Abstract
Invasive fungal infections due to Aspergillus species have become a major cause of morbidity and mortality among immunocompromised patients. Aspergillus terreus, a less common pathogen, appears to be an emerging cause of infection at our institution, the University of Alabama hospital in Birmingham. We therefore investigated the epidemiology of A. terreus over the past 6 years by using culture data; antifungal susceptibility testing with amphotericin B, voriconazole, and itraconazole; and molecular typing with random amplification of polymorphic DNA-PCR (RAPD-PCR). During the study period, the percentage of A. terreus isolates relative to those of other Aspergillus species significantly increased, and A. terreus isolates frequently were resistant to amphotericin B. Molecular typing with the RAPD technique was useful in discriminating between patient isolates, which showed much strain diversity. Further surveillance of A. terreus may better define epidemiology and determine whether this organism is becoming more frequent in relation to other Aspergillus species.
Invasive fungal infections due to Aspergillus species have become a major cause of morbidity and mortality among immunocompromised patients. Aspergillus fumigatus is most frequently isolated from clinical specimens, but other important species include A. flavus, A. niger, and A. terreus. A. terreus appears to be emerging as a cause of opportunistic infections (8, 9, 20) and is of concern because of in vitro resistance to amphotericin B (18). At our institution, the University of Alabama hospital in Birmingham, an increase in the frequency of A. terreus isolates and invasive infections due to A. terreus has been noticed (4). Although several recent studies have discussed clinical cases of invasive A. terreus disease and strain typing of A. terreus environmental and clinical isolates (7, 9, 11, 19, 20), questions about the epidemiology of A. terreus remain unanswered. We therefore were interested in studying the epidemiology of A. terreus at our tertiary care university hospital with the use of clinical data, antifungal susceptibility testing, and molecular genotyping using the random amplification of polymorphic DNA-PCR (RAPD-PCR) method.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
MATERIALS AND METHODS
We identified cultures positive for A. terreus and other Aspergillus species at the laboratory of the University of Alabama hospital over a 6-year period (1996 to 2001) to investigate the frequency of isolation of A. terreus from clinical samples. The Laboratory Information System was utilized to identify the number of Aspergillus isolates. A subgroup of 41 patients with cultures positive for A. terreus was selected for a more focused epidemiologic study that included medical record review. In addition, 23 of 41 patients had isolates stored and available for molecular typing and susceptibility testing. Data collected from medical records included demographics, information on underlying disease, identification of the clinical disease entity or condition due to A. terreus (invasive aspergillosis, chronic necrotizing pneumonia [semi-invasive aspergillosis], aspergilloma, allergic bronchopulmonary aspergillosis, colonization, or contamination), and dates and sources of cultures positive for A. terreus. Colonization was defined as having a culture positive for A. terreus without evidence of clinical or histopathologic disease. Contamination was defined as isolation of Aspergillus but no apparent connection with clinical condition or relevance to the patient (15). Invasive infection was defined as the recovery of A. terreus from a sterile site or a biopsy specimen or from a contiguous nonsterile site with compatible clinical and radiographic findings. Chronic necrotizing pneumonia, aspergilloma, and allergic bronchopulmonary aspergillosis were classified on the basis of current guidelines (16). Patient locations at the time of culture, dates of hospital admission and discharge, and locations within the hospital throughout hospital stays were determined for 23 patients whose isolates were available for typing in order to investigate potential clustering of cases or a potential hospital source of isolates. Strain relatedness was determined by molecular genotyping with the use of RAPD-PCR. Antifungal susceptibility of A. terreus isolates to amphotericin B, itraconazole, and voriconazole was determined.
Molecular genotyping.
