Summary
In adult skin, stem cells in the hair follicle bulge cyclically regenerate the follicle, whereas a distinct stem cell population maintains the epidermis. The degree to which all bulge cells have equal regenerative potential is not known. We found that Sonic hedgehog (Shh) from neurons signals to a novel population of cells in the telogen bulge marked by the Hedgehog response gene Gli1. Gli1-expressing bulge cells function as multipotent stem cells in their native environment and repeatedly regenerate the anagen follicle. Shh-responding perineural bulge cells incorporate into healing skin wounds where, notably, they can change their lineage into epidermal stem cells. The perineural niche (including Shh) is dispensable for follicle contributions to acute wound healing and skin homeostasis, but is necessary to maintain bulge cells capable of becoming epidermal stem cells. Thus nerves cultivate a microenvironment where Shh creates a molecularly and phenotypically distinct population of hair follicle stem cells.
Introduction
The maintenance and repair of adult tissues is dependent on stem cells that undergo self-renewal while also producing progeny fated for proliferation and differentiation. Moreover, signals from the microenvironment or “niche” often regulate tissue specific stem cells (Greco and Guo, 2010). Mammalian skin relies on at least two populations of stem cells for its maintenance. Cells in the basal keratinocyte layer replenish the interfollicular epidermis (IFE), whereas the hair follicle is cyclically regenerated by stem cells in the bulge region (Blanpain and Fuchs, 2009; Cotsarelis, 2006). The hair follicle cycles through predictable phases of growth (anagen), apoptotic regression (catagen), and quiescence (telogen), and regeneration of the anagen follicle can be experimentally induced by depilation (Plikus et al., 2008). As Hedgehog (Hh) signaling has been demonstrated to regulate quiescent stem cells in the adult forebrain (Ahn and Joyner, 2005; Balordi and Fishell, 2007), we tested whether Hh also regulates stem cells in the adult hair follicle.
The hair follicle bulge houses stem cells that regenerate the follicle during anagen, are slow cycling, can generate new follicles in recipient skin when cografted with dermal cells, and are multipotent and highly colonogenic in vitro (Blanpain et al., 2004; Claudinot et al., 2005; Cotsarelis et al., 1990; Morris and Potten, 1994; Oshima et al., 2001; Taylor et al., 2000). The adult bulge is part of the outer root sheath (ORS), an epithelial cylinder that is contiguous with the basal layer of the adjacent epidermis and surrounds the inner layers of the follicle. In adult mice, the bulge is convex because it surrounds the bulbous club hair, a retained hair shaft from prior telogen cycles (Cotsarelis, 2006). The bulge along with the isthmus (a narrowing of the ORS above the bulge) and the infundibulum (follicle opening) comprise the non-cycling portion of the hair follicle (See Figure 1). The incompletely overlapping expression domains of proposed follicle stem cell markers in the bulge such as CD34 (Trempus et al., 2003) and Lgr5 (Jaks et al., 2008) suggests the bulge contains molecularly distinct subdomains (Watt and Jensen, 2009). Whether all subdomains within the bulge have equal regenerative potential is not clear.
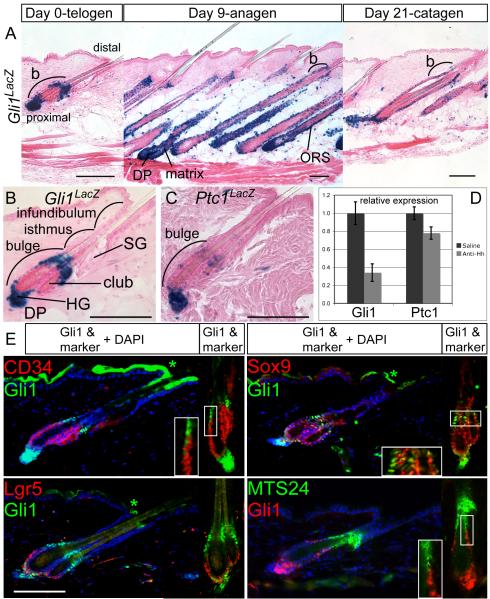
Figure 1.
Hh-responding cells are localized in molecularly distinct subdomains of the telogen hair follicle. Also see Figures S1, S2.
(A) X-gal staining in adult Gli1LacZ/+ skin at day 0, 9, and 21 after depilation of telogen hair to induce regeneration of the anagen follicle. b; bulge, Scale bars=100μm.
(B, C) X-gal staining of Gli1LacZ/+ and Ptc1LacZ/+ telogen follicles showing Hh-response genes in the upper bulge, lower bulge, HG, and DP. Scale bars=100μm.
(D) Relative expression levels of Gli1 and Ptc1 mRNA assessed by RT-qPCR in wild type telogen skin treated with Hh-neutralizing antibody. Error bars=SEM. club; club hair.
(E) Immunostaining of Gli1LacZ and the progenitor cell markers indicated. Blue; DAPI. *Non-specific staining. Scale bar=100μm.
Below the telogen bulge, the epithelial hair germ (HG) separates the bulge from the dermal papilla (DP), a mesenchymal condensation at the base of each follicle. The HG is the initial site of proliferation at the transition from telogen to anagen, and stem cells from the bulge, in turn, replenish the HG (Greco et al., 2009). Regeneration of the lower follicle during anagen includes an elongation of the ORS and regeneration of the matrix, a germinal epithelium that surrounds the enlarged DP and produces the cylindrical inner root sheath (IRS) and hair shaft (HS). Multiple signaling pathways have been implicated in regulating anagen regrowth, including Hh (Jaks et al., 2008; Oro and Higgins, 2003; Wang et al., 2000). However, little is known about Hh signaling in the quiescent telogen hair follicle.
Hh signaling regulates proliferation and developmental patterning in many tissues, including the hair follicle (Fuccillo et al., 2006; Varjosalo and Taipale, 2008). Hh signaling is mediated by three Gli transcription factors. When Hh ligand is present, full-length Gli2 and Gli3 accumulate and stimulate transcription of Hh target genes. Gli1 is induced by activator forms of Gli2 and Gli3, and its expression requires Hh signaling (Bai et al., 2002; Bai et al., 2004). Whereas Gli1 expression is a useful reporter of Hh signaling, Gli1 itself is dispensable in mouse development (Bai et al., 2002). In embryonic skin, loss of Gli2 or Sonic Hedgehog (Shh) prevents the initial hair follicle elongation (Chiang et al., 1999; Mill et al., 2003; St-Jacques et al., 1998), possibly because Shh is a mitogen that promotes keratinocyte proliferation (Adolphe et al., 2004; Fan and Khavari, 1999). Similarly, Hh blockade in adult skin with a neutralizing antibody inhibits regeneration of the anagen follicle (Wang et al., 2000). Moreover, expression studies of Gli1 and Ptc1, another Hh target gene, identified Hh responding cells in the adult ORS, matrix, and DP during the proliferative anagen phase (Oro and Higgins, 2003). Although it appears that Shh is required in transient amplifying cells for follicle expansion, it is unclear whether long-lived follicle stem cells are a direct target of Hh signaling.
