Abstract
Objectives
To determine if an incomplete response to and/or inadequate antiplatelet effect of aspirin contribute to saphenous vein graft (SVG) occlusion after coronary artery bypass graft (CABG) surgery.
Background
Thrombosis is the predominant cause of early SVG occlusion. Aspirin, which inhibits cyclooxygenase-1 activity and thromboxane generation in platelets, reduces early SVG occlusion by half.
Methods
Aspirin-responsiveness and platelet reactivity were characterized 3 days and 6 months after CABG in 229 subjects on aspirin monotherapy by; platelet aggregation to arachidonic acid, ADP, collagen and epinephrine; PFA-100 closure time (CT) using collagen/epinephrine (CEPI) and collagen/ADP (CADP) agonist cartridges; VerifyNow Aspirin assay, and; urine levels of 11-dehydro-thromboxane B2 (UTXB2). SVG patency was determined 6 months after surgery by computed tomography coronary angiography.
Results
Inhibited arachidonic acid-induced platelet aggregation, indicative of aspirin-mediated cyclooxygenase-1 suppression, occurred in 95% and >99% of subjects 3 days and 6 months after surgery, respectively. Despite this, 73% and 31% of subjects at these times had elevated UTXB2. Among tested parameters, only UTXB2 and PFA-100 CADP CT measured 6 months after surgery correlated with outcome. By multivariate analysis, CADP CT ≤88 seconds (OR 2.85, P=0.006), target vessel diameter ≤1.5 mm (OR 2.38, P=0.01) and UTXB2 ≥450 pg/mg creatinine (OR 2.59, P=0.015) correlated with SVG occlusion. CADP CT and UTXB2 in combination further identified subjects at particularly high- and low-risk for SVG occlusion.
Conclusions
Aspirin-insensitive thromboxane generation measured by UTXB2 and shear-dependent platelet hyper-reactivity measured by PFA-100 CADP CT are novel independent risk factors for early SVG thrombosis after CABG surgery.
Keywords: vein graft, thrombosis, aspirin, thromboxane, platelet
INTRODUCTION
Coronary artery bypass graft (CABG) surgery is a commonly used revascularization strategy in patients with severe coronary artery disease. Saphenous vein grafts (SVGs) are the most frequently used conduits for this procedure. Unlike arterial grafts, SVGs are susceptible to occlusive thrombosis during the first post-operative year which exposes patients to increase risks of death, myocardial infarction and repeat revascularization (1–3).
Aspirin (ASA) is the pharmacologic agent most convincingly shown to prevent SVG thrombosis, reducing its incidence during the first post-operative year by nearly 50% (4). The principal effect of ASA is to suppress agonist-induced platelet activation by irreversibly inhibiting platelet cyclooxygenase-1 (COX-1), a critical enzyme regulating the generation of thromboxane A2 (TXA2) from arachidonic acid (5). As a strong platelet agonist, TXA2 not only mediates activation of the platelet in which it was formed, but its release activates adjacent quiescent platelets and causes arterial vasoconstriction by binding to cellular thromboxane receptors.
Recent studies indicate that some patients, particularly those with cardiovascular disease, exhibit an incomplete or inadequate antiplatelet effect by ASA characterized by persistent TXA2 generation and high platelet reactivity assessed by ex vivo platelet assays (6). Such patients, variably referred to as being ASA resistant, ASA non-responsive or more recently as having high on-treatment platelet reactivity, are at increased risk for adverse cardiac events compared to those with suppressed platelet reactivity and TXA2 generation (7–9). Patients undergoing CABG surgery have been reported to have decreased ASA responsiveness early in the post-operative period (10,11), though the effect of this on clinical outcome remains unclear. The goal of the present study was to determine if an incomplete response to and/or an inadequate antiplatelet effect of ASA contribute to early SVG occlusion after CABG surgery.
METHODS
Patient Population
The Reduction in Graft Occlusion Rates (RIGOR) study is a multicenter study of patients undergoing first-time CABG surgery designed to investigate the effects of antiplatelet factor 4/heparin antibody induction on SVG occlusion. A detailed description of the study and its principal findings has been previously reported (12). A second prospectively-defined objective of the study was to determine whether ASA resistance and/or platelet hyper-reactivity also contribute to early SVG thrombosis.
