Abstract
Current thinking suggests that despite the heterogeneity of myeloid-derived suppressor cells (MDSC), all Gr-1+CD11b+ cells can become suppressive when exposed to inflammatory stimuli. In vitro evaluation shows MDSC from multiple tissue sites have suppressive activity, and in vivo inhibition of MDSC function enhances T cell responses. However, the relative capacity of MDSC present at localized inflammatory sites or in peripheral tissues to suppress T cell responses in vivo has not been directly evaluated. We now demonstrate that during a tissue specific inflammatory response, MDSC inhibition of CD8 T cell proliferation and IFN-γ production is restricted to the inflammatory site. Using a prostate specific inflammatory model and a heterotopic prostate tumor model, we show that MDSC from inflammatory sites or from tumor tissue possess immediate capacity to inhibit T cell function, whereas those isolated from peripheral tissues (spleens and liver) are not suppressive without activation of iNOS by exposure to IFN-γ. These data show MDSC are important regulators of immune responses in the prostate during acute inflammation and the chronic inflammatory setting of tumor growth and that regulation of T cell function by MDSC during a localized inflammatory response is restricted in vivo to the site of an ongoing immune response.
Keywords: Myeloid derived suppressor cells, Immune Regulation, Inflammation, Prostate Inflammation
Introduction
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells identified in mice as Gr-1+CD11b+ cells with the ability to suppress T cell proliferation [1–2]. Expanded populations of MDSC with the capacity to inhibit T cell proliferation in vitro have been identified in the blood, spleen, bone marrow, liver, tumor and sites of ongoing inflammatory conditions in mice [3–6]. Current thinking suggests that MDSC acquire suppressive function only after exposure to factors present in inflammatory or tumor microenvironments [7–8]. However, while it is understood that inflammatory factors and/or activated T cells are necessary for activation of suppressive function in MDSC; current in vitro studies to evaluate the suppressive potential of MDSC utilize long term culture with activated T cells, allowing the acquisition of suppressive function that may not have been present at the time of isolation.
In vivo studies examining the function of MDSC in tumor bearing mice have clearly demonstrated MDSC promote tumor growth by inhibiting anti-tumor immune responses [9–14]. Indeed, removal of MDSC through chemotherapeutic drugs or drug inhibitors of Arg1 and iNOS results in increased IFN-γ production from splenic or tumor-draining lymph node CD8 T cells in response to tumor antigens [9, 11]. Furthermore, a recent study by Bronte and colleagues demonstrates tumor-induced splenic MDSC regulate T cell function during normal immune responses [14]. Similarly, MDSC play an important role during benign inflammatory conditions in vivo [15–18]. MDSC shape the immune response to viral antigens, influence antibody production during sepsis, and down-regulate T cell responses to auto-antigens [15–18]. Thus, while it is clear that MDSC regulate cell mediated immune responses in vivo during cancer and inflammatory responses, it is unclear whether MDSC present in tissues peripheral to a local inflammatory site functionally regulate T cell responses.
To characterize the suppressive capacity of MDSC from inflammatory or peripheral sites during a localized inflammatory response, we used the Prostate Ovalbumin-Expressing Transgenic (POET-3) mouse model of prostate inflammation. POET-3 mice provide an animal model where a CD8 T cell dependent inflammatory response to ovalbumin is induced locally in the prostate [19]. Herein we demonstrate that during a tissue specific inflammatory response the suppressive activity of MDSC in vivo is restricted to cells present in the inflammatory environment. Furthermore, in vitro we demonstrate only MDSC from acute or chronic (tumor-induced) inflammatory environments possess the immediate capacity to regulate T cell proliferation. In support of these data, in vivo depletion of Gr-1+ cells during acute prostate inflammation specifically increased T cell function at the inflammatory site while T cell function in the spleen was not affected. Together these data demonstrate that in vivo regulation of T cell function by MDSC during a tissue specific inflammatory response is localized to the inflammatory site.
Results
Gr-1+CD11b+ cells are expanded during acute prostate inflammation
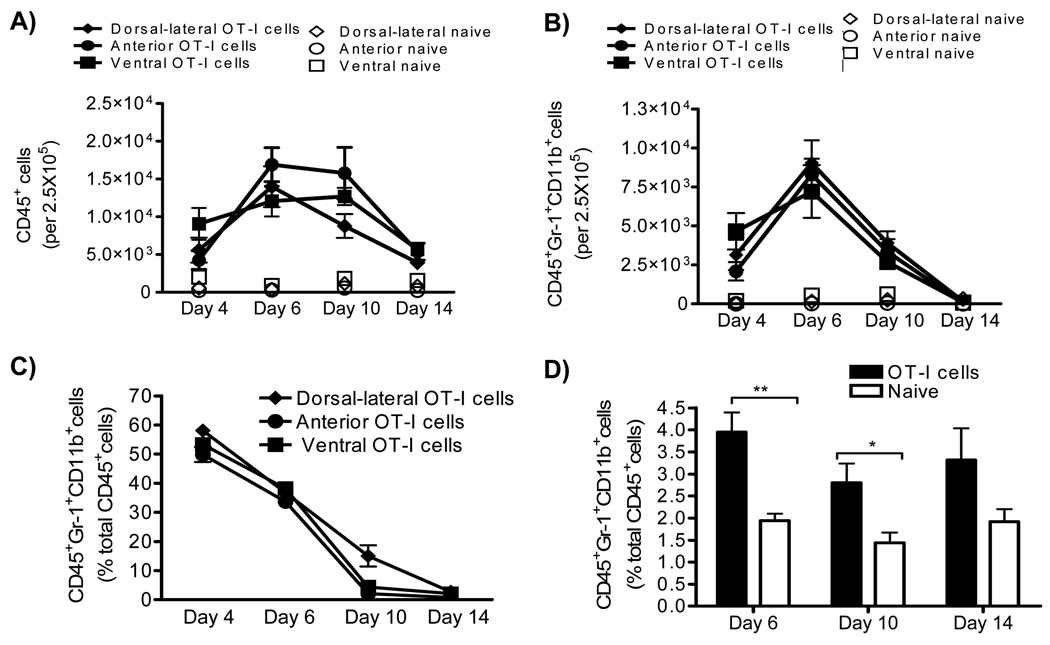
To determine if MDSC were expanded during prostate inflammation, we used the Prostate Ovalbumin-Expressing Transgenic (POET-3) mouse model of prostate inflammation [19]. Adoptive transfer of activated Thy1.1+OT-I T cells induced prostate inflammation as measured by histological analysis (Supplementary Fig. 1A) and by the presence of CD45+ leukocytes in prostate tissue (Fig. 1A). The peak of the acute inflammatory response was observed between day 6 and 10 before declining between day 10 and 14 (Fig. 1A). Importantly, all lobes of the prostate show similar levels and kinetics of CD45+ infiltration (Fig. 1B), demonstrating the inflammatory response is not restricted to one area of the prostate as has been seen in other rodent models of prostate inflammation [20]. Further flow cytometry analysis demonstrated recruitment of Gr-1+CD11b+ cells into inflamed prostates, with the peak infiltration occurring at day 6, followed by a rapid loss of these cells within 14 days (Fig. 1B, C). Remarkably, Gr-1+CD11b+cells comprise approximately 50% of all CD45+ leukocytes in inflamed prostates at day 4, and approximately 35% at day 6 (Fig. 1C). Importantly, the percentages of Gr-1+CD11b+cells among the CD45+ population were similar in all prostate lobes (Fig. 1C). Immunohistochemical analysis showed the distribution of Gr-1+CD11b+ cells in prostate tissue (Supplementary Fig 1B). Naïve prostate tissue contained only trace numbers of Gr-1+CD11b+ cells, preventing further study of these cells (Fig. 1B).