In addition to maintaining the cycling follicle, bulge cells can also be recruited to the IFE after wounding (Claudinot et al., 2005). Keratin 15 (K15) expressing bulge cells migrate to the healing epidermis during reepithelialization, but they do not persist in the regenerated epidermis (Ito et al., 2005). In contrast, genetic fate mapping of the entire telogen follicle, including the isthmus and infundibulum, indicates there is a population of cells outside of the K15 domain that can establish long-term progenitors in the regenerated epidermis (Levy et al., 2007). Identifying which follicle cells have the capacity to become epidermal stem cells after wounding and the signals that regulate them remains an important question for regenerative medicine.
We tested whether Gli1 marks cells in the telogen follicle capable of regenerating the cycling hair follicle and becoming epidermal stem cells after wounding. Strikingly, we found that Gli1 was expressed in two distinct domains within the telogen bulge. We then used Genetic Inducible Fate Mapping (GIFM) (Joyner and Zervas, 2006) to mark and follow Gli1-expressing cells in vivo. We found that Gli1-GIFM marked a population of stem cells that regenerated the anagen follicle, self-renewed for the life of the animal, and contributed to multiple lineages within the follicle. Surprisingly, we discovered that neurons in the dorsal root ganglion were the source of Shh signaling to Gli1 positive (+), K15 negative (−) cells in the upper bulge. Significantly, unlike K15(+) bulge cells, labeled Gli1-GIFM cells not only migrated to healing wounds, they also established stem cells that maintained the regenerated epidermis. Finally we found that the ability of the upper bulge to become epidermal stem cells was lost in denervated skin, establishing the perineural niche as a necessary regulator of this unique population of bulge stem cells.
Results
Hh signaling genes are expressed in the telogen bulge
To identify cells in the adult hair follicle with the potential to undergo Hh signaling, expression of the requisite transcriptional mediators Gli2 and Gli3 was assessed using Gli2LacZ/+ (n=4) and Gli3LacZ/+ (n=3) knock-in mice (Bai and Joyner, 2001) (Gli3LacZ allele to be described elsewhere). Mice 8-11 weeks old in which the dorsal hair follicles are synchronized in telogen (hereafter called adult telogen), were depilated to induce anagen regeneration, and biopsies were obtained at different stages of the hair cycle (Figure S1). During telogen, Gli2LacZ and Gli3LacZ were detected in similar distributions within the proximal follicle, including the bulge and HG. In anagen, staining in both Gli2LacZ/+ and Gli3LacZ/+ skin was seen in the ORS from the bulge down and in the follicle matrix. During catagen, Gli2LacZ expression was maintained in the regressing follicle, whereas Gli3LacZ staining was barely detectable in the follicle epithelium. Throughout the hair cycle, both reporter alleles were expressed robustly in the DP and diffusely in the dermis, subcutaneous tissue, and arrector pili muscle. In contrast to the cycling hair follicle, the normal IFE, follicular infundibulum, and SG lacked both Gli2LacZ and Gli3LacZ X-gal staining, showing that they are missing the transcription factors that transduce Hh signaling.
We next used Gli1LacZ/+ mice (n=5) (Bai et al., 2002) to identify follicle cells that were receiving Hh signaling (Figure 1). As anticipated, Gli1LacZ expression throughout the hair cycle was limited to domains that also expressed Gli2LacZ or Gli3LacZ. Interestingly, in the telogen follicle epithelium, Gli1LacZ staining was restricted to two distinct domains: the upper bulge and a lower portion of the bulge plus the adjacent HG (Figure 1). The middle region of the telogen bulge lacked Gli1LacZ expression, despite having Gli2LacZ and Gli3LacZ expression, suggesting a lack of Hh signaling in that region. During anagen, Gli1LacZ was expressed more broadly in the ORS from the bulge down and also in the matrix. The regressing catagen follicle continued to broadly express Gli1LacZ. In addition, at all stages, the DP stained for Gli1LacZ, and there were scattered Gli1(+) cells throughout the dermis and subcutaneous fat that included arrector pili muscles, nerves, and blood/lymph vessels. Although, Gli1LacZ was absent from the IFE, expression of all three Gli reporter alleles was detected in the specialized sensory epithelium of touch domes (data not shown). The spatiotemporal pattern of Gli1LacZ staining confirms the distribution of Hh-responding cells as detected by PtcLacZ staining during anagen and catagen (Oro and Higgins, 2003). It also reveals previously undescribed Hh-responding cells in the telogen bulge. We found that extended X-gal staining of PtcLacZ/+ telogen skin recapitulated the same expression pattern seen with Gli1LacZ (Figure 1C), confirming the distribution of Hh-responding cells in the telogen bulge.
Gli1 expression in the telogen skin is driven by Hh signaling
To confirm that Gli1 expression is a reporter of Hh signaling in adult skin, mice were treated with a neutralizing antibody against Shh. Wild type mice in adult telogen received injections of either 200μg antibody (n=3) or saline (n=3) twice daily for 5 days. The relative levels of Gli1 and Ptc1 mRNA were then determined by quantitative RT-PCR (RT-qPCR) from shaved, full-thickness skin with the subcutaneous fat removed. Significantly, anti-Hh antibody treatment reduced levels of Gli1 mRNA three-fold (Figure 1D), demonstrating a dependence on Hh signaling. There was a smaller reduction in Ptc1 expression.
RT-qPCR was also used to assay expression of the Hh ligands Sonic, Indian, and Desert Hedgehog. Shh was reported to be expressed in sorted Lgr5(+), CD34(−) cells, thought to be HG cells isolated from telogen skin (Jaks et al., 2008). In contrast, our RT-qPCR results and published in situ hybridization studies (Greco et al., 2009; Oro and Higgins, 2003) failed to detect Shh in telogen skin. Similarly, Ihh expression has been reported in mouse sebaceous glands by immunostaining (Niemann et al., 2003), yet we were unable to detect Ihh mRNA by RT-qPCR. We did detect Dhh in both treated and untreated skin. Potential sources for Dhh in telogen skin include Schwann cells on myelinated nerves (Sharghi-Namini et al., 2006) and the DP (Driskell et al., 2009). Dhh expression by DP cells could account for Gli1(+) cells in the surrounding lower bulge, HG and DP. If Dhh is a prominent Hh ligand in telogen skin, it also could explain the incomplete inhibition of Gli1 expression in treated animals, because the antibody used has only moderate avidity for Dhh binding (Wang et al., 2000).
