Abstract
Background and Purpose
To identify a minimally-acceptable CPP threshold above which the risk of brain tissue hypoxia (BTH) and oxidative metabolic crisis is reduced for patients with SAH.
Methods
We studied thirty poor-grade SAH patients who underwent brain multimodality monitoring (3042 hours). Physiological measures were averaged over 60 minutes for each collected microdialysis sample. Metabolic crisis was defined as a lactate/pyruvate ratio (LPR) >40 with a brain glucose concentration ≤0.7 mmol/L. BTH was defined as PbtO2 <20 mm Hg. Outcome was assessed at 3 months with the modified Rankin Scale.
Results
Multivariable analyses adjusting for admission Hunt-Hess grade, intraventricular hemorrhage, systemic glucose, and end-tidal CO2 revealed that CPP ≤70 mm Hg was significantly associated with an increased risk of BTH (OR=2.0; 95%-CI: 1.2–3.3, P=0.007) and metabolic crisis (OR=2.1; 95%-CI 1.2–3.7, P=0.007). Death-or-severe-disability at 3 months was significantly associated with metabolic crisis (OR 5.4; 95%-CI: 1.8–16, P=0.002) and BTH (OR 5.1; 95%-CI: 1.2–23, P=0.03) after adjusting for admission Hunt-Hess grade.
Conclusions
Metabolic crisis and BTH are associated with mortality and poor functional recovery after SAH. CPP levels below 70 mm Hg was associated with metabolic crisis and BTH and may increase the risk of secondary brain injury in poor-grade SAH patients.
Keywords: Subarachnoid hemorrhage, cerebral perfusion pressure, brain tissue oxygen tension, cerebral microdialysis
Introduction
Brain tissue oxygen pressure (PbtO2)1 and microdialysis monitoring2, 3 are used to indicate impending ischemia from vasospasm, and findings are related to outcome4, 5 for patients with poor-grade subarachnoid hemorrhage (SAH) patients. SAH patients with cerebral vasospasm are more vulnerable to infarction when brain oxygen tension is dependent on cerebral perfusion pressure, a phenomenon referred to as oxygen autoregulation failure.6, 7 Brain tissue hypoxia and elevation of the lactate pyruvate ratio (LPR) after SAH have been linked to an increased risk of symptomatic vasospasm, cerebral infarction, hospital mortality, and poor functional outcome after SAH.3, 4, 8–10
Blood pressure is often manipulated after SAH to improve cerebral perfusion and is a critical component of hypertensive-hypervolemic therapy used to delayed cerebral ischemia (DCI) from vasospasm.1 However in poor-grade SAH there is limited ability to detect delayed cerebral ischemia (DCI) clinically.11 Therefore it would be of interest to know if there is an ideal maintenance cerebral perfusion pressure (CPP) threshold above which the risk of cerebral ischemia is minimized. Guidelines exist that recommend maintaining CPP between 50 and 70 mm Hg after severe traumatic brain injury,12 but it has been difficult to generate data to support specific CPP targets in postoperative SAH patients.13 Using simultaneous PbtO2 and microdialysis multimodality neuromonitoring we sought to identify a CPP threshold that is associated with reduced risk of brain tissue hypoxia and oxidative metabolic crisis in comatose patients with SAH.
Materials and Methods
Study Population
Between May 2006 and December 2009.106 poor-grade patients were consecutively admitted to the neurological ICU at Columbia University Medical Center. Thirty patients who underwent a minimum of 12 hours of simultaneous intracranial pressure (ICP), PbtO2 and microdialysis monitoring as part of their clinical care. were included in the present analysis. Of the 76 patients who were excluded, 57 did not undergo monitoring because of early death or decisions to limit aggressive care, 10 were excluded because they had PbtO2 or microdialysis data but not both (due to technical problems), 5 had contraindications for the procedure to place the probes (coagulaopathy or platelet dysfunction), 4 were monitored for less than 12 hours, and 1 had the probes placed in infracted tissue.
This observational study was approved by the Columbia University Medical Center Institutional Review Board. The diagnosis of SAH was established on the basis of an admission computed tomographic (CT) in all cases. DCI was defined as clinical deterioration, cerebral infarction, or both due to vasospasm after adjudication of all relevant clinical information in weekly meetings by the study team.14 Outcome was assessed at 3 months with the modified Rankin Scale (mRS).