Figure 1. Acute prostate inflammation expands Gr-1+CD11b+ cells.
(A–C) Acute prostate inflammation in POET-3 mice after adoptive transfer of OT-I cells as measured by analysis of infiltrating CD45+ and CD45+Gr-1+CD11b+ cells (mean ± SD) (D) CD45+Gr-1+CD11b+ cells are increased in the spleens of POET-3 mice during acute prostate inflammation (mean ± SD). The unpaired t test p-value is reported, ** p≤.01, *p≤.05. Data are derived from no less than three independent experiments, no less than five mice per group.
Kinetics of Gr-1+CD11b+ accumulation in spleens showed increases relative to naïve control mice at days 4, 6 and 10 but the difference lost significance by day 14 as a result of variability among the mice evaluated (Fig. 1D). These data demonstrate acute prostate inflammation is characterized by a rapid but transient accumulation of Gr-1+CD11b+ cells at the inflammatory site, and an approximately 2-fold increase in sites distal to inflammation such as the spleen.
Arg1 and iNOS expression is restricted to Gr-1+CD11b+ cells at inflammatory site
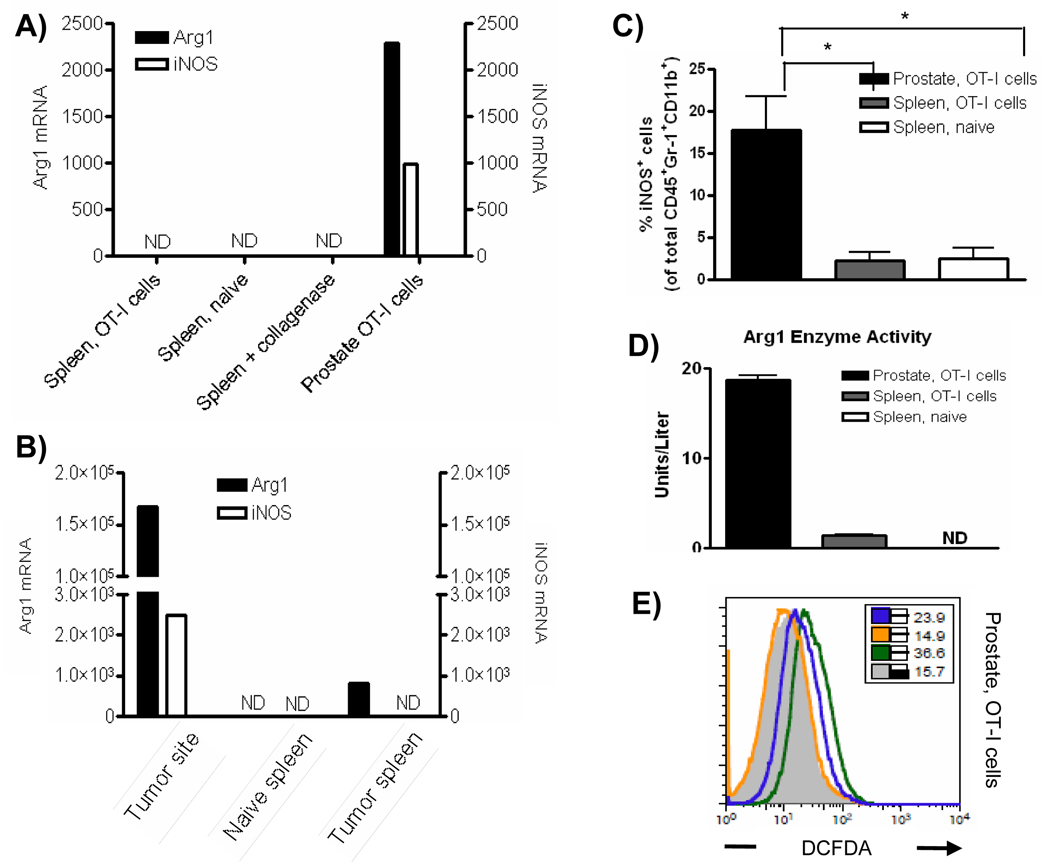
The suppressive function of MDSC can largely be attributed to Arg1 and iNOS expression, and inflammation can significantly increase expression of these enzymes [21–24]. We therefore hypothesized that Arg1 and iNOS mRNA levels would be higher in Gr-1+CD11b+ cells isolated from prostates than in the spleens of mice with prostate inflammation. Neither Arg1 nor iNOS mRNAs were detectable in freshly isolated cells from the spleen (Fig. 2A). In contrast, both Arg1 and iNOS mRNAs were highly expressed in freshly isolated CD45+Gr-1+CD11b+ cells from inflamed prostates (Fig. 2A). Enhanced expression of Arg1 and iNOS in CD45+Gr-1+CD11b+ cells from the inflammatory site was not an artifact of treatment with collagenase D, as splenocytes incubated with this enzyme were not observed to express detectable Arg1 or iNOS mRNA (Fig. 2A). Since the POET-3 inflammation model represents an acute inflammatory response, we evaluated Arg1 and iNOS expression in RM-1 prostate tumor bearing mice as a means of evaluating a chronic inflammatory response. As reported for multiple tumor models, RM-1 tumors expanded the percentage of Gr-1+CD11b+ cells in the spleens of tumor-bearing mice and recruited them to the tumor site (Supplemental Fig. 2) As observed in POET-3 mice, Gr-1+CD11b+ cells from RM1 tumor bearing mice had elevated Arg1 and iNOS mRNA (Fig. 2B). Analysis of the transcription factor C/EBPβ, which controls the immunosuppressive activity of MDSC through its ability to regulate Arg1 and iNOS expression [25–27] showed expression in Gr-1+CD11b+ cells from prostates and spleens of mice with prostate inflammation and from naïve mice; expression was highest cells from inflamed prostates (Supplementary Fig. 3A).
Figure 2. Gr-1+CD11b+ cells isolated the inflammatory site express Arg1 and iNOS.
(A, B) Quantitative RT-PCR analysis of Arg1 and iNOS mRNA expression in freshly isolated CD45+ Gr-1+CD11b+ cells from POET-3 (A) or from RM-1 tumor-bearing (B) mice. Samples were performed in duplicate and the data presented are the average value, ND no detected expression. (C) The percentage of iNOS expressing cells among the total CD45+ Gr-1+CD11b+ population. Data are compiled from two independent experiments. The students t test p-value is reported, *p≤.05. (D) Arg1 activity in freshly isolated CD45+ Gr-1+CD11b+ cells was measured in duplicates as described in materials and methods (mean ± SD), ND no expression detected. (E) Production of reactive oxygen and nitrogen species by CD45+Gr-1+CD11b+ cells was measured by incubation with DCFDA in the presence or absence of inhibitors as described in materials and methods. Inset numbers show MFI of empty FL1 channel (gray solid histogram); DCFDA (green line); nor-NOHA and DCFDA (orange line); LNMMA and DCFDA (blue line). For all experiments cells were pooled from five mice per group and data are representative of no less than three independent experiments.
To determine if mRNA expression correlates with protein expression, the level of intracellular iNOS was examined in CD45+Gr-1+CD11b+ cells isolated from the spleen and inflamed prostates. In agreement with the RT-PCR data, CD45+Gr-1+CD11b+ cells from the inflammatory site express elevated iNOS protein; however, iNOS protein was detected only in a fraction of the total CD45+Gr-1+CD11b+ cells from inflamed prostates (Fig. 2C, Supplementary Fig. 4A). Further analysis showed no detectable expression of iNOS in CD45+CD11b− cells, demonstrating the specificity of the antibody (Supplementary Fig. 4B). Production of nitric oxide (NO) by iNOS can lead to the nitration of proteins in inflammatory tissues and is indicative of NO mediated cell damage [28]. Therefore, we asked if nitrated proteins could be detected in inflamed prostate tissue. Indeed, nitrotyrosine residues were present only in inflamed prostate tissue, confirming the activity of iNOS during acute prostate inflammation (Supplemental Fig. 5).
