Abstract
Objectives To evaluate the association between thyroid autoantibodies and miscarriage and preterm birth in women with normal thyroid function. To assess the effect of treatment with levothyroxine on pregnancy outcomes in this group of women.
Design Systematic review and meta-analysis.
Data sources Medline, Embase, Cochrane Library, and SCISEARCH (inception-2011) without any language restrictions. We used a combination of key words to generate two subsets of citations, one indexing thyroid autoantibodies and the other indexing the outcomes of miscarriage and preterm birth.
Study selection Studies that evaluated the association between thyroid autoantibodies and pregnancy outcomes were selected in a two stage process. Two reviewers selected studies that met the predefined and explicit criteria regarding population, tests, and outcomes.
Data synthesis Odds ratios from individual studies were pooled separately for cohort and case-control studies with the random effects model.
Results 30 articles with 31 studies (19 cohort and 12 case-control) involving 12 126 women assessed the association between thyroid autoantibodies and miscarriage. Five studies with 12 566 women evaluated the association with preterm birth. Of the 31 studies evaluating miscarriage, 28 showed a positive association between thyroid autoantibodies and miscarriage. Meta-analysis of the cohort studies showed more than tripling in the odds of miscarriage with the presence of thyroid autoantibodies (odds ratio 3.90, 95% confidence interval 2.48 to 6.12; P<0.001). For case-control studies the odds ratio for miscarriage was 1.80, 1.25 to 2.60; P=0.002). There was a significant doubling in the odds of preterm birth with the presence of thyroid autoantibodies (2.07, 1.17 to 3.68; P=0.01). Two randomised studies evaluated the effect of treatment with levothyroxine on miscarriage. Both showed a fall in miscarriage rates, and meta-analysis showed a significant 52% relative risk reduction in miscarriages with levothyroxine (relative risk 0.48, 0.25 to 0.92; P=0.03). One study reported on the effect of levothyroxine on the rate of preterm birth, and noted a 69% relative risk reduction (0.31, 0.11 to 0.90).
Conclusion The presence of maternal thyroid autoantibodies is strongly associated with miscarriage and preterm delivery. There is evidence that treatment with levothyroxine can attenuate the risks.
Introduction
Miscarriage, the loss of a pregnancy before 24 weeks of gestation, affects up to one in five women who conceive, making it the commonest complication of pregnancy.1 Preterm birth, delivery of a baby between 24 and 37 completed weeks of gestation, occurs in 6-10% of pregnancies.2 Up to 85% of neonatal deaths are attributable to preterm birth (especially those delivered before 28 weeks). Of those who survive, around 10% have long term disability.3 The cost of preterm birth is £93m a year in the United Kingdom.3 This includes healthcare costs (including neonatal care), education, and costs to the parents.
There is evidence that thyroid autoimmunity is an important risk factor for miscarriage and preterm birth.4 The presence of thyroid autoantibodies is relatively common in women of reproductive age. In an “unselected” population of women, the prevalence ranges from 6% to 20%,4 5 being even higher in women with a history of recurrent pregnancy loss, at around 17-33%,6 7 8 and in women with a history of subfertility, at around 10-31%.9 10 11 In the developed world, thyroid autoimmunity is the main cause of hypothyroidism, which itself results in poor obstetric outcomes. Even in women with biochemically normal thyroid function, studies have reported an association between the presence of thyroid autoantibodies, particularly thyroid peroxidase antibodies and adverse pregnancy outcomes, including miscarriage, preterm birth, and adverse neurodevelopmental sequelae in children.12 13 The exact mechanisms of these associations are unknown, though two have been proposed. Firstly, the presence of thyroid autoantibodies in women with normal thyroid function could be associated with a subtle deficiency in the availability of thyroid hormones (a fall in circulating free thyroid hormones within the reference range) or a lower capacity of the thyroid gland to adequately rise to the demand for augmented synthesis of thyroid hormones required in pregnancy.14 Given that minor perturbations in thyroxine concentrations within the normal range can lead to an association between thyroid autoantibodies and adverse pregnancy outcomes, trials have been conducted to evaluate the effects of supplementation with levothyroxine on pregnancy outcomes in women with normal thyroid function who tested positive for thyroid autoantibodies. Secondly, thyroid autoantibodies might be an indicator of an underlying enhanced global autoimmune state. This itself can have a direct adverse effect on placental or fetal development.14
Given the importance of the potential association between thyroid autoantibodies and adverse pregnancy outcomes, we systematically reviewed and meta-analysed the association between thyroid autoantibodies and miscarriage and preterm birth. Given the possible role of levothyroxine in improving pregnancy outcomes, we also reviewed the available randomised evidence on the effect of treatment with levothyroxine on pregnancy outcomes.
