Abstract
Serotonin1A (5-HT1A) receptors are reported altered in the brain of subjects with major depressive disorder (MDD). Recent studies have identified transcriptional regulators of the 5-HT1A receptor and have documented gender-specific alterations in 5-HT1A transcription factor and 5-HT1A receptors in female MDD subjects. The 5′ repressor element under dual repression binding protein-1 (Freud-1) is a calcium-regulated repressor that negatively regulates the 5-HT1A receptor gene. This study documented the cellular expression of Freud-1 in the human prefrontal cortex (PFC) and quantified Freud-1 protein in the PFC of MDD and control subjects as well as in the PFC of rhesus monkeys chronically treated with fluoxetine. Freud-1 immunoreactivity was present in neurons and glia and was co-localized with 5-HT1A receptors. Freud-1 protein level was significantly decreased in the PFC of male MDD subjects (37%, p=0.02) relative to gender-matched control subjects. Freud-1 protein was also reduced in the PFC of female MDD subjects (36%, p=0.18) but was not statistically significant. When the data was combined across genders and analysed by age, the decrease in Freud-1 protein level was greater in the younger MDD subjects (48%, p=0.01) relative to age-matched controls as opposed to older depressed subjects. Similarly, 5-HT1A receptor protein was significantly reduced in the PFC of the younger MDD subjects (48%, p=0.01) relative to age-matched controls. Adult male rhesus monkeys administered fluoxetine daily for 39 wk revealed no significant change in cortical Freud-1 or 5-HT1A receptor proteins compared to vehicle-treated control monkeys. Reduced protein expression of Freud-1 in MDD subjects may reflect dysregulation of this transcription factor, which may contribute to the altered regulation of 5-HT1A receptors observed in subjects with MDD. These data may also suggest that reductions in Freud-1 protein expression in the PFC may be associated with early onset of MDD.
Keywords: Major depression, prefrontal cortex, 5-HT1A receptors, serotonin, transcription factor
Introduction
Evidence from in-vivo imaging and human postmortem brain studies have suggested that 5-HT1A receptors are altered in specific brain regions of subjects with major depressive disorder (MDD). A number of positron emission tomography (PET) imaging studies have reported reductions in 5-HT1A receptor-binding potential (BP) in various cortical regions of MDD patients. For example, Drevets and colleagues, using the selective 5-HT1A receptor antagonist, [11C]WAY-100635, reported that unipolar and bipolar depressed subjects with a familial form of illness exhibited reduced BP in the medial temporal cortex and hippocampus compared to healthy controls (Drevets et al. 1999, 2000). Sargent et al. (2000) also reported decreased 5-HT1A receptor BP in the frontal, temporal and limbic cortices in both medicated and unmedicated male subjects with MDD. Bhagwagar et al. (2004) noted that the decrease in 5HT1A BP throughout the cortex and limbic structures persisted in MDD despite clinical remission. In a cohort of female subjects, Moses-Kolko and colleagues found that 5-HT1A receptor BP was significantly decreased in the mesiotemporal, subgenual cingulate and lateral orbitofrontal cortices of postpartum depressed subjects relative to postpartum control subjects (Moses-Kolko et al. 2008). A recent study extended these previous findings by reporting decreased 5-HT1A receptor BP in several cortical areas including prefrontal, cingulate and insular of drug-naive primary-care patients with MDD compared to healthy control subjects (Hirvonen et al. 2008).
Although several studies utilizing human postmortem tissue have also reported alterations in 5-HT1A receptors in specific brain regions of suicide or subjects with MDD, the results have not been consistent. For example, Matsubara et al. (1991) reported an increase in 5-HT1A receptor-binding sites in the prefrontal cortex (PFC) [Brodmann areas (BA) 8, 9] of nonviolent suicide victims that were not psychiatrically characterized. Moreover, Arango et al. (1995) reported elevated binding to 5-HT1A receptors in the ven-trolateral PFC only of uncharacterized suicide victims, but as clarified via a personal communication by Arango and quoted in a recent review by Savitz et al. (2009), the elevation in 5-HT1A receptor binding previously reported was found in the suicide cases with comorbid alcoholism. In contrast to these studies, Lopez-Figueroa et al. (2004) found decreased 5-HT1A receptor mRNA levels in the dorsolateral PFC in MDD and Hsiung et al. (2003) reported reductions in 5-HT1A receptor signalling in the occipital cortex of depressed suicide victims. However, other studies have reported no significant changes in agonist-labelled 5-HT1A receptor-binding sites in the PFC of depressed suicide victims (Arranz et al. 1994; Dillon et al. 1991; Lowther et al. 1997; Stockmeier et al. 1997). Although a recent study by Stockmeier et al. (2009) reported that 5-HT1A receptor-binding sites labelled with the antagonist, [3H]MPPF, were significantly decreased in the outer layers of the orbitofrontal cortex of both male and female subjects with MDD relative to control subjects.
Previous studies have identified two novel transcription factors that regulate the expression of the 5-HT1A receptor gene. Nuclear deformed epidermal autoregulatory factor-1 (Deaf-1) or the human homo-log, NUDR, can function as a transcriptional inducer or repressor of 5-HT1A receptor depending on the synaptic localization of 5-HT1A receptors (Albert & Lemonde, 2004; Czesak et al. 2006; Lemonde et al. 2003). The 5′ repressor element under dual repression binding protein-1 (Freud-1) also known as CC2D1A (coiled-coil C2 domain-1A), the other transcription factor, binds to the 5′ 14-bp element (FRE) of the 5-HT1A receptor promoter and acts as a transcriptional repressor of the 5-HT1A receptor gene (Ou et al. 2003). Studies in rodents have found that both NUDR and Freud-1 are regionally located in the dorsal raphe nucleus, PFC and hippocampus and are co-localized with 5-HT1A receptors in neurons in these brain structures (Lemonde et al. 2003; Ou et al. 2003).
A recent report from our group measured NUDR and 5-HT1A receptor protein levels in the PFC of female and male MDD subjects and gender-matched control subjects, and found that the protein concentrations of NUDR and 5-HT1A receptors were significantly decreased in BA 10 of the PFC of only female MDD subjects relative to female controls (Szewczyk et al. 2009). Given this gender-specific alteration in NUDR and its target protein, 5-HT1A receptors in depressed female subjects, we were interested in investigating whether expression of Freud-1, a 5-HT1A receptor transcription factor, may also be altered in subjects with MDD. Therefore, the present study assesses the cellular localization of Freud-1 in the human PFC and quantifies Freud-1 protein levels in BA 10 of the PFC of female and male subjects diagnosed with MDD and in psychiatrically normal gender-matched control subjects.
Methods and materials
Subjects
All procedures in our study were approved by the Institutional Review Board of the University of Mississippi Medical Center and University Hospitals of Cleveland. Human brain specimens were obtained in the course of routine autopsies conducted at the Cuyahoga County Coroner’s Office, Cleveland, OH, after obtaining written consent from the legally defined next-of-kin. Blood and urine samples from all subjects were examined by the coroner’s office for psychotropic medications and substances of abuse. Subjects included 13 female and 12 male subjects diagnosed with MDD. The 13 female and 12 male control subjects never met criteria for an Axis I illness and had no history of a neurological disorder. Each depressed subject was matched with a control subject for gender and as closely as possible for age and post-mortem interval (PMI). Some of the pairs were also matched for race. The demographics for each subject are summarized in Table 1. The subjects included in the present study are the same as to those examined in a previously published study by our group (Szewczyk et al. 2009) except for two female control subjects, one female MDD subject, one male control subject and one male MDD subject.
Table 1.