Arg1 enzyme activity was used to evaluate protein expression in freshly isolated CD45+Gr-1+CD11b+ cells from spleens or inflamed prostates. Gr-1+CD11b+ cells from the spleens of mice with prostate inflammation express low but detectable levels of Arg1 enzyme activity (Fig. 2D), whereas those from inflamed prostates express higher levels (Fig. 2D). Arg1 activity was not detected in CD45+Gr-1+CD11b+ cells from the spleens of naïve mice (Fig. 2D). Similar to the POET-3 model, Arg1 and iNOS protein levels were elevated only in Gr-1+CD11b+ cells isolated from prostate tumors (Supplemental Fig. 6).
Arg1 and iNOS metabolize L-arginine to produce reactive oxygen species (ROS) and reactive nitrogen species (RNS) such as superoxide (O2−), peroxynitrite (ONOO−) and hydrogen peroxide (H2O2) [1–2, 29–31]. Fluorescence of the oxidation sensitive dye, DCFDA, showed that only CD45+Gr-1+CD11b+ cells isolated from inflamed prostates expressed elevated ROS and RNS (Fig. 2E, Supplementary Fig. 7). To examine the contribution of both Arg1 and iNOS to DCFDA fluorescence, selective inhibitors of these enzymes were used. Treatment with LNMMA, a selective iNOS inhibitor only partially decreased DCFDA fluorescence, whereas treatment with nor-NOHA, a selective Arg1 inhibitor, resulted in a total loss of DCFDA fluorescence in CD45+Gr-1+CD11b+ cells from the inflammatory site (Fig. 2 E, Supplementary Fig. 7) [32–33]. These data demonstrate ROS and RNS are elevated only in CD45+Gr-1+CD11b+ cells from inflamed prostates, suggesting Gr-1+CD11b+ cells present at the inflammatory site are functionally distinct from those in the spleen.
Gr-1+CD11b+ cells from inflamed prostates have a distinct phenotype compared to cells from the spleen
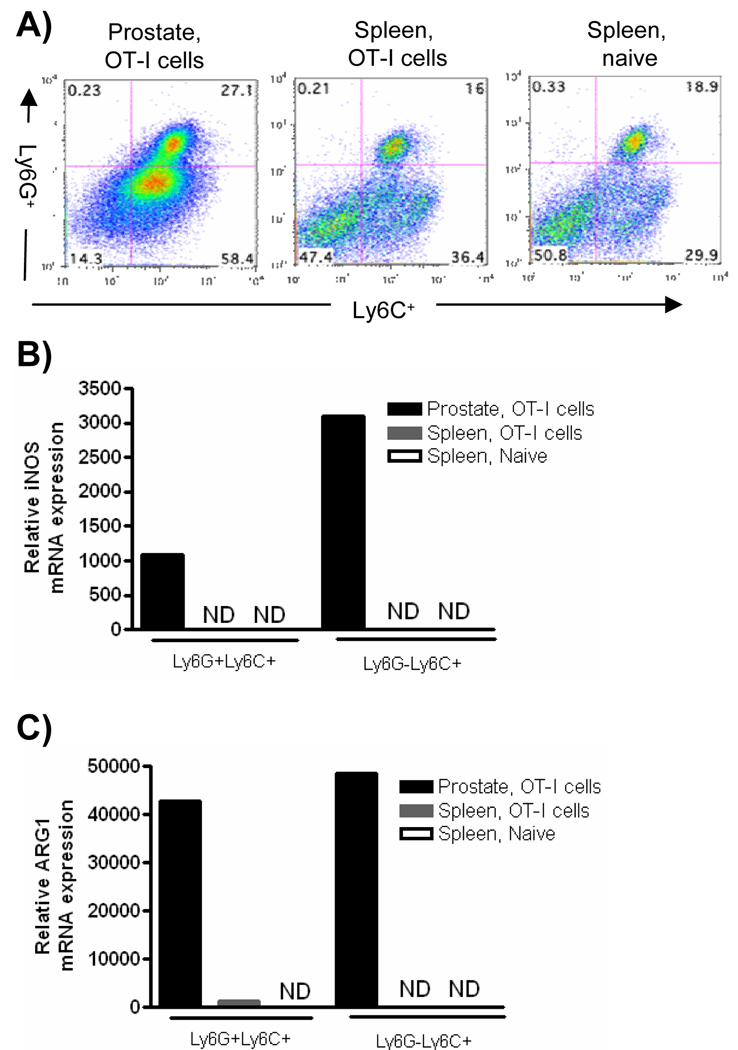
Given that Gr-1+CD11b+ cells from the inflammatory site expressed increased levels of Arg1 and iNOS, we next asked if there were phenotypic differences in the populations of cells from the spleen and inflammatory site. The Gr-1 antibody RB6-8C5 recognizes two epitopes, Ly6G and Ly6C, and MDSC have recently been subdivided into either monocytic or granulocytic subsets based on their Ly6C and Ly6G expression [4–5]. Therefore we next examined the morphology of the cells by flow cytometry to determine which subset(s) of MDSC were expanded during prostate inflammation. CD11b+ cells from inflamed prostates and spleens were found to compose a mixture of Ly6G+Ly6C+ and Ly6C+Ly6G− cells, with the predominant population being the monocytic Ly6C+Ly6G− cell fraction (Fig. 3A). Importantly, the ratio of the Ly6G+Ly6C+ to the Ly6C+Ly6G− cells among the total CD11b+ population was similar in the prostate and spleen of inflamed mice, suggesting that functional differences between the spleen and prostate were not due to the differential expansion of MDSC subsets (Fig. 3A). Monocytic and granulocytic subpopulations of MDSC have different mechanisms of suppression [4–5]. Therefore, we next determined the level of Arg1 and iNOS in the CD11b+ subsets. Arg1 and iNOS were expressed only in cells from the inflammatory environment, with the monocytic population of cells having the highest iNOS mRNA expression, while both subsets of cells expressed Arg1 (Fig. 3B, C). Further, cells isolated from the inflammatory environment expressed the highest levels of C/EBPβ, with expression being predominately associated with the Ly6C+Ly6G+ subset (Supplementary Fig. 3B).
Figure 3. Expression of iNOS and Arg1 are elevated in CD11b+ subsets from the inflammatory site.
Prostate and spleen tissues were harvested from POET-3 mice 6 days after adoptive transfer of OT-I cells or from naïve controls. (A) Cells were stained for flow cytometry. Plots are gated on CD45+CD11b+ cells. (B, C) Subpopulations of CD45+CD11b+ cells were sorted from spleens and prostate tissue and the levels of iNOS (B) or Arg1 (C) were evaluated with RT-PCR. Samples were performed in duplicate and the data presented are the average values, ND no detected expression. For all experiments data are from pooled samples of 5 mice per group and are representative of no less than three independent experiments.
Further phenotype analysis revealed that CD45+Gr-1+CD11b+ cells from inflamed prostates expressed lower levels of CD71 than cells from spleens, suggesting a non-proliferating phenotype (Supplemental Fig. 8A). PDL-1 was equivalently expressed on CD45+Gr-1+CD11b+ cells from all tissues. Levels of F4/80, MHC II, MHC I, CD80 and CD86 were lower on cells from the inflammatory site, suggesting that cells from inflamed prostates are of a lower maturation status compared to cells in the periphery (Supplemental Fig. 8A). No expression of CD115, IL-4Rα, CD34 or CTLA-4 was detected on cells from prostate or spleen tissue (data not shown).