Methods
This systematic review was conducted with a prospective protocol with widely recommended methods.15
Identification of studies
We searched Medline (1951-2011), Embase (1974-2011), the Cochrane Library (2011), and SCISEARCH (1974-2011) for relevant citations and examined the reference lists of all known primary and review articles to identify cited articles not captured by the electronic searches. Language restrictions were not applied. We used a combination of MeSH and text words to generate two subsets of citations, one indexing thyroid autoantibodies (“thyroid autoimmune antibodies”, exp thyroid/ AND exp antibodies, thyroid AND autoimmune AND antibodies) and the other indexing outcomes (“miscarriage”, “abortion”, “pregnancy loss”, “preterm”, “premature”, “early labo(u)r”, “pret$”). These subsets were combined with “AND” to generate a subset of citations relevant to our research question.
Study selection and data extraction procedures
The electronic searches were scrutinised and two independent reviewers (ST and EK) obtained full manuscripts of all citations that were likely to meet the predefined selection criteria. Studies were selected if they included women with normal thyroid function who tested positive for thyroid autoantibodies, and the outcomes included adverse maternal and fetal outcomes. We excluded articles in which women were known to have overt biochemical hypothyroidism or hyperthyroidism. When disagreements occurred, they were resolved by consensus and discussion with a third reviewer (AC). In cases of duplicate publication, we selected the most recent and complete versions.
Information was extracted from each selected article on study characteristics, quality, and test results. Data were used to construct 2×2 tables of thyroid autoantibody results (test positive if concentrations were above a threshold as defined in the primary study, and test negative if these were below the threshold) and pregnancy outcomes (miscarriage and preterm delivery). We also extracted data on mean age and serum concentrations of thyroid stimulating hormone and free thyroxine. For the randomised trials, we extracted data on study population, quality of the methods, details of the interventions, and outcomes.
Assessment of quality of methods
We used the Newcastle-Ottawa scale16 to assess methodological quality of the selected studies, with the components of study design that are related to internal validity. Information on adequacy of definition of cases or cohorts, representativeness of the sample, selection and evaluation of controls, comparability, ascertainment of exposure, and outcome were evaluated for cohort and case-control studies.16 The study was considered to have low risk of bias if it scored a maximum of 4 for selection, 2 for comparability, and 3 for assessment of outcome or ascertainment of exposure. Any study that scored 1 or zero for selection or zero for comparability or for outcome assessment was categorised as having a high risk of bias. Studies that scored in between were rated as having medium risk of bias.
We assessed quality of randomised trials evaluating the effectiveness of levothyroxine according to appropriate randomisation, concealment of allocation, blinding, analysis by intention to treat, and follow-up rates. A quality score for the randomised trials was given on Jadad’s scale.17
Data synthesis