Demographic characteristic of the subjects
| Control subjects |
Major depressive subjects |
Age of onset (yr) |
Duration of MDD (yr) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pair | Sex/race/ age (yr) |
PMI (h) |
pH | Cause of death | Sex/race/ age (yr) |
PMI (h) |
pH | Cause of death | Episodes S/M |
||
| 1 | F/W/23 | 11 | 6.8 | MVA | F/W/25 | 17 | 6.5 | Hanging | M | 18 | 7 |
| 2 | F/W/38 | 11 | 6.5 | HCD | F/W/38 | 12 | 6.4 | Drug overdose | M | 25 | 13 |
| 3 | F/W/44 | 32 | 6.7 | Heart | F/W/36 | 25 | 6.8 | Chronic asthmatic bronchitis |
S | 20 | 16 |
| 4 | F/W/46 | 24 | 6.3 | Homicide | F/W/38 | 24 | 6.5 | Drug overdose | M | 30 | 8 |
| 5 | F/B/49 | 20 | 6.8 | Cardiac arrhythmia |
F/W/44 | 24 | 6.7 | SIGSW | M | 32 | 12 |
| 6 | F/W/49 | 29 | 6.6 | Heart | F/W/48 | 24 | 6.1 | CO poisoning | M | 34 | 14 |
| 7 | F/B/45 | 9 | 6.9 | Heart | F/W/50 | 23 | 6.8 | Hanging | M | 25 | 25 |
| 8 | F/W/50 | 27 | 6.7 | Heart | F/W/50 | 28 | 6.5 | ACD | M | 30 | 20 |
| 9 | F/B/58 | 12 | 6.2 | HCD | F/W/63 | 18 | 6.3 | Heart | M | 20 | 43 |
| 10 | F/W/65 | 26 | 6.2 | MVA | F/W/73 | 17 | 6.6 | Aortic aneurysm | M | 72 | 1 |
| 11 | F/W/78 | 11 | 6.4 | Heart disease | F/W/72 | 19 | 6.6 | Drowning | S | 33 | 39 |
| 12 | F/B/80 | 21 | 6.8 | HCD | F/W/78 | 25 | 6.9 | Fall from height | M | 45 | 33 |
| 13 | F/W/83 | 25 | 6.7 | Heart | F/W/87 | 24 | 6.6 | Aortic aneurysm | M | 46 | 41 |
| 14 | M/W/27 | 17 | 6.9 | Homicide | M/B/30 | 18 | 6.9 | SIGSW chest | M | 27 | 3 |
| 15 | M/B/27 | 11 | 6.8 | Asthma | M/W/36 | 11 | 6.9 | Undetermined | M | 35 | 1 |
| 16 | M/W/44 | 6 | 6.7 | Aortic aneurysm | M/W/45 | 8 | 6.7 | Multiple knifing | S | 43 | 2 |
| 17 | M/W/47 | 25 | 6.1 | Pulmonary embolism |
M/B/46 | 17 | 6.3 | Homicide | S | 45 | 1 |
| 18 | M/W/47 | 17 | 6.9 | Heart | M/W/47 | 11 | 6.8 | SIGSW head | M | 20 | 27 |
| 19 | M/W/50 | 26 | 6.9 | MCI | M/W/42 | 44 | 6.7 | Hanging | M | 42 | 1 |
| 20 | M/B/53 | 23 | 6.8 | Electrocution | M/W/54 | 23 | 6.2 | CO poisoning | M | 51 | 3 |
| 21 | M/W/58 | 21 | 6.8 | Heart disease | M/W/53 | 29 | 6.7 | SIGSW head | M | 28 | 25 |
| 22 | M/W/59 | 6 | 6.8 | Heart | M/W/62 | 5 | 6.7 | Hanging | M | 43 | 19 |
| 23 | M/W/71 | 24 | 6.8 | Cardiac rupture | M/W/74 | 24 | 6.9 | Hanging | M | 72 | 2 |
| 24 | M/W/73 | 22 | 6.7 | Heart | M/W/74 | 25 | 6.7 | SIGSW head | M | 50 | 24 |
| 25 | M/W/82 | 16 | 6.7 | Aneurysm | M/W/82 | 12 | 6.5 | CO poisoning | M | 73 | 9 |
F, Female; M, male; W, white; B, black; PMI, post-mortem interval in hours; Episodes – S, single; M, multiple; HCD, hypertensive cardiac disease; MVA, motor vehicle accident; ACD, Atherosclerotic cardiovascular disease; MCI, myocardial infarction; SIGSW, single gunshot wound.
Retrospective, informant-based psychiatric assessments were performed for all depressed and control subjects as previously described (Stockmeier et al. 2004). About 3 months after the death of the subjects, a trained interviewer administered the Schedule for Affective Disorders and Schizophrenia – Lifetime Version (SADS-L; Spitzer, 1978) to knowledgeable next-of-kin of 18 of the depressed subjects, as previously described (Stockmeier et al. 1997). The Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID; First et al. 1996) was administered to next-of-kin of the seven remaining depressed subjects. Axis I psychopathology was independently assessed by a clinical psychologist and psychiatrist and consensus diagnosis was reached in conference using information from the interview, previous hospitaliz-ations and doctors’ records, and the coroner’s office. Responses from the subjects evaluated with the SADS-L were also recorded in the SCID, and regardless of the structured diagnostic interview used, all subjects met criteria for MDD based on DSM-IV (APA, 1994). Twenty-three subjects met DSM-IV criteria for a major depressive episode within the last month of life and MDD was in partial remission for one subject.
All 13 female depressed subjects met DSM-IV criteria for an Axis I diagnosis of MDD. Twelve female subjects met criteria for MDD within the last month of life and one female subject was in partial remission during the last month. Eleven female subjects had multiple depressive episodes during their life and two female subjects had a single episode. Alcohol dependence in one female subject and alcohol abuse in another were in remission at the time of death. Among the 13 female depressed subjects, 11 had a prescription for an antidepressant medication at the time of death, although post-mortem toxicology screening revealed only the presence of diazepam in one subject. The mean (±S.E.M.) age of onset of depression was 32.0± 4.2 yr and the average duration of illness was 23.7 ± 4.3 yr.
Eleven male depressed subjects met DSM-IV criteria for an Axis I diagnosis of MDD, and they also met MDD criteria in their last month of life. One depressed male subject met clinical criteria for dysthymia. Nine male subjects had multiple depressive episodes during their life and two male subjects had a single episode. Two male subjects had a history of alcohol abuse and one of benzodiazepine abuse, although these disorders were in remission at the time of death. Among the 12 depressed male subjects, eight had a prescription for an antidepressant medication at the time of death; however, post-mortem toxicology screening revealed only the presence of temazepam in one subject. The mean (±S.E.M.) age of onset of depression was 45.3 ± 4.8 yr and the average duration of illness was 9.9 ± 3.1 yr.
Non-human primate studies
See Supplementary material (available online).
Tissue sampling
The study was carried out on blocks of tissue which were dissected approximately 1–2 cm from the frontal pole of the right hemisphere. The tissue blocks consisted of BA 10; however, some portions of the blocks also contained adjacent cortical areas. In order to ensure that sections or tissue punches were collected consistently from BA 10, two 30-µm-thick sections were cut from each cortical block, stained for Nissl and examined cytoarchitectonically, as previously described (Rajkowska & Goldman-Rakic, 1995; Rajkowska et al. 1999).
Immunohistochemistry
Immunohistochemistry was used to examine the expression and laminar distribution of Freud-1 in the human PFC of normal control subjects. Frozen 30-µm sections from PFC (BA 10) were fixed in 4% para-formaldehyde [in 0.05 M phosphate-buffered saline (PBS)] for 1 h at room temperature, preincubated in 5% normal horse serum in PBS for 30 min and then incubated for 24 h at 4 °C in the same solution containing rabbit anti-Freud-1 polyclonal antibody (1:1000, Ou et al. 2003). Sections were washed in PBS and incubated for 4 h at room temperature in biotiny-lated horse anti-rabbit IgG (1:200; Vector Laboratories, USA) in PBS buffer. After incubation, the sections were processed using the Vectastain ABC immunoperoxidase kit (Vector Laboratories) for 24 h at 4 °C. Antibody distribution was visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB, 0.05%; Sigma, USA).