Patients ≥18 years of age undergoing first-time CABG surgery with implantation of ≥1 SVG were eligible for enrollment. While patients with an anticipated requirement for postoperative oral anticoagulation or non-ASA antiplatelet therapy were excluded, those placed on such agents for unforeseen postoperative conditions remained in the study. All subjects were administered ASA (300–325 mg) within 24 hours of surgery and were given a supply of 325 mg enteric-coated ASA at discharge and instructed to take one pill daily unless directed otherwise by their physician. Pill counts were performed at each post-operative encounter. ASA responsiveness and platelet reactivity were measured in subjects enrolled at the Johns Hopkins Hospital 2–4 days after CABG surgery and in all subjects 6 months after CABG surgery at the time SVG patency was assessed by multidetector computed tomography coronary angiography (CTA).
Measurement of Platelet Reactivity and ASA Responsiveness
Platelet Aggregometry
Platelet rich plasma was prepared from blood collected in 3.2% citrate by centrifugation at 100 rpm for 10 minutes and the platelet count was adjusted to 180,000/mm3 by the addition of platelet-poor plasma. Undiluted samples with a platelet count of <100,000/mm3 were excluded from analysis. Impedance platelet aggregometry was performed by stimulation with arachidonic acid (0.5 mM), ADP (5, 10 and 20 µM ADP), epinephrine (50 µM) and collagen (1 µg/mL) using a Chrono-Log Model 560CA aggregometer (Chrono-Log, Havertown, PA). The maximum aggregation response within 5 minutes was recorded in ohms. Subjects were considered ASA responsive if arachidonic acid-induced platelet aggregation was ≤1 ohm (normal range in our laboratory for ASA-naïve subjects: 5–17 ohms).
Shear-dependent Platelet Activation
The closure time (CT) of whole blood collected in 3.8% citrate under shear was measured by the Platelet Function Analyzer-100® (PFA-100) device (Siemens Healthcare Diagnostics, Newark, DE) as previously described (13). Samples were tested with the collagen/epinephrine (CEPI) agonist cartridge, which is sensitive to the effects of ASA, and the collagen/ADP (CADP) agonist cartridge, which assesses global platelet reactivity but is not affected by ASA. Samples from subjects with a platelet count <50,000/m3 were excluded from analysis. Samples with non-closure were assigned a CT value of 300 seconds, the maximum measurable by the device. ASA responsiveness by the PFA-100 assay was defined as a CEPI CT >193 seconds, the upper limit of the normal range in our laboratory for ASA-naïve patients (13).
VerifyNow Aspirin Assay
The VerifyNow® Aspirin assay (Accumetrics, Inc., San Diego, CA) measures arachidonic acid-induced platelet aggregation in whole blood containing fibrinogen-coated beads (14). Samples from subjects with a platelet count <50,000/m3 were excluded from analysis. ASA responsiveness by this assay was defined as an Aspirin Reaction Units (ARU) value <550 according to the manufacturer’s instruction.
Measurement of TXA2 Generation
TXA2 generation was quantified by measuring the concentration of its stable metabolite 11-dehydro-thromboxane B2 (UTXB2) in urine by ELISA and expressed as a ratio to urinary creatinine as previously described (13). ASA responsiveness by this assay was defined as UTXB2 <400 pg/mg creatinine according to established criteria (15).
Assessment of SVG Patency
SVG patency was assessed 6 months after CABG surgery by CTA as previously described (12). Data from clinically-driven invasive coronary angiograms could be used for the primary endpoint analysis if performed within 6 weeks of the anticipated 6-month follow up visit or if it was the only assessment of SVG patency prior to an adverse clinical endpoint. Each segment of “Y-grafts” and “skip grafts” were considered as separate SVGs according to the Society of Thoracic Surgeons (STS) criteria. Reconstructed images were analyzed by two blinded reviewers and classified as patent (containing stenoses of 0–75%), significantly diseased (containing stenoses of 76–99%) or occluded (containing a 100% stenosis). There was 98% concordance in assessment of SVG patency between reviewers. In cases of discordance, a third reviewer adjudicated all SVGs in that patient.
Statistical Analysis
Data from subjects who survived the index hospitalization, underwent angiographic assessment of SVG patency and were maintained throughout the study period on ASA as the only antiplatelet agent were analyzed for the outcome of SVG occlusion versus patency. For statistical purposes, the small number of SVGs classified as significantly diseased were considered as patent. Demographic differences between patients with and without occluded SVGs were evaluated using a student’s t test for mean values or Wilcoxon rank sum for medians as appropriate for the data distribution. Proportions were compared using a Chi-square or Fisher’s exact test and comparisons among groups were done with ANOVA, McNemar or Kruskal-Wallis testing, as appropriate.