Twenty-three clinical isolates from twenty-three patients were subjected to molecular genotyping. Reactions were carried out three times to confirm reproducibility. In brief, isolates were grown on Sabouraud's dextrose agar slants and spores were harvested and inoculated into Vogel's medium at a final concentration of 107 spores/ml before incubation at 37°C for 48 h. Mycelium collected by filtration was freeze-dried with liquid nitrogen, ground to a powder, and added to microcentrifuge tubes. After centrifugation, the pellets were resuspended in extraction buffer (2, 5). DNA was isolated by heating in a boiling water bath, extracting once with chloroform-isoamyl alcohol (1:1) and twice with isopropanol, and precipitating with ethanol. RAPD-PCR was performed using 100 ng of A. terreus DNA as a template, primers R108 (GTATTGCCCT; 50% G+C content) and CII (GCGCACGG; 88% G+C content) obtained from Oligos, Etc., Portland, Oreg., and Stoffel DNA polymerase in a Perkin-Elmer model 480 thermocycler (Perkin-Elmer, Norwalk, Conn.) (3). The following conditions were used: 1 cycle of 94°C for 5 min, 30°C for 1 min, and 72°C for 1 min; 35 cycles of 94°C for 1 min, 36°C for 1 min, and 72°C for 1 min; 5 cycles of 94°C for 1 min, 36°C for 1 min, and 72°C for 5 min; and then refrigeration at 4°C (2). Aliquots of PCR products were analyzed by electrophoresis in a 1.5% agarose gel with ethidium bromide at 0.5 μg/ml and photographed under UV light (GelDoc 2000; Bio-Rad). Analysis of the DNA band patterns was done with Gelcompar II software (Applied Maths, Kortjik, Belgium). Highly related strains (groups) were defined as those having at least 90% homology on the basis of banding patterns.
Antifungal susceptibility testing. (i) Organisms.
A total of 23 A. terreus isolates from 23 patients were available for antifungal susceptibility testing. Sources of isolates included 22 respiratory specimens (sputum, bronchoalveolar lavage fluid, transbronchial biopsy specimen, or tracheal aspirate) and 1 wound specimen. Isolates were grown on potato flake agar and identified as A. terreus by colony color and typical microscopic morphology, including the presence of aleuriospores (17). All isolates were grown on potato flake agar slants and stored at −70°C until testing.
(ii) Antifungal drugs.
Voriconazole (Pfizer Pharmaceutical Group, New York, N.Y.), itraconazole (Janssen Research Foundation, Beerse, Belgium), and amphotericin B (Sigma Chemical Co., St. Louis, Mo.) were obtained as reagent-grade powders from their manufacturers, and stock solutions were prepared in dimethyl sulfoxide. All drugs were diluted in RPMI 1640 medium (Sigma Chemical Co.) buffered to pH 7.0 with morpholinepropanesulfonic acid (MOPS) buffer and dispensed into 96-well microdilution trays. Trays containing an aliquot of 0.1 ml of appropriate drug solution (2× final concentration) in each well were sealed and stored at −70°C until use. The final ranges of drug concentrations tested were 0.008 to 8 μg/ml for voriconazole and itraconazole and 0.016 to 16 μg/ml for amphotericin B.
(iii) Susceptibility testing.
MICs were determined by NCCLS M38-A broth microdilution methodology (13). In brief, isolates were grown on potato dextrose agar slants at 35°C for 7 days. The slants were covered with 1 ml of sterile 85% saline and gently scraped with a sterile pipette. The resulting suspensions were transferred to separate tubes, and heavy particles were allowed to settle. Turbidities of the conidial suspensions were measured at 530 nm and adjusted to optical densities ranging from 0.09 to 0.11 (80 to 82% transmittance). Final concentrations of stock inocula ranged from 0.5 to 5 × 106 CFU/ml and were verified by plating onto Sabouraud dextrose agar. Drug-free controls were included in each tray. The microdilution trays were incubated at 35°C for 48 h. Following incubation, MIC endpoints were determined as the lowest drug concentrations that prevented any discernible growth (optically clear) compared to that of the drug-free controls. Quality control was measured by inclusion of the following strains: Candida parapsilosis (ATCC 22019) and C. krusei (ATCC 6258). All readings were within the recommended limits based on NCCLS M38-A methodology (13).
