib | Isolate 147-0552T | |
| Growth at 45°C | + | − | − |
| Growth at 37°C | + | + | + |
| Growth at 24°C | + | + | + |
| Colony morphology | Rough/smooth | White smooth | White smooth |
| Pigmentation | Late | − | − |
| Nitrate | + | + | + |
| SQ catalase | − | − | >45 mm |
| 68°C catalase | − | − | − |
| Tween hydrolysis | + | − | + |
| Tellurite reduction | + | NAc | + |
| 5% NaCl | + | + | − |
| Iron uptake | +/− | NA | + |
| Arylsulfatase activity at 3 days | − | − | − |
| MacConkey agar (no crystal violet) | + | NA | − |
| Urease | NA | + | + |
| Arabinose | + | + | + |
| Inositol | + | + | − |
| Mannitol | + | + | + |
| Sorbitol | + | − | − |
| Trehalose | +/− | − | − |
| Xylose | + | + | − |
| Citrate | +/− | +/− | − |
Data are from references 3 and 7.
Data are from reference 15.
NA, not available.
Rough/smooth, either rough or smooth; +/−, usually positive.
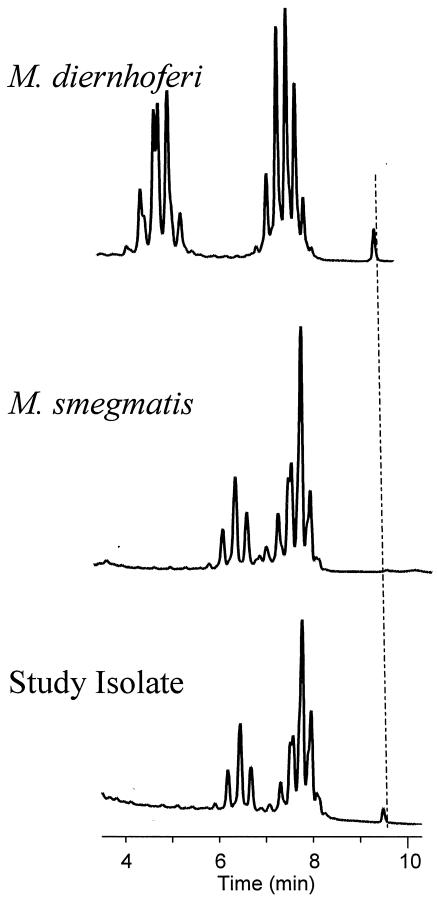
Mycolic acids characteristic of mycobacteria were detected in isolate 147-0552T by HPLC. The mycolates eluted from the C18 nonpolar analysis column between 6 and 8 min of real time, with relative values of 1.54 to 3.30 min and a range of 1.76 min. Overall, these times were consistent with α-, α-′, and methoxy mycolates. This result was compared to that for mycolic acids from M. diernhoferi, which emerged from the column in 4 to 8.25 min of real time, with relative values of 1.43 to 5.67 min and a range of 4.24 min. Mycolic acids in this time range are reliably α-, α-′, keto, and wax ester mycolates. Notably, the study isolate produced a mycolic acid profile identical to the reference pattern for M. smegmatis but different from the HPLC reference pattern for M. diernhoferi (Fig. 1).
FIG. 1.
Representative mycolic acid HPLC pattern standards of M. smegmatis ATCC 19420T and M. diernhoferi ATCC 19340T compared with that of mycolic acids for the study isolate 147-0552T. Dashed line, alignment of the internal standard. The bottom scale reflects the actual or real time of elution of the mycolates.
The five isolates demonstrated identical DNA sequence similarity for the first 500 bp of the 16S rRNA gene, confirming that they represented multiple isolations of the same species. An initial similarity search of two public databases, GenBank (BLAST; National Center for Biotechnology Information) and Ridom (http://www.ridom-rdna.de/), with the first 500 bp revealed that the closest match was M. diernhoferi, with a similarity value of 99%. The almost complete sequence (1,455 nucleotides) of the 16S rRNA gene for one of the isolates (isolate 147-0552T) was determined. A further evolutionary comparison with the16S rRNA gene sequence (GenBank accession number AY266138) confirmed the unknown strain to be 99.1% similar to M. diernhoferi (GenBank accession number AF480599).