Odds ratios from individual studies were pooled separately for the cohort and case-control studies with the random effects model. Heterogeneity of treatment effects was evaluated graphically with forest plots and statistically with χ2 and I2 tests. We generated the pooled mean difference in age and weighted mean difference in serum thyroid stimulating hormone between groups with positive and negative results for thyroid autoantibodies. All analyses were performed with Stata 10.0 and Revman 5 statistical software.18 19
Results
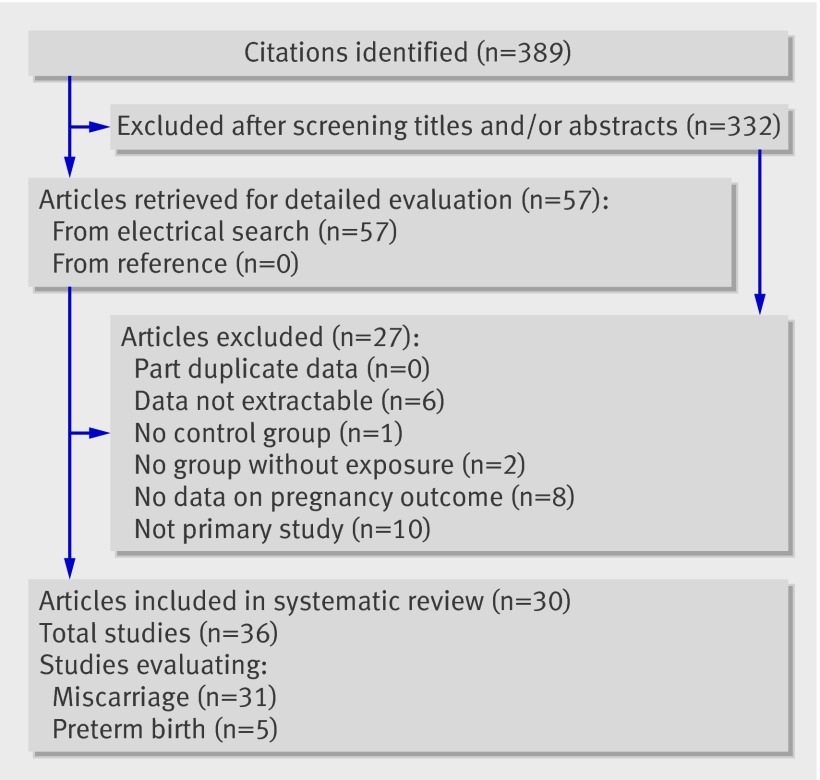
Figure 1 summarises the processes of literature identification and selection of studies evaluating the relation between thyroid autoantibodies and adverse pregnancy outcomes. From the 389 citations identified from electronic and hand searches, we included 30 primary articles with 36 studies (31 for miscarriage and five for preterm birth) in the systematic review.
Fig 1 Flow chart of study selection in review of association between thyroid autoantibodies and adverse pregnancy outcome
Thyroid autoantibodies and miscarriage
Thirty one studies (12 126 women)6 7 8 9 10 11 12 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 evaluated the association between thyroid autoantibodies and miscarriage. Nineteen studies were cohort studies, and 12 were case-control studies. Thirteen studies (three cohort, 10 case-control) evaluated the association in women with recurrent miscarriage, nine studies (seven cohort, two case-control) in women with subfertility and nine cohort studies in unselected or other populations. The studies were judged to have low risk of bias for assessment of outcome (19/19 cohort studies) and ascertainment of exposure (12/12 case-control studies) on the Newcastle-Ottawa scale.16 Nearly half the cohort studies (9/19) had low selection bias and the others (10/19) had medium selection bias. All the case-control studies had medium selection bias. Eight of the 19 (42%) cohort studies had established good comparability of the groups compared with 16% (2/12) of the case-control studies.
Appendix 1 on bmj.com provides details of the characteristics of the women in the included studies. All studies tested for thyroid peroxidase antibodies. Studies varied in the frequency and timing of the autoantibody testing, ranging from testing before pregnancy, in early pregnancy, and after delivery or miscarriage. The commonest threshold concentration of thyroid peroxidase for a diagnosis of positive thyroid autoantibodies was >100 U/ml. The various thresholds used to define positivity for thyroid autoantibodies are in appendix 1 on bmj.com.
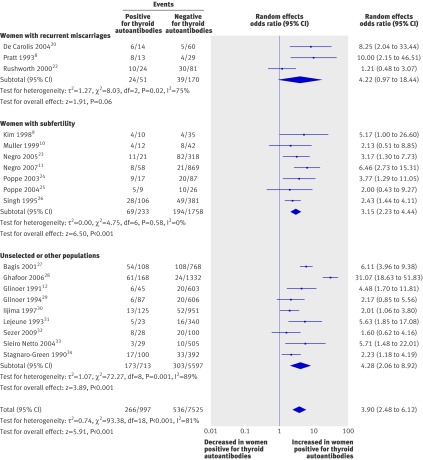
Twenty eight of the 31 (19/19 cohort and 9/12 case-control) studies showed a positive association between thyroid autoantibodies and spontaneous miscarriage. Meta-analysis of the cohort studies found more than tripling in the odds of miscarriage in the presence of thyroid autoantibodies (odds ratio 3.90, 95% confidence interval 2.48 to 6.12; P<0.001) (fig 2). The odds of miscarriage with thyroid autoantibodies was increased for women with recurrent miscarriages (4.22, 0.97 to 18.44; P=0.06), women with subfertility (3.15, 2.23 to 4.44; P<0.001), and unselected or other populations (4.28, 2.06 to 8.92; P<0.001). There was significant unexplained heterogeneity for studies in women with recurrent miscarriage (I2=75%) and the unselected population (I2=89%). There was no evidence of heterogeneity for studies in women with subfertility (I2=0%) (fig 2).
