Abstract
Acute phosphate nephropathy occurs whenever a patient with renal dysfunction is exposed to high doses of phosphate. Bowel purgative agents are a common source of high doses of sodium phosphate and are widely used as bowel preparation agents prior to colonoscopy due to their efficacy and tolerability. Oral sodium phosphate (OSP) preparations used to prepare patients for colonoscopy may be a cause of acute and chronic kidney disease (CKD). CKD associated with OSP agents is the result of nephrocalcinosis, or calcium phosphorus crystal deposition in the renal parenchyma leading to interstitial disease. It is often irreversible and progressive in nature. The authors report a case of CKD which presented with non-specific symptoms weeks after use of an OSP agent as part of a bowel preparation regimen. Renal biopsy confirmed nephrocalcinosis.
Background
Acute phosphate nephropathy (APN) occurs whenever a patient with renal dysfunction is exposed to high doses of phosphate. Chronic kidney disease (CKD) due to APN may present insidiously weeks to months after the dose of OSP, which may mean that this problem is more prevalent than is currently recognised. We report a case of CKD which presented with non-specific symptoms weeks after use of an OSP agent as part of a bowel preparation regimen.
Case presentation
A 69-year-old female was found on a routine laboratory test to have a serum creatinine of 1.6 mg/dl. One year previously she had a serum creatinine of 0.9 mg/dl. Her medical history was significant for (1) chronic back pain, for which she had taken nambutone 500 mg twice daily for many years, (2) hypertension, well controlled with amlodipine 5 mg daily, (3) depression, treated with paroxetine 20 mg daily, (4) diverticulosis and (5) prior cholecystectomy, hysterectomy and appendectomy. She had intermittently taken omeprazole 20 mg daily for gastro-oesophageal reflux and trazadone 25 mg at night for insomnia. She had never smoked and worked as a bookkeeper. Both her parents suffered from cardiac ailments, but neither were reported to have had kidney disease.
Physical examination revealed an asymptomatic woman weighing 146 lb, with a blood pressure of 150/70 mm Hg and heart rate of 88 bpm. She had a normal thoracic and abdominal examination and no oedema.
Three months prior to presentation the patient had undergone colonoscopy for intermittent abdominal pain with a sodium phosphate preparation. The colonoscopy proved unremarkable. A renal ultrasound revealed normal sized kidneys with no hydronephrosis or echogenicity. Her urinalysis revealed no blood or protein. Serum calcium was 9.2 mg/dl, phosphorus was 2.8 mg/dl and other serum electrolytes were within the normal range. She was mildly anaemic with a haemoglobin of 11.2 g/dl, but white cell and platelet counts were normal.
Investigations
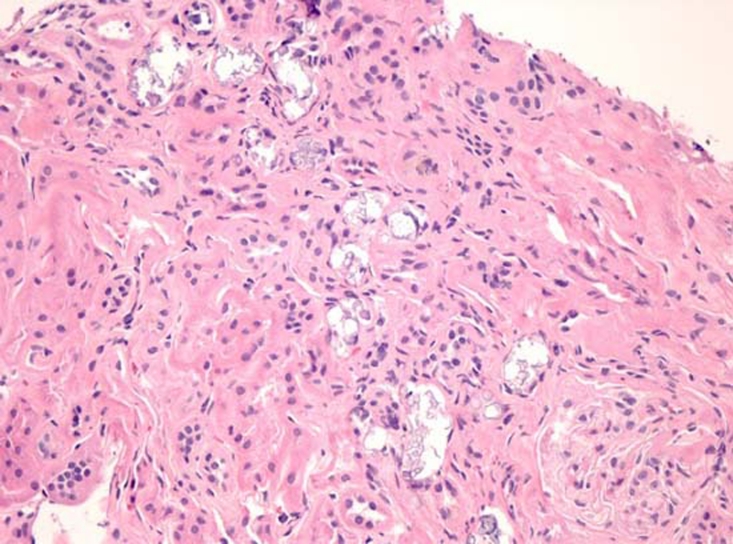
Following her initial examination, a renal biopsy was performed which found acute and chronic tubulointerstitial disease with extensive calcifications and moderate arteriolosclerosis (figure 1). There were no immune deposits.
Figure 1.
Renal cortex with numerous tubular and interstitial calcifications accompanied by tubular atrophy and fibrosis. (H&E, original magnification×100).
Outcome and follow-up
After her initial evaluation the patient’s nambutone was stopped. Her creatinine remained elevated, ranging from 1.4 to 1.7 mg/dl.