DISCUSSION
Nontuberculous mycobacteria are uncommon causes of catheter-associated infections that are becoming more frequently recognized (8, 11, 14). Several reports have described infections in neutropenic and nonneutropenic cancer patients. Mycobacteria of the M. fortuitum complex, i.e., M. fortuitum, M. chelonae, and M. abscessus, represent the etiologic agents in most of these infections. These organisms can cause exit site infections, tunnel or pocket infections, and catheter-related bacteremia in immunocompetent and immunocompromised patients. In this report, the repeated isolation of an acid-alcohol-fast microorganism from central line and peripheral blood cultures of a 6-year-old female patient with relapsed pre-B-cell acute lymphocytic leukemia indicated involvement in the infection as a clinically significant etiologic agent.
Identical nucleotide sequences of the first 500 bp of the 5′ 16S rRNA genes for the five isolates indicated that a single species was present. Analysis of the almost complete 16S rRNA gene sequence of strain 147-0552T revealed a close nucleotide similarity of 99.1% with M. diernhoferi. This represented a sequence difference between M. diernhoferi and the clinical isolate of 13 nucleotides. Compared to that of M. diernhoferi, the 5′ 16S rRNA end of the gene had a 5-bp difference; additionally 4 bp near the middle and 4 bp at the 3′ 16S rRNA end of the gene were different. A difference of 13 bp between genotypically similar species for the 16S rRNA gene is confirmation of a new species (12, 13). Furthermore, four of the mismatches were in hypervariable region A of the 5′ 16S rRNA gene, a result suggestive of a novel species. The helix 18, hypervariable region B sequence was identical to that of M. diernhoferi and was characteristic of those of most other rapidly growing mycobacteria. Further evidence for a novel species was the remaining eight mismatches of M. diernhoferi that were in a region of the 16S rRNA gene normally considered conserved for a species. Additionally, analysis of the mycolic acid profile by HPLC demonstrated major peak differences between the isolate and M. diernhoferi but similarity to M. smegmatis ATCC 19420T (Fig. 1) and closely related strain M. goodii ATCC 700504T (3). Biochemical results of positive semiquantitative catalase and negative 5% NaCl tolerance results complemented this discrimination of the isolate from both M. diernhoferi and M. smegmatis (Table 1).
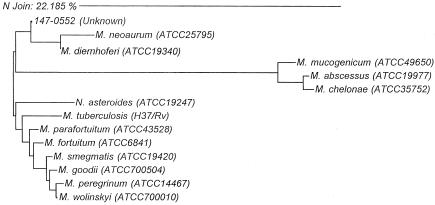
The uniqueness of the clinical isolate was verified by phylogenetic analysis of the almost complete 16S rRNA gene that appeared on the evolutionary tree as a branch with that of M. diernhoferi (Fig. 2). M. smegmatis was demonstrated to be a distantly related species. 16S rRNA gene sequence and phylogenetic analysis have been shown to be powerful approaches for studying genetics and evolutionary relatedness of mycobacterial species (9). This application has been used extensively to study the phylogeny of bacteria and was recently used to describe a novel Mycobacterium species found in cats (1). This molecular approach provided evidence to demonstrate the uniqueness of the organism. Moreover, combining 16S rRNA gene sequencing, biochemical assays, and HPLC mycolic acid analysis verified the unique properties of the isolate.
FIG. 2.
Phylogenetic relationships of study isolate 147-0552T with genetically related Mycobacterium species and Nocardia asteroides. The analysis was based on the full 16S rRNA gene sequences. The scale indicates relative phylogenetic distance.