Fig 2 Association between thyroid autoantibodies and miscarriage in cohort studies
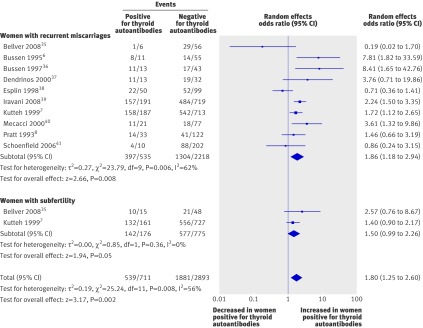
Meta-analysis of the 12 case-control studies also showed an increase in the odds of miscarriage in women with normal thyroid function and thyroid autoantibodies (1.80, 1.25 to 2.60; P=0.002) (fig 3). The odds of miscarriage was increased in two subgroups: women with recurrent miscarriage (1.86, 1.18 to 2.94; P=0.008) and women with subfertility (1.50, 0.99 to 2.26; P=0.05).
Fig 3 Association between thyroid autoantibodies and miscarriage in case-control studies
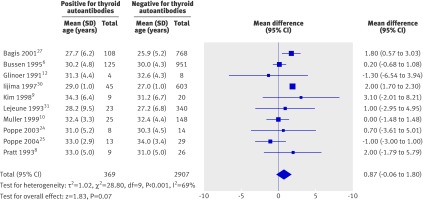
As increasing maternal age is an “independent” risk factor for miscarriage,42 we examined the difference in the mean age of women who were positive or negative for thyroid autoantibodies through a meta-analysis of the 10 studies that reported age. There was no significant difference between the groups (weighted mean difference 0.87 years, −0.06 to 1.80 years; P=0.07, fig 4).
Fig 4 Comparison of age between women postive and negative for thyroid autoantibodies in included studies
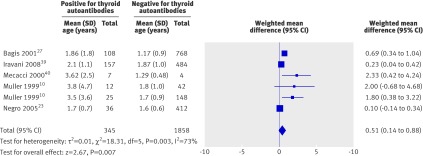
To explore the hypothesis that women who are positive for thyroid autoantibodies have relative hypothyroidism, we meta-analysed the serum thyroid stimulating hormone concentrations in six studies. The weighted mean difference for thyroid stimulating hormone was significantly higher in the thyroid autoantibody positive group compared with the negative group by 0.51 mIU/L (95% confidence interval 0.14 to 0.88; P=0.007), giving credibility to this hypothesis (fig 5). There were insufficient data on serum free thyroxine (fT4) and free triiodothyronine (T3) concentrations for meta-analysis.
Fig 5 Comparison of serum thyroid stimulating hormone (TSH) concentration between women positive and negative for thyroid autoantibodies in included studies
Thyroid autoantibodies and preterm birth
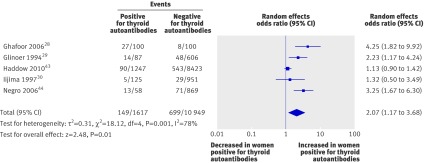
Five studies28 29 30 43 44 including a total of 12 566 women evaluated the association between thyroid autoantibodies and preterm birth. All were cohort studies and had low risk of bias for selection and outcome assessment on the Newcastle-Ottawa scale.16 Three studies were judged to have medium risk of bias and two as high risk of bias for comparability of cohorts. All showed a positive association between the presence of thyroid autoantibodies and preterm births. Meta-analysis showed an increase in the odds of preterm birth in the presence of thyroid autoantibodies (2.07, 1.17 to 3.68; P=0.01) (fig 6).
Fig 6 Association between thyroid autoantibodies and preterm births
Effect of levothyroxine treatment on pregnancy outcomes
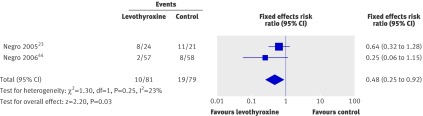
Two randomised studies, including a total of 187 women, evaluated the effect of levothyroxine treatment on pregnancy outcomes.23 44 Both studies were in women with normal thyroid function with thyroid autoantibodies. One study was in unselected women44 and the other in women scheduled to have in vitro fertilisation treatment.23 One used levothyroxine at a dose of 1 µg/kg/day,23 and the other used a titrated dose, with a mean levothyroxine dose of 49.7 µg/day (SD 14 µg) in the treatment group.44 The Jadad score17 for quality of the studies was 5/5 and 3/5. Both studies showed a reduction in miscarriage rates (36% and 75% relative reductions), and when the results were pooled, there was a significant 52% relative risk reduction in miscarriages with levothyroxine (0.48, 0.25 to 0.92; P=0.03) (fig 7). One of the two studies reported on preterm birth44; this study (n=115) found a 69% relative risk reduction in preterm births with levothyroxine (0.31, 0.11 to 0.90).