Gli1 expression identifies molecularly distinct subdivisions of the telogen bulge
To further define the Gli1(+) populations in the telogen follicle, Gli1LacZ expression was compared to the expression of additional bulge and progenitor cell markers by immunostaining (Figure 1E, 2D). Two isthmus markers MTS24 (Nijhof et al., 2006) and Lrig1 (Jensen et al., 2009) were expressed immediately above the upper Gli1 expression domain, although both markers had occasional overlap with Gli1(+) cells along the boundary. This result localized the upper Gli1 domain to the uppermost bulge. Interestingly, the upper Gli1 domain resided primarily in a gap between the isthmus markers and the bulge-associated markers K15 and CD34. Thus the upper bulge is a newly described Gli1(+) K15(−) CD34(−) domain in the telogen follicle that is most easily visualized as the gap of K15(−) cells between the K15(+) bulge cells and cells expressing a low level of K15 in the follicular isthmus.
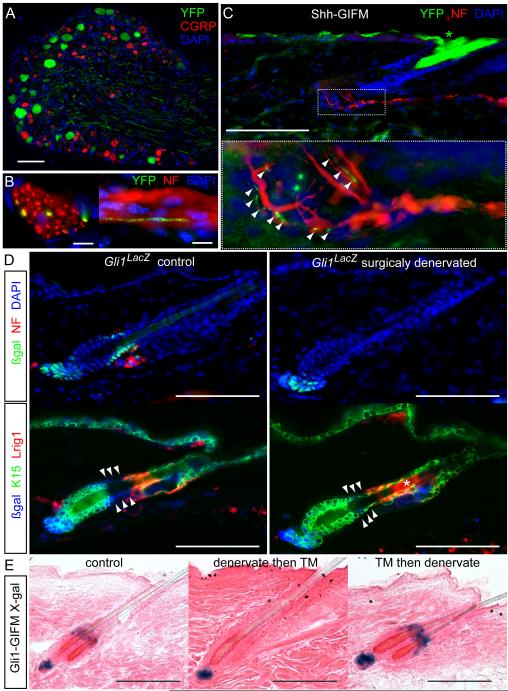
Figure 2.
Shh from sensory nerves signals to K15(−) hair follicle epithelium in the upper telogen bulge. Also see Figures S3, S4.
(A-C) Immunostaining in adult Shh-GIFM mice after TM induction. A) DRG. Scale bar=100μm. B) Axial and longitudinal sections through cutaneous nerves. NF; neurofilament. Scale bars=10μm. C) Hair follicle. Inset; magnification of boxed area, arrowheads; YFP(+) nerve endings on hair follicle. Scale bar=100μm.
(D) Immunostaining of Gli1LacZ/+ skin showing changes in the telogen bulge after denervation. Arrowheads; normally K15(−) upper bulge. Scale bar=100μm.
(E) X-gal staining of Gli1-GIFM telogen follicles. Upper bugle cells are not labeled in skin that is denervated then induced with TM (denervate then TM). Labeled upper bulge cells persist in skin denervated after TM induction (TM then denervate). Scale bars=100μm.
Co-staining of CD34 and K15 in the telogen bulge (Figure S1) confirmed that both genes were co-expressed in the basal layer with a common upper border that we designate as the upper limit of the middle bulge. This border anatomically corresponds to the distal limit of the K15(+) CD34(−) companion layer that resides between the basal layer and the club hair in the middle and lower bulge. Of note, in some follicles Gli1LacZ expression in the upper domain extended down into the uppermost middle bulge. We define the lower bulge as the Gli1(+) K15(+) CD34(+) region that envelops the proximal club hair. Below the lower bulge, the HG was Gli1(+) K15(+) CD34(−).
Other stem cell markers also showed overlap with the Gli1 populations in the follicle (Figure 1E). Lgr5 overlapped with Gli1LacZ in the HG and the lower bulge basal layer, but also extended into the Gli1(−) middle bulge in many follicles. Sox9, a marker of adult stem cells in multiple tissues including the hair follicle (Nowak et al., 2008), showed brightly stained cells scattered among dimly stained cells in all levels of the bulge including overlap with Gli1(+) cells in the upper bulge, lower bulge and HG. Overall, the combined expression of Gli1LacZ and other markers divides the telogen follicle into a complex molecular map of progenitor cell domains (see Figure 7), such that each domain has a unique gene expression profile.
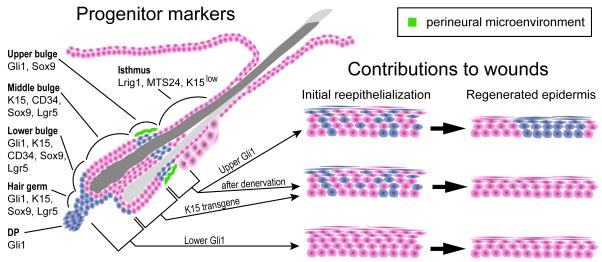
Figure 7.
Schematic summarizing expression domains of stem cell markers and the functionally distinct perineural subdomain in the telogen hair follicle.
Expression of Gli1LacZ and other markers define molecularly distinct zones in the telogen follicle, including regionalization of the bulge into the upper, middle, and lower bulge. Gli1(+) cells in the upper bulge (upper Gli1) receive Shh signaling from follicle-associated nerve endings and are functionally distinct from the cells in the middle bulge, lower bulge and HG in their ability to become epidermal stem cells during wound healing.
A more global characterization of sorted dorsal skin keratinocytes by microarray mRNA expression analysis confirmed a distinctive gene signature in Gli1(+) cells (GFP expressing cells in Gli1eGFP mice – see Figure S2). Skin epithelium was isolated from Gli1eGFP/+ mice in adult telogen, and viable ∝6 Integrin(+) basal keratinocytes were sorted into three cohorts: GFP(+), GFP(−) CD34(+) (middle bulge cells), and GFP(−) CD34(−) (predominately IFE). Expression analysis identified multiple genes differentially regulated in the Gli1(+) cells relative to the two other skin populations (Figure S2, Table S1). As expected, Gli1 expression was up in the GFP(+) cells (p=0.0026) and CD34 expression was up in the GFP(−) CD34(+) cells (p=0.00023) relative to the other sorted cells. Of note, both Sox9 and Lgr5 were preferentially expressed in GFP(−) CD34(+) and GFP(+) cells relative to IFE cells (p<0.00005). A trend toward elevated K15 expression in the bulge populations relative to the IFE cells was not significant (p=0.049), likely because the IFE expresses K15. These findings are consistent with the observed immunostaining patterns, and further establish that Gli1(+) keratinocytes are molecularly distinct from CD34(+) middle bulge cells and the IFE. A full list of differentially expressed genes is in Table S1.
Sensory nerves are the source of Shh that signals to the upper bulge
As Dhh produced by DP cells could explain the Gli1 expression in the lower telogen follicle, we sought the Hh source for the upper bulge. Since no Shh mRNA was detected in telogen skin by RT-qPCR, we hypothesized that Shh protein could be transported to the follicle by the sensory nerves that wrap around the upper Gli1(+) domain (Figure 2). To test this, we generated Shh-GIFM mice heterozygous for the ShhCreER allele (Harfe et al.) and homozygous for the Rosa26floxSTOP-YFP (R26RYFP) reporter allele (Srinivas et al.). Indeed, adult mice (n=5) that received tamoxifen (TM) contained YFP labeled sensory neurons in the dorsal root ganglions (DRGs) that were CGRP(−) (Figures 2 and S3). Furthermore, YFP immunostaining in the peripheral process of Shh-expressing neurons was seen within cutaneous nerves, including nerve fibers contacting the upper bulge region of hair follicles (Figure 2C).