Gr-1+CD11b+ cells from inflamed prostates suppress T cell proliferation in vitro
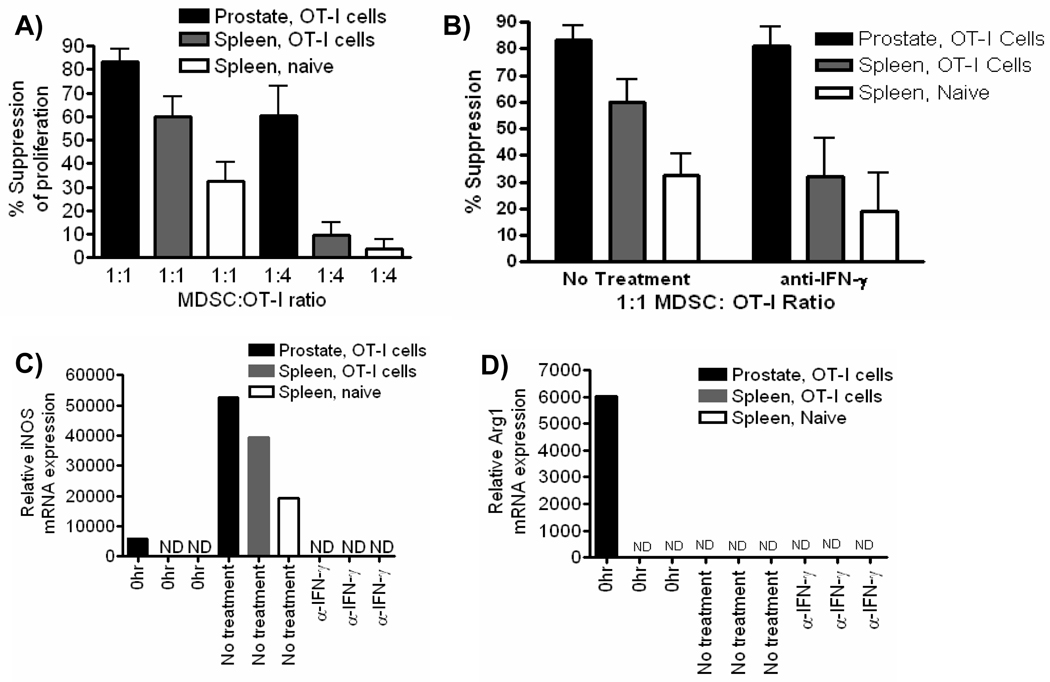
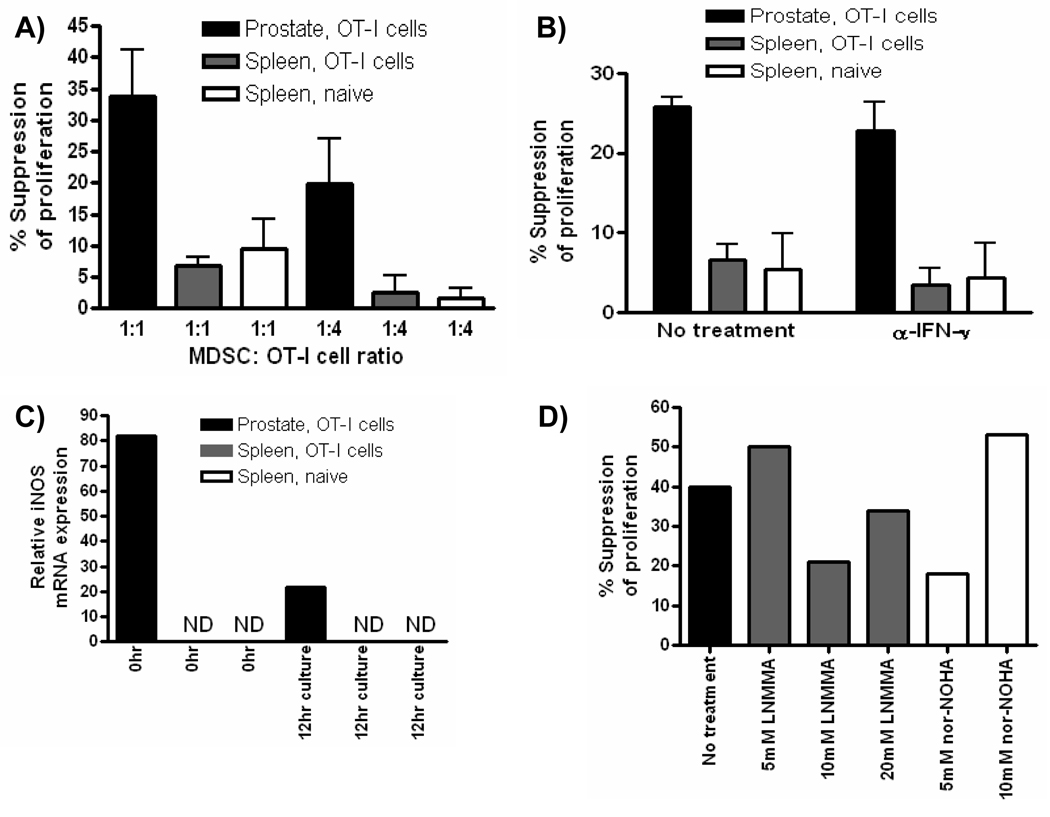
Given that Arg1 and iNOS are associated with suppressive function of MDSC, we next tested the ability of Gr-1+CD11b+ cells from inflamed prostates and spleens to suppress antigen-specific CD8 T cell proliferation [34]. Gr-1+CD11b+ cells isolated from inflamed prostates and spleens of inflamed or control mice strongly inhibit T cell activation at a 1:1 ratio (Fig. 4A). MDSC isolated from inflamed prostates remained significantly suppressive at a lower MDSC: OT-I cell ratio suggesting MDSC from the inflammatory site have a stronger suppressive activity compared to MDSC from the spleen (Fig. 4A). The regulatory function of MDSC has been shown to be dependent upon IFN-γ, either by its direct ability to induce iNOS, or through its ability to induce MDSC to produce cytokines such as IL-4 and IL-13 that induce Arg1 expression [4, 24, 35–37]. Therefore, we next asked if the suppressive function was dependent upon IFN-γ. When IFN-γ was neutralized the suppressive function of prostate Gr-1+CD11b+ cells was not significantly changed, whereas the function of cells isolated from spleens of mice receiving OT-I cells or from naïve controls was strongly inhibited (Fig. 4B). These data demonstrate a selective dependence of splenic Gr-1+CD11b+ cells on IFN-γ for in vitro measured suppressive function, suggesting inflammatory cytokines such as IFN-γ modulate MDSC function in vivo, resulting in functional differences between MDSC isolated from different environments within the same host.
Figure 4. Gr-1+CD11b+ cells from inflamed prostates suppress T cell proliferation in vitro.
(A, B) Suppressive function of CD45+ Gr-1+CD11b+ cells from prostates and spleens of mice with acute prostate inflammation or from the spleens of control mice was measured by BrdU incorporation in Thy1.1+ OT-I cells after 48 (A) or 72 (B) hours. Neutralizing IFN-γ antibody was added to wells at the beginning of culture where indicated. Data are compiled from two independent experiments (mean ± SD). (C–D) Quantitative RT-PCR analysis of iNOS (C) and Arg1 (D) mRNA expression in prostate and spleen CD45+ Gr-1+CD11b+ cells prior to being placed in suppression assays (0Hr) and on cells sorted from culture wells at the end of the suppression assay. ND, no expression detected. The data presented are from one representative experiment, and all experiments were independently repeated no fewer than two times. For all experiments, data are from pooled samples of no less than 5 mice per group.