Fig 7 Effect of levothyroxine treatment in reducing miscarriage in women with normal thyroid function and thyroid autoantibodies
Discussion
Association of thyroid autoantibodies with adverse pregnancy outcomes
Our systematic review and meta-analysis of the published literature showed that in women with normal thyroid function, there is a strong association between the presence of thyroid autoantibodies and poor obstetric outcomes, for both miscarriage and preterm birth. The odds of miscarriage more than tripled and that of preterm birth doubled in women with thyroid autoantibodies.
Given the high prevalence of thyroid autoantibodies in women of reproductive age group, these increases in miscarriage and preterm birth are clinically highly relevant at the individual and population level. We showed that the increase in miscarriage rates in women positive for thyroid autoantibodies could not be accounted for by confounding factors such as a difference in age. Our review of the effectiveness of treatment with levothyroxine has provided preliminary evidence on its efficacy in reducing the rates of miscarriages and preterm births.
Five reviews have examined the association between thyroid autoantibodies and miscarriage,4 5 14 45 46 and one has examined the association with preterm birth.47 Several new studies have emerged since these reviews were completed, however, and the existing reviews have used limited meta-analytical methods, necessitating our review.
Pathogenesis of adverse pregnancy outcomes with thyroid autoantibodies
The frequent presence of thyroid autoantibodies in several non-thyroidal autoimmune diseases supports a hypothesis of global immune dysfunction being relevant to these clinical outcomes.48 There is evidence that there is an alteration in cytokine expression by peripheral T lymphocytes in women positive for thyroid antibodies outside of pregnancy.49 Pregnancy is an inflammatory process involving a shift in the regulation of cytokine networks within the local placental-decidual environment.50 Dysregulation of local inflammatory processes can be associated with miscarriage and premature delivery.51 The presence of thyroid autoantibodies can reflect a generalised activation of the immune system and specifically a dysregulated activity of the immune system at the fetal-maternal interface. Thyroid hormones can directly influence angiogenic growth factor and cytokine production52 53 as well as trophoblast proliferation, survival, and invasion.54 55 Furthermore, the presence of thyroid autoantibodies might be a marker of underlying subtle alteration in thyroid reserve. A reduction in the functional reserve of the thyroid gland associated with reduced adaptation to the physiological changes of pregnancy could contribute to minor changes in circulating thyroid hormone concentrations within the reference range.56 The increase in thyroid stimulating hormone concentrations in euthyroid women with thyroid autoantibodies supports this hypothesis. There were insufficient data to identify any differences in the concentrations of triiodothyronine (T3) between the two groups. We postulate that treatment with levothyroxine might correct any relative deficiency of thyroid hormones and impact on both systemic immune regulation and the local placental-decidual environment.
Clinical implications of the findings
The prevalence of the thyroid autoantibodies in the included studies varied between 5.4% and 31%. The miscarriage rates ranged from 2.4% to 42.9%. The association between thyroid autoantibodies and miscarriage was consistently strong across varying rates of thyroid autoantibodies and miscarriages. The individual studies used various assays for thyroid peroxidase antibodies, each with different detection limits and thresholds for test positivity, which were usually predetermined by the assay manufacturer. The effect size does not seem to be related to the threshold concentrations of thyroid peroxidase antibodies.
Although 28 of the 31 miscarriage studies and all five preterm studies showed a positive association, there was still unexplained heterogeneity in the meta-analyses for both miscarriage and preterm birth. The clinical heterogeneity between the studies on the population, test assay platforms, and thresholds used and the quality features of the studies are likely to have contributed to the statistical heterogeneity observed.
The strength of inference on the effectiveness of levothyroxine is weakened by the small number of studies. There have been only two small randomised trials involving a total of 187 women. One of them was not placebo controlled. To obtain a definitive answer on the role of levothyroxine in reducing miscarriages and preterm births a large placebo controlled randomised trial is needed with live births as the primary outcome. The two randomised studies included in the review did not find any safety concerns for the mother or the baby; specifically there were no instances of hyperthyroidism (resulting from overtreatment with levothyroxine). As the randomised trials were small and the follow-up was only to the end of pregnancy, however, these trials were not suitable for assessing rare or long term adverse events. Furthermore, the two trials on this topic have been conducted in the same research unit, with possible implications to the generalisability of the findings.