Immunofluorescence
Immunofluorescence was used to examine the cellular expression of Freud-1 and co-localization with 5-HT1A receptor in the human PFC. Frozen 20-µm sections from PFC (BA 10) were subjected to double-fluorescent immunolabelling procedure to detect in individual sections simultaneously Freud-1 and the neuronal marker – neuronal nuclei (NeuN), Freud-1 and the astrocytic marker – glial fibrillary acidic protein (GFAP), and Freud-1 and the 5-HT1A receptor. Each section was incubated overnight with the rabbit anti-Freud-1 poly-clonal antibody (1:100) and a mouse anti-GFAP monoclonal antibody (1:1000; Chemicon, USA), a mouse anti-NeuN monoclonal antibody (1:1000; Chemicon) or a mouse anti-5-HT1A polyclonal antibody (1:100; Affinity Bioreagent, USA), respectively. After washes in TBS, sections were incubated for 90 min with a mixture of a goat anti-mouse antibody conjugated with the fluorochrome Cy5 (1:200; Jackson Immuno-chemicals, USA) and a goat anti-rabbit antibody conjugated to fluorochrome Cy2 (1:200; Jackson Im-munochemicals) and washed again before cover-slipping. Omission of the primary or the secondary antibody resulted in the absence of immunostaining. The cellular localization of immunofluorescence was analysed using a Nikon confocal microscope.
Western blot
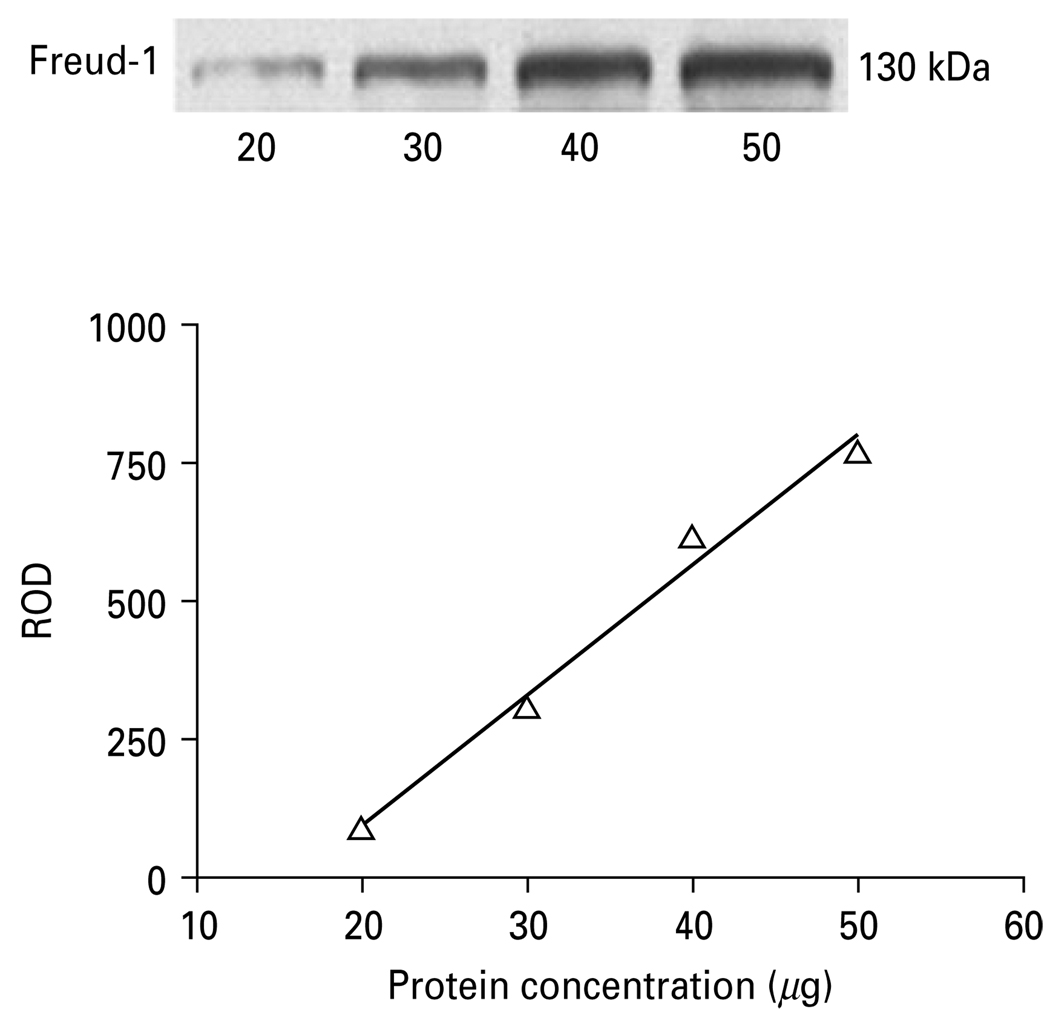
Immunolabelling of Freud-1 and the 5-HT1A receptor were determined in the tissue punches of PFC (BA10) from both human and monkey cortical blocks. Equal volumes of protein samples containing mostly membrane and nuclear fraction (30 µg protein) were resolved on 12.5% sodium dodecyl sulfate–polyacrylamide gel and blotted on nitrocellulose membrane. The blots were incubated overnight at 4 °C with affinity-purified primary antibody that was generated to an amino-terminal peptide from Freud-1 (1:10000) and with rabbit anti-5-HT1A receptor polyclonal antibody (1:2000; Aviva System Biology, USA). Antibody specificity experiments revealed that pre-incubation of the primary 5-HT1A receptor antibody with a specific 5-HT1A peptide (Aviva System Biology), and preincubation of the primary Freud-1 antibody with a specific Freud-1 peptide completely blocked immunoreactivity. As a control for transfer and sample loading, anti-actin monoclonal antibody was used (1:5000; Chemicon). Immunoreactivity of Freud-1 and 5-HT1A receptor was investigated in the pairs of depressed and control subjects matched for age, gender and PMI as well as in fluoxetine- and vehicle-treated monkeys. Each subject pair was immunoblotted in duplicate. The relationship between optical density values and the concentration of Freud-1 and 5-HT1A receptor immunoreactivity was determined by loading increasing concentrations of sample onto gels and immunoblotting with anti-Freud-1 or anti-5-HT1A receptor antibody. Relative optical density values of immunoreactive bands were measured and presented as a function of protein concentration. The relationship between optical density and protein concentrations was linear (Fig. 1).
Fig. 1.
Relationship between the relative optical density (ROD) values of Freud-1 immunoreactivity and increasing total protein concentrations (20, 30, 40, 50 µg) of human prefrontal cortex.
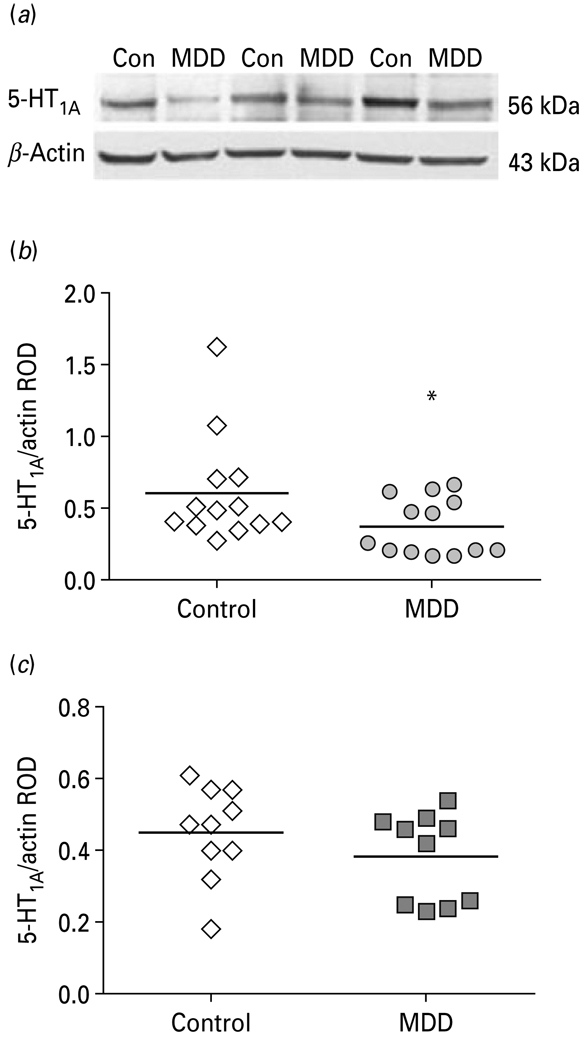
Relative optical density of Freud-1 and 5-HT1A receptor bands were analysed using imaging software (MCID Elite 7.0; Imaging Research, Canada) and normalized by the optical density of the corresponding β-actin band. The anti-Freud-1 antibody detected a major band on the gel with a molecular weight of 130 kDa, corresponding to the long isoform which is the major isoform in human cells (Rogaeva & Albert, 2007). However, we did observe a doublet in the human prefrontal cortical samples consisting of the major 130-kDa band and faint lower molecular weight band (Fig. 4a). These observations are consistent with our previous studies (Rogaeva & Albert, 2007). We have not identified the modification that causes this, but the upper isoform seems to be more nuclear localized. The anti-5-HT1A receptor antibody detected a 56-kDa band as previously described (Szewczyk et al. 2009).