RESULTS
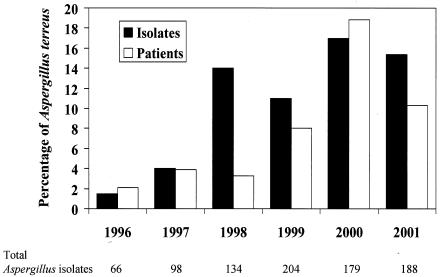
Eight hundred sixty-nine Aspergillus isolates were identified by the University of Alabama at Birmingham Clinical Microbiology Laboratory during the 6-year study period. The most common species was A. fumigatus, making up 60% of isolates, followed by A. niger (18%), A. terreus (12%), and A. flavus (9%). One hundred seven isolates of A. terreus from 41 patients were identified (1 isolate from 1 patient, 1996; 4 isolates from 2 patients, 1997; 19 isolates from 2 patients, 1998; 23 isolates from 6 patients, 1999; 31 isolates from 19 patients, 2000; and 29 isolates from 11 patients, 2001). As shown in Fig. 1, the percentage of A. terreus isolates relative to the total number of Aspergillus isolates (1.5% in 1996 to 15.4% in 2001; P < 0.001; chi-square test for linear trend [EPI-INFO 6]) and the percentage of patients with A. terreus isolates relative to the total number of patients with Aspergillus isolates (2.1% in 1996 to 10.2% in 2001; P = 0.001) significantly increased during the study period.
FIG. 1.
Graph depicting the frequency of isolation of A. terreus isolates relative to that of all Aspergillus species during the study period (black bars) and the percentage of patients with A. terreus isolates relative to the total number of patients with Aspergillus species (white bars). Total numbers of Aspergillus isolates are listed below the x axis. The y axis represents percentages of A. terreus isolates or patients.
Clinical data were collected from 41 patients with positive A. terreus cultures. Isolates from 34 (83%) of 41 patients were cultured from the respiratory tract, those from three patients (7.3%) were from skin and soft tissues, and isolates from one patient each were from blood, a toenail, the ear canal, and peritoneal fluid. The mean age of patients was 54 years, and 66% were male. Twenty (48.7%) of 41 patients were immunocompromised, with 11 (26.8%) having received solid organ or hematopoietic stem cell transplants. Twenty-four (58.5%) of 41 patients were colonized with A. terreus, and in three patients (7.3%), isolates were considered to be contaminants. Of 14 patients (34%) with infection, 11 had invasive aspergillosis, 1 had aspergilloma, 1 had external otitis, and 1 had onychomycosis. Among the 11 patients with invasive aspergillosis, 5 (45.4%) had disseminated disease and the deaths of 8 patients (72.7%) were attributed to A. terreus infection. Throughout the study period, among patients who had typing data available, there appeared to be time-related clusters of hospitalized patients with positive A. terreus cultures (Table 1); however, these patients were not consistently located in the same hospital units or areas.
TABLE 1.
Results of RAPD typing of A. terreus isolates
| Date (mo/day/yr) of isolation | Patient no. | R108-identified typea | CII-identified typea | Combined typeb |
|---|---|---|---|---|
| 5/26/96 | 1 | M | A | I |
| 2/26/97 | 2 | O | E | II |
| 2/26/97 | 6 | L | E | III |
| 7/3/97 | 22 | I | C | IV |
| 4/03/98 | 7 | Q | J | V |
| 11/20/98 | 8 | P | B | VI |
| 2/27/99 | 9 | K | F | VII |
| 3/12/99 | 10 | N | F | VIII |
| 11/5/99 | 3 | C | I | IX |
| 1/10/00 | 4 | L | E | III |
| 1/20/00 | 5 | K | E | X |
| 8/5/00 | 11 | E | D | XI |
| 9/7/00 | 19 | A | G | XII |
| 9/11/00 | 20 | G | C | XIII |
| 10/3/00 | 16 | A | H | XIV |
| 10/9/00 | 17 | F | D | XV |
| 10/11/00 | 18 | A | G | XII |
| 10/25/00 | 12 | B | D | XVI |
| 11/10/00 | 13 | H | D | XVII |
| 12/6/00 | 15 | J | K | XVIII |
| 12/7/00 | 21 | D | L | XIX |
| 12/23/00 | 23 | A | G | XII |
| 1/29/01 | 14 | D | L | XIX |
| Total no. of types | 17 | 12 | 19 |
Antifungal susceptibilities.
The activities of amphotericin B, itraconazole, and voriconazole against 23 patient isolates of A. terreus are shown in Table 2. Itraconazole and voriconazole were highly active against A. terreus (100% susceptible at an MIC of ≤1 μg/ml), while only 13% of isolates were susceptible to amphotericin B at an MIC of ≤1 μg/ml.
TABLE 2.
