Abstract
The authors describe an HIV-positive patient with nodular regenerative hyperplasia of the liver with non-cirrhotic portal hypertension. Despite stopping the culprit drug, didanosine, the radiologic changes persisted for years. When evaluating liver pathologies, antiretroviral drugs must be included in the differential diagnosis, even when they have been stopped years ago.
Background
Highly active antiretroviral therapy (HAART) has resulted in substantial decrease of HIV type-1 associated morbidity and mortality. HAART is based on the prescription of two nucleoside analogues in concert with either a non-nucleoside analogue or a protease inhibitor. In the current paradigm, HIV-infected patients need to take HAART for their entire life time.
However, the long-term use of HAART has revealed its downsides particularly toxicity. Some of the side effects associated with HAART may manifest within the first years of intake, other side effects at later time points.
Didanosine (DDI), one of the first nucleoside analogues, was introduced in the early 90s. While side effects of DDI such as lactate acidosis, hepatitis and polyneuropathy were described early after its introduction,1 2 only recently a new hepatic disease in HIV-infected persons with non-cirrhotic portal hypertension (NCPH) has been linked to DDI.3–5 Symptoms manifest years after its intake. The syndrome presents mostly with the typical signs of portal hypertension, oesophageal varices, variceal bleeding, ascites and splenomegaly. Portal vein thrombosis is noted in many cases. Liver enzymes and alkaline phosphatase are often mildly elevated. Hepatic function is usually preserved.5
The patho-mechanism of DDI causing NCPH is hypothetical. DDI is a guanosine nucleoside analogue (2’,3’-dideoxyinosine).2 All nucleoside analogues act similarly on HIV replication; they are incorporated instead of the natural nucleosides into the retroviral nascent DNA strand during reverse transcription, thereby acting as a chain terminator of HIV DNA. However, nucleoside analogues may also be incorporated into the mitochondrial DNA (mtDNA) by the human mitochondrial polymerase γ, this time acting as chain terminator of the mtDNA. Depletion of mtDNA, in turn, may lead to impaired mitochondrial function. This mitochondrial damage might injure hepatic endothelial cells. However, the pathogenesis of NCPH in HIV-infected persons is probably multifactorial. Besides DDI hyper-coagulability has been identified as an associated factor.4 In non-HIV-infected patients besides certain drugs, such as azathioprine and 6-thioguanine, autoimmune, myeloproliferative or lymphoproliferative diseases are associated with NCPH.6
In this case report, we describe an HIV-positive patient on longstanding antiretroviral therapy presenting with NCPH and striking radiologic features. Even after interruption of the culprit drug, DDI, the marked radiologic changes persisted for more than 7 years.
Case presentation
Herewith, we report on a homosexual 47-year-old man, diagnosed HIV-positive in November 1988 while presenting with fever and cervical lymphadenopathy.
In December 1992, 2×250 mg zidovudine (azidothymidine (AZT))/day was started when the CD4 T cell count fell to 190/mm3 (figure 1A). Because of progressive CD4 T cell loss, AZT was replaced by 2×200 mg DDI/day in April 1993. Prophylaxis against pneumocystis jiroveci pneumonia was installed with trimethoprim/sulfamethoxazol (160 mg/800 mg) 3 times a week.
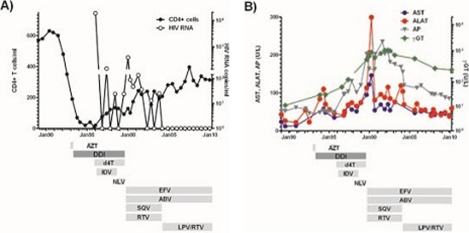
Figure 1.
CD4 T cell counts, HIV RNA (A) and liver enzymes (B) over years in an HIV-infected patient treated with highly active antiretroviral treatment (HAART). ABV, abacavir; AZT, azidothymidine; DDI, didanosine (2’-3’-dideoxyinosine); d4T, stavudine (2’-3’-didehydro-2’-3’-dideoxythymidine); EFV, efavirenz; IDV, indinavir; LPV/RTV, lopinavir/ritonavir; NLV, nelfinavir; RTV, ritonavir; SQV, saquinavir.