Clinical Management
All patients received mechanical ventilation, external ventricular drainage, daily interruption of sedation, prophylactic oral nimodipine, and intravenous hydration with 0.9% saline at 1 ml/kg/hr and 250 ml of 5% albumin solution every 2 hours to maintain central venous pressure >5 cm H2O according to a standardized management protocol.15 Cerebral perfusion pressure was targeted to avoid levels below 50 mm Hg at all times. Clinical deterioration from DCI was treated with hypertensive hypervolemic therapy (HHT) to maintain systolic blood pressure (SBP) between 160–220 mm Hg as required to reverse the neurological deficit. Strict normothermia16 was maintained with a surface (Arctic Sun, Medivance, Louisville, CO) or endovascular (Alsius Thermogard, Zoll Circulation, Boston MA) cooling device. Intracranial pressure was maintained below < 20 mm Hg using a stepwise management strategy (cerebrospinal fluid drainage, sedation, CPP optimization, hyperventilation to PCO2 30–34 mm Hg, osmotherapy with mannitol and hypertonic saline, and mild hypothermia (35 °C).17 The hemoglobin threshold for blood transfusion was 7 mg/dl in the absence of ongoing myocardial or cerebral ischemia and 10 mg/dl if either condition was present. All patients were ventilated to achieve an arterial oxygen saturation ≥ 95% and PCO2 of 30 to 40 mm Hg. PbtO2 measurements were excluded from this analysis when the fraction of inspired oxygen exceeded 50%.
Data Acquisition
A high resolution data acquisition system (BedmasterEX, Excel Medical Electronics, Jupiter, FL) was used to acquire digital data every 5 seconds from General Electric (GE) Solar 8000i monitors. Heart rate (HR), arterial blood pressure, end-tidal CO2 (ETCO2), CPP and ICP were continuously monitored in all patients. ICP was measured using an intraparenchymal probe (CamIno, Integra Neurosciences, Plainsboro, NJ), PbtO2 was measured with a flexible polarographic Licox Clark-type probe (Licox GMBH, Kiel, Germany), and a CMA 70 microdialysis catheter with 10 mm membrane length (CMA Microdialysis®, Stockholm, Sweden) was used to monitor cerebral metabolism.18 Probes were placed via a single burr hole at the bedside using a triple-lumen bolt in the frontal lobe ipsilateral to lateralized aneurysms when possible, or in the right frontal lobe in the case of midline aneurysms.19 Two measures of the adequacy of autoregulation, the pressure reactivity index (PRx)20 and the oxygen (PbtO2) pressure reactivity index (ORx), were calculated post-hoc.21
Statistical Analysis
MAP, CPP, ICP, ETCO2, and PbtO2 measures were averaged over 60 minutes preceding each microdialysis measurement. Metabolic crisis was defined as a lactate/pyruvate ratio greater than 40 and brain glucose less than 0.7 mmol/L.22 Brain tissue hypoxia (BTH) was dichotomized as brain tissue oxygen tension less than 20 mm Hg based on data demonstrating a higher risk of poor outcome below this threshold.23–28 Univariate comparisons of pooled data were carried out using a generalized linear model (GLM) using a binomial distribution and logit link function and extended by generalized estimating equations (GEE) using the autoregressive process (AR-1)29 to handle repeated observations within subject. SPSS 17 software® (SPSS Inc., Chicago, IL, USA) was used for data analysis. A P value <0.05 was considered statistically significant.
Results
Baseline Characteristics
30 patients were mechanically ventilated and comatose (GCS<9) at the initiation of neuromonitoring. A total of 3042 hours of data were recorded and analyzed (Table 1). Twelve patients (40%) developed delayed cerebral ischemia (DCI) from vasospasm: 3 experienced clinical deterioration only, 5 had clinical deterioration with cerebral infarction, and four developed cerebral infarction only.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Demographics | |
| Age | 46 (37–57) |
| Gender | 19 (63%) |
| Non-White | 20 (67%) |
| Admission Hunt-Hess Score | |
| 2–3 | 5 (17%) |
| 4 | 10 (33%) |
| 5 | 15 (50%) |
| Tissue Status of Neuromonitoring Probes | |
| Normal (ipsilateral) | 12 (40%) |
| Normal (contralateral) | 9 (30%) |
| Peri-lesional (adjacent to focal lesion) | 9 (30%) |
| Monitoring | |
| SAH Day at Start of Monitoring | 3 (2–4) |
| Monitoring Hours | 110 (87–184) |
| Metabolic Crisis Hours | 3 (0–34) |
| Brain Tissue Hypoxia Hours | 11 (1–32) |
| Temperature Modulation | |
| None | 7 (23%) |
| Normothermia | 13 (43%) |
| Hypothermia (< 36 °C for ICP control) | 10 (33%) |
| Interventions Received | |
| Hypertonic Saline for ICP control | 25 (83%) |
| Mannitol for ICP control | 22 (73%) |
| External Ventricular Drain | 26 (87%) |
| Insulin Infusion | 29 (97%) |
| Vasopressor Infusion | 29 (97%) |
| HHT Therapy (for delayed cerebral ischemia) | 19 (63%) |
| Intra-Arterial Vasodilators | 15 (50%) |
| Angioplasty | 7 (23%) |
| Vasospasm | |
| Clinical deterioration | 12 (40%) |
| Infarct | 9 (30%) |
| 3 Month Rankin Scale Score | |
| 2–3 | 7 (23%) |
| 4 | 9 (30%) |
| 5 | 7 (23%) |
| 6 | 7 (23%) |
Data are shown as number (%) for categorical variables and median (IQR) for continuous variables. Tissue Status= Peri-lesional refers to probe positioning within 2 cm of a focal lucency or hemorrhage on CT; or Normal: 1=Ipsilateral; 2=Contralateral); Admission Start Day=Day post-injury monitoring started.