It is well known that CD8 T cells produce IFN-γ when stimulated, and that IFN-γ can directly modulate levels of iNOS or Arg1 through a positive feedback mechanism [1, 23–24, 36]. Therefore, we next asked if the expression of these enzymes was induced in splenic Gr-1+CD11b+ cells during the suppression assay itself. Neither Arg1 nor iNOS mRNA were detected in freshly isolated splenic Gr-1+CD11b+ cells prior to being placed in culture with activated T cells (Fig. 4C, D). However, iNOS mRNA was induced in splenic Gr-1+CD11b+ cells, in an IFN-γ dependent manner (Fig. 4C). In contrast, the constitutive expression of Arg1 and iNOS mRNA in Gr-1+CD11b+ cells from inflamed prostates was high before (0hr) co-culture with T cells, and iNOS levels were further elevated by the presence of IFN-γ during the suppression assay (Fig. 4C). Arg1 mRNA was not detected in any group of cells after culture with OT-I cells, most likely due to the strong production of IFN-γ during the assay (Fig. 4D). Consistent with previous reports on the role of IFN-γ in MDSC, these data demonstrate T cell derived IFN-γ converts precursor splenic Gr-1+CD11b+ cells into functional MDSC during conventional suppression assays [8, 36]. Furthermore, these data suggest spleen Gr-1+CD11b+ cells have differential regulatory capacity compared to cells from the inflammatory site in vivo.
Gr-1+CD11b+ cells from the inflammatory site possess immediate capacity to regulate T cell proliferation
To test the hypothesis that precursor spleen Gr-1+CD11b+ cells have differential regulatory capacity compared to cells present at the inflammatory site, we developed a novel short term in vitro suppression assay to evaluate the immediate capacity of Gr-1+CD11b+ cells to inhibit T cell proliferation. In this assay, sorted populations of Gr-1+CD11b+ cells were cultured with pre-activated OT-I T cells for 12 hours to minimize exposure of Gr-1+CD11b+ cells to IFN-γ. Freshly isolated Gr-1+CD11b+ cells from the spleens of mice with prostate inflammation or from control mice showed little suppressive activity in short term suppression assays (Fig. 5A). In contrast, Gr-1+CD11b+ cells from inflamed prostates strongly suppressed proliferation of pre-activated effector T cells at both at 1:1 and 1:4 ratios (Fig. 5A). Importantly, suppressive function of MDSC from the inflammatory site does not require in vitro IFN-γ production during the suppression assay (Fig. 5B). To confirm that exposure to IFN-γ had not induced iNOS mRNA in MDSC during the assay RT-PCR was performed on freshly isolated cells and on cells recovered at the end of the suppression assay. iNOS mRNA was present only in cells from inflamed prostates and was not detectable in cells from the spleen at any time point tested (Fig. 5C). Furthermore, freshly isolated Gr-1+CD11b+ cells from the liver of mice with prostate inflammation do not express Arg1 and iNOS and can not suppress antigen activated CD8 T cell proliferation in the 12 hr assay, further confirming that Gr-1+CD11b+ cells from peripheral tissues are not functional MDSC (Supplementary Fig. 9). These data demonstrate that freshly isolated Gr-1+CD11b+ cells from the inflammatory site but not peripheral tissues suppress proliferation of effector T cells directly ex vivo, supporting the hypothesis that the suppressive function of MDSC is acquired in vivo at the inflammatory site.
Figure 5. Gr-1+CD11b+ cells from the inflammatory site possess immediate capacity to regulate T cell proliferation.
(A, B) The suppressive function of CD45+ Gr-1+CD11b+ cells from prostates and spleens of mice with acute prostate inflammation or from the spleens of control mice was measured by BrdU incorporation in Thy1.1+ OT-I cells after 12 hours. Neutralizing IFN-γ antibody was added to wells at the beginning of culture where indicated. Data are compiled from two independent experiments (mean ± SD). (C) Quantitative RT-PCR analysis of iNOS mRNA expression in prostate and spleen CD45+ Gr-1+CD11b+ cells prior to being placed in suppression assays (0Hr) and on cells sorted from culture wells at the end of the suppression assay (12 hour culture). Samples were performed in duplicate, ND, no expression detected. (D) The suppressive function of CD45+ Gr-1+CD11b+ cells from prostates of mice with acute prostate inflammation was measured by BrdU incorporation in Thy1.1+ OT-I cells (1:2 MDSC:OT-I cell ratio) after 12 hours. LNMMA or nor-NOHA were added at the indicated concentrations at the beginning of the culture. The data presented are from one representative experiment. All experiments were independently repeated at least two times and data are from pooled samples of no less than five mice per group.
Our RT-PCR data suggested that both Arg1 and iNOS were involved in the suppressive function of MDSC isolated from inflamed prostates. When the ability of MDSC from inflamed prostates to inhibit antigen activated T cell proliferation was tested both the iNOS (LNMMA) and Arg1 (nor-NOHA) inhibitors partially blocked the suppressive function prostate-derived MDSC (Fig. 5D). Importantly, the partial suppression was not due to insufficient concentrations of inhibitors as higher levels did not result in increased inhibition of suppression (Fig. 5D).
Gr-1+CD11b+ cells isolated from prostate tumors site possess immediate capacity to regulate T cell proliferation
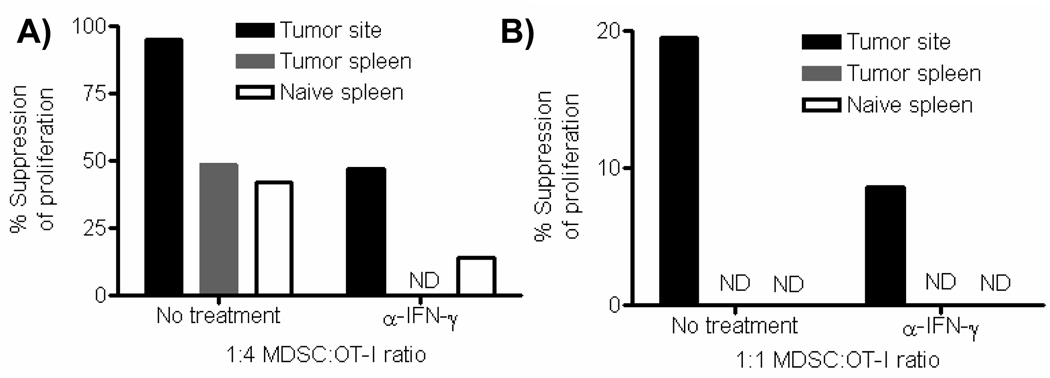
The POET-3 model provided strong evidence that the function of MDSC was restricted to the inflammatory site. However, it remained possible that this phenomenon was restricted to acute tissue specific inflammation and would not pertain to chronic inflammatory conditions. When the suppressive function of Gr-1+CD11b+ cells was tested using conventional suppression assays, cells from both the tumor site and spleen or prostate tumor-bearing mice were strongly suppressive (Figure 6A). Importantly, MDSC from the tumor site were more suppressive than cells from the spleen and were only partially dependent upon IFN-γ for suppressive function (Figure 6A). Similar to data observed in our acute inflammatory model, iNOS mRNA was induced in Gr-1+CD11b+ cells from the spleen of tumor-bearing mice, and sustained in cells from the tumor site by IFN-γ (Supplementary Fig. 10). As observed in the acute inflammatory model, only Gr-1+CD11b+ cells isolated from the tumor site were able to suppress proliferation of pre-activated CD8 T cells during short term suppression assays (Fig. 6B). Thus, as in the prostate inflammatory model, these data demonstrate that during a chronic inflammatory response generated by solid tumors, only Gr-1+CD11b+ cells present in the tumor microenvironment possess the immediate capacity to regulate T cell activation.