In November 1993, the patient had epigastric pain and elevated transaminases were measured for the first time (figure 1B). Sonographically hepatosplenomegaly without any sign of portal hypertension was documented. Because of hyperamylasemia (p-amylase 146 U/l) incriminated to DDI, DDI was reduced to 2×100 mg/day. Because of progressive CD4 T cell loss and despite manifest hepatitis the dose of DDI was increased to 2×200 mg/day again in March 1994. In January 1995, the patient presented with thrush stomatitis and HIV-associated thrombopenia, enforcing the MD in charge to add AZT 2×250 mg/day to DDI. Because of AZT suspected leucopenia, AZT was replaced by stavudine (d4T) 2×100 mg/day in November 1995; indinavir 3×800 mg/day was added to the regimen in 1996 until September 1998.
In 1999, the patient complained of prolonged episodes of dysphagia, ptosis and weight loss; (body mass index 22.2 March 1994; 18.3 kg/m2 August 1999). Liver transaminases were at that time substantially increased and ultrasound of the abdomen showed a pathologically hyperechogenic, inhomogeneous structure of the liver, irregular blood vessels and retrograde portal vein flow, consistent with portal hypertension. AZT and DDI were stopped August 1999 because of nucleoside analogues’ high likelihood to be at the origin of the patient’s symptoms. Subsequently, a regimen consisting of 600 mg efavirenz/day, 2×400 mg ritonavir/day, 2×400 mg saquinavir/day and 2×300 mg abacavir/day was prescribed in September 1999.
Other causes of hepatopathy, such as hepatitis B and C infection, hemochromatosis, Wilson disease, α 1-antitrypsin deficiency, autoimmune hepatitis and non-alcoholic steatohepatitis were excluded. The patient consumed alcohol very rarely.
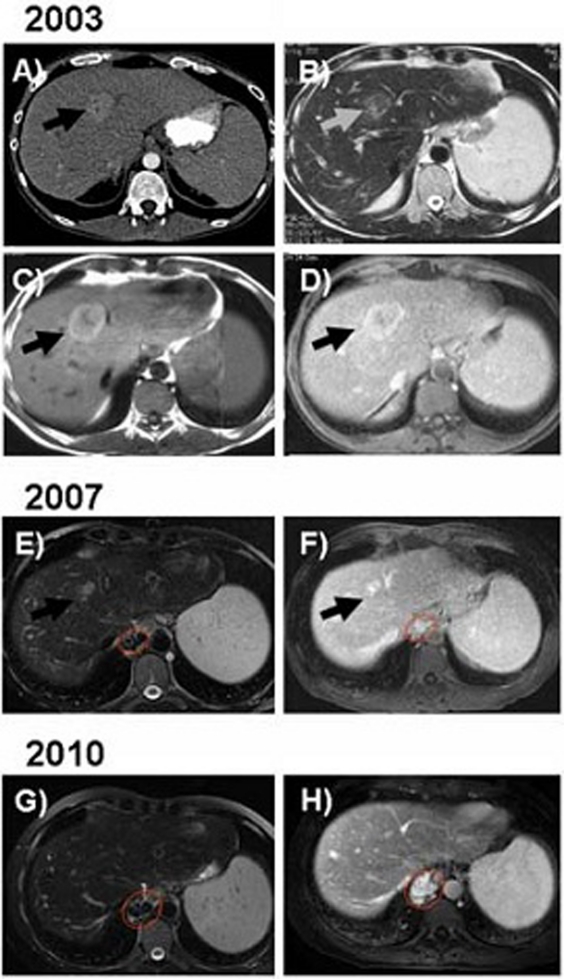
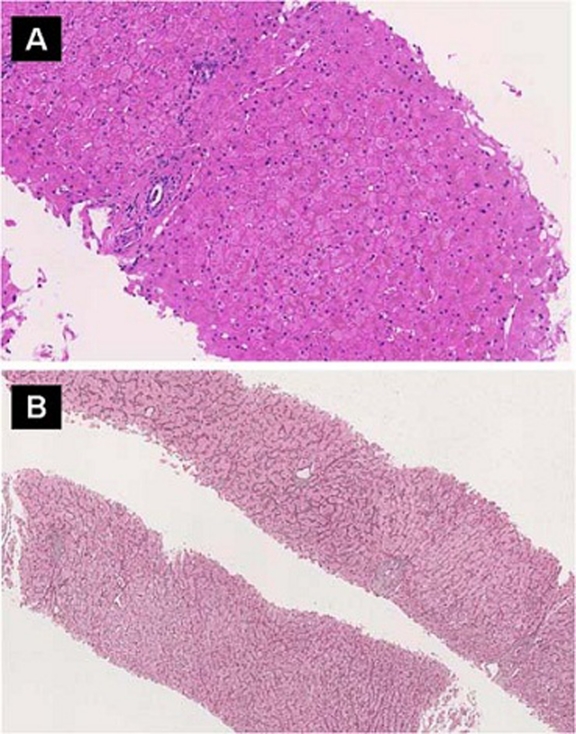
In August 2000, the patient suffered from episodes of melaena and anaemia. Endoscopy of the upper and lower gastrointestinal tract in November 2000 revealed oesophageal varices grade I. In 2003 the patient was again hospitalised due to upper gastrointestinal bleeding. Upper endoscopy was performed showing persistent oesophageal varices grade I. CT and MRI were performed which demonstrated hepatosplenomegaly as signs of portal hypertension. In addition, several focal liver lesions were noted with the largest of these lesions located in liver segment IV (figure 2A–D). Histopathology of the liver showed a preserved architecture of the lobules, no inflammatory infiltrates or steatosis or mallory bodies (figure 3).