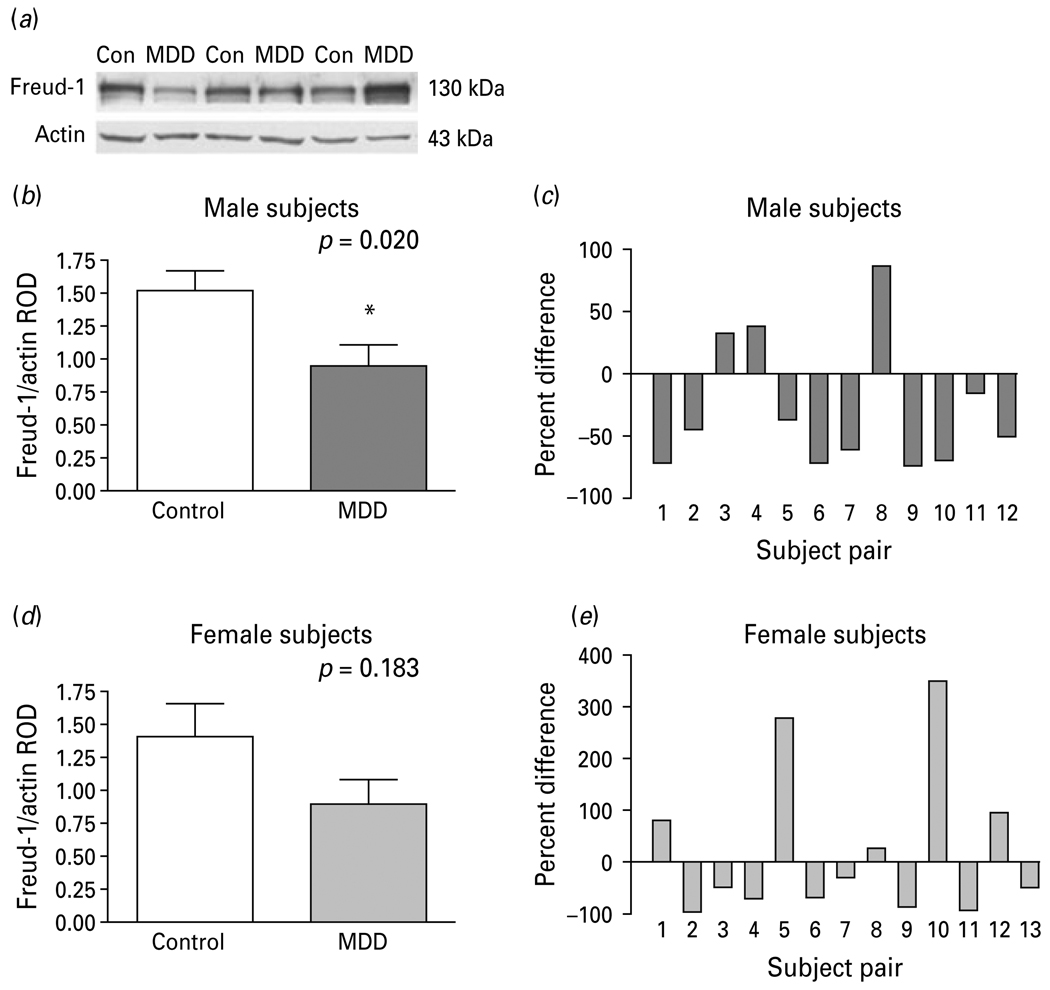
Fig. 4.
(a) Representative Western blots showing the immunolabelling of Freud-1 and β-actin in prefrontal cortex (PFC) of three pairs of control (Con) and matched major depressive disorder (MDD) subjects. (b) Freud-1 protein levels in PFC of 12 male (b, c) and 13 female (d, e) control and MDD subjects expressed as the mean ± S.E.M. of Freud-1/actin relative optical density (ROD). Data were analysed using a maximum-likelihood mixed-models test. Male subject groups: control subjects (1.52 ± 0.16) compared to the matched MDD subjects (0.96 ± 0.15, t21=2.53, * p=0.020). Female subject groups: control subjects (1.41 ± 0.25) compared to the matched MDD subjects (0.90 ± 0.18, t21=1.38, p=0.183). Freud-1 levels in PFC of male (c) and female (d) depressive subjects expressed as percent difference from paired control subjects.
Statistical analysis
Data were analysed using a matched-pairs design. Each subject was measured at least twice so that there are two sources of potentially correlated observations, replicates within subjects and subjects within pairs. That is, data are considered multilevel with subjects representing the first level and pairs representing the second level. Statistical tests were initially performed separately for male and female groups, and then subject groups combined and further analysed. A maximum-likelihood mixed-models test was used to estimate parameters of the models, assuming pairs and subjects within pairs were random components (SAS, Little et al. 1996). As a first step, unadjusted models were fit to compare depressives vs. controls without adjusting for potential confounders. Gender-specific adjusted models included the main effect for comparing depressives vs. controls and covariates for age, PMI, pH and cause of case death (suicide vs. non-suicide). Based on previous studies by our group documenting reduced levels of the astrocytic marker, GFAP in the PFC of MDD subjects aged <60 yr at the time of death (Si et al. 2004) and significantly lower density of pyramidal neurons in layers IIIc and V of the PFC in elderly depressed subjects aged ≥60 years (Rajkowska et al. 2005), we used this age criteria to categorize our MDD and control subjects. Since age 58 yr was the closest age of our subjects to 60 yr, age was dichotomized as <58 years vs. ≥58 years. Interactions between the main effect, depressives vs. controls, and each of the potentially confounding covariates were investigated and dropped from the model if they were not statistically significant. Results for the depressed and control groups are reported as mean ± standard error based on the mixed model. Results for main effects are considered significant if p<0.05. The non-human primate immunoblot data were analysed using an unpaired Student’s t test.