In vitro susceptibilities of 23 isolates of A. terreus to amphotericin B, itraconazole, and voriconazole
| Antifungal agent | MIC (μg/ml)a
|
% of isolates susceptible at an MIC (μg/ml) of:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | 0.25 | 0.5 | 1 | 2 | 4 | |
| Amphotericin B | 1-4 | 2 | 4 | 0 | 0 | 13 | 87 | 100 |
| Itraconazole | 0.06-1 | 0.5 | 1 | 48 | 83 | 100 | 100 | 100 |
| Voriconazole | 0.25-1 | 0.5 | 0.5 | 30 | 96 | 100 | 100 | 100 |
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
Molecular genotyping.
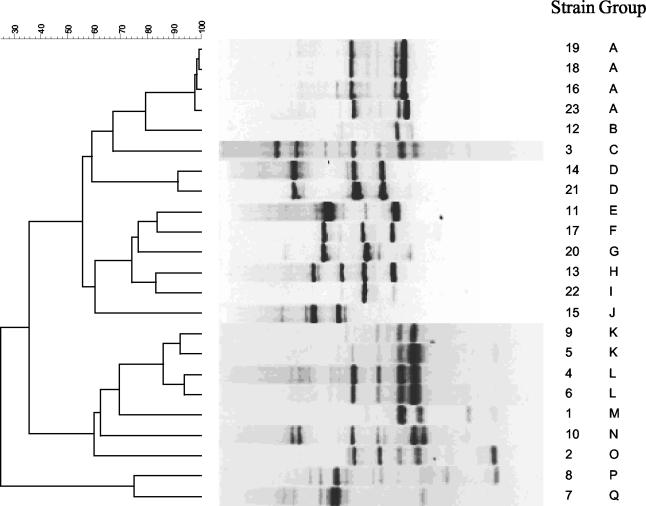
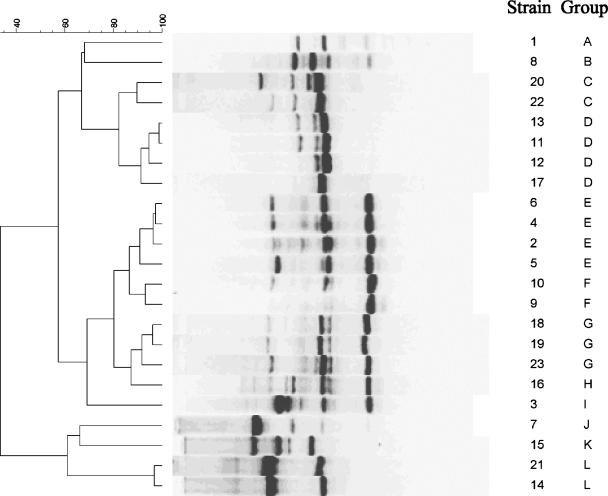
Twenty-three isolates from 23 patients were genotyped using RAPD-PCR methodology with two primers previously shown to have good discriminatory power for Aspergillus species (2, 5). Seventeen distinct strains were identified with the R108 primer (Fig. 2) and 12 distinct strains were identified with the CII primer (Fig. 3). By combining the results of the two primers, 19 distinct strains were identified (Table 1).
FIG. 2.
Dendrogram and gel band patterns of RAPD fingerprints of 23 A. terreus isolates typed with primer R108. The scale at the top represents percent similarity. The patient numbers and strain types are listed to the right of the figure and correspond to those in Table 1.
FIG. 3.
Dendrogram and gel band patterns of RAPD fingerprints of 23 A. terreus isolates typed with primer CII. The scale at the top represents percent similarity. The patient numbers and strain types are listed to the right of the figure and correspond to those in Table 1.
Several patients' isolates were highly similar (>90% homology) and were placed in three groupings: type III (patients 4 and 6), type XII (patients 18, 19, and 23), and type XIX (patients 14 and 21). Patients 4 and 6 were hospitalized and their specimens were cultured almost 3 years apart (Table 1). Patients 18, 19, and 23 had A. terreus cultures that were temporally related, but they were not hospitalized at the same time nor were they in the same ward or unit. Patients 14 and 21 were hospitalized at the same time but were in different locations.