What is already known on this topic
Thyroid autoantibodies are relatively common in women of reproductive age
Thyroid autoimmunity might be associated with adverse pregnancy outcomes
What this study adds
In women with normal thyroid function and thyroid autoantibodies the risk of miscarriage is more than tripled and the risk of preterm birth is doubled
Treatment with levothyroxine can halve the risk of miscarriage in women with normal thyroid function and thyroid autoantibodies
Contributors: AC conceived the review. EK, ST, and AT performed the search, study selection, and data extraction. ST, AT, and AC analysed the results. ST, AC, JF, and MDK drafted the manuscript. All authors provided input into the development of the manuscript. All authors have approved the final manuscript. AC is guarantor.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. The authors have been funded by the NIHR (National Institute of Health Research, UK) EME Programme (09-100-10) to conduct a multicentre placebo-controlled randomised trial on the pregnancy effects of levothyroxine treatment in thyroid antibody positive women with normal thyroid function (the TABLET trial).
Ethical approval: Not required.
Data sharing: No additional data available.
Cite this as: BMJ 2011;342:d2616
Web Extra. Extra material supplied by the author
Appendix 1: Clinical characteristics of included studies
References
- 1.NHS Direct Wales Encyclopaedia. Miscarriage. 2010. www.nhsdirect.wales.nhs.uk/encyclopaedia/m/article/miscarriage/.
- 2.Creasy RK. Preventing preterm birth. N Engl J Med 1991;325:727-9. [DOI] [PubMed] [Google Scholar]
- 3.Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009;123:e312-27. [DOI] [PubMed] [Google Scholar]
- 4.Stagnaro-Green A, Glinoer D. Thyroid autoimmunity and the risk of miscarriage. Best Pract Res Clin Endocrinol Metab 2004;18:167-81. [DOI] [PubMed] [Google Scholar]
- 5.Poppe K, Velkeniers B, Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nat Clin Pract Endocrinol Metab 2008;4:394-405. [DOI] [PubMed] [Google Scholar]
- 6.Bussen S, Steck T. Thyroid autoantibodies in euthyroid non-pregnant women with recurrent spontaneous abortions. Hum Reprod 1995;10:2938-40. [DOI] [PubMed] [Google Scholar]
- 7.Kutteh WH, Yetman DL, Carr AC, Beck LA, Scott RT Jr. Increased prevalence of antithyroid antibodies identified in women with recurrent pregnancy loss but not in women undergoing assisted reproduction. Fertil Steril 1999;71:843-8. [DOI] [PubMed] [Google Scholar]
- 8.Pratt D, Novotny M, Kaberlein G, Dudkiewicz A, Gleicher N. Antithyroid antibodies and the association with non-organ-specific antibodies in recurrent pregnancy loss. Am J Obstet Gynecol 1993;168:837-41. [DOI] [PubMed] [Google Scholar]
- 9.Kim CH, Chae HD, Kang BM, Chang YS. Influence of antithyroid antibodies in euthyroid women on in vitro fertilization-embryo transfer outcome. Am J Reprod Immunol 1998;40:2-8. [DOI] [PubMed] [Google Scholar]
- 10.Muller AF, Verhoeff A, Mantel MJ, Berghout A. Thyroid autoimmunity and abortion: a prospective study in women undergoing in vitro fertilization. Fertil Steril 1999;71:30-4. [DOI] [PubMed] [Google Scholar]
- 11.Negro R, Formoso G, Coppola L, Presicce G, Mangieri T, Pezzarossa A, et al. Euthyroid women with autoimmune disease undergoing assisted reproduction technologies: the role of autoimmunity and thyroid function. J Endocrinol Invest 2007;30:3-8. [DOI] [PubMed] [Google Scholar]
- 12.Glinoer D, Soto MF, Bourdoux P, Lejeune B, Delange F, Lemone M, et al. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab 1991;73:421-7. [DOI] [PubMed] [Google Scholar]
- 13.Glinoer D, Delange F. The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid 2000;10:871-87. [DOI] [PubMed] [Google Scholar]
- 14.Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage. Eur J Endocrinol 2004;150:751-5. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomised studies in meta-analysis. www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 17.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
- 18.Review Manager (RevMan) [computer program]. Version 5.0. Cochrane Collaboration, 2008.