Relationship of CPP to Brain Tissue Oxygenation and Energy Metabolism
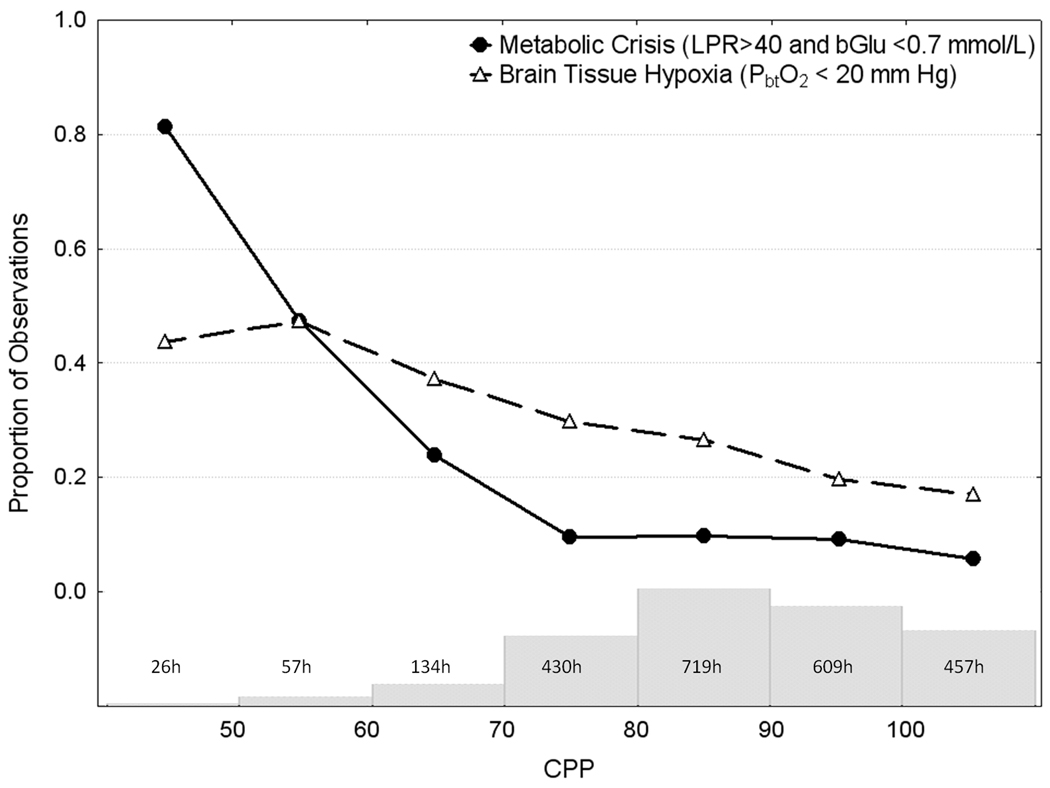
The probability of BTH increased steadily and significantly from 19% to 47% as CPP declined from 110 to 50 mm Hg. In contrast, the probability of metabolic crisis remained below 10% when CPP was between 70 and 110 mm Hg, but doubled significantly to 24% when CPP was between 60 to 70 mm Hg continuing to rise to over 80% when CPP was < 50 mm Hg (Figure 1). Compared to CPP > 90 mm Hg, the CPP threshold associated with significant increases in the risk of both BTH and metabolic crisis was <70 mm Hg (Table 3).
Figure 1.
Depicts observed probability of cerebral metabolic crisis and brain tissue hypoxia over the range of spontaneous cerebral perfusion pressures (CPP). The labeled histogram is the number of patients hours spent in each 10 mm Hg range of CPP. See Table 3 for the results of a multivariable model (GEE) that correspond to this figure.