Figure 2.
(A) Contrast enhanced CT performed in 2003 demonstrates a focal liver lesion located in segment IV with contrast enhancement in the arterial phase of the contrast administration. In addition splenomegaly is noted as a sign of portal hypertension. (B) On a MR imaging performed at the same time as figure A the lesion is iso-slightly hypointense on T2-weighted (B) as well as hyperintense on T1-weighted imaging (C). Following intravenous administration of gadolinium-based contrast agent the lesion is hyperintense (D). MR imaging 4 years later (2007) shows that the focal liver lesion decreased in size both on unenhanced T2-weighted imaging (E) as well as on contrast-enhanced sequences (F). MRI performed 8 years later (17 June 2010) showed that the focal liver lesion previously located in liver segment IV disappeared completely (G and H). However, splenomegaly as well as oesophageal varices as sign of portal hypertension remained unchanged. Black resp. grey arrow points to the focal lesion. Circled in red are the oesophageal varices.
Figure 3.
(A) Liver biopsy showed mostly unremarkable liver parenchyma without any significant inflammation or fibrosis (HE staining). (B) Reticulin staining revealed a discrete nodularity and areas with thick cords adjacent to atrophic cords.
While the incriminated drugs were stopped, transaminases tended to decrease over the next years (figure 1B). In April 2007, MRI of the liver was repeated (figure 2E). Although MRI revealed persistent hepatosplenomegaly and oesophageal varices (arrow), decrease of the liver lesion in liver segment IV was noted (figure 2F). Another MRI performed in 2010 demonstrated near complete regression of the lesion (figure 2G,H). Despite the persistent prominent liver changes, the patient is now in good health.
Discussion
In this paper we report on a 47-year-old HIV-infected man, developing striking imaging findings of the liver suggestive of nodular regenerative hyperplasia (NRH) associated with the intake of DDI. Regression of the radiographic findings was very protracted with eventual disappearance 10 years after interruption of DDI. The liver enzymes which were at the maximum 5–10 fold increased showed a rapid decrease after interruption of DDI but their normalisation is still ongoing. Clinically the patient recovered very quickly after stopping DDI. Thus, DDI-associated NRH may present with striking imaging findings and the radiological signs and abnormal liver enzymes may persist years beyond the time DDI is taken.
While the patient certainly benefitted from the antiretroviral therapy (ART) that was started in the early 90s and also included DDI, he developed a myasthenia-like syndrome which was associated with steadily increasing liver enzymes over the years. These symptoms vanished rapidly after interruption of the ART consisting of DDI and d4T. DDI was the most likely culprit; indeed, long-time exposure of DDI has recently been linked to NRH. Furthermore, all other conditions which are associated with NRH6 were excluded.
NRH is one of the histological manifestations of NCPH. Due to vascular flow abnormalities, a local regenerative hyperplasia of hepatocytes and atrophy of the intervening cells result in the characteristic nodule formation. Between the nodules usually there is no or little fibrosis although there may be periportal or perisinusoidal fibrosis. Sinusoidal dilatation is often found.6 7 It must be clearly distinguished from hepatitis with necrosis as well as from cirrhosis. Final proof for the diagnosis of NRH requires a liver histology. However, NRH can easily be missed on a liver needle biopsy in which the nodularity is difficult to appreciate. A reticulin stain can be helpful in demonstrating the intermixture of hypertrophic and atrophic areas. NRH usually becomes evident on an open laparoscopic biopsy allowing sampling larger pieces of liver tissue.4 6
The imaging findings in this patient over time are consistent with the diagnosis of NRH associated with DDI. Although the regenerative nodules were visible as enhancing liver lesions on contrast-enhanced CT in this patient, MRI usually better displays the changes associated with alteration of liver structure. On T1-weighted images NRH nodules are hyperintense, and hypo- to isointense on T2-weighted images.8–10 Following administration of intravenous contrast agents, the lesions are enhancing.