- 19.StataCorp. Stata statistical software: release 10. StataCorp, 2007.
- 20.De Carolis C, Greco E, Guarino MD, Perricone C, Dal LA, Giacomelli R, et al. Anti-thyroid antibodies and antiphospholipid syndrome: evidence of reduced fecundity and of poor pregnancy outcome in recurrent spontaneous aborters. Am J Reprod Immunol 2004;52:263-6. [DOI] [PubMed] [Google Scholar]
- 21.Pratt DE, Kaberlein G, Dudkiewicz A, Karande V, Gleicher N. The association of antithyroid antibodies in euthyroid nonpregnant women with recurrent first trimester abortions in the next pregnancy. Fertil Steril 1993;60:1001-5. [DOI] [PubMed] [Google Scholar]
- 22.Rushworth FH, Backos M, Rai R, Chilcott IT, Baxter N, Regan L. Prospective pregnancy outcome in untreated recurrent miscarriers with thyroid autoantibodies. Hum Reprod 2000;15:1637-9. [DOI] [PubMed] [Google Scholar]
- 23.Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod 2005;20:1529-33. [DOI] [PubMed] [Google Scholar]
- 24.Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, et al. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab 2003;88:4149-52. [DOI] [PubMed] [Google Scholar]
- 25.Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Devroey P, van Steirteghem A, et al. Impact of ovarian hyperstimulation on thyroid function in women with and without thyroid autoimmunity. J Clin Endocrinol Metab 2004;89:3808-12. [DOI] [PubMed] [Google Scholar]
- 26.Singh A, Dantas ZN, Stone SC, Asch RH. Presence of thyroid antibodies in early reproductive failure: biochemical versus clinical pregnancies. Fertil Steril 1995;63:277-81. [PubMed] [Google Scholar]
- 27.Bagis T, Gokcel A, Saygili ES. Autoimmune thyroid disease in pregnancy and the postpartum period: relationship to spontaneous abortion. Thyroid 2001;11:1049-53. [DOI] [PubMed] [Google Scholar]
- 28.Ghafoor F, Mansoor M, Malik T, Malik MS, Khan AU, Edwards R, et al. Role of thyroid peroxidase antibodies in the outcome of pregnancy. J Coll Physicians Surg Pak 2006;16:468-71. [PubMed] [Google Scholar]
- 29.Glinoer D, Riahi M, Grun JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab 1994;79:197-204. [DOI] [PubMed] [Google Scholar]
- 30.Iijima T, Tada H, Hidaka Y, Mitsuda N, Murata Y, Amino N. Effects of autoantibodies on the course of pregnancy and fetal growth. Obstet Gynecol 1997;90:364-9. [DOI] [PubMed] [Google Scholar]
- 31.Lejeune B, Grun JP, de Nayer P, Servais G, Glinoer D. Antithyroid antibodies underlying thyroid abnormalities and miscarriage or pregnancy induced hypertension. Br J Obstet Gynaecol 1993;100:669-72. [DOI] [PubMed] [Google Scholar]
- 32.Sezer K, Kamel N, Unlu C, Celik HK. Impact of first trimester and postpartum period thyroid autoantibodies on abortus incidence in Turkish pregnant women. Gynecol Endocrinol 2009;25:387-91. [DOI] [PubMed] [Google Scholar]
- 33.Sieiro Netto L, Medina CC, Micmacher E, Mamede Da Costa S, Nazar L, Galvao D, et al. Influence of thyroid autoimmunity and maternal age on the risk of miscarriage. Am J Reprod Immunol 2004;52:312-6. [DOI] [PubMed] [Google Scholar]
- 34.Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Alvarez-Marfany M, Davies TF. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990;264:1422-5. [PubMed] [Google Scholar]
- 35.Bellver J, Soares SR, Alvarez C, Munoz E, Ramirez A, Rubio C, et al. The role of thrombophilia and thyroid autoimmunity in unexplained infertility, implantation failure and recurrent spontaneous abortion. Hum Reprod 2008;23:278-84. [DOI] [PubMed] [Google Scholar]
- 36.Bussen SS, Steck T. Thyroid antibodies and their relation to antithrombin antibodies, anticardiolipin antibodies and lupus anticoagulant in women with recurrent spontaneous abortions (antithyroid, anticardiolipin and antithrombin autoantibodies and lupus anticoagulant in habitual aborters). Eur J Obstet Gynecol Reprod Biol 1997;74:139-43. [DOI] [PubMed] [Google Scholar]