Table 3.
Multivariable Predictors of Brain Tissue Hypoxia and Metabolic Crisis
| Variable | Metabolic Crisis | Brain Tissue Hypoxia | ||
|---|---|---|---|---|
| OR (95%-CI) | P Value | OR (95%-CI) | P Value | |
| Admission Hunt and Hess grade 5 | 5.1 (1.6–16) | .006 | - | |
| IVH on admission CT | 5.4 (1.5–20) | .009 | - | |
| Hourly Systemic Glucose < 6.6 mmol/L | 1.4 (1.1–1.8) | .016 | - | |
| End-tidal CO2 ≤ 35 | - | 1.2 (0.9–1.5) | .071 | |
| CPP > 90 mm Hg | CPP Odds Ratio Reference Group | |||
| CPP 80–90 mm Hg | 1.1 (0.8–1.5) | .406 | 1.2 (1.0–1.5) | .023 |
| CPP 70–80 mm Hg | 1.4 (0.9–2.1) | .159 | 1.6 (1.1–2.3) | .012 |
| CPP 60–70 mm Hg | 2.1 (1.2–3.7) | .007 | 2.0 (1.2–3.3) | .007 |
| CPP 50–60 mm Hg | 2.8 (1.5–5.0) | .001 | 2.8 (1.4–5.3) | .003 |
| CPP < 50 mm Hg | 6.7 (2.6–17) | .000 | 3.5 (1.5–7.9) | .003 |
Multivariable logistic regression model (GEE) adjusted for the variables listed. OR (95%-CI) = Odds Ratio (95% Confidence Interval); Metabolic Crisis=Lactate / Pyruvate Ratio > 40 and brain glucose concentration < 0.7 mmol/L; Brain Tissue Hypoxia PbtO2<20 mm Hg; CPP=cerebral perfusion pressure modeled as six level factor using a CPP > 90 as the reference group.
Predictors of Brain Tissue Hypoxia
Univariate analysis logistic regression (GEE) revealed that patients were 22% more likely to experience BTH for every 10 mm Hg decline in CPP (OR: 1.22; 95%-CI: 1.07–1.39; P=0.002). Mean arterial pressure and end tidal CO2 were significantly associated with brain tissue hypoxia in a univariate analysis (Table 2). In a multivariable GLM (GEE) model CPP was the strongest predictor of brain tissue hypoxia after adjusting for end tidal CO2 (Table 3).
Table 2.
Univariate Predictors of Brain Tissue Hypoxia and Metabolic Crisis
| Variable | Metabolic Crisis | Brain Tissue Hypoxia | ||
|---|---|---|---|---|
| OR (95%-CI) | P Value | OR (95%-CI) | P Value | |
| Age >47 years | 0.5 (0.1–1.6) | .232 | 0.8 (0.2–2.7) | .762 |
| Non-white | 1.9 (0.6–6.8) | .295 | 1.8 (0.5–6.3) | .378 |
| SAH day | 1.1 (0.9–1.2) | .506 | 1.1 (0.9–1.2) | .437 |
| Peri-lesional probe location | 2.4 (0.7–8.8) | .175 | 0.8 (0.2–5.1) | .763 |
| Hunt -Hess grade 5 | 3.9 (1.2–13) | .021 | 0.9 (0.3–2.8) | .843 |
| Apache II subscore >10 | 1.0 (0.9–1.1) | .946 | 1.1 (0.9–1.2) | .293 |
| SAH sum score ≥15 | 0.8 (0.2–3.9) | .775 | 1.9 (0.6–6.0) | .244 |
| IVH present | 4.2 (1.0–17) | .046 | 2.3 (0.8–6.4) | .110 |
| ICH present | 10 (2.1–46) | .003 | 0.2 (0.1–2.6) | .198 |
| Bicaudate index >.2 | 4.8 (1.4–16) | .011 | 1.6 (0.5–5.1) | .415 |
| CPP ≤ 70 mm Hg | 1.6 (1.2–2.1) | .001 | 1.4 (1.1–1.8) | .005 |
| ICP >20 mm Hg | 1.0 (1.0–1.1) | .001 | 1.0 (0.9–1.0) | .319 |
| ETCO2 <35 mm Hg | 1.0 (0.7–1.4) | .972 | 1.3 (1.1–1.6) | .014 |
| ORx > 0.2 | 1.0 (0.9–1.1) | .590 | 0.9 (0.8–1.1) | .097 |
| PRx > 0.2 | 1.0 (0.9–1.2) | .864 | 1.1 (1.0–1.2) | .074 |
| Serum glucose < 6.6 mmol/L | 1.3 (1.0–1.6) | .026 | 1.0 (0.9–1.1) | .831 |
OR (95%-CI) = Odds Ratio (95% Confidence Interval); Metabolic Crisis=Lactate / Pyruvate Ratio > 40 and brain glucose concentration < 0.7 mmol/L; Brain Tissue Hypoxia PbtO2<20 mm Hg; Age cutoff at median (47 years old); SAH ≥15=SAH sum score, scaled 0=no blood, 30=all cisterns and fissures completely filled42 ; IVH=intraventricular blood present43 ; bicaudate index=presence of cerebral edema44 ; ORx= Oxygen PbtO2 Pressure Reactivity Index; PRx=Pressure Reactivity Index.