Results
Laminar and cellular localization of Freud-1 immunoreactivity
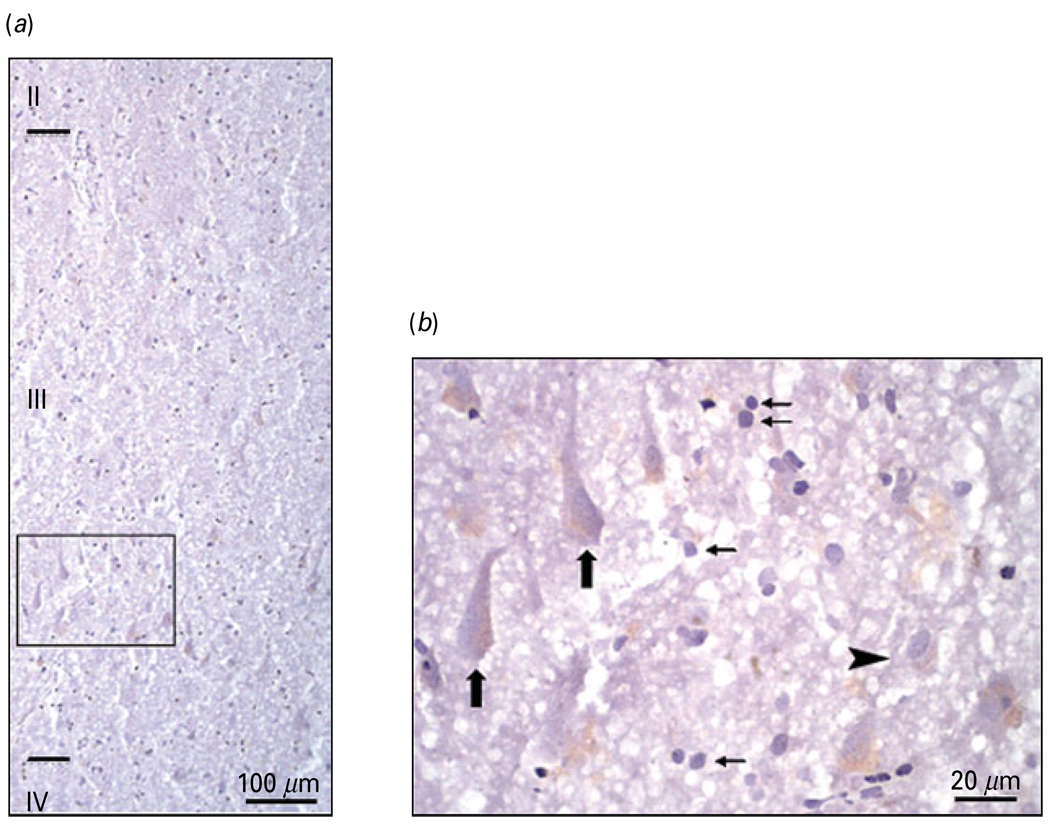
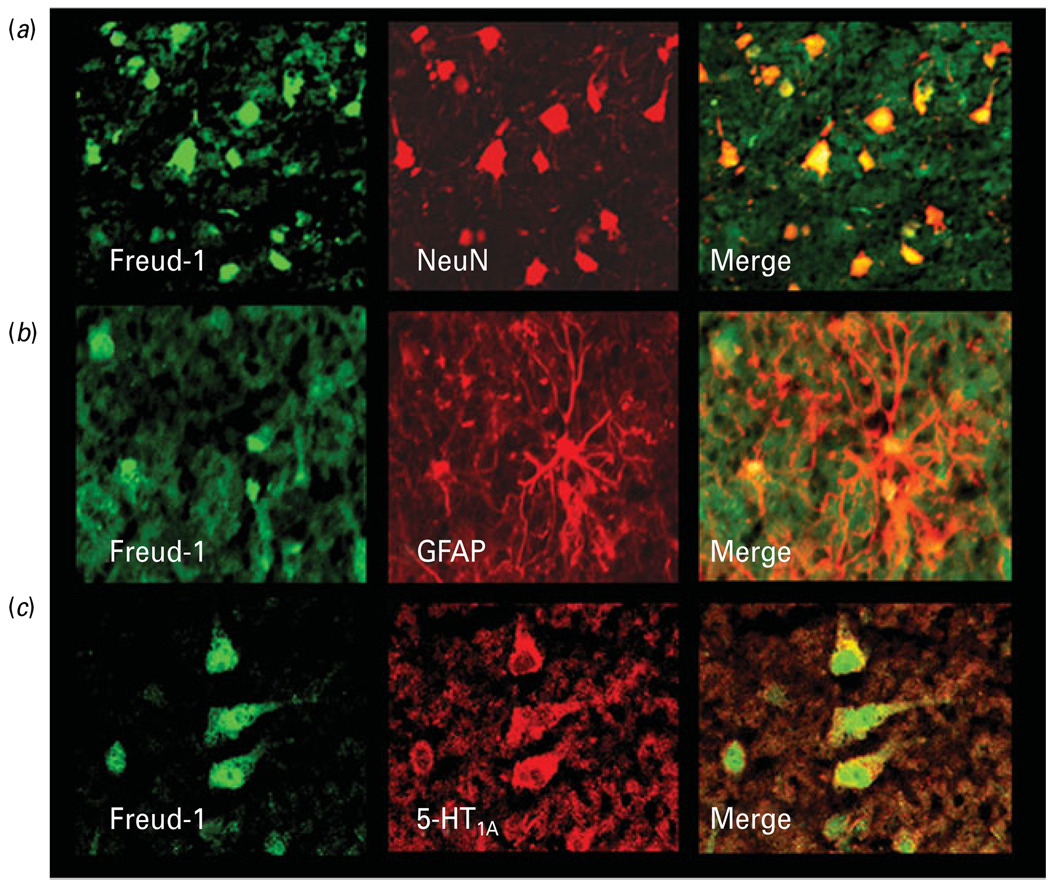
Freud-1 immunoreactivity was observed in cellular profiles in both prefrontal grey and white matter of PFC (BA 10). In the grey matter, immunoreactivity was spread across all cortical layers with the highest abundance in superficial layer II and upper layer III (Fig. 2a). Freud-1 immunoreactivity was found in both neurons and glia localized to nuclear and perinuclear areas of neuronal cell bodies (Fig. 2b). Freud-1 im-munoreactivity in cortical neurons appeared in both pyramidal neurons and smaller non-pyramidal inter-neurons (Figs 2b and 3a). Double-fluorescence immunostaining performed for Freud-1 and neuronal marker NeuN as well as for Freud-1 and astrocytic marker GFAP showed that the majority of Freud-1-positive cortical cells represent neurons (Fig. 3a). However, some glial cells expressing GFAP also expressed Freud-1 immunoreactivity (Fig. 3b). Co-labelling of Freud-1 and 5-HT1A receptor revealed that most cortical cells expressing 5-HT1A receptor immunoreactivity also expressed Freud-1 (Fig. 3c).
Fig. 2.
Laminar and cellular localization of Freud-1 immunoreactivity (IR) in the human prefrontal cortex (PFC) (BA 10). (a) Distribution of Freud-1 IR in the upper cortical layers of BA 10. (b) High-power micrograph of Freud-1 IR [box in panel (a)] showing the presence of immunoreactive product in both neurons and the glial cell nuclei located in layer III. Large arrows identify Freud-1 IR localized to pyramidal neurons, small arrows identify Freud-1 IR is localized to glial cells and arrowhead identifies Freud-1 IR localized to non-pyramidal neuron.
Fig. 3.
Colocalization of Freud-1 immunoreactivity (IR) in neurons and glia. (a) Co-labelling of Freud-1 IR (green) with the neuronal marker NeuN (red). Freud-1 protein (yellow) is localized to neuronal nuclei and perinuclear areas. (b) Co-labelling of Freud-1 IR (green) with the astrocytic marker GFAP (red). Freud-1 (yellow) is localized in glial cell nuclei. (c) Colocalization of Freud-1 IR (green) and immunoreactivity for 5-HT1A receptor (red). Freud-1 IR (yellow) is present in the cytoplasm of a majority of cells expressing 5-HT1A receptor immunoreactivity.
Protein level of Freud-1
Figure 4a displays representative immunoblots of Freud-1 and β-actin protein from matched control and MDD subjects. The results of the Western blot analyses of Freud-1 protein in the PFC (BA 10) of depressed and control subjects are summarized in Fig. 4(b–e). The level of Freud-1 protein for each subject was determined as the ratio of the optical density of Freud-1 band to the optical density of the β-actin band. There was no significant difference between control and depressed subjects in the mean level of β-actin (t24=1.59, p=0.123; Fig. S4). The mean protein level of Freud-1 in the PFC was significantly decreased by 37% in the male depressed subjects (0.96 ± 0.15, t21=2.53, p=0.020) compared to the matched male control group (1.52 ± 0.16; Fig. 4b). Comparison of individual matched pairs revealed that the mean Freud-1 protein level was decreased in nine of the 12 depressed male subjects compared to the matched control subjects (Fig. 4c). Although the mean Freud-1 protein level in the PFC of the female depressed subjects (0.90 ± 0.18, t21=1.38, p=0.183) was also reduced relative to matched female control subjects (1.41 ± 0.25; Fig. 4d), the difference was not found to be statistically significant. Eight of the 13 depressed female subjects had lower levels of Freud-1 protein compared to the matched control subjects (Fig. 4e).