Univariate analyses were performed on a per graft basis for the odds of occlusion versus patency using those variables deemed biologically plausible or supported by the literature. Categorical covariates were tested for collinearity using Chi-square or Fisher’s exact tests, while continuous variables were tested using Pearson’s correlation coefficients. Highly correlated covariates (e.g. rho ≥ 0.7) were eliminated based upon clinical significance. Variables with statistical significance of P ≤0.15 in the univariate analysis or those supported by the literature were entered into a multilevel random effects model. A four-level model was explored initially; analyzing SVG outcome with random intercepts for patient, surgeon, hospital and multi-segmented SVGs (i.e. skip and Y-grafts) considered as single grafts. Because random intercepts on surgeon, hospital and multi-segmented SVGs were not significant, the analysis thereafter included clustering on patient alone. Stepwise backwards elimination of variables was performed to generate several potential models. Akaike Information Criterion (AIC) was utilized for model comparison.
Analyses were performed using Stata/MP 10.0 for Windows (College Station, TX). Differences were considered significant when P<0.05.
Results
Study population characteristics
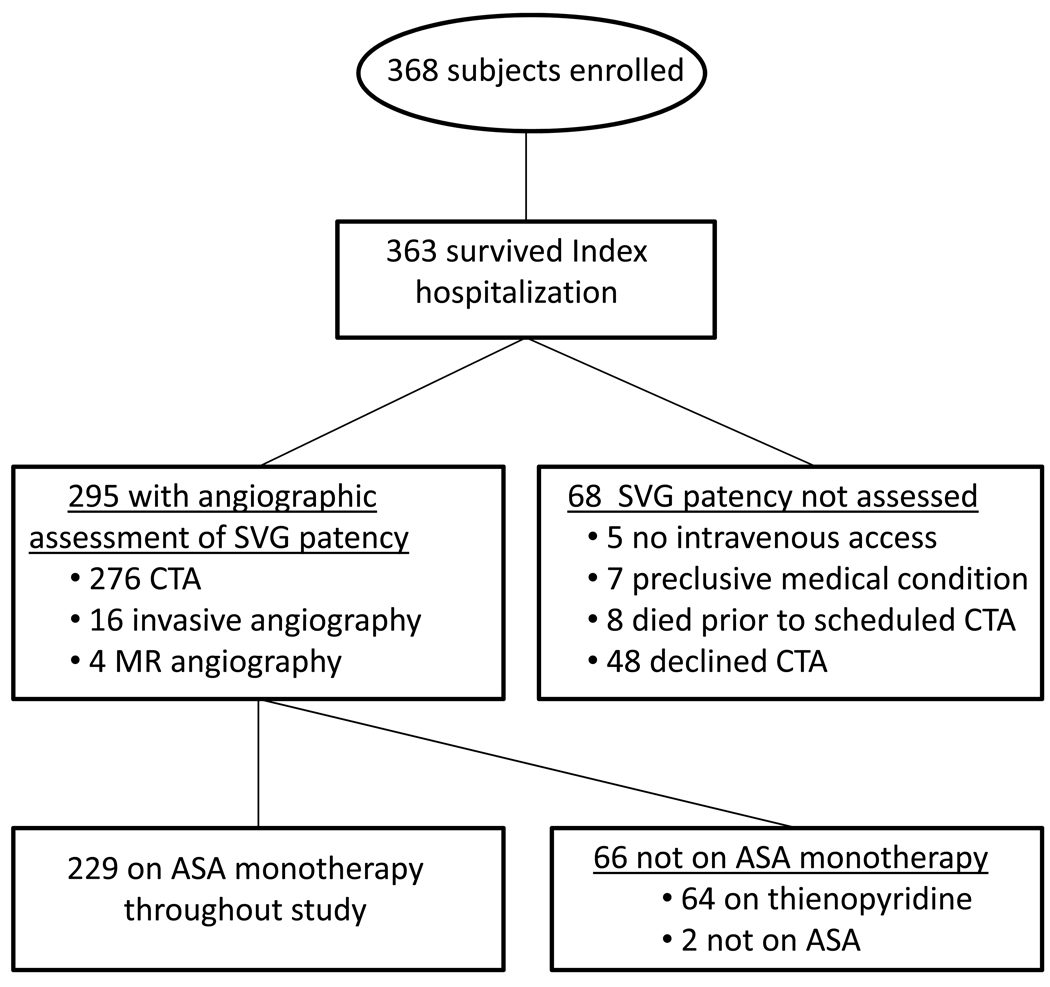
The RIGOR study enrolled 368 subjects undergoing first-time CABG surgery with implantation of ≥1 SVG. The study population was representative of patients undergoing isolated CABG surgery based on comparison with the STS National Database (12). Two hundred twenty-nine subjects who survived the index hospitalization, underwent angiographic assessment of SVG patency and were maintained on ASA throughout the study period as the only antiplatelet agent were included in the present analysis (Figure 1). In addition to major differences in antiplatelet regimens and angiographic follow-up, subjects included in the analysis were more likely to be men, to be referred electively for CABG surgery, to have a slightly lower median euroSCORE and prevalences of current tobacco use and history of atrial fibrillation than excluded subjects (Supplement Table 1). The baseline, operative and post-operative characteristics of subjects with at least one occluded SVG were very similar to those with patent SVGs except that the former group underwent implantation of ≥4 SVGs more frequently (Table 1). Of the 497 SVGs implanted in these subjects (median 2, range 1–6 SVGs/subject), 94 (19%) were totally occluded when assessed a median of 189 days (interquartile range [IQR], 182–203 days) after CABG surgery. Only 9 SVGs (2%) contained high grade (>75%) stenoses, consistent with the concept that thrombosis and not atherosclerosis or neointima hyperplasia is the predominant cause of early SVG failure. Seventy subjects (31%) had occlusion of ≥1 SVG and 21 subjects (9%) had occlusion of all implanted SVGs.