Figure 6. Gr-1+CD11b+ cells isolated from prostate tumors possess immediate capacity to regulate T cell proliferation.
(A) Suppressive function of CD45+ Gr-1+CD11b+ cells from tumors and spleens were measured by BrdU incorporation in Thy1.1+ cells after 72 hours. Neutralizing IFN-γ antibody was added to wells at the beginning of culture where indicated. (B) The suppressive function of CD45+ Gr-1+CD11b+ cells from tumors and spleens to suppress effector T cell proliferation was measured by BrdU incorporation in Thy1.1+ cells after 12 hours. Neutralizing IFN-γ antibody was added to wells at the beginning of culture where indicated. All experiments were repeated twice, and the values presented are from one representative experiment.
In vivo regulatory function of MDSC is restricted to cells present at the inflammatory site
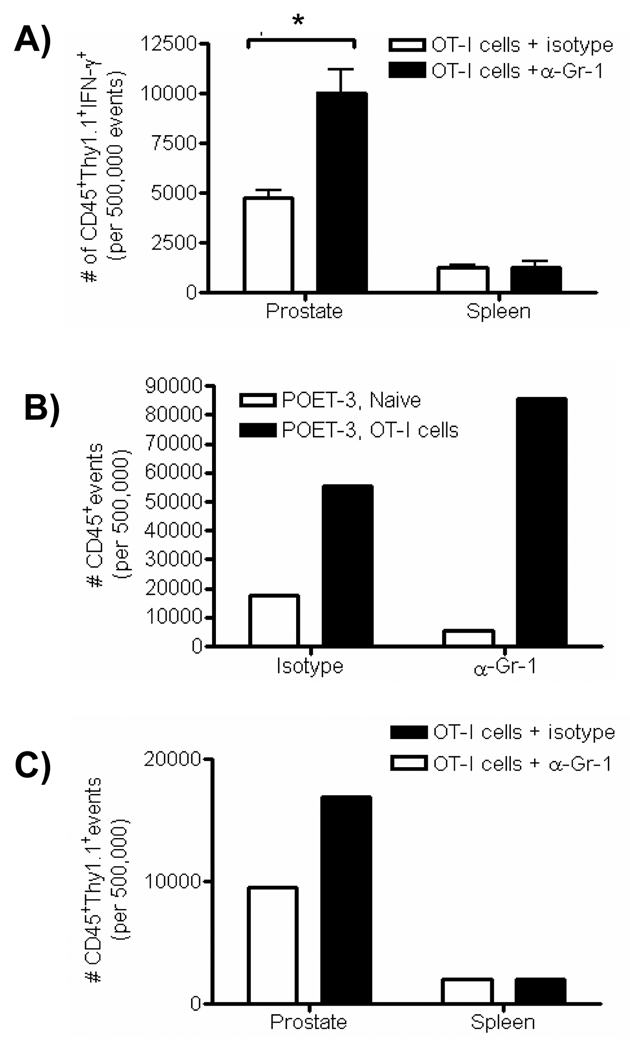
Our in vitro data suggested that in vivo regulatory function of MDSC was restricted to the inflammatory site during a tissue specific inflammatory process. Therefore, to test this hypothesis, we asked whether MDSC from either inflamed prostates or spleens of mice with prostate inflammation were able to inhibit T cell function in vivo using anti-Gr-1 to block MDSC function [38]. To accomplish this task, the congenic markers, Thy 1.1 and Thy 1.2, were used to identify T cells. Inflammation was induced by the injection of Thy 1.1+ OT-I cells in the presence or absence of anti-Gr-1 treatment. Six days later Thy1.1+ OT-I cells were isolated from inflamed prostates and spleens of POET-3 (Thy1.2) mice and tested for IFN-γ production in vitro. Thy 1.1+ OT-I cells from inflamed prostates of mice given anti-Gr-1 antibody showed a significant increase in their ability to produce IFN-γ compared to isotype control treated mice, whereas Thy1.1+ cells from the spleen were not affected (Fig. 7A). High dose anti-Gr-1 treatment has been reported to deplete subsets of CD8 T cells [39–40]. Therefore we chose a dose where depletion of CD8 T cells was not observed (Supplemental Fig. 11). Consistent with the impact of anti-Gr-1 on T cell function in vitro, an increase in prostate inflammation as measured by infiltration of CD45+ cells (Fig. 7B) was observed in anti-Gr-1 treated mice compared to isotype treated control animals. Notably, the total number of infiltrating OT-I cells (Thy1.1+ cells) into inflamed prostates of anti-Gr-1 treated mice was almost double that of isotype treated controls (Fig. 7C). In contrast, the number of Thy1.1+ cells remained unchanged in the spleen relative to isotype controls (Fig. 7C). These data demonstrate that during localized inflammatory responses, inhibition of MDSC function with anti-Gr-1 enhances T cell function at the inflammatory site in vivo but has no effect on T cell function in the spleen, supporting our conclusion that MDSC are only active at the site of inflammation.
Figure 7. In vivo regulatory function of MDSC is restricted to cells present at the inflammatory site.
(A–C) Prostate and spleen tissue was harvested from POET-3 mice receiving OT-I cells and anti-Gr-1 or isotype control antibody. (A) Cells were re-stimulated with SIINFEKL peptide (5µg/mL) for 5 hours and the absolute number of IFN-γ producing Thy1.1+ OT-I cells was measured in CD45+Gr-1+CD11b+ cells (mean ±SD, n=4). The two tailed t test is reported, * p≤.05. (B) Prostate inflammation was monitored by assessing the absolute number of CD45+ cells in prostate tissue of POET-3 mice receiving anti-Gr-1 or isotype control antibody injections. Prostate tissue was pooled from four mice per group. The data are representative of no less than three independent experiments. (C) The absolute number of CD45+Thy1.1+ cells in prostate or spleen tissue in POET-3 mice receiving anti-Gr-1 or control antibody injections. Prostate and spleen tissues were pooled from four mice per group. The data are representative of no less than three independent experiments.
DISCUSSION
Data reported herein demonstrate that MDSC are important regulators of immune responses in the prostate during acute inflammation and during prostate tumor growth. Importantly, both in vitro and in vivo experiments demonstrate that the ability of MDSC to inhibit CD8 T cell proliferation and IFN-γ production is restricted to the site of inflammation.
Acute prostate inflammation resulted in accumulation of Gr-1+CD11b+ cells into inflamed prostates, with MDSC composing approximately 40% of all CD45+ cells at the peak of the inflammatory response. These data are supported by other inflammatory models such as experimental autoimmune encephalomyelitis (EAE), where at the peak of clinical disease over 30% of infiltrating cells in the CNS were CD11b+ Ly6Chigh cells [37]. However, in contrast to our study, the function of MDSC at the inflammatory site was not examined.
The expression of Arg1 and iNOS by MDSC is necessary for inhibition of T cell responses [2, 41–42]. Analysis of Arg1 and iNOS enzyme activity demonstrates that expression is restricted to isolated cells from the inflammatory site, not in peripheral tissues such as the spleen and liver. Likewise, Arg1 and iNOS also were up-regulated in MDSC isolated from prostate tumors. Previous studies reported similar for data in that CD11b+ cells infiltrating rat kidney allografts and tumor; however, suppressive capacity was not evaluated in either model [43–44].