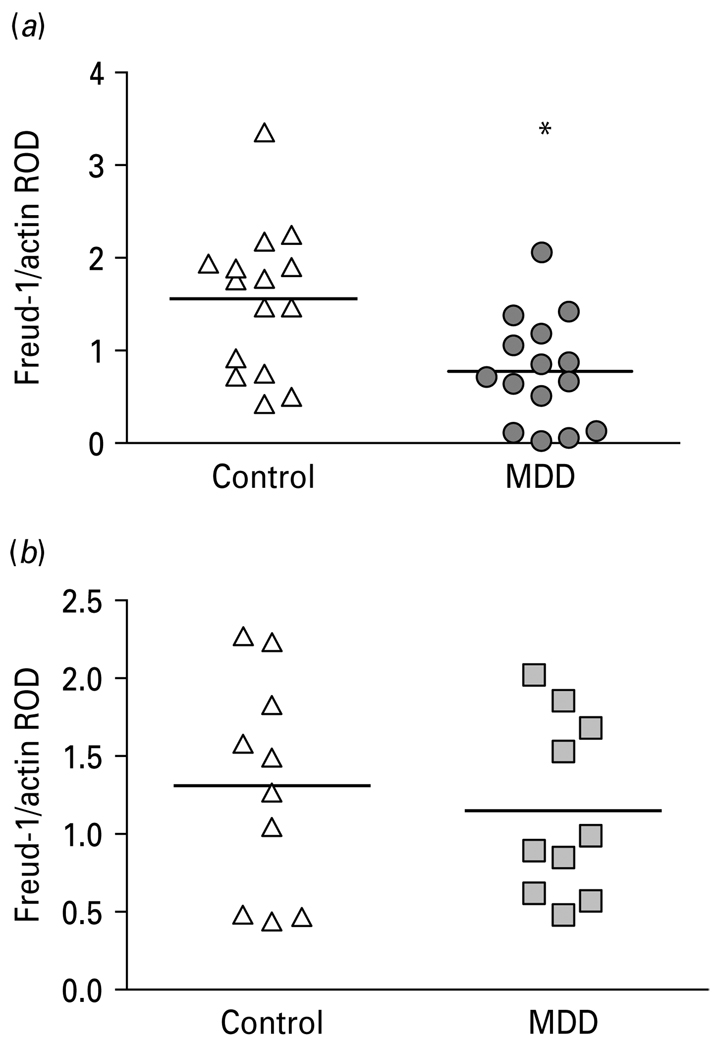
Given that Freud-1 is localized to neurons and glial cells in the PFC and that previous studies have documented age-dependent changes in neuronal and glia cell density in the PFC of depressed subjects, we categorized our subjects based on these previous studies (Si et al. 2004; Rajkowska et al. 2005) using an age cutoff of 58 yr and further analysed Freud-1 protein levels in two age groups, those aged <58 yr and those aged ≥58 yr across both genders. Mean Freud-1 protein levels in the PFC were significantly reduced by 50% in the younger MDD subjects (0.78 ± 0.15; age <58 yr; t21=2.84, p=0.010) relative to the age-matched control subjects (1.56 ± 0.20; Fig. 5a), whereas, Freud-1 protein levels were unchanged in the older MDD subjects (1.15 ± 0.18; age ≥58 yr; t21=0.53, p=0.602; Fig. 5b) relative to age-matched controls (1.32 ± 0.22).
Fig. 5.
(a) Scatterplot of Freud-1 protein levels normalized to actin relative optical density (ROD) in prefrontal cortex (PFC) of younger (<58 yr) controls and major depressive disorder (MDD) subjects. (b) Scatterplot of Freud-1 protein levels normalized to actin ROD in PFC of older (≥58 yr) control and MDD subjects. Data were analysed using a maximum-likelihood mixed-models test. Younger subject groups: control subjects (1.56 ± 0.20, mean ± S.E.M.) compared to the matched MDD subjects (0.78 ± 0.15; age <58 yr; t21=2.84, * p=0.010). Older subject groups: control subjects (1.32 ± 0.22) compared to matched MDD subjects (1.15 ± 0.18; age ≥58 yr; t21=0.53, p=0.602).
Since protein data on the 5-HT1A receptor was available on these same subjects from our previous study (Szewczyk et al. 2009), but was not analysed by age classification, we analysed the 5-HT1A receptor in the young and old MDD and age-matched control groups. Mean 5-HT1A receptor protein levels in the PFC were significantly reduced by 33 % in the younger MDD subjects (0.37 ± 0.07; age <58 yr; t18 = 2.58, p = 0.019) relative to the age-matched control subjects (0.55 ± 0.10; Fig. 6a), whereas, 5-HT1A receptor protein levels were unchanged in the older MDD subjects (0.39 ± 0.05; age ≥58 yr; t18 = 0.76, p = 0.459; Fig. 6b) relative to age-matched controls (0.45 ± 0.06). However, the correlation between Freud-1 and 5-HT1A receptor protein levels was not statistically significant (Spearman’s p = 0.26, p = 0.080).
Fig. 6.
(a) Representative Western blots showing the immunolabelling of 5-HT1A receptor and β-actin in prefrontal cortex (PFC) of three pairs of control (Con) and matched MDD subjects. (b) Scatterplot of 5-HT1A receptor protein levels normalized to actin relative optical density (ROD) in PFC of younger (<58 yr) controls and major depressive disorder (MDD) subjects. (c) Scatterplot of 5-HT1A receptor protein levels normalized to actin ROD in PFC of older (≥58 yr) control and MDD subjects. Data were analysed using a maximum-likelihood mixed-models test. Younger subject groups: control subjects (0.55 ± 0.10, mean ± S.E.M.) compared to the matched MDD subjects (0.37 ± 0.07; age <58 yr; t18=2.58, * p=0.019). Older subject groups: control subjects (0.45 ± 0.06) compared to matched MDD subjects (0.39 ± 0.05; age ≥58 yr; t18=0.76, p=0.459).
The mean 5-HT1A receptor protein level for the three HTR1A genotypes of the C(−1019)G polymorphism (C/C, n = 10; C/G, n = 13; G/G, n = 3) were similar in magnitude (0.44 ± 0.09, 0.51 ± 0.10, 0.50 ± 0.07, respectively) and not found to be significantly different (ANOVA, p = 0.4843).
Effects of covariates on Freud-1
The effect of potential confounding variables such as age, PMI, brain pH, cause of death (suicide or non-suicide), onset of depression or duration of depression on protein levels of Freud-1 was evaluated in the male and female subject groups using the adjusted mixed-models test. There were no statistically significant differences in age, PMI and pH between male and female groups and there were no significant changes in the results after adjusting for age, PMI, brain pH as well as cause of death. Moreover, there was no significant correlation between age at death and Freud-1 levels among the control subjects (r=−0.10, p=0.6338; Fig. S3). There was a statistically significant difference between female and male MDD groups in the onset of depression (t23=2.08, p=0.048) and duration of depression (t23=2.56, p=0.017). However, there is no statistical evidence to support effects of these potentially confounding variables on changes in Freud-1 protein levels related to depression.