Discussion
Every year approximately 14 million colonoscopies are performed in the USA. The ability to safely cleanse the bowel prior to colonoscopy is necessary to optimise the diagnostic accuracy of that test. In some cases multiple bowel preparations are required to ensure adequate preparation. Sodium phosphate bowel preparations have a number of potentially adverse effects, including abdominal discomfort, nausea, vomiting and dizziness.1 Other bowel preparations include mannitol ingestion and saline lavage, both of which resulted in intolerable risks or side effects including flammable gas production and severe electrolyte abnormalities. More recently polyethylene glycol solution has been used; this agent has been found to be both efficacious and safe but the large volume and unpalatable taste have led to decreased compliance. Sodium phosphorus preparations are effective and palatable, leading to increased compliance and better bowel preparation.2
Sodium phosphorus preparations work as osmotic purgatives which obligate water excretion into the intestinal lumen, leading to peristalsis and colonic evacuation.1 The usual dose of OSP is two doses, 8–12 h apart.3 Forty-five millilitres of OSP contains 5 g of sodium and 17 g of phosphate; in the usual two doses, there are 11.5 g of elemental phosphorus. Forty-five millilitres of OSP may cause a loss of up to 1.6 l of fluid.4 This loss of volume, in conjunction with limited oral intake dictated by precolonoscopy protocols, may exacerbate some of the electrolyte abnormalities and the risk of renal failure among patients receiving these agents.
Different types of electrolyte imbalances and renal injuries may result from the use of OSPs; these abnormalities result from either fluid and electrolyte loss in stool or from the sequelae of hyperphosphatemia.1 Both hyper and hyponatraemia may occur as the result of OSP use, stemming from either too much free water loss or free water retention due to non-osmotic release of antidiuretic hormone.1 5 Additionally, OSP preparations reliably cause an increase in serum phosphorus, even among patients with normal renal function. One study found average phosphorus levels among patients with normal renal function ranged from 3.7–7.3 mg/dl after OSP administration.6 Another study found phosphorus levels greater than 8 mg/dl among 28% of patients treated with OSP.7 High phosphorus levels can lead to symptomatic hypocalcaemia, with subsequent weakness and tetany.8 Additionally, hyperphosphatemia may be the aetiology of renal dysfunction seen with use of OSP preparations.
APN occurs after the use of OSP agents. There are two types of APN noted with use of OSP; acute kidney injury (AKI) and chronic kidney dysfunction.3 AKI with systemic symptoms and hypocalcaemia may occur within hours to days after OSP use. This type of renal failure is typically short-lived and can be managed with phosphorus binding agents and conservative therapy. Most patients suffering from AKI due to APN will have significant improvement or total recovery of renal function.9 The chronic form of kidney damage due to OSP agents may present weeks after the initial exposure, and may be identified as the result of an investigation of mild or non-specific symptoms such as fatigue. Patients with CKD due to APN may see some improvement in their renal dysfunction, but may develop progressive CKD.3 Biopsies among patients with CKD due to APN generally find nephrocalcinosis. Nephrocalcinosis is a tubulointerstitial disease with prominent tubular calcifications with tubular atrophy and interstitial fibrosis.10 The tubular injury is felt to be secondary to calcium phosphorus crystal deposition, secondary to increased phosphorus delivery to the nephron and intratubular phosphate concentration in the setting of elevated serum phosphorus levels.3 The majority of calcium phosphate precipitation is usually found in the collecting duct and distal tubule.10 Volume depletion that accompanies use of OSP agents likely exacerbates this process. Volume depletion leads to increased reabsorption of water and sodium in the proximal tubule (which is impermeable to phosphorus) and water reabsorption without phosphorus reabsorption in the loop of Henle, resulting in higher relative concentration of phosphorus and calcium in the distal tubule.11
A dilemma posed by the use of OSP agents is how to adjust or monitor their use, given their efficacy and tolerability make them a preferred choice for bowel preparation prior to colonoscopy. The incidence of APN following OSP ingestion is unknown, but could be substantial in populations at risk. Thus, identification of patients at risk of acute or CKD with OSP use prior to administration is likely important. One study found that patients with CKD or advanced age prior to use of OSP agents were at increased risk of negative renal sequelae.6 12–14 Some studies have suggested that females are at greater risk than males for developing APN.15 Drugs that impact intravascular volume and renal perfusion may increase the risk of kidney injury after OSP use, including diuretics, ace inhibitors, angiotensin receptor blockers (ARBs) and non-steroidal anti-inflammatory agents.1 13 15 The dose of OSP likely impacts outcomes; more frequent doses (every 6 h, for example) compared to doses every 12–24 h lead to higher serum phosphorus levels,16 which may impact the risk of calcium phosphate deposition in the kidney. Similarly, ongoing inflammatory bowel disease at the time of OSP use may increase the absorption of phosphorus in the gut,17 possible contributing to higher serum phosphorus levels and increased calcium phosphate deposition in the renal parenchyma.
APN results in an acute or chronic decline in renal function among patients who have received OSP agents as part of a bowel preparation regimen. Those patients who develop CKD as the result of APN can have permanent and/or progressive renal dysfunction.3 CKD due to APN may present insidiously weeks to months after the dose of OSP, which may mean that this problem is more prevalent than is currently recognised.12 In response to well-documented kidney injury associated with OSP agents, the FDA has recently imposed a requirement for OSP manufacturers to label their products with a black box warning regarding the use of these agents. Their statement includes recognition of increased risk of APN in patients ‘who are over age 55; who are hypovolemic or have decreased intravascular volume; who have baseline kidney disease, bowel obstruction or active colitis; and who are using medications that affect renal perfusion or function (such as diuretics, angiotensin converting enzyme inhibitors, ARBs, and possibly non-steroidal anti-inflammatory drugs (NSAIDs)).’18 This warning, coupled with improved understanding of APN, including recognition of associated risk factors, may help reduce the incidence of this serious iatrogenic disease in the future.