Predictors of Brain Metabolic Crisis
There was a threshold effect of CPP on the likelihood of metabolic crisis, occurring 10% of the time when mean hourly CPP was > 70 mm Hg compared to 24% when CPP was ≤ 70 mm Hg (P<0.001). In univariate analysis metabolic crisis was significantly associated with an admission Hunt-Hess grade of 5, CT evidence of intraventricular or intraparenchymal clot, hydrocephalus, and low systemic glucose concentrations (Table 2). After adjusting for other significant factors a multivariable logistic regression (GEE) analysis revealed that CPP was the strongest predictor of metabolic crisis, but only when CPP was below 70 mm Hg (Table 3).
3-Month Outcome
Seven patients (23%) had died by day 90. Among the survivors seven patients (23%) had mild-or-moderate disability (mRS 2 or 3, able to ambulate but unable to carry out all activities on their own), nine (30%) had moderate-to-severe disability (mRS 4, unable to ambulate independently), and seven (23%) had severe disability (mRS 5, bedbound)(Table 1). Logistic regression (GEE) revealed that after adjusting for admission Hunt-Hess grade, death or severe disability (modified Rankin ≥ 4) at three months was significantly associated with metabolic crisis (OR 5.4; 95%-CI: 1.8–16, P=0.002) and brain tissue hypoxia (OR 5.1; 95%-CI: 1.2–23, p=0.03). Mortality at three months was not significantly associated with either metabolic crisis (OR 0.9; 95%-CI: 0.3–3.2, P=0.991) or brain tissue hypoxia (OR 1.6; 95%-CI: 0.3–7, P=0.557).
Discussion
In this study of 30 poor-grade SAH patients we found that death or severe disability at three months was significantly associated with brain metabolic crisis and tissue hypoxia, which confirm what has been observed previously.3, 4, 8–10 Abnormalities of PbtO2, brain glucose, and LPR were all more likely with lower CPP, with a statistically-significant threshold effect occurring with levels below 70 mm Hg. These findings suggest that maintaining CPP above this level might minimize the risk of secondary ischemic injury in comatose SAH patients.
Studies have shown that brain oxygen tension and cerebral metabolism do not always tightly correlate despite the capacity for both methods to identify tissue hypoperfusion.30, 31 Brain oxygen tension is a complicated multidimensional parameter that under specific circumstances is a useful surrogate marker for regional CBF.32 PbtO2 is best characterized as an interaction between oxygen delivery, diffusion, and demand at the capillary, tissue, and cellular level.33 This can make changes in PbtO2 difficult to interpret in isolation.34, 35
In contrast to the linear reduction in PbtO2 that occurred with declining CPP, an ischemic pattern of cerebral metabolism occurred when CPP dropped below 70 mm Hg. When cerebral autoregulation mechanisms are intact, the arterial response to a reduction in CPP is vasodilatation. Small arterioles less than 100 µm in diameter begin to dilate when mean arterial pressure is less than 90 mm Hg, and can expand to 140% to 170% of their original diameter36 in order to maintain adequate cerebral blood flow (CBF) and protect against ischemia.37
The sharp increase in the frequency of metabolic crisis below CPP levels of 70 mm Hg that we observed, combined with the gradual reduction in PbtO2 that occurred across the entire range of CPP, suggests that both blunting and a rightward shift of pressure autoregulation can occur in poor-grade SAH patients. Our data also suggest that brain oxygen metabolism behaves differently from glucose metabolism, such that mild reductions in CBF and substrate delivery result in a graded decrease in PbtO2, as opposed to having more of a threshold effect in terms of triggering anaerobic glucose metabolism. Brain oxygen tension monitoring may be best suited to determine autoregulation status and to identify patients that are particularly vulnerable to hypoperfusion events, whereas cerebral metabolism monitoring may be better suited to detect a safe lower limit of CPP on a patient-specific basis.38 Further studies are needed to confirm these observations.