To test whether cutaneous neurons deliver Shh to the telogen hair follicle and stimulate Gli1 expression, we physically severed the nerves in Gli1LacZ/+ mice (n=7). As predicted, surgical denervation of back skin eliminated LacZ staining in the upper telogen bulge in 93.6% of follicles (N=187) within two weeks (Figure 2, S4), but not the lower bulge, HG or DP. Follicles with persistent Gli1LacZ staining in the upper bulge after denervation consistently remained associated with neurofilament(+) processes, indicating incomplete denervation.
In the two weeks following denervation surgery, there was no obvious change in follicle cytoarchitecture, no histological evidence of necrosis, no apoptosis detected by immunostaining for cleaved Caspase 3, and no loss of quiescence as assayed by EdU incorporation (Figures 2, S4) – suggesting the cells of the upper bugle remained in place after loosing Shh signaling. To confirm this, we performed Gli1-GIMF by administering TM to 8 week old Gli1CreER/+; R26RLacZ/LacZ mice (Ahn and Joyner, 2004; Soriano, 1999) to label the Gli1-expressing cells during adult telogen (N=6). After 5 days, back skin was denervated and after two additional weeks biopsies were taken. Staining in control and denervated skin was seen in the upper bulge, lower bulge, HG, and DP of hair follicles, mirroring the telogen expression pattern seen with the Gli1LacZ and Gli1eGFP alleles. Importantly, in denervated skin the distribution and amount of labeling remained unchanged relative to control skin, proving the persistence of upper bulge cells even when deprived of their Hh source (Figure 2E). Together these results demonstrate that in contrast to the afferent sensory inputs and hypothesized trophic factors that signal from the follicle to the nerve (Botchkarev et al., 1997), there is retrograde Shh signaling from nerves to the follicle epithelium in the upper bulge.
Perineural niche maintains the molecular phenotype of the upper bulge
Despite the normal appearing cytoarchitecture of the bulge after denervation, immunostaining of Gli1LacZ/+ skin revealed a loss of the K15(−) gap in the upper bulge (Figure 2D). In contrast, Lrig1 staining appeared normal, suggesting that the isthmus remained unchanged. Similarly MTS24 staining in the isthmus was unchanged (data not shown). However, the upper bulge did not adopt a complete middle bulge phenotype after denervation, as CD34 staining remained absent in the upper bulge region (data not shown). The shift from Gli1(+) K15(−) cells to Gli1(−) K15(+) cells in the upper bulge after denervation suggests that the perinural microenvironment, including Shh signaling, is necessary to maintain the molecular profile associated with an upper bulge identity.
Gli1-expressing cells expand to regenerate the hair follicle
To test whether Hh-responding cells in the telogen follicle have stem cell properties, we administered TM to Gli1-GIFM mice in adult telogen (n=6) 4-6 days prior to inducing a new anagen cycle by hair depilation. Staining of skin taken on the day of depilation showed the expected staining pattern (Figure 3), and 100% of follicles (n=512) contained labeled cells (Figure S5). Strikingly, 3 days post depilation (dpd), the early anagen follicles showed staining throughout the regenerating portion of the follicle epithelium and DP. By 9 dpd, the amount of labeled cells had expanded even further in the regenerated anagen ORS, matrix, IRS, and HS, with staining in the regenerated portion of 100% of follicles (n=531). In catagen (21 dpd), labeling in all components of the hair follicle from the bulge down, including the DP, was maintained. Importantly, Gli1-GIFM-labeled cells did not contribute to the normal IFE, infundibulum, or sebaceous gland. Occasional labeling was seen in these skin compartments where the skin was traumatized during depilation, consistent with prior reports that trauma can mobilize bulge cells to alternate fates (Claudinot et al., 2005).
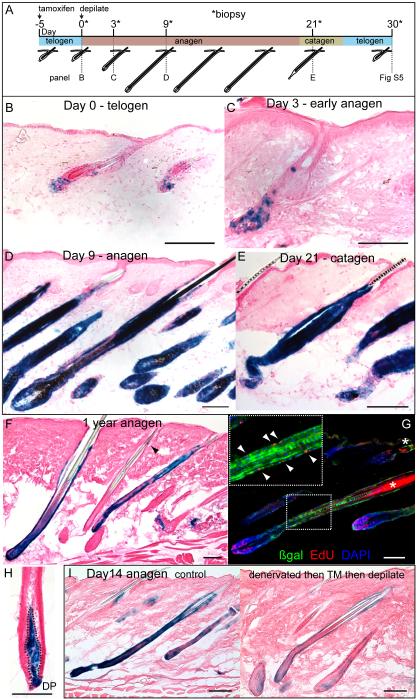
Figure 3.
Gli1(+) cells in the telogen hair follicle regenerate the anagen follicle and self renew for normal lifespan of the animal. Also see Figure S5.
(A) Scheme of experiment with TM induction of Gli1-GIFM mice in telogen phase of hair cycle prior to depilation.
(B-E) X-gal staining of Gli1-GIFM skin throughout hair cycle showing expansion of initially labeled cells during anagen regrowth. Scale bars=100μm.
(F, H) X-gal staining of fate-mapped cells in Gli1-GIFM skin 1 year after labeling during telogen. Labeled cells retain the ability to regenerate the anagen follicle. Arrowhead; labeling in bulge region, outline; DP. Scale bars=100μm.
(G) Immunostaining anagen follicle in same mice given EdU, showing proliferating GIFM labeled cells. Arrowheads; Edu(+) ßgal(+) cells in ORS. Scale bar=100μm.
(I) X-gal staining of control and denervated Gli1-GIFM skin induced with TM during telogen and collected 14 days after anagen onset. Scale bars=100μm.
In the subsequent telogen (30 dpd), labeled cells were found in the upper bulge, lower bulge, HG, and DP, with scattered staining also found in the middle bulge and isthmus in some follicles (Figure S5), demonstrating redistribution of labeled bulge cells after hair cycling. These Gli1-GIFM results demonstrate that Gli1(+) cells in the telogen hair follicle expand to regenerate all lineages of the follicular epithelium, a function of bulge stem cells. Additionally, the commingling of Gli1-GIFM-labeled cells and unlabeled cells in regenerated anagen hair follicles shows that multiple progenitor cells contribute to the regeneration of each follicle. Furthermore, Hh-responding cells are also potential progenitors in the cycling DP.