This case illustrates that MRI might be a useful non-invasive examination to diagnose DDI-associated NRH. MRI appears to have a reasonable sensitivity of 77% and a specificity of 72% in diagnosing NRH as assessed in a multicentre study investigating hepatic changes by liver biopsy and MRI in patients treated with 6-thioguanine, a drug well known to cause NRH.11
Long-term prognosis of patients with NRH is not well known. It is inconclusive whether NRH is a reversible process once the presumed cause is removed. One report described an impressive reversibility of azathioprine-induced NRH changes depicted by MRI 12 months after stopping the culprit drug.12 So far, there are no long-term reports of HIV-infected patients with NCPH. The clinical recovery of the patient described and the regression of radiological findings while protracted are indices of a rather favourable prognostic.
In summary, here we presented a case of long-term toxicity of DDI causing NRH. This is the first report about the radiologic features of DDI-associated NRH. The radiologic imaging and persistent elevated liver enzymes clearly document that the DDI associated liver changes might persist more than 10 years beyond the interruption of DDI. Whenever liver disease is difficult to assign to a distinct pathology in HIV-infected patients, a careful drug history must be taken and in particular, diligence must be given also to drugs which have been given years before.
Learning points.
-
▶
DDI-associated NRH may persists years after interruption of DDI and must therefore be included in the differential diagnosis of liver diseases in HIV-infected patients with drug history of DDI.
-
▶
Interruption of DDI results in regression of liver fibrosis with overall a good prognosis.
-
▶
MRI is a useful and reasonable sensitive examination to diagnose DDI-associated NRH.
Acknowledgments
The authors would especially express their thanks to the patient who agreed that his case was put together for scientific communication.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Lai KK, Gang DL, Zawacki JK, et al. Fulminant hepatic failure associated with 2’,3’-dideoxyinosine (ddI). Ann Intern Med 1991;115:283–4 [DOI] [PubMed] [Google Scholar]
- 2.Perry CM, Noble S. Didanosine: an updated review of its use in HIV infection. Drugs 1999;58:1099–135 [DOI] [PubMed] [Google Scholar]
- 3.Mallet V, Blanchard P, Verkarre V, et al. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS 2007;21:187–92 [DOI] [PubMed] [Google Scholar]
- 4.Saifee S, Joelson D, Braude J, et al. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol 2008;6:1167–9 [DOI] [PubMed] [Google Scholar]
- 5.Kovari H, Ledergerber B, Peter U, et al. Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis 2009;49:626–35 [DOI] [PubMed] [Google Scholar]
- 6.Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology 2006;44:7–14 [DOI] [PubMed] [Google Scholar]
- 7.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 1990;11:787–97 [DOI] [PubMed] [Google Scholar]
- 8.Casillas C, Martí-Bonmatí L, Galant J. Pseudotumoral presentation of nodular regenerative hyperplasia of the liver: imaging in five patients including MR imaging. Eur Radiol 1997;7:654–8 [DOI] [PubMed] [Google Scholar]
- 9.Horita T, Tsutsumi A, Takeda T, et al. Significance of magnetic resonance imaging in the diagnosis of nodular regenerative hyperplasia of the liver complicated with systemic lupus erythematosus: a case report and review of the literature. Lupus 2002;11:193–6 [DOI] [PubMed] [Google Scholar]
- 10.Rha SE, Lee MG, Lee YS, et al. Nodular regenerative hyperplasia of the liver in Budd-Chiari syndrome: CT and MR features. Abdom Imaging 2000;25:255–8 [DOI] [PubMed] [Google Scholar]
- 11.Seiderer J, Zech CJ, Reinisch W, et al. A multicenter assessment of liver toxicity by MRI and biopsy in IBD patients on 6-thioguanine. J Hepatol 2005;43:303–9 [DOI] [PubMed] [Google Scholar]
- 12.Seiderer J, Zech CJ, Diebold J, et al. Nodular regenerative hyperplasia: a reversible entity associated with azathioprine therapy. Eur J Gastroenterol Hepatol 2006;18:553–5 [DOI] [PubMed] [Google Scholar]