Patients who had a poor outcome (death or severe disability) at three months were significantly more likely to have a greater burden of BTH and metabolic crisis during monitoring, even after adjusting for admission Hunt and Hess grade. However, this association does not prove causality: although it is feasible that low CPP exacerbates tissue ischemic injury and directly contributes to poor neurological outcome, it might also be possible that these abnormalities are the result of brain injury that has already occurred in patients who are more hemodynamically unstable and already destined to have a poor outcome. Nevertheless, our study clearly demonstrates a relationship between cerebral hypoxia and metabolic crisis with poor outcome after SAH.
This study has a number of important limitations. We did not have access to direct measurements of CBF, which can be measured with thermodilution tissue probes (Hemedex, Inc). This would have allowed us to gain a better understanding of the state of pressure autoregulation in our patients. We could not incorporate detailed FiO2 or PaO2/FiO2 measurements, all of which may influence brain oxygenation, into the regression model for predicting PbtO2 because of technical limitations. In order to minimize this problem, however, we excluded hourly data collected when FiO2 was equal to or above 50%. We recorded an hourly average CPP of < 70 mm Hg for 217 hours (7%) of the observed monitoring. At the time of this study our clinical practice was to maintain cerebral perfusion pressure (CPP) > 50 mm Hg and ICP < 20 mm Hg, in accordance with Brain Trauma Foundation guidelines. In the case of symptomatic vasospasm, which occurred in 27% of patients, we directed further increases in CPP at reversal of the clinical deficit, to levels ranging between 90–120 mm Hg. Wide variances in blood pressure are common in poor-grade SAH patients. Individual physiological responses to cerebral perfusion drops below 70 mm Hg were not analyzed and it is unknown from this study if these fluctuations are harmful and should also be avoided. Patient-specific responses of PbtO2 and cerebral metabolism are also not analyzed in this study and might lead to different conclusions: confirmation of the importance of maintaining CPP above 70 mm Hg in a larger validation cohort is needed. If confirmed, a randomized controlled trial of PbtO2-targeted CPP management strategy might be justified.
Advanced multimodality neuromonitoring provides a platform to devise strategies to identify personalized clinical thresholds for physiological variables that can influence brain perfusion such as CPP and end tidal CO2.39, 40 Our findings indicate that maintaining a minimum CPP level above 70 mm Hg may prove to be a useful clinical guideline for minimizing the risk of secondary brain injury after poor-grade SAH in unmonitored patients. Ideally, however, multimodality monitoring with real-time data acquisition can allow for the individualized blood pressure and ventilation targets.41 Intervention studies that combine patient-specific targets and group design elements are needed to determine whether such findings have purely prognostic value or represent modifiable factors that can be manipulated to improve patient outcome.
Acknowledgments
Sources of Funding
The project described was supported by a research grant from the Charles A. Dana Foundation (SAM) and by Grant Number KL2 RR024157 (JMS) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from NIH Roadmap website.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Raabe A, Beck J, Keller M, Vatter H, Zimmermann M, Seifert V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. Journal of Neurosurgery: Pediatrics. 2005:103. doi: 10.3171/jns.2005.103.6.0974. [DOI] [PubMed] [Google Scholar]
- 2.Skjoth-Rasmussen J, Schulz M, Kristensen SR, Bjerre P. Delayed neurological deficits detected by an ischemic pattern in the extracellular cerebral metabolites in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;100:8–15. doi: 10.3171/jns.2004.100.1.0008. [DOI] [PubMed] [Google Scholar]
- 3.Unterberg AW, Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR. Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2001;94:740–749. doi: 10.3171/jns.2001.94.5.0740. [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishna R, Stiefel M, Udoetuk J, Spiotta A, Levine JM, Kofke WA, Zager E, Yang W, Leroux P. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;109:1075–1082. doi: 10.3171/JNS.2008.109.12.1075. [DOI] [PubMed] [Google Scholar]