DISCUSSION
Our study confirms that A. terreus is an increasingly common clinical isolate, but our data also do not support a common source for this phenomenon. At our institution, A. terreus appears to be an emerging pathogen and constitutes a growing proportion of Aspergillus isolates in our hospital over the last several years. By comparison, in a recent 1-year, multicenter epidemiologic survey in the United States, of 1,477 Aspergillus isolates, only 17 (1%) were A. terreus (15). Chandrasekar and colleagues have reported an increase in Aspergillus species over the past decade, but the number of A. terreus isolates did not appear to increase during the study period (6). Recent surveillance data from other institutions would be helpful to determine whether our observed trend over the past 6 years is representative of trends at other sites.
In parallel with the increased number of clinical isolates, there has been a growing number of patients with invasive disease caused by A. terreus at our institution, especially among immunocompromised patients (4). Of 41 patients with positive cultures, nearly one-half were immunocompromised, and 11 (27%) of 41 had invasive infection. Of the 11 patients with invasive disease, 8 died of the infection with disseminated disease or overwhelming pneumonia. Perfect and colleagues reported that among 17 patients with A. terreus isolates, invasive disease was seen in 47% (15). Other recent reports have found that between 3 and 13% of cases of invasive aspergillosis may be due to A. terreus and that disease is rapidly progressive in immunocompromised patients (9, 20). Therefore, an isolate of A. terreus from an immunocompromised patient is of concern and should be considered a potential pathogen.
In isolates we studied, as has been previously reported, MICs of amphotericin B were higher for A. terreus than for A. fumigatus (18). Amphotericin B resistance may in part explain the rapid disease progression and poor clinical response seen among some patients, as until recently amphotericin B had been considered standard primary treatment for invasive aspergillosis (16). MICs of voriconazole and itraconazole for A. terreus isolates were similar to those in other reports (17, 18).
The molecular genotyping of Aspergillus species has proven useful in many epidemiologic situations (11, 12, 19). One of the most widely used genotyping techniques is RAPD-PCR, a relatively technically simple and rapid procedure. Although RAPD-PCR has been criticized for a lack of reproducibility, this method has been used with success for A. terreus isolates (5, 19). Birch and Denning used five primers to test 14 isolates and found primers R108 and CII to be highly discriminatory (5). Symoens and colleagues tested 43 isolates of environmental and clinical origins with the primers NS3 and NS7 (19). Among epidemiologically unrelated isolates, the primers were highly discriminatory. In addition, they found RAPD-PCR to be a useful tool in demonstrating the clonal origin of contamination of the environment in a hematology unit. Among our patients, each primer was highly discriminatory, and when the primers were used in combination, 19 different strains were found. Three groups of patients had highly related (≥90% homology) strains, but only two patients in one group were hospitalized at the same time. Moreover, the patients were in different hospital locations during their stay. No common exposures could be detected to suggest nosocomial acquisition; however, certain factors present may not have been identified in the chart review. Environmental isolates were not obtained during the study period but may have been useful in identifying potential nosocomial exposures.
It remains unclear why the frequencies of A. terreus isolates and infections are increasing at our institution. Multiple studies have linked hospital construction and renovation to Aspergillus species outbreaks (7, 10, 14). Hospital construction has been prevalent at our institution over the past decade but not in areas where these patients were housed. Lass-Florl and colleagues suggested potted plants as a source of A. terreus, and there are also concerns about Aspergillus species contaminating water and foodstuffs (1, 11). It is important that targeted surveillance of Aspergillus species, especially A. terreus, be continued in order to better define environmental patterns.
In conclusion, A. terreus has become more common at our institution over the past 6 years. It is an important pathogen because of relative amphotericin B resistance and the potential to cause rapidly progressive invasive infections in immunocompromised patients. Molecular genotyping of isolates with RAPD-PCR was helpful in discriminating patient isolates, and when results of this procedure are combined with other clinical and epidemiologic data, it appears that A. terreus isolates at our institution are highly diverse and are unlikely to come from a common source. Surveillance of A. terreus at other institutions may be helpful to better define epidemiology and determine whether this organism is becoming more frequent in relation to other Aspergillus species.