To test the relative contributions to anagen regeneration of the upper and lower Gli1(+) cells in the telogen follicle, we surgically denervated back skin of 6 week old Gli1-GIFM mice. Two weeks later, during adult telogen, the mice were given TM to label Gli1-expressing cells. After 5 days, the mice were depilated. Skin biopsied on the day of depilation confirmed that reporter expression was predominantly absent in the upper bulge, whereas the lower bulge, HG and DP all had similar staining to control skin (Figure 2E). Biopsies taken 14 dpd showed, on average, reduced staining throughout the regenerated anagen follicle compared to non-denervated skin (Figure 3I), suggesting that the upper bulge normally contributes to anagen regeneration. DP staining in anagen was unaffected by denervation.
To test if denervation alters the ability of the upper bulge cells to contribute to the regenerating anagen follicle, we gave TM to 8 week old Gli1-GIFM mice (N=4) in adult telogen to label the Gli1(+) cells, including the upper bulge. 5 days later back skin was denervated and after another 2 weeks mice were depilated. As reported above, denervation did not alter the amount or distribution of labeling in the telogen follicle (Figure 2E). Likewise, staining in anagen skin taken 14dpd was indistinguishable from control skin (data not shown). These results confirm that denervation does not impact progression of the hair cycle (Maurer et al., 1998), and demonstrates that the perineural niche (including Shh) is dispensable in maintaining upper bulge cells that contribute to anagen follicle regeneration, at least through one cycle of regeneration.
Gli1-expressing follicular stem cells continue to regenerate the anagen follicle through multiple hair cycles
To test for the stem cell property of long-term self-renewal, Gli1-GIFM-marked cells were assayed for their ability to persist through multiple rounds of follicle regeneration. Gli1-GIFM mice were induced with TM during adult telogen and were serially depilated every 5 to 7 weeks for 6 hair cycles (n=3), or were allowed to cycle spontaneously for 13 months (n=6) (at least 3 hair cycles based on the number of retained club hairs). In both groups of mice, telogen follicles showed labeled cells in the DP and epithelium, but after multiple hair cycles labeled cells were preferentially distributed in the proximal isthmus and upper half of the bulge, with only rare staining in the more proximal bulge and HG (Figure S5). After a subsequent depilation to induce anagen, labeled cells were seen throughout regenerated portions of hair follicles and the DP in both groups of mice (Figures 3, S5). Whereas 99.2% of anagen follicles (n=550) at 1 year continued to show staining in the bulge region, only 16.0% had labeling in the regenerated portions of the follicle epithelium. Moreover, compared with the initial anagen cycle after TM administration, follicles that showed staining in the regenerated portion at 1 year had a larger proportion of unlabeled cells on average. Incorporation of EdU during the final anagen confirmed the proliferative capacity of many of the labeled cells (Figure 3G). These experiments demonstrate that progeny of the original Gli1-GIFM-marked cells in the telogen follicle include self-renewing stem cells that retain the ability to regenerate the anagen follicle for up to a year or through 7 hair cycles, and are preferentially retained in the upper telogen follicle.
Gli1-GIFM labeling in the DP also persisted for multiple hair cycles, because 87.6% of anagen follicles at 1 year (n=550) showed DP staining. Whereas the amount of labeling in a given DP was variable in both initial and long-term Gli1-GIFM skin, it was common to find DPs where the majority of cells were labeled even after 1 year (Figure 3H). Thus, the progenitor cells maintaining the cycling DP must express Gli1.
Even after multiple hair cycles, Gli1-GIFM cells labeled during telogen did not contribute regularly to normal IFE, follicular infundibulum, or the sebaceous gland. The occasional staining seen in these skin compartments appeared more frequent in the serially depilated mice than those allowed to cycle spontaneously, supporting the idea that trauma can alter the lineage commitments of these cells. Thus, Gli1(+) stem cells in the telogn follicle primarily maintain the cycling hair follicle and can do so for the normal lifespan of the animal.
Gli1-expressing follicle cells are multipotent
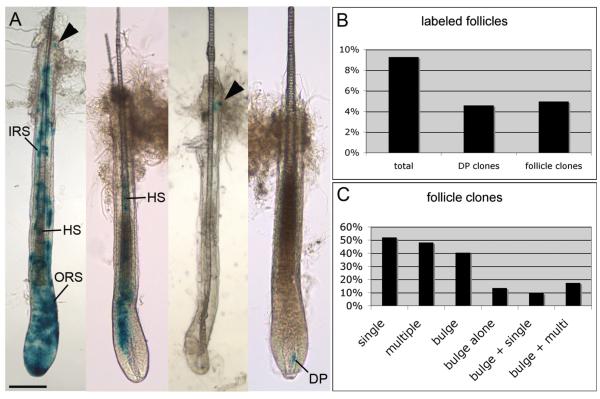
Most adult stem cells have the ability to contribute to multiple lineages within the tissue they maintain. To assay the multipotency of Gli1(+) cells in the telogen hair follicle, we performed in vivo clonal analysis. By reducing the dose of TM administered to Gli1-GIFM mice in adult telogen to 0.2mg, less than 10% of follicles had marked cells. Thus, any labeling in a follicle is likely to have arisen from a single labeled cell (Legue and Nicolas, 2005). Five days after TM administration, mice were depilated and biopsies were taken in anagen (12 dpd). Quantification of individually dissected whole mount stained follicles (N=1046) showed that 9.3% of follicles were labeled (4.6% with DP labeling, 5.0% with epithelium labeling, 0.3% with both), validating the low probability of multiple clones occurring in the same follicle. A lineage compartment analysis assigned stained cells in a given follicle to one or more of five groups based on anatomic location and cell shape: DP, ORS, IRS, HS, or bulge (Figure 4).
Figure 4.
Gli1(+) cells in the telogen hair follicle are multipotent.
(A) Whole mount X-gal staining in anagen follicles isolated from Gli1-GIFM skin induced at clonal frequency during telogen. Examples of labeling in multiple epithelial lineages, a single lineage (HS), bulge only, and in the DP. Arrowhead; labeling in bulge region. Scale bar=100μm.
(B) Frequency of anagen follicles with labeling after clonal Gli1-GIFM during telogen. follicle clones; any epithelial lineage.
(C) Frequency of staining in epithelial lineage compartments among labeled follicles. single; restricted to one lineage compartment, multiple (multi); at least two lineages.
Among the epithelial clones, 51.9% were restricted to a single lineage compartment, and 48.1% demonstrated multipotency with staining in two or more compartments. Of the epithelial clones, 40.4% had at least one stained cell in the bulge region (17.3% had bulge staining and 2 or 3 other lineages, 9.6% had bulge staining and one other lineage, and 13.5% had only one or two labeled cells in the bulge). However, these numbers likely underestimate actual bulge staining as damage to the bulge region occasionally occurred during follicle dissection. Nevertheless, at least 26.9% of epithelial clones appeared to have undergone asymmetric cell division producing a bulge cell plus at least one differentiated lineage. Taken together, clonal Gli1-GIFM demonstrates that single Gli1(+) cells in the telogen follicle have the ability to generate a new bulge cell while contributing to multiple lineages in the hair follicle as would a self-renewing multipotent stem cell. Moreover, the observed staining patterns support the idea that not every bulge stem cell is recruited during a given anagen regeneration, and not every stem cell division is an asymmetric division.