- 5.Sarrafzadeh A, Haux D, Kuchler I, Lanksch WR, Unterberg AW. Poor-grade aneurysmal subarachnoid hemorrhage: Relationship of cerebral metabolism to outcome. J Neurosurg. 2004;100:400–406. doi: 10.3171/jns.2004.100.3.0400. [DOI] [PubMed] [Google Scholar]
- 6.Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38:981–986. doi: 10.1161/01.STR.0000257964.65743.99. [DOI] [PubMed] [Google Scholar]
- 7.Bijlenga P, Czosnyka M, Budohoski KP, Soehle M, Pickard JD, Kirkpatrick PJ, Smielewski P. "Optimal cerebral perfusion pressure" In poor grade patients after subarachnoid hemorrhage. Neurocrit Care. doi: 10.1007/s12028-010-9362-1. [DOI] [PubMed] [Google Scholar]
- 8.Meixensberger J, Vath A, Jaeger M, Kunze E, Dings J, Roosen K. Monitoring of brain tissue oxygenation following severe subarachnoid hemorrhage. Neurol Res. 2003;25:445–450. doi: 10.1179/016164103101201823. [DOI] [PubMed] [Google Scholar]
- 9.Persson L, Valtysson J, Enblad P, Warme PE, Cesarini K, Lewen A, Hillered L. Neurochemical monitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg. 1996;84:606–616. doi: 10.3171/jns.1996.84.4.0606. [DOI] [PubMed] [Google Scholar]
- 10.Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR, Unterberg AW. On-line microdialysis following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2001;77:141–144. doi: 10.1007/978-3-7091-6232-3_30. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt JM, Wartenberg KE, Fernandez A, Claassen J, Rincon F, Ostapkovich ND, Badjatia N, Parra A, Connolly ES, Mayer SA. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg. 2008;109:1052–1059. doi: 10.3171/JNS.2008.109.12.1052. [DOI] [PubMed] [Google Scholar]
- 12.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. X. Brain oxygen monitoring and thresholds. J Neurotrauma. 2007;24 Suppl 1:S65–S70. doi: 10.1089/neu.2007.9986. [DOI] [PubMed] [Google Scholar]
- 13.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the stroke council, american heart association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 14.Frontera J, Fernandez A, Schmidt J, Claassen J, Wartenberg K, Badjatia N, Connolly E, Mayer S. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke. 2009;40:1963. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 15.Komotar R, Schmidt J, Starke R, Mayer S, Claassen J, Wartenberg K, Lee K, Badjatia N, Connolly E., Jr Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64:397. doi: 10.1227/01.NEU.0000338946.42939.C7. [DOI] [PubMed] [Google Scholar]
- 16.Badjatia N, Fernandez L, Schmidt JM, Lee K, Claassen J, Connolly ES, Mayer SA. Impact of induced normothermia on outcome after subarachnoid hemorrhage: A case-control study. Neurosurgery. 2010;66:696–700. doi: 10.1227/01.NEU.0000367618.42794.AA. discussion 700-691. [DOI] [PubMed] [Google Scholar]
- 17.Mayer S, Chong J. Critical care management of increased intracranial pressure. Journal of Intensive Care Medicine. 2002;17:55. [Google Scholar]
- 18.Helbok R, Schmidt JM, Kurtz P, Hanafy KA, Fernandez L, Stuart RM, Presciutti M, Ostapkovich ND, Connolly ES, Lee K, Badjatia N, Mayer SA, Claassen J. Systemic glucose and brain energy metabolism after subarachnoid hemorrhage. Neurocrit Care. 2010;12:317–323. doi: 10.1007/s12028-009-9327-4. [DOI] [PubMed] [Google Scholar]
- 19.Stuart RM, Schmidt M, Kurtz P, Waziri A, Helbok R, Mayer SA, Lee K, Badjatia N, Hirsch LJ, Connolly ES, Claassen J. Intracranial multimodal monitoring for acute brain injury: A single institution review of current practices. Neurocrit Care. 2010;12:188–198. doi: 10.1007/s12028-010-9330-9. [DOI] [PubMed] [Google Scholar]
- 20.Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006;34:1783–1788. doi: 10.1097/01.CCM.0000218413.51546.9E. [DOI] [PubMed] [Google Scholar]
- 22.Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, Ostapkovich ND, Levine JM, Le Roux P, Mayer SA. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: A microdialysis study. Crit Care Med. 2008 doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- 23.Fleckenstein W, Maas AI, Nollert G, de Long DA. Oxygen pressure in cerebrospinal fluid. Berlin: Blackwell; 1990. [Google Scholar]
- 24.Hoffman WE, Charbel FT, Edelman G. Brain tissue oxygen, carbon dioxide, and ph in neurosurgical patients at risk for ischemia. Anesth Analg. 1996;82:582–586. doi: 10.1097/00000539-199603000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Kiening KL, Unterberg AW, Bardt TF, Schneider GH, Lanksch WR. Monitoring of cerebral oxygenation in patients with severe head injuries: Brain tissue po2 versus jugular vein oxygen saturation. J Neurosurg. 1996;85:751–757. doi: 10.3171/jns.1996.85.5.0751. [DOI] [PubMed] [Google Scholar]