- 37.Dendrinos S, Papasteriades C, Tarassi K, Christodoulakos G, Prasinos G, Creatsas G. Thyroid autoimmunity in patients with recurrent spontaneous miscarriages. Gynecol Endocrinol 2000;14:270-4. [DOI] [PubMed] [Google Scholar]
- 38.Esplin MS, Branch DW, Silver R, Stagnaro-Green A. Thyroid autoantibodies are not associated with recurrent pregnancy loss. Am J Obstet Gynecol 1998;179:1583-6. [DOI] [PubMed] [Google Scholar]
- 39.Iravani AT, Saeedi MM, Pakravesh J, Hamidi S, Abbasi M. Thyroid autoimmunity and recurrent spontaneous abortion in Iran: a case-control study. Endocr Pract 2008;14:458-64. [DOI] [PubMed] [Google Scholar]
- 40.Mecacci F, Parretti E, Cioni R, Lucchetti R, Magrini A, La Torre P, et al. Thyroid autoimmunity and its association with non-organ-specific antibodies and subclinical alterations of thyroid function in women with a history of pregnancy loss or preeclampsia. J Reprod Immunol 2000;46:39-50. [DOI] [PubMed] [Google Scholar]
- 41.Shoenfeld Y, Carp HJ, Molina V, Blank M, Cervera R, Balasch J, et al. Autoantibodies and prediction of reproductive failure. Am J Reprod Immunol 2006;56:337-44. [DOI] [PubMed] [Google Scholar]
- 42.Nybo Anderson A. Maternal age and fetal loss: population based register linkage study. BMJ 2000;320:1708-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haddow JE, Cleary-Goldman J, McClain MR, Palomaki GE, Neveux LM, Lambert-Messerlian G, et al. Thyroperoxidase and thyroglobulin antibodies in early pregnancy and preterm delivery. Obstet Gynecol 2010;116:58-62. [DOI] [PubMed] [Google Scholar]
- 44.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006;91:2587-91. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf) 2011;74:513-9. [DOI] [PubMed] [Google Scholar]
- 46.Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, et al. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol 2010;162:643-52. [DOI] [PubMed] [Google Scholar]
- 47.Stagnaro-Green A. Maternal thyroid disease and preterm delivery. J Clin Endocrinol Metab 2009;94:21-5. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura H, Usa T, Motomura M, Ichikawa T, Nakao K, Kawasaki E, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest 2008;31:861-5. [DOI] [PubMed] [Google Scholar]
- 49.Colin IM, Isaac J, Dupret P, Ledant T, D’Hautcourt JL. Functional lymphocyte subset assessment of the Th1/Th2 profile in patients with autoimmune thyroiditis by flowcytometric analysis of peripheral lymphocytes. J Biol Regul Homeost Agents 2004;18:72-6. [PubMed] [Google Scholar]
- 50.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308:1592-4. [DOI] [PubMed] [Google Scholar]
- 51.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF III, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16:206-15. [DOI] [PubMed] [Google Scholar]
- 52.Mascanfroni I, Montesinos MM, Susperreguy S, Cervi L, Ilarregui JM, Ramseyer VD, et al. Control of dendritic cell maturation and function by triiodothyronine. FASEB J 2008;22:1032-42. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo H, Maruo T, Murata K, Mochizuki M. Human early placental trophoblasts produce an epidermal growth factor-like substance in synergy with thyroid hormone. Acta Endocrinol (Copenh) 1993;128:225-9. [DOI] [PubMed] [Google Scholar]
- 54.Barber KJ, Franklyn JA, McCabe CJ, Khanim FL, Bulmer JN, Whitley GS, et al. The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. J Clin Endocrinol Metab 2005;90:1655-61. [DOI] [PubMed] [Google Scholar]
- 55.Oki N, Matsuo H, Nakago S, Murakoshi H, Laoag-Fernandez JB, Maruo T. Effects of 3,5,3’-triiodothyronine on the invasive potential and the expression of integrins and matrix metalloproteinases in cultured early placental extravillous trophoblasts. J Clin Endocrinol Metab 2004;89:5213-21. [DOI] [PubMed] [Google Scholar]
- 56.Glinoer D. Miscarriage in women with positive anti-TPO antibodies: is thyroxine the answer? J Clin Endocrinol Metab 2006;91:2500-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Clinical characteristics of included studies