MDSC are a heterogeneous population of cells and multiple markers have been identified that correspond with increased regulatory capacity. This, along with our data demonstrating differential expression of regulatory enzymes raised the possibility that different populations of cells were expanded in the inflammatory site and spleen. Subset analyses showed that Ly6G and Ly6C populations were similar in the spleen, inflamed prostate and RM-1 tumors. Further marker analysis showed that Gr-1+CD11b+ cells from the inflammatory site have a more immature phenotype than cells from the spleen, as evaluated by decreased expression of MHC I, MHC II, CD80, CD86 and F4/80 on cells from inflamed prostates. Given the high expression of ROS and RNS by cells from the inflammatory site, it is not surprising that these cells have a lower maturation status. Production of ROS can inhibit the differentiation of myeloid cells via its ability to modulate the expression of genes through regulation of signaling pathways such as NF-κB, MAPK and AKT [45–46].
Using standard in vitro suppression assays, Gr-1+CD11b+ cells from both the spleen and inflammatory site or tumor site were able to inhibit T cell activation; however, cells from the inflammatory site were more potent suppressors and not dependent upon IFN-γ produced in vitro. Interestingly, cells from the spleen of inflamed mice were consistently more suppressive than cells from spleens of naïve mice, suggesting that low levels of inflammatory cytokines may result in partial in vivo priming. Similarly, Gr-1+CD11b+ cells from the tumor site, which represents a chronic inflammatory setting, were more potent suppressors than cells isolated from the spleen of tumor-bearing mice, although in this model partial dependence upon IFN-γ was observed. These data demonstrate Gr-1+CD11b+ cells from inflamed prostates or from prostate tumors can suppress T cell function in vitro, confirming that MDSC can be important regulators of immune responses in the prostate. However, in contrast to our data studies by others comparing the function of Gr-1+CD11b+ cells from spleen, bone marrow and blood of tumor-bearing mice reported no functional difference in their ability to prevent CD4 or CD8 T cell activation [47]. These conflicting results may be explained by the activation status of the responder T cells used or the length of co-culture as it is well know that activated T cells can induce regulatory potential in MDSC. However, in contrast to previously published results, our study is the first to directly demonstrate long term culture with activated T cells can convert precursor splenic Gr-1+CD11b+ cells into functional MDSC through IFN-γ induction of iNOS.
When the suppressive function of MDSC was tested in assays where their exposure to T cells was minimized, only MDSC isolated from an acute or chronic inflammatory site were able to inhibit T cell proliferation. While other studies have investigated MDSC function from specific sites such as the liver, spleen, bone marrow, lymph node and tumor, none simultaneously investigate MDSC function from other sites distal to the local inflammatory response in a manner directly testing immediate function after isolation [3, 8, 11, 36, 48–49]. When the suppressive capacity of Gr-1+CD11b+ cells from peripheral tissues (spleen and liver) was directly compared to cells from the inflammatory site, only cells from the inflammatory site were able to inhibit antigen activated T cell proliferation. Furthermore, using Arg1 and iNOS inhibitors, the ability of prostate MDSC to inhibit antigen activated OT-I cells was partially dependant on both enzymes. Similarly, Gabrilovich and colleagues recently demonstrated Gr-1+CD11b+ cells freshly isolated from the spleen of tumor-bearing mice lack iNOS or Arg1 expression[44]. Comparative suppression studies between spleen and tumor MDSC showed differential ability to regulate antigen specific responses; however, the suppression assays evaluated MDSC function after more than 72 hours in culture with T cells. Given our data showing 48 hours in culture with activated T cells is sufficient to induce suppressive function in splenic Gr-1+CD11b+ cells, the immediate suppressive capacity of MDSC was not examined in the study by Gabrilovich et al.
The data presented in our study are the first to show during a localized inflammatory response a site specific difference in the ability of MDSC to down-regulate T cell function directly ex vivo. Furthermore, given that MDSC comprise a dominant population at the site of inflammation, the short-term suppressor assay, which tests the ability of MDSC to control pre-activated T cells, may better reflect the in vivo function of MDSC at inflammatory sites.
The ability of MDSC to suppress T cell function during normal immune responses in vivo has been demonstrated in several experimental models [15–18]. However, none of these previous studies examined the function of T cells at a site of localized inflammation in comparison to peripheral tissues in vivo. Using a tissue specific inflammatory model where OVA antigen (and the resulting inflammatory response) is restricted to the prostate, we found that MDSC inhibited T cell function only at the inflammatory site, demonstrating a restricted in vivo regulatory pattern for MDSC. The in vitro studies reported herein provide compelling data supporting the hypothesis that MDSC are functional in vivo only at the site of inflammation or within a tumor. The in vivo studies monitoring T cell expansion and function support the in vitro data; however, the absence of OVA antigen presentation in the spleen limits ones ability to definitively conclude that MDSC lack the capacity to regulate in the absence of additional signals. Functionally, the in vivo demonstration that MDSC do not regulate T cell expansion and function in the spleen supports our conclusion that MDSC regulation is restricted to the site of inflammation, providing a mechanism by which this occurs. Additionally, there is extensive evidence demonstrating that MDSC gain function in the presence of activated T cells due to exposure to IFN-γ, and that regulation is antigen-specific [50]. In addition, previous studies where spleen Gr-1+CD11b+ cells are adoptively transferred into tumor bearing hosts, T cell function is diminished in vivo, showing transferred MDSC inhibit T cell expansion [25]. In the POET model, OVA is not present in the spleen and accordingly, OT-I cells are not activated in this tissue. Therefore, obtaining direct evidence to support the absence of capacity to regulate in the spleen is complicated by the fact that if OVA is presented in the spleen, OT-I cells will respond by producing IFN-γ and activate function in MDSC. This conclusion is supported by data presented in this manuscript where spleen-derived MDSC are activated by IFN-γ in a 72 hr. assay. Thus current published data together with the data presented in this manuscript support the conclusion that MDSC are active only at the site of inflammation. Because of the apparent need for cytokine activation, these data further suggest that MDSC function to control activated T cells not the priming event.
Materials & Methods
Mice and in vivo experiments
Prostate ovalbumin expressing transgenic-3 mice (POET-3) were generated as previously described [19]. Rag−/−Thy1.1+ OT-I (OT-I) mice were generated by breeding Rag−/− mice (Jackson Laboratories) to Thy1.1+OT-I mice. Male C57BL/6 mice were obtained from the Transgenic Mouse Facility at Purdue University. All animals used were male mice between 8–12 weeks of age. All protocols for the reported animal studies were approved by The Lab Animal Program at Purdue University. To induce prostate inflammation, splenocytes were isolated from OT-I mice and cultured at 5×105/mL with 1 µg/mL SIINFEKL (Ova peptide 257–264, American Peptide) for 48hrs. Live cells were purified by Fico/Lite (Atlanta Biologicals) and 5×106 cells were injected i.v. into POET-3 mice. For RM-1 tumor studies, 5×105 RM-1 cells were injected s.c. on the flank area. Mice were sacrificed when tumor sizes reached 20 mm in diameter. Measurements were performed with a caliper by measuring the largest diameter and its perpendicular length.