- 26.Maas AI, Fleckenstein W, de Jong DA, van Santbrink H. Monitoring cerebral oxygenation: Experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien) 1993;59:50–57. doi: 10.1007/978-3-7091-9302-0_9. [DOI] [PubMed] [Google Scholar]
- 27.Meixensberger J, Dings J, Kuhnigk H, Roosen K. Studies of tissue po2 in normal and pathological human brain cortex. Acta Neurochir Suppl (Wien) 1993;59:58–63. doi: 10.1007/978-3-7091-9302-0_10. [DOI] [PubMed] [Google Scholar]
- 28.Zauner A, Doppenberg EM, Woodward JJ, Choi SC, Young HF, Bullock R. Continuous monitoring of cerebral substrate delivery and clearance: Initial experience in 24 patients with severe acute brain injuries. Neurosurgery. 1997;41:1082–1091. doi: 10.1097/00006123-199711000-00011. discussion 1091-1083. [DOI] [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 30.Meixensberger J, Kunze E, Barcsay E, Vaeth A, Roosen K. Clinical cerebral microdialysis: Brain metabolism and brain tissue oxygenation after acute brain injury. Neurol Res. 2001;23:801–806. doi: 10.1179/016164101101199379. [DOI] [PubMed] [Google Scholar]
- 31.Johnston AJ, Steiner LA, Coles JP, Chatfield DA, Fryer TD, Smielewski P, Hutchinson PJ, O'Connell MT, Al-Rawi PG, Aigbirihio FI, Clark JC, Pickard JD, Gupta AK, Menon DK. Effect of cerebral perfusion pressure augmentation on regional oxygenation and metabolism after head injury. Crit Care Med. 2005;33:189–195. doi: 10.1097/01.ccm.0000149837.09225.bd. discussion 255-187. [DOI] [PubMed] [Google Scholar]
- 32.Jaeger M, Soehle M, Schuhmann MU, Winkler D, Meixensberger J. Correlation of continuously monitored regional cerebral blood flow and brain tissue oxygen. Acta Neurochir (Wien) 2005;147:51–56. doi: 10.1007/s00701-004-0408-z. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal G, Hemphill JC, 3rd, Sorani M, Martin C, Morabito D, Obrist WD, Manley GT. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36:1917–1924. doi: 10.1097/CCM.0b013e3181743d77. [DOI] [PubMed] [Google Scholar]
- 34.Carrera E, Schmidt JM, Oddo M, Fernandez L, Claassen J, Seder D, Lee K, Badjatia N, Connolly ES, Jr, Mayer SA. Transcranial doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009;65:316–323. doi: 10.1227/01.NEU.0000349209.69973.88. discussion 323-314. [DOI] [PubMed] [Google Scholar]
- 35.Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23:507–538. doi: 10.1016/j.ccc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- 37.Rosner MJ, Becker DP. Origin and evolution of plateau waves. Experimental observations and a theoretical model. J Neurosurg. 1984;60:312–324. doi: 10.3171/jns.1984.60.2.0312. [DOI] [PubMed] [Google Scholar]
- 38.Nordstrom CH. Assessment of critical thresholds for cerebral perfusion pressure by performing bedside monitoring of cerebral energy metabolism. Neurosurg Focus. 2003;15:E5. doi: 10.3171/foc.2003.15.6.5. [DOI] [PubMed] [Google Scholar]
- 39.Wartenberg K, Schmidt J, Fernandez A, Frontera J, Claassen J, Ostapkovich N, Badjatia N, Palestrant D, Parra A, Mayer S. Multiterritorial symptomatic vasospasm after subarachnoid hemorrhage: Predictors, associated complications, and impact on outcome. Stroke. 2007;38:463. [Google Scholar]
- 40.De Georgia MA, Deogaonkar A. Multimodal monitoring in the neurological intensive care unit. Neurologist. 2005;11:45–54. doi: 10.1097/01.nrl.0000149993.99956.09. [DOI] [PubMed] [Google Scholar]
- 41.Meixensberger J, Jaeger M, Vath A, Dings J, Kunze E, Roosen K. Brain tissue oxygen guided treatment supplementing icp/cpp therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2003;74:760–764. doi: 10.1136/jnnp.74.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hijdra A, van Gijn J, Nagelkerke NJ, Vermeulen M, van Crevel H. Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1250–1256. doi: 10.1161/01.str.19.10.1250. [DOI] [PubMed] [Google Scholar]
- 43.Brouwers PJ, Dippel DW, Vermeulen M, Lindsay KW, Hasan D, van Gijn J. Amount of blood on computed tomography as an independent predictor after aneurysm rupture. Stroke. 1993;24:809–814. doi: 10.1161/01.str.24.6.809. [DOI] [PubMed] [Google Scholar]
- 44.Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: Frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–1232. doi: 10.1161/01.str.0000015624.29071.1f. [DOI] [PubMed] [Google Scholar]