Figure 1. Schematic of RIGOR study enrollment and analysis criteria.
Abbreviations: ASA = aspirin; CTA = multidetector computed tomography coronary angiography; MR = magnetic resonance; SVG, saphenous vein graft
Table 1.
Baseline, operative and post-operative characteristics of subjects stratified by SVG patency.
| ≥1 Occluded SVG (n=70) |
Patent SVGs (n=159) |
|
|---|---|---|
| Age, yrs (median, IQR) | 63 (55–72) | 63 (57–71) |
| Men | 52 (74%) | 135 (85%) |
| White race | 57 (81%) | 144 (91%) |
| Body Mass Index, kg/m2 ( median, IQR) | 30 (27–34) | 29 (26–32) |
| Medical history | ||
| Hypertension | 53 (76%) | 134 (84%) |
| Hyperlipidemia | 56 (80%) | 135 (85%) |
| Diabetes | 20 (29%) | 64 (40%) |
| Heart failure | 6 (9%) | 24 (15%) |
| Peripheral/cerebrovascular disease | 13 (19%) | 30 (19%) |
| Atrial fibrillation | 1 (1%) | 6 (4%) |
| Current tobacco use | 20 (29%) | 32 (20%) |
| Creatinine, mg/dL (median, IQR) | 1.0 (0.9–1.2) | 1.0 (0.8–1.1) |
| Myocardial infarction | 28 (40%) | 61 (38%) |
| Within 7 days | 16 (23%) | 29 (18%) |
| Prior percutaneous coronary intervention | 11 (16%) | 34 (21%) |
| Preoperative ejection fraction | ||
| ≤30% | 4 (6%) | 16 (10%) |
| 30–50% | 22 (31%) | 54 (34%) |
| ≥50% | 44 (63%) | 89 (56%) |
| Number of coronary arteries with >50% stenosis | ||
| 2 | 11 (16%) | 21 (13%) |
| 3 | 59 (84%) | 135 (85%) |
| Left main with >50% stenosis | 27 (39%) | 59 (37%) |
| Elective CABG surgery | 32 (46%) | 66 (42%) |
| Isolated CABG surgery | 64 (91%) | 142 (89%) |
| euroSCORE (median, IQR) | 4 (2–5) | 3 (2–5) |
| Cardiopulmonary bypass | 70 (100%) | 156 (98%) |
| Duration, minutes (median, IQR) | 80 (65–95) | 75 (60–96) |
| Arterial graft implanted | 69 (99%) | 154 (97%) |
| Number of SVGs per subject | ||
| 1 | 14 (20%) | 47 (30%) |
| 2 | 23 (33%) | 73 (46%) |
| 3 | 21 (30%) | 32 (20%) |
| ≥4 | 11 (17%)* | 7 (4%) |
| SVG skip graft implanted | 13 (19%) | 18 (11%) |
| SVG patency assessment, post-operative day (median, IQR) | 188 (182–198) | 189 (183–202) |
| Medications at time of SVG patency assessment | ||
| ASA | 70 (100%) | 159 (100%) |
| <325 mg/day | 3 (4%) | 16 (10%) |
| Non-ASA antiplatelet agent | 0 (0%) | 0 (0%) |
| Oral anticoagulation | 3 (4%) | 10 (6%) |