Flow cytometry
Prostate and tumor tissue was placed in a solution of 2 µg/mL Collagenase D (Roche Diagnostics) in RPMI containing 10% FBS. Tissue was minced and placed at 37°C for 1 hr for digestion followed by passing through a 70 µm filter. Spleens were removed and ground between frosted microscope slides in PBS. Red blood cells were lysed with ACK buffer and passed through a 70 µm filter. Single cell suspensions were then incubated with TruStain FcX antibody (BioLegend) then stained with directly conjugated antibodies (eBioscience; BioLegend) according to manufacturer instructions. Flow-cytometric analysis was performed on a FACSCanto (BD Biosciences) and data were analyzed using FlowJo software (Tree Star).
Arg1 assay
For analysis of Arg1 enzyme function, CD45+Gr-1+CD11b+ cells from freshly isolated tissues were sorted and re-suspended at a concentration of 1×107 per mL in lysis buffer. The level of Arg1 activity was assessed in supernatants of cell lysates according to manufacturer instructions (BioAssay Systems). Units per liter are defined as 1 unit of Arg1 able to convert 1 µm of L-arginine to ornithine and urea per minute at pH 9.5 and 37°C.
Detection of iNOS
For detection of iNOS protein by flow cytometry, an intracellular staining kit was used according to manufacturer instructions (BD Biosciences) using FITC mouse anti-iNOS (BD Biosciences) or FITC mouse IgG2a isotype control (BioLegend) at a 1:100 dilution.
Quantitative real-time PCR
Total RNA was prepared from FACS sorted CD45+Gr-1+CD11b+ cells using the RNAeasy kit (Qiagen, Valencia, CA, USA). cDNA was synthesized using qscript flex cDNA synthesis kit (Quanta Biosciences). Quantitative RT-PCR was carried out using TaqMan primer and probe sets for mouse Arg1, iNOS, C/EBPβ and 18s rRNA (Applied Biosystems). Relative mRNA expression = 2 − (Ct of gene − Ct of 18s rRNA), where Ct is the threshold cycle value. Data were normalized to 18s RNA and are representative of three independent experiments.
T cell suppression assay
CD45+Gr-1+CD11b+ cells were pooled from 5 mice per group and sorted from tissues using the iCyte Reflection (iCyte) cell sorter and seeded at 1×105 per well. Thy1.1+OT-I spleen cells were added at varying ratios in the presence of SIINFEKL peptide (1mg/mL). To monitor proliferation, BrdU (BD Biosciences) was added 24 hours prior to analysis of proliferation. In effector suppression assays OT-I cells were pre-activated for 24–48 hours with SIINFEKL (1mg/mL), purified by Fico/Lite gradient and added at 1×105 cells per well. BrdU was added directly to OT-I cells at the time of incubation with MDSC and cells were harvested for analysis of proliferation after 12 hours. To evaluate suppression, the percentage of BrdU+ Thy1.1+ cells was analyzed by flow cytometry. Where indicated neutralizing IFN-γ antibody, LNMMA or nor-NOHA were added at the beginning of culture (10µg/mL, clone H22; R&D systems). The percentage suppression of proliferation is calculated as (1 − proliferation with MDSC proliferation without MDSC) × 100. Where neutralizing IFN-γ antibody is used the percentage suppression of proliferation with is calculated as (1 − proliferation with MDSC with inhibitor proliferation without MDSC with inhibitor) × 100. The percent dependence on IFN-γ is expressed as [1 − (% Suppression with MDSC and with inhibitor % Suppression with MDSC and without inhibitor)] × 100.
In vivo depletion experiments
POET-3 mice received 100µg IV anti-Gr-1 depletion antibody clone RB6-8C5 (a kind gift of Dr. John Harty, The University of Iowa) or isotype control antibody (SFR8, eBiosciences) four hours prior to adoptive transfer of Thy1.1+ cells as described above. Mice received additional injections of antibody at day 2 and day 4, and on day 6 prostate and spleen tissue were harvested and single cell suspensions were prepared. Intracellular IFN-γ (Biolegend) staining of prostate and spleen tissue was performed according to manufacturer instructions (BD) after re-stimulation with SIINFEKL peptide (5ug/mL) for 5 hours.
Supplementary Material
Acknowledgements
We thank Dr. James F. Leary and Mike Zordan (Flow Cytometry and Cell Separation Facility) for their expertise and assistance in cell sorting. We also thank Paul Snyder and the School of Veterinary Medicine Tissue Core for assistance with the histology. This work was supported by NIH grant DK084454.
List of abbreviations
- POET-3
Prostate Ovalbumin Expressing Transgenic-3
- MDSC
myeloid derived suppressor cell
- Arg1
Arg1-1
- iNOS
inducible nitric oxide synthase
- LNMMA
NG-Monomethyl-:-arginine monoacetate salt
- nor-NOHA
Nw-hydroxyl-nor-L-argenine
- DCFDA
5-(and-6)carboxy-2,7-dichlorodihydrofluoresceindiacetate
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 5.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Grone HJ, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 8.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 9.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 12.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 14.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 15.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 16.Cauley LS, Miller EE, Yen M, Swain SL. Superantigen-induced CD4 T cell tolerance mediated by myeloid cells and IFN-gamma. J Immunol. 2000;165:6056–6066. doi: 10.4049/jimmunol.165.11.6056. [DOI] [PubMed] [Google Scholar]
- 17.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marhaba R, Vitacolonna M, Hildebrand D, Baniyash M, Freyschmidt-Paul P, Zoller M. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol. 2007;179:5071–5081. doi: 10.4049/jimmunol.179.8.5071. [DOI] [PubMed] [Google Scholar]
- 19.Lees JR, Charbonneau B, Hayball JD, Diener K, Brown M, Matusik R, Cohen MB, Ratliff TL. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate. 2006;66:578–590. doi: 10.1002/pros.20307. [DOI] [PubMed] [Google Scholar]
- 20.Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 2007;10:15–29. doi: 10.1038/sj.pcan.4500930. [DOI] [PubMed] [Google Scholar]
- 21.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 22.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coccia EM, Stellacci E, Marziali G, Weiss G, Battistini A. IFN-gamma and IL-4 differently regulate inducible NO synthase gene expression through IRF-1 modulation. Int Immunol. 2000;12:977–985. doi: 10.1093/intimm/12.7.977. [DOI] [PubMed] [Google Scholar]
- 24.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 25.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ. Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol. 2004;172:7565–7573. doi: 10.4049/jimmunol.172.12.7565. [DOI] [PubMed] [Google Scholar]
- 27.Teng X, Li D, Catravas JD, Johns RA. C/EBP-beta mediates iNOS induction by hypoxia in rat pulmonary microvascular smooth muscle cells. Circ Res. 2002;90:125–127. doi: 10.1161/hh0202.103647. [DOI] [PubMed] [Google Scholar]
- 28.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration--functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 30.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 31.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenu JP, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher JL. Effects of the new arginase inhibitor N(omega)-hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide. 1999;3:427–438. doi: 10.1006/niox.1999.0255. [DOI] [PubMed] [Google Scholar]
- 33.Reif DW, McCreedy SA. N-nitro-L-arginine and N-monomethyl-L-arginine exhibit a different pattern of inactivation toward the three nitric oxide synthases. Arch Biochem Biophys. 1995;320:170–176. doi: 10.1006/abbi.1995.1356. [DOI] [PubMed] [Google Scholar]
- 34.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 36.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 38.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J Immunol. 2004;173:1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki J, Tsuji T, Chamoto K, Takeshima T, Sendo F, Nishimura T. Successful elimination of memory-type CD8+ T cell subsets by the administration of anti-Gr-1 monoclonal antibody in vivo. Cell Immunol. 2003;224:98–105. doi: 10.1016/j.cellimm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 42.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 43.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, Thebault P, Renaudin K, Vanhove B. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180:7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 44.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1{alpha} regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010 doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 46.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 47.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.