The novel organism is closely related to M. diernhoferi, a rapidly growing, nonphotochromogenic mycobacterium originally isolated from soil (2) and reported to be phenotypically similar to M. parafortuitum (15). There are only a few reports in the literature of M. diernhoferi, which has not been associated with human or animal infection. In our case, the repeated isolation of the same species in large numbers from otherwise sterile sites indicates the medical significance of the species. It was speculated that the immunocompromised status of the patient facilitated infection with this organism. Until more cases are described, the potential for this novel mycobacterium species to cause mycobacteriosis in the immunocompetent host is unknown. Since the mycobacteria were isolated in the Microbiology Laboratory of Hackensack University Medical Center, it is proposed the isolate be included in the genus Mycobacterium as “Mycobacterium hackensackense.”
Acknowledgments
We thank Hans G. Trüper for his assistance in naming the species and Milagros Rabassa for technical assistance.
REFERENCES
- 1.Appleyard, G. D., and E. G. Clark. 2002. Histologic and genotypic characterization of a novel Mycobacterium species found in three cats. J. Clin. Microbiol. 40:2425-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bönicke, R., and S. E. Juhasz. 1965. Mycobacterium diernhoferi n. sp., eine in der Umgebung des Rindes häufig vorkommende neue Mycobacterium-Species. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 197:292-294. [PubMed] [Google Scholar]
- 3.Brown, B. A., B. Springer, V. A. Steingrube, R. W. Wilson, G. E. Pfyffer, M. J. Garcia, M. C. Menendez, B. Rodriguez-Salgado, K. C. Jost, Jr., S. H. Chiu, G. O. Onyi, E. C. Bottger, and R. J. Wallace, Jr. 1999. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int J. Syst Bacteriol. 49:1493-1511. [DOI] [PubMed] [Google Scholar]
- 4.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, W. R., M. M. Floyd, V. Silcox, G. Cage, E. Desmond, P. S. Duffey, L. S. Guthertz, W. M. Gross, K. C. Jost, Jr., S. L. Ramos, L. Thibert, and N. Warren. 1999. Mycolic acid pattern standards for HPLC identification of mycobacteria. Centers for Disease Control and Prevention, Atlanta, Ga.
- 6.Butler, W. R., and L. S. Guthertz. 2001. Mycolic acid analysis by high-performance liquid chromatography for identification of Mycobacterium species. Clin. Microbiol. Rev. 14:704-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Della-Latta, P., and I. Weitzman. 1998. Mycobacteriology, p. 169-202. In H. D. Isenberg (ed.), Essential procedures for clinical microbiology. ASM Press, Washington, D.C.
- 8.Levendoglu-Tugal, O., J. Munoz, A. Brudnicki, M. Fevzi Ozkaynak, C. Sandoval, and S. Jayabose. 1998. Infections due to nontuberculous mycobacteria in children with leukemia. Clin. Infect. Dis. 27:1227-1230. [DOI] [PubMed] [Google Scholar]
- 9.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes. Tentative standard M24-T2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa. [PubMed]
- 11.Rodgers, G. L., J. E. Mortensen, D. Blecker-Shelly, M. C. Fisher, and S. S. Long. 1996. Two case reports and review of vascular catheter-associated bacteremia caused by nontuberculous Mycobacterium species. Pediatr. Infect. Dis. J. 15:260-264. [DOI] [PubMed] [Google Scholar]
- 12.Rogall, T., T. Wolters, T. Flohr, and E. C. Böttger. 1990. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int. J. Syst. Bacteriol. 40:323-330. [DOI] [PubMed] [Google Scholar]
- 13.Springer, B., L. Stockman, K. Teschner, G. D. Roberts, and E. C. Bottger. 1996. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J. Clin. Microbiol. 34:296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suryanavayan, K., J. Campbell, and A. E. Eskenazi. 2002. Nontuberculous mycobacterial infections in pediatric acute leukemia. J. Pediatr. Hematol. Oncol. 24:558-560. [DOI] [PubMed] [Google Scholar]
- 15.Tsukamura, M., H. J. Van Der Meulen, and W. O. K. Grabow. 1983. Numerical taxonomy of rapidly growing, scotochromogenic mycobacteria of the Mycobacterium parafortuitum complex: Mycobacterium austroafricanum sp. nov. and Mycobacterium diernhoferi sp. nov., nom. rev. Int. J. Syst. Bacteriol. 33:460-469. [Google Scholar]