Gli1-expressing follicle cells contribute to wound healing and become epidermal stem cells
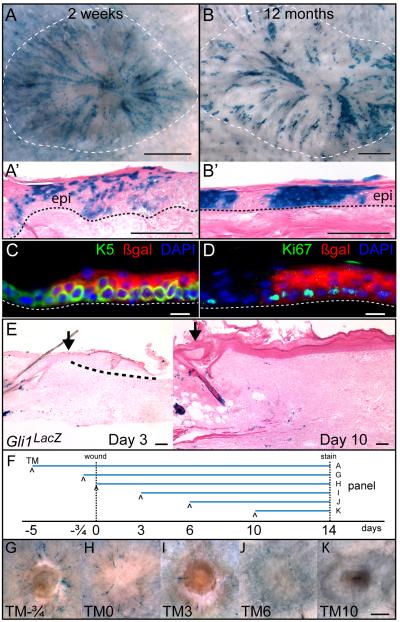
Given that Gli1-GIFM-marked cells in the telogen follicle do not normally contribute to the IFE but appeared to be recruited there after minor injury, we tested the potential of Gli1(+) follicle cells to contribute to healing wounds. Gli1-GIFM mice in adult telogen (n=8) were induced with TM, and 5 days later, 8-10mm diameter full-thickness skin wounds were excised. Two weeks after surgery, the reepithelialized wound contained many labeled cells arrayed in radial streams extending from adjacent follicles toward the wound center, with labeled cells scattered within both basal and suprabasal layers of the regenerating epidermis (Figures 5, S6). Despite the fact that Gli1-GIFM can mark cells in the arrector pili muscles, touch domes, epineurium and vascular adventitia in the skin, close examination of sectioned and whole mount stained tissue failed to identify labeled cells migrating from these structures to the healing epidermis. Ito et al. reported a similar radial staining pattern when a transgene containing the K15 promoter was used to fate map K15-CrePR-expressing bulge cells prior to wounding (Ito et al., 2005). However, labeled K15-GIFM cells from the telogen bulge do not persist in healed wounds, with no epidermal staining remaining beyond 50 days. In stark contrast, Gli1-GIFM-marked cells established long-term progenitors that maintain the regenerated epidermis. Twelve months after wounding (n=4), labeled cells in the Keratin 5(+) basal epidermis generated solid areas of marked proliferative clones extending to the skin surface (Figure 5).
Figure 5.
Wound healing converts Gli1(+) hair follicle cells into epidermal stem cells. Also see Figure S6.
(A-B) X-gal staining of whole mount healed wounds and sections of regenerated epidermis in Gli1-GIFM mice induced during telogen, 2 weeks and 12 months after full-thickness skin wounding. Dashed line; wound area, dotted line; epidermal basement membrane, epi; regenerated epidermis. A, B: scale bars=1mm, A’, B’: scale bars=100μm.
(C-D) Immunostaining of regenerated epidermis in Gli1-GIFM mice 12 months after wounding. dotted line; epidermal basement membrane. scale bars=10μm.
(E) X-gal staining in Gli1LacZ/+ skin, 3 days and 10 days after wounding. Arrow; edge of wound, dashed line; regenerating epidermal tongue. Scale bars=100μm.
(F) Experimental scheme to test when Gli1-GIFM cells exit follicle into healing wound.
(G-K) Whole mount X-gal staining 2 weeks after wounding in Gli1-GIFM mice induced with TM on different days relative to wounding. Scale bar=1mm.
The healing epidermis does not contain Hh responding cells
The above Gli1-GIFM results demonstrate that Hh responding cells in the telogen follicle can contribute to the regenerating epidermis. However, they do not address if epithelial cells respond to Hh signaling as they migrate into a wound. To determine this, 10mm wounds in Gli1LacZ/+ mice were assessed 1 day (n=3), 3 days (n=3) and 10 days after (n=5) surgery, before the wound was fully closed. In sectioned tissue, the unperturbed skin adjacent to the wound showed the expected X-gal staining in follicles. However, no staining was seen in the epithelial tongue advancing over the wound at any point (Figure 5E and data not shown). Sparse Gli1(+) cells were seen scattered at the periphery of the granulation tissue in the regenerating dermis at day 10, but mostly around blood vessels. Thus Hh signaling does not appear to be active in the regenerating epidermis, but may be involved with neovascularization of the dermis.
Hair follicles contribute only transiently to wound healing
The radial pattern of labeled cells running from adjacent follicles to the center of the acutely regenerated epidermis (Figure 5A, S6) suggests two alternative modes of follicle cell contribution to healing wounds. Either bulge cells continually migrate out of the follicle into the regenerating epidermis as it covers the wound, or cells are recruited out of the follicle over a limited period of time and then proliferate to regenerate the epidermis. To determine the time interval when bulge cells contribute to wound healing, 8mm full-thickness skin wounds were excised from Gli1-GIFM mice in adult telogen, and TM was administered 18 hours before wounding or on day 0, 3, 6, or 10 after wounding (n=3 for each time point), to induce reporter recombination for a 6-36 hour period after TM (Joyner and Zervas, 2006). Wounds were then stained on day 14 after wounding (Figure 5G-K). Staining in the regenerated epidermis of mice given TM 18 hours before wounding was comparable to those given TM 5 days before wounding. Mice given TM at the time of wounding had fewer stained cells in the wound than those labeled before wounding. Similarly, giving TM on day 3 resulted in only rare staining. No wound staining was seen with the later TM time points. Thus, the ability of Gli1-GIFM-marked bulge cells to join the regenerating epidermal progenitors was maximal only in the first day after wounding; it then diminished over 4-5 days and was absent after day 6.
Perineural niche is necessary for upper bulge cells to become epidermal stem cells
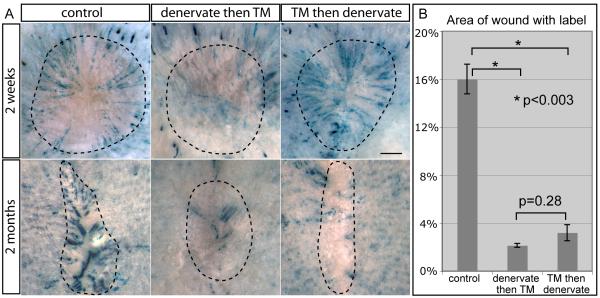
Because the Gli1(+) cells in the lower bulge and HG are K15(+), it is most likely the Gli1(+) K15(−) upper bulge cells that become epidermal stem cells after wounding. We used Gli1-GIFM to assess the contribution of the upper Gli1(+) follicle domain to wound healing. As described above, when TM is given to Gli1-GIFM mice 2 weeks after skin denervation, the upper bugle is primarily not labeled (Figure 2E). Interestingly, two weeks after wounding such mice (n=4), very few labeled cells were detected in the reepithelialized wound (Figure 6), demonstrating that the lower bulge, HG and non-epithelial Gli1(+) cells are inefficient at migrating into healing wounds. This is especially true when considering that some staining will be from the follicles that maintain upper bulge Gli1 expression after denervation due to incomplete nerve ablation.