Effects of chronic fluoxetine on Freud-1 and 5-HT1A receptor in monkey PFC
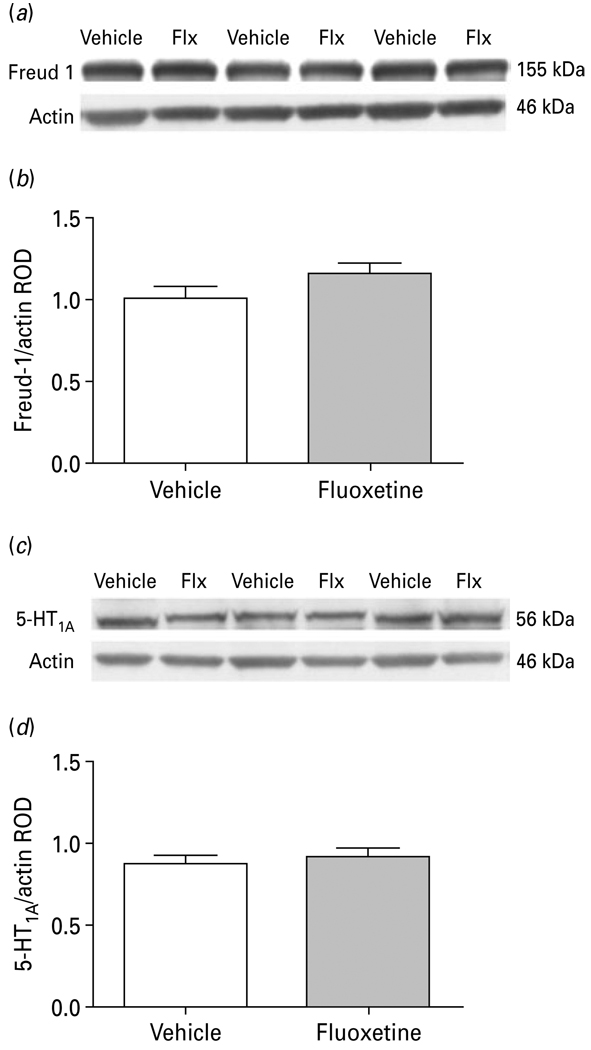
To investigate whether possible confounding effects of antidepressant medication may account for the Freud-1 and 5-HT1A receptor protein changes in the PFC of subjects diagnosed with MDD, rhesus monkeys were treated with either vehicle or fluoxetine for 39 wk. No significant differences were detected in Freud-1 (vehicle: 1.01 ± 0.07; fluoxetine: 1.16 ± 0.07; t11=1.503, p=0.161) or in 5-HT1A (vehicle: 0.88 ± 0.05; fluoxetine: 0.92 ± 0.06; t11=0.536, p=0.602) protein levels in the PFC of fluoxetine-treated monkeys compared to vehicle-treated controls (Fig. 7).
Fig. 7.
(a) Representative Western blots showing the immunolabelling of Freud-1 and β-actin in prefrontal cortex (PFC) of vehicle- and fluoxetine (Flx)-treated monkeys. (b) Freud-1 protein levels in PFC of six vehicle- and seven fluoxetine-treated monkeys expressed as the mean ± S.E.M. of Freud-1/actin relative optical density (ROD). (c) Representative Western blots showing the immunolabelling of 5-HT1A receptor and β-actin in PFC of vehicle and fluoxetine-treated monkeys. (d) 5-HT1A receptor protein levels in PFC of six vehicle- and seven fluoxetine-treated monkeys expressed as the mean ± S.E.M. of 5-HT1A/actin ROD.
Discussion
The results of our study found that the concentration of Freud-1 protein was significantly decreased in the PFC (BA 10) in male MDD subjects compared to gender-matched male control subjects. In contrast, although Freud-1 protein levels were also reduced in the female subjects diagnosed with MDD relative to the gender-matched control subjects, the data did not reach statistical significance. In addition, the decrease in Freud-1 expression was greatest in the younger MDD subjects (<58 yr) compared to older MDD subjects (≥58 yr). Similarly, 5-HT1A receptor protein expression was reduced to a greater extent in the younger MDD subjects relative to age-matched control subjects.
Freud-1 has been shown to act as a transcriptional repressor of the 5-HT1A receptor gene in both serotonin raphe cells and in non-serotonergic post-synaptic neurons. Freud-1 binds to the 5′ 14-bp element (FRE) of the 5-HT1A promoter and negatively regulates basal expression of the 5-HT1A receptor in neurons. Freud-1 exists as two isoforms, a short form (Freud-1S) and a long form (Freud-1L). Previous studies identified Freud-1S as the predominant isoform in rodent cells, whereas Freud-1L is most abundant in human cells and is the major isoform that mediates repression of the human 5-HT1A receptor gene (Rogaeva & Albert, 2007).
As discussed earlier, both PET imaging and human post-mortem brain studies have documented alterations in 5-HT1A receptors in several cortical and forebrain brain regions of subjects with major depression (for reviews see Albert & Lemonde, 2004; Drevets et al. 2007; Savitz et al. 2009; Stockmeier, 2003). While the findings from the in-vivo imaging studies are relatively consistent with the majority of the reports revealing decreased BP of 5-HT1A receptors in cortical regions of depressed subjects, the postmortem findings are much more disparate with reports of increased, decreased and no change in 5-HT1A receptor-binding sites in depressed subjects (for reviews see Stockmeier, 2003; Albert & Lemonde, 2004; Savitz et al. 2009). Many of the previous postmortem studies included depressed subjects that had committed suicide and the majority of the subjects examined in the present study also committed suicide. However, we did not find any significant difference in 5-HT1A receptor or Freud-1 protein levels when suicide vs. non-suicide was analysed as a covariate. Possible differences between our findings and those of previous post-mortem 5-HT1A receptor studies may be related to gender influences since our study included 13 female MDD subjects whereas the majority of previous post-mortem studies only included male subjects. Further, methodological differences may be considered because the present study used immunblotting to measure 5-HT1A protein compared to previous receptor autoradiography studies measuring 5-HT1A binding sites.
We recently examined NUDR and 5-HT1A receptor protein expression in the same PFC (BA 10) homogenates obtained from the majority of the same cohort of MDD subjects and gender-matched controls that were assayed for Freud-1 in the present report. Both NUDR and 5-HT1A receptor protein levels were significantly reduced in the PFC of only depressed females relative to gender-matched controls, but were unchanged in males with MDD (Szewczyk et al. 2009). It is interesting that the cortical Freud-1 protein changes in MDD subjects are different with respect to gender. In contrast to NUDR and 5-HT1A receptor, Freud-1 was significantly reduced in the male group of MDD subjects but these same male subjects failed to show a change in cortical 5-HT1A receptor expression compared to gender-matched controls (Szewczyk et al. 2009). Since Freud-1 is a repressor of 5-HT1A receptors, reduced levels of Freud-1 in the male MDD subjects may reflect an adaptive response to maintain normal levels of 5-HT1A receptors. Similarly, while Freud-1 was not significantly decreased in the female MDD subjects, a trend for reduced expression of Freud-1 was observed, but perhaps this adaptive response in Freud-1 is not sufficient to override the defect in NUDR expression and the corresponding reduction in 5-HT1A receptors in females with MDD (Szewczyk et al. 2009). It is interesting that the decrease in cortical Freud-1 expression in depressed male subjects is consistent with a recent report of decreased Freud-1 mRNA and protein expression in the PFC of male rats exposed to chronic restraint stress (Iyo et al. 2009) which suggest that Freud-1 may play a role in stress responses.
Our immunofluorescence experiments revealed that Freud-1 is localized to both neurons and to glial cells in the human PFC, but the decrease in Freud-1 expression in males with MDD detected by immunoblotting cannot distinguish the cellular specificity of this alteration in the PFC. However, the observation that both Freud-1 and 5-HT1A receptor levels were significantly decreased in the younger MDD subjects and not in the older MDD subjects may provide some insights into the cellular characteristics of these changes. For example, several post-mortem studies indicate smaller size and/or decreased density of neurons and glia cells in the PFC of depressed subjects (Cotter et al. 2002; Miguel-Hidalgo et al. 2000; Rajkowska & Miguel-Hidalgo, 2007; Rajkowska, 2002; Rajkowska et al. 1999, 2005, 2007). Moreover, glia pathology seems to be more prevalent in younger MDD subjects and neuronal pathology of pyramidal projection neurons seems to be more severe in elderly MDD subjects (Miguel-Hidalgo et al. 2000; Rajkowska et al. 2005; Si et al. 2004). Taking these findings into consideration, and the fact that Freud-1 and 5-HT1A receptors (Doherty & Pickel, 2001; Patel & Zhou, 2005) are also present on glial cells, the diminished expression of Freud-1 and 5-HT1A receptor proteins observed in our study in the young depressed subjects may reflect alterations in glial cell density and possibly non-pyramidal GABA-containing neurons in the PFC of this cohort of depressed subjects. On the other hand, the reductions in Freud-1 and 5-HT1A receptor observed in young depressed subjects may be related to neuronal deficits as smaller sizes of neuronal cell bodies were reported in the PFC of younger subjects with MDD (Cotter et al. 2002; Rajkowska et al. 1999). The greater decrease of Freud-1 in younger (<58 yr) depressed subjects suggests that reduced expression of Freud-1 may be an early event to compensate for diminished expression of 5-HT1A receptor levels but is not sufficient to override other negative regulators of 5-HT1A or it raises further questions regarding the cellular pathology in the PFC of early onset major depression. It is possible that Freud-1 dysregulation could contribute to the development of depression susceptibility.