Learning points.
-
▶
APN has been reported primarily as a cause of AKI. It is a cause of CKD as well
-
▶
It is currently recognised that APN may be a much more common cause of CKD
-
▶
Inquiry regarding the use of OSPs should be included in the work up of CKD
-
▶
OSPs should not be administered to patients at risk for developing renal disease.
Acknowledgments
The authors would like to thank Donald Houghton, Department of Pathology, Oregon Health and Sciences University.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Heher EC, Thier SO, Rennke H, et al. Adverse renal and metabolic effects associated with oral sodium phosphate bowel preparation. Clin J Am Soc Nephrol 2008;3:1494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johanson JF, Popp JW, Jr, Cohen LB, et al. A randomized, multicenter study comparing the safety and efficacy of sodium phosphate tablets with 2L polyethylene glycol solution plus bisacodyl tablets for colon cleansing. Am J Gastroenterol 2007;102:2238–46 [DOI] [PubMed] [Google Scholar]
- 3.Sica DA, Carl D, Zfass AM. Acute phosphate nephropathy–an emerging issue. Am J Gastroenterol 2007;102:1844–7 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed M, Raval P, Buganza G. Oral sodium phosphate catharsis and acute renal failure. Am J Gastroenterol 1996;91:1261–2 [PubMed] [Google Scholar]
- 5.Cohen CD, Keuneke C, Schiemann U, et al. Hyponatraemia as a complication of colonoscopy. Lancet 2001;357:282–3 [DOI] [PubMed] [Google Scholar]
- 6.Gumurdulu Y, Serin E, Ozer B, et al. Age as a predictor of hyperphosphatemia after oral phosphosoda administration for colon preparation. J Gastroenterol Hepatol 2004;19:68–72 [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DA, Ghormley J, Flora K. Effect of oral sodium phosphate colon preparation on serum electrolytes in patients with normal serum creatinine. Gastrointest Endosc 1996;43:467–9 [DOI] [PubMed] [Google Scholar]
- 8.Ma KK, Ng CS, Mui LM, et al. Severe hyperphosphatemia and hypocalcemia following sodium phosphate bowel preparation: a forgotten menace. Endoscopy 2003;35:717. [DOI] [PubMed] [Google Scholar]
- 9.Gonlusen G, Akgun H, Ertan A, et al. Renal failure and nephrocalcinosis associated with oral sodium phosphate bowel cleansing: clinical patterns and renal biopsy findings. Arch Pathol Lab Med 2006;130:101–6 [DOI] [PubMed] [Google Scholar]
- 10.Markowitz GS, Nasr SH, Klein P, et al. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol 2004;35:675–84 [DOI] [PubMed] [Google Scholar]
- 11.Asplin JR, Mandel NS, Coe FL. Evidence of calcium phosphate supersaturation in the loop of Henle. Am J Physiol 1996;270:F604–13 [DOI] [PubMed] [Google Scholar]
- 12.Markowitz GS, Stokes MB, Radhakrishnan J, et al. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol 2005;16:3389–96 [DOI] [PubMed] [Google Scholar]
- 13.Russmann S, Lamerato L, Marfatia A, et al. Risk of impaired renal function after colonoscopy: a cohort study in patients receiving either oral sodium phosphate or polyethylene glycol. Am J Gastroenterol 2007;102:2655–63 [DOI] [PubMed] [Google Scholar]
- 14.Khurana A, McLean L, Atkinson S, et al. The effect of oral sodium phosphate drug products on renal function in adults undergoing bowel endoscopy. Arch Intern Med 2008;168:593–7 [DOI] [PubMed] [Google Scholar]
- 15.Brunelli SM, Lewis JD, Gupta M, et al. Risk of kidney injury following oral phosphosoda bowel preparations. J Am Soc Nephrol 2007;18:3199–205 [DOI] [PubMed] [Google Scholar]
- 16.Rostom A, Jolicoeur E, Dubé C, et al. A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol-based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc 2006;64:544–52 [DOI] [PubMed] [Google Scholar]
- 17.Beyea A, Block C, Schned A. Acute phosphate nephropathy following oral sodium phosphate solution to cleanse the bowel for colonoscopy. Am J Kidney Dis 2007;50:151–4 [DOI] [PubMed] [Google Scholar]
- 18.Federal Drug Administration 2008. Oral Sodium Phosphate (OSP) Products for Bowel Cleansing (marketed as Visicol and OsmoPrep, and Oral Sodium Phosphate Products Available Without a Prescription) FDA ALERT http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm103354.htm (accessed 12 November 2008).