Figure 6.
Perineural niche is required to maintain bulge cells that convert to epidermal stem cells after wounding.
(A) Whole mount X-gal staining 2 weeks and 2 months after wounding in Gli1-GIFM skin with TM induction during telogen and denervation prior to or following TM. Dashed line; wound area. Scale bars=1mm.
(B) Percent of regenerated epidermis with labeled cells 2 months after wounding. Error bars=SEM.
We then denervated Gli1-GIFM mice (n=4) that had been given TM 5 days before, to test the requirement of the perineural niche in wound healing. Two weeks after denervation, dorsal skin was wounded. Two weeks after wounding, healing was complete and the amount and distribution of labeled cells in the newly healed wounds of denervated mice was similar to control skin (Figure 6), suggesting the perineural niche (including Shh) is not required for overall wound healing or maintaining follicle cells that contribute to acute wound healing. Despite the unaltered ability of upper bulge cells to migrate into wounds after denervation, subsequent retention of labeled cells in the regenerated epidermis was significantly reduced. Two months after wounding, the regenerated epidermis in control wounds had multiple well-demarcated areas of solid staining. Quantification of staining in photographs of whole-mount stained wounds revealed that 16.0% of the regenerated epidermis was labeled in control skin, whereas only 3.2% of the wound area was stained in denervated skin (p<0.003). Hence, ablating cutaneous nerves not only changes the expression of Gli1 and K15 in upper bulge cells of the telogen follicle, it markedly reduces their distinctive ability to become epidermal stem cells in regenerated wounds.
Discussion
Our study demonstrates that, like the brain, hair follicles are maintained by adult stem cells that express the Hh response gene Gli1. We found two distinct domains of Gli1(+) epithelial stem cells in the quiescent telogen follicle, and demonstrated that Gli1(+) cells can regenerate the anagen follicle, self-renew for the life of the animal, and contribute to multiple lineages in the cycling follicle. In addition, we saw that Gli1-expressing mesenchymal cells maintain the DP. Finally, we showed that Gli1(+) K15(−) stem cells in the upper bulge receive Shh from sensory neurons, and are dependant on a novel perineural stem cell niche for their distinctive ability to become epidermal stem cells after wounding. Our Gli1-GIFM studies taken together with previous studies demonstrate that at least some molecularly distinct stem cell subdomains in the bulge have differing capacities to alter their stem cell lineage commitments in vivo (Figure 7). These results have important implications regarding the complexity of adult epithelial stem cell populations and their roles in tissue regeneration and repair, as well as for studies of wound healing and skin disease.
Gli1-GIFM allowed us to study Hh-responding hair follicle stem cells in the context of their native environment. Two other markers that overlap with Gli1 expression in the lower telogen bulge and HG have been studied in intact skin using GIFM. The K15-CrePR transgene was used to label a subset of bulge cells in the telogen follicle and follow their contribution to anagen regeneration, but persistence of labeling after the first hair cycle was not assessed (Morris et al., 2004). More recently, Lgr5-GIFM identified anagen-regenerating cells capable of self-renewal for multiple hair cycles (Jaks et al., 2008). Consistent with our results, gene expression studies on sorted Lgr5(+) skin cells indicated that at least some of these cells receive Hh signaling during telogen. Neither K15 nor Lgr5 expression, however, overlaps with Gli1 in the upper bugle, distinguishing the region as a molecularly distinct, Shh-responding subdomain in the telogen follicle. GIFM studies have also been done using Lgr6, a marker expressed primarily in the isthmus of the adult telogen follicle, but also in the IFE (Snippert et al., 2010). Labeled Lgr6-GIFM cells maintained the IFE, isthmus and SG with rare contributions to the cycling adult hair follicle, suggesting that Lgr6(+) stem cells largely reside outside the adult telogen bulge. However, some overlap with the upper bulge is suggested by a trend of Lgr6 expression being increased in GFP(+) cells (p=0.064) relative to the other sorted cells from Gli1eGFP/+ skin.
Observing stem cells in their native environment allows one to study both their role in homeostasis and their response to pathological states such as wounding. We found that Gli1(+) stem cells in the telogen bulge regenerate the cycling hair follicle throughout life. However, when exposed to additional signals from a fresh wound, Gli1(+) upper bulge cells will break lineage boundaries and move into the epidermis. The potency of the skin wound environment to alter stem cell behavior was recently illustrated by its ability to reprogram transplanted thymic epithelial cells into both hair follicle and epidermal stem cells (Bonfanti et al., 2010). Intriguingly, it is a perineural microenvironment in the follicle that instills Gli1(+) upper bugle cells with the capacity to be similarly reprogrammed into epidermal stem cells. This illustrates the importance of environmental cues in both maintaining stem cell plasticity and altering stem cell behavior (Watt and Jensen, 2009). These environmental effects should be considered when interpreting stem cell assays where cells are removed from their native environment. Moreover, understanding the environmental signals involved may help in reprogramming cells for stem cell based therapies.
Like many hair follicle stem cell markers (K15, Lgr5, and Sox9), Gli1 is expressed in the bulge and HG during the quiescent telogen phase and also in the proliferative ORS during anagen. Hh signaling has been proposed to regulate both stem cell maintenance and, at higher signaling levels, cell proliferation in many adult epithelia (Jiang and Hui, 2008). In anagen skin, Shh is expressed at high levels in the follicle matrix and acts as a mitogen that drives anagen regeneration (Gat et al., 1998; Oro and Higgins, 2003). Here we found that removal of the neural source of lower-level Hh signaling in the upper telogen bugle changes the expression profile and biological potential of the stem cells. Thus, Shh appears to have multiple roles in the hair follicle, including regulation of a subpopulation of quiescent stem cells.
Shh is a critical intercellular signaling molecule during development that classically signals to adjacent cell populations after release into the extracellular space. However in the developing optic nerve, Shh protein is transported down neuronal axons where it signals to distant astrocytes (Wallace and Raff, 1999). A recent study suggests this atypical mode of Shh signaling from neurons to distant astrocytes also occurs in the adult brain (Garcia et al., 2010). We now report retrograde transportation of Shh down the peripheral process of adult bipolar sensory neurons, and signaling to a completely different tissue type – the follicle epithelium. Moreover, outside of the nervous system, the perineural microenvironment has not been widely considered as a stem cell niche, although proper embryonic innervation has been implicated as necessary for normal organogenesis in some epithelial structures including the skin (Knox et al., 2010; Peters et al., 2002). We have now demonstrated functional dependence of an adult epithelial stem cell population on a Shh-expressing perineural niche for maintaining lineage plasticity during wound healing.