REFERENCES
- 1.Anaissie, E. J., and S. F. Costa. 2001. Nosocomial aspergillosis is waterborne. Clin. Infect. Dis. 33:1546-1548. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, M. J., K. Gull, and D. W. Denning. 1996. Molecular typing by random amplification of polymorphic DNA and M13 Southern hybridization of related paired isolates of Aspergillus fumigatus. J. Clin. Microbiol. 34:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aufauvre-Brown, A., J. Cohen, and D. W. Holden. 1992. Use of randomly amplified polymorphic DNA markers to distinguish isolates of Aspergillus fumigatus. J. Clin. Microbiol. 30:2991-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baddley, J. W., T. A. Stroud, D. Salzman, and P. G. Pappas. 2001. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 32:319-324. [DOI] [PubMed] [Google Scholar]
- 5.Birch, M., and D. W. Denning. 1999. Molecular typing of Aspergillus terreus by random amplification of polymorphic DNA. Eur. J. Clin. Microbiol. Infect. Dis. 18:838-841. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar, P. H., J. L. Cutright, and E. K. Manavathu. 2001. Aspergillus: rising frequency of clinical isolation and continued susceptibility to antifungal agents, 1994-1999. Diagn. Microbiol. Infect. Dis. 41:211-214. [DOI] [PubMed] [Google Scholar]
- 7.Flynn, P. M., B. G. Williams, S. V. Hetherington, B. F. Williams, M. A. Giannini, and T. A. Pearson. 1993. Aspergillus terreus during hospital renovation. Infect. Control Hosp. Epidemiol. 14:363-365. [DOI] [PubMed] [Google Scholar]
- 8.Hara, K. S., J. H. Ryu, J. T. Lie, and G. D. Roberts. 1989. Disseminated Aspergillus terreus infection in immunocompromised hosts. Mayo Clin. Proc. 64:770-775. [DOI] [PubMed] [Google Scholar]
- 9.Iwen, P. C., M. E. Rupp, A. N. Langnas, E. C. Reed, and S. H. Hinrichs. 1998. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-year experience and review of the literature. Clin. Infect. Dis. 26:1092-1097. [DOI] [PubMed] [Google Scholar]
- 10.Krasinski, K., R. S. Holzman, B. Hanna, M. A. Greco, M. Graff, and M. Bhogal. 1985. Nosocomial fungal infection during hospital renovation. Infect. Control 6:278-282. [DOI] [PubMed] [Google Scholar]
- 11.Lass-Florl, C., P. Rath, D. Niederwieser, G. Kofler, R. Wurzner, A. Krezy, and M. P. Dierich. 2000. Aspergillus terreus infections in haematological malignancies: molecular epidemiology suggests association with in-hospital plants. J. Hosp. Infect. 46:31-35. [DOI] [PubMed] [Google Scholar]
- 12.Leenders, A., A. van Belkum, S. Janssen, S. de Marie, J. Kluytmans, J. Wielenga, B. Lowenberg, and H. Verbrugh. 1996. Molecular epidemiology of apparent outbreak of invasive aspergillosis in a hematology ward. J. Clin. Microbiol. 34:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Oren, I., N. Haddad, R. Finkelstein, and J. M. Rowe. 2001. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am. J. Hematol. 66:257-262. [DOI] [PubMed] [Google Scholar]
- 15.Perfect, J. R., G. M. Cox, J. Y. Lee, C. A. Kauffman, L. de Repentigny, S. W. Chapman, V. A. Morrison, P. G. Pappas, J. W. Hiemenz, D. A. Stevens, and the Mycoses Study Group. 2001. The impact of culture isolation of Aspergillus species: a hospital based survey of aspergillosis. Clin. Infect. Dis. 33:1824-1833. [DOI] [PubMed] [Google Scholar]
- 16.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. D. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for disease caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 17.Sutton, D. A., A. W. Fothergill, and M. G. Rinaldi. 1998. Guide to clinically significant fungi. Williams & Wilkins, Baltimore, Md.
- 18.Sutton, D. A., S. E. Sanche, S. G. Revankar, et al. 1999. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J. Clin. Microbiol. 37:2343-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symoens, F., J. P. Bouchara, S. Heinemann, and N. Nolard. 2000. Molecular typing of Aspergillus terreus isolates by random amplification of polymorphic DNA. J. Hosp. Infect. 44:273-280. [DOI] [PubMed] [Google Scholar]
- 20.Tritz, D. M., and G. L. Woods. 1993. Fatal disseminated infection with Aspergillus terreus in immunocompromised hosts. Clin. Infect. Dis. 16:118-122. [DOI] [PubMed] [Google Scholar]