It is unlikely that Freud-1 protein levels were influenced by antidepressant medication since toxicology screens were negative for antidepressants for all depressed subjects. However, since several of the depressed subjects had a prescription for an anti-depressant at the time of death, questions may be raised regarding the compliance of these patients and the possible long-term effects of antidepressants on Freud-1 expression. To address this issue and to directly examine the effects of chronic antidepressant treatment on cortical Freud-1 and 5-HT1A receptor expression, we treated rhesus monkeys daily with the serotonin-selective reuptake inhibitor (SSRI), fluoxetine, for 39 wk and then measured protein levels in PFC (BA 10) homogenates. We found that chronic fluoxetine administration did not alter protein levels of either Freud-1 or 5-HT1A receptors in monkey PFC. While there have been no previous studies examining the effects of antidepressants on Freud-1, the lack of change in 5-HT1A receptors is consistent with previous rodent studies reporting no changes in post-synaptic 5-HT1A receptor-binding sites following chronic fluoxetine or other SSRIs (Hensler, 2002; Hensler et al. 1991; Le Poul et al. 2000). The non-human primate data suggest that the decrease in Freud-1 and 5-HT1A receptor proteins in MDD subjects do not result from the patients’ antidepressant medication and are probably related to the pathophysiology of MDD.
Although Freud-1 has been shown to function as a transcriptional repressor of 5-HT1A receptors, it has also been reported that Freud-1 binds to the promoter of the dopamine D2 receptor and acts as a negative regulator of D2 receptor expression (Rogaeva et al. 2007). Therefore, the reduced levels of Freud-1 in MDD subjects may also influence expression of D2 receptors in the PFC of the depressed subjects.
In summary, this report presents evidence of reduced protein expression of Freud-1, a novel tran-scriptional repressor of the 5-HT1A receptor, in the PFC of male subjects with MDD and the reduction in Freud-1 was most pronounced in younger MDD subjects across both genders. These data provide a basis for future experiments to explore the role of Freud-1 in the developmental pathophysiology of MDD.
Supplementary Material
Acknowledgements
We acknowledge Dr Abiye Iyo for genotyping the serotonin transporter from the non-human primate samples and Tarsha Harris for technical assistance in punching tissue samples and preparation of proteins for assays. The assistance of James Overholser, Ph.D., George Jurjus, M.D., Herbert Meltzer, M.D., Bryan Roth, M.D., Ph.D., Lisa Konick and Lesa Dieter in the psychiatric assessments is gratefully acknowledged. We also thank Lisa Konick and Nicole Herbst for their work in collecting tissues. We are deeply appreciative of the assistance of the next-of-kin of the deceased and gratefully acknowledge the assistance of the Cuyahoga County Coroner’s Office, Cleveland, Ohio. This study was supported by grants from the National Institute of Mental Health: MH63187, MH67996, MH61578, MH60451, National Center for Research Resources: RR17701 and the Canadian Institutes of Health Research: FRN36437.
Footnotes
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/pnp).
Statement of Interest
None.
References
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Research. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arranz B, Eriksson A, Mellerup E, Plenge P, et al. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biological Psychiatry. 1994;35:457–463. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, et al. Persistent reduction in brain serotonin 1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Molecular Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cerebral Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Czesak M, Lemonde S, Peterson EA, Rogaeva A, et al. Cell-specific repressor or enhancer activities of deaf-1 at a serotonin 1A receptor gene polymorphism. Journal of Neuroscience. 2006;26:1864–1871. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Research. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Pickel VM. Targeting of serotonin 1A receptors to dopaminergic neurons within the parabrachial subdivision of the ventral tegmental area in rat brain. Journal of Comparative Neurology. 2001;433:390–400. doi: 10.1002/cne.1147. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biological Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, et al. Serotonin type-1A receptor imaging in depression. Nuclear Medicine and Biology. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Medicine and Biology. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (version 2) Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Kovachich GB, Frazer A. A quantitative autoradiographic study of serotonin1A receptor regulation: effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology. 1991;4:131–144. [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Lepola A, et al. Decreased brain serotonin 5-HT1A receptor availability in medication naïve patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. International Journal of Neuropsychopharmacology. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- Hsiung SC, Adlersberg M, Arango V, Mann JJ, et al. Attenuated 5-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, akt and mitogen-activated protein kinase. Journal of Neurochemistry. 2003;87:182–194. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- Iyo AH, Kieran N, Chandran A, Lenicov FR, et al. Differential regulation of the serotonin 1A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience. 2009;163:1119–1127. doi: 10.1016/j.neuroscience.2009.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. Journal of Neuroscience. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, Laporte AM, et al. Differential adaption of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39:110–122. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Little RC, Milleken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute Inc.; 1996. [Google Scholar]
- Lopez-Figueroa L, Norton CS, Lopez-Figueroa MO, Rmellini-Dodel D, et al. Serotonin 5-HT1A, 5-HT1B and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biology Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lowther S, De Paermentier F, Cheetham SC, Crompton MR, et al. 5-HT1A receptor binding sites in postmortem brain samples from depressed suicides and controls. Journal of Affective Disorders. 1997;42:199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. Journal of Neural Transmission: General Section. 1991;85:181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser J, et al. GFAP immunoreactivity in the dorsolateral prefrontal cortex separates young from old adults with major depressive disorder. Biological Psychiatry. 2000;48:860–872. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Wisner KL, Price JC, Berga SL, et al. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertility and Sterility. 2008;89:685–692. doi: 10.1016/j.fertnstert.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Lemonde S, Jafar-Nejad H, Bown CD, et al. Freud-1: a neuronal calcium-regulated repressor of the 5-HT1A receptor gene. Journal of Neuroscience. 2003;23:7415–7425. doi: 10.1523/JNEUROSCI.23-19-07415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Zhou FC. Ontogeny of 5-HT1A receptor expression in the developing hippocampus. DevelopmentalBrain Research. 2005;157:42–57. doi: 10.1016/j.devbrainres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Seminars in Clinical Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurological Disorders Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, et al. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biological Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, et al. Reduction in calbindin-immunoreactive GABA interneurons in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva A, Albert PR. The mental retardation gene CC2D1A/Freud-1 encodes a long isoform that binds conserved DNA elements to repress gene transcription. European Journal of Neuroscience. 2007;26:965–974. doi: 10.1111/j.1460-9568.2007.05727.x. [DOI] [PubMed] [Google Scholar]
- Rogaeva A, Ou XM, Jafar-Nejad H, Lemonde S, et al. Differential repression by Freud-1/CC2D1A at a polymorphic site in the dopamine-D2 receptor gene. Journal of Biological Chemistry. 2007;282:20897–20905. doi: 10.1074/jbc.M610038200. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Archives of General Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Progress in Neurobiology. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, et al. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Endiott J. Schedule for Affective Disorders and Schizophrenia (SADS) 3rd edn. New York: New York State Psychiatric Institute; 1978. [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. Journal of Psychiatric Research. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, et al. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, et al. Cellular changes in the postmortem hippocampus in major depression. Biological Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Howley E, Shi X, Sobanska A, et al. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. Journal of Psychiatric Research. 2009;43:887–894. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. International Journal of Neuropsychopharmacology. 2009;12:155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.