Lgr5-GIFM-marked cells from the lower follicle were found in the upper bugle and isthmus after multiple hair cycles (Jaks et al., 2008), showing that progeny from cells in the lower telogen follicle can redistribute upward after cycling through anagen and catagen. Similarly, we found that Gli1-GIFM-marked cells are preferentially retained in the isthmus and upper portions of the bulge after multiple hair cycles. This raises the possibility that the upper bulge and isthmus are important for long-term maintenance of the cycling follicle. As these regions of the follicle reside within or around the perineural niche, it is tempting to speculate that nerves play an additional role in maintaining long-lived follicle stem cells. This could explain the clinical hair loss seen in some patients with chronic peripheral neuropathies.
Comparative expression analysis of adult hair follicle stem cell markers illustrates that the telogen follicle contains multiple distinct domains, each with unique molecular signatures (Watt and Jensen, 2009)(Figure 7). Moreover, our study illustrates a difference between the lineage reprogramming potential of nerve-regulated Gli1(+) K15(−) upper bulge cells and that of the K15(+) bulge cells. It will be important to fully elucidate the complexity of the stem cell pool in the adult hair follicle, and identify any hierarchical organization among the stem cell populations, especially considering how the follicle bulge cells redistribute over the course of a hair cycle. Our molecular map of the telogen follicle will facilitate future studies to further dissect the genetic and functional anatomy of the bulge, and identify cell populations useful for regenerative medicine.
Experimental Procedures
Mice
A new Gli1eGFP allele was generated by gene targeting in embryonic stem cells (Figure S2). Gli1eGFP/+, Gli1LacZ/+, Gli2LacZ, Gli3LacZ/+, PtcLacZ/+, Gli1CreER/+, and Rosa26 reporter mice were housed and bred on an outcrossed Swiss Webster background in the animal facility at Memorial Sloan-Kettering Cancer Center (MSKCC). TM (Sigma) was dissolved in corn oil (20mg/ml) and administered by gavage (for Gli1-GIFM 10mg single dose, for Shh-GIFM 10mg daily for 3 days). EdU (200mg/kg) was injected IP 1 hr prior to sacrifice for anagen skin. EdU (100mg/kg) was injected IP every 12 hours for 6 doses, with the last dose 1 hr prior to sacrifice for telogen skin. Depilation was achieved by manual plucking of dorsal trunk skin followed by wax strip depilation. Dorsal trunk skin wounds were allowed to heal uncovered by secondary intention. All experiments were performed in accordance MSKCC IACUC approved protocols.
Tissue processing
Skin was fixed in 4% paraformaldehyde (PFA) for 20 minutes (for X-gal) or overnight (for immunostaining). Tissue was wholemount stained or cryoprotected overnight in 30% sucrose, embedded in frozen OCT, and 12μm sections were obtained. X-gal-stained slides were counterstained with nuclear fast red and eosin. DRGs were dissected after intracardiac perfusion with 55mL of cold 4% PFA and post-fixed for 2 hours before cryoprotection, OCT embedding, and 10μm sectioning.
Anti-Hh injections
Black Swiss mice obtained from Taconic Farms, Inc were confirmed to be in telogen phase by age and inspection of skin and were injected with 5E1 anti-Hh antibody (Wang et al., 2000), generated by the MSKCC monoclonal antibody core facility. Dorsal trunk skin was flash frozen for RNA extraction and RT-qPCR with TaqMan assays (Applied Biosystems, Inc) by the MSKCC Genomics Core Laboratory.
Gene expression analysis of flow-sorted cell populations
Keratinocytes from dorsal trunk skin were isolated using published methods (Jensen et al., 2010). After isolation, staining, and sorting, gene expression was assessed using MouseRef-8 Beadarrays (Illumina, Inc.). RNA isolation, labeling and hybridization performed by the MSKCC Genomics Core Laboratory.
Surgical denervation
Dorsal cutaneous nerves were severed using microsurgery as described (Maurer et al., 1998). A midline incision allowed denervation of the right back with contralateral skin as a control. For wounding assays, a flank incision was used and the entire back was denervated with a sham surgery performed on littermate control mice.
Supplementary Material
Acknowledgments
We thank Abhishek Patel, Rowena Turnbull, Daniel Stephen and Jason Chan for technical assistance. We thank Dr. Richard Boyd for the anti-MTS24 antibody, Dr. Michael Wegner for the anti-Sox9 antibody, Dr. Louis Reichardt for the anti-TrkA antibody, and Dr. Thomas Jessell for the anti-Runx1 and anti-Runx3 antibodies. This work was supported by NIH grant R01CA128158 and the Tri-Institutional Stem Cell Initiative (to ALJ); and by NIH grant F32AR55435, the Dermatology Foundation, and the Charles A. Dana Foundation (to IB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphe C, Narang M, Ellis T, Wicking C, Kaur P, Wainwright B. An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development. 2004;131:5009–5019. doi: 10.1242/dev.01367. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y. Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature. 2010;466:978–982. doi: 10.1038/nature09269. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Eichmuller S, Johansson O, Paus R. Hair cycle-dependent plasticity of skin and hair follicle innervation in normal murine skin. J Comp Neurol. 1997;386:379–395. doi: 10.1002/(sici)1096-9861(19970929)386:3<379::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Khavari PA. Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol. 1999;147:71–76. doi: 10.1083/jcb.147.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci. 2010;30:13597–13608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1586–1594. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Driskell RR, Watt FM. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat Protoc. 2010;5:898–911. doi: 10.1038/nprot.2010.39. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: Establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235:2376–2385. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. Faseb J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Maurer M, Peters EM, Botchkarev VA, Paus R. Intact hair follicle innervation is not essential for anagen induction and development. Arch Dermatol Res. 1998;290:574–578. doi: 10.1007/s004030050354. [DOI] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, Hui CC. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17:282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Potten CS. Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif. 1994;27:279–289. doi: 10.1111/j.1365-2184.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Niemann C, Unden AB, Lyle S, Zouboulis Ch, C., Toftgard R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11873–11880. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhof JG, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, Willemze R, Mullenders LH, Watt FM, de Gruijl FR, et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Peters EM, Botchkarev VA, Muller-Rover S, Moll I, Rice FL, Paus R. Developmental timing of hair follicle and dorsal skin innervation in mice. J Comp Neurol. 2002;448:28–52. doi: 10.1002/cne.10212. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharghi-Namini S, Turmaine M, Meier C, Sahni V, Umehara F, Jessen KR, Mirsky R. The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J Neurosci. 2006;26:6364–6376. doi: 10.1523/JNEUROSCI.0157-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Wallace VA, Raff MC. A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development. 1999;126:2901–2909. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- Wang LC, Liu ZY, Gambardella L, Delacour A, Shapiro R, Yang J, Sizing I, Rayhorn P, Garber EA, Benjamin CD, et al. Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. 2000;114:901–908. doi: 10.1046/j.1523-1747.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.