| ACE inhibitor/ARB | 43 (61%) | 110 (62%) |
| Beta-blocker | 62 (89%) | 128 (81%) |
| Lipid lowering agent | 60 (86%) | 145 (91%) |
P value =0.008;
ASA = aspirin; CABG =coronary artery bypass graft; ACE =angiotensin converting enzyme; ARB =angiotensin receptor blocker
Prevalence of ASA non-responsiveness after CABG surgery
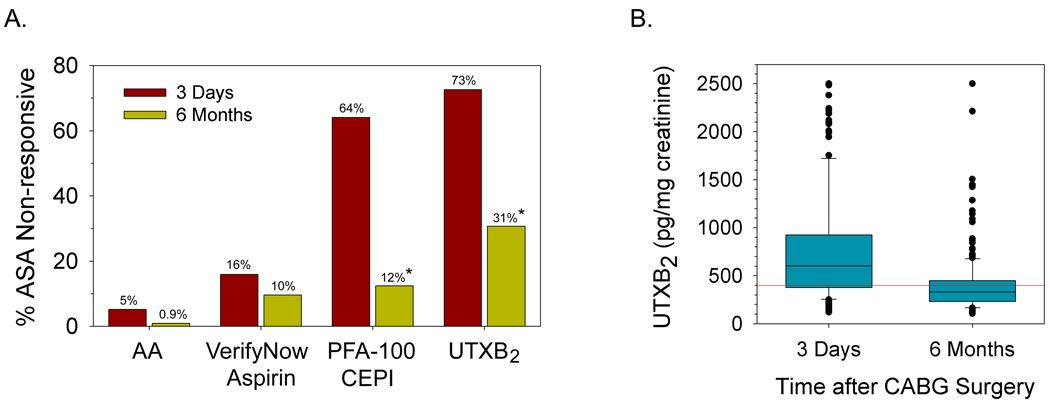
Platelet function testing was performed on 168 subjects a median of 3 days (IQR, 3–4 days) and on all 229 subjects a median of 189 days (IQR, 182–203 days) after CABG surgery. UTXB2 was measured in 219 and 228 subjects at these same respective time points. Of the assays performed, four have definable thresholds limits for ASA responsiveness. The prevalence of ASA non-responsiveness varied widely by assay and by time after CABG surgery (Figure 2A). By arachidonic acid-induced platelet aggregation, a specific measure of platelet COX-1 activity, ASA non-responsiveness was present in 5% of subjects (median aggregation 5 ohms, IQR 2–10 ohms) 3 days and in <1% of subjects (median aggregation 12 ohms, range 8–15 ohms) 6 months after surgery. Concurrent non-steroidal anti-inflammatory drug use did not correlate with the presence of ASA non-responsiveness defined by this method at either time point (data not shown). ASA non-responsiveness was also significantly more prevalent immediately after CABG surgery when defined by PFA-100 CT using the CEPI agonist cartridge, but remained relatively constant over time when assessed by the VerifyNow Aspirin assay. Despite a high degree of platelet COX-1 inhibition by ASA, persistent TXA2 generation was observed in 73% and 31% of subjects 3 days and 6 months after CABG surgery respectively, as evidenced by often significantly elevated levels of UTXB2 (Figure 2B).
Figure 2. Prevalence of ASA non-responsiveness by assay and time after CABG surgery.
A) Percentage of subjects with ASA non-responsiveness 3 days and 6 months after CABG surgery. ASA non-responsiveness was defined by impedance aggregometry as arachidonic acid-induced platelet aggregation (AA) >1 ohm, by Verify Now Aspirin test result ≥550 ARU, by PFA-100 CEPI CT ≤193 seconds and by UTXB2 ≥400 pg/mg creatinine. * P <0.001 for 6 month versus 3 day values. B) UTXB2 in subjects 3 days and 6 months after CABG surgery. Median values with interquartile ranges (boxes), 5% and 95% confidence intervals (bars) and individual outliers are shown. P <0.001 for difference between groups. Red line denotes threshold for ASA non-responsiveness (400 pg/mg creatinine).
Association with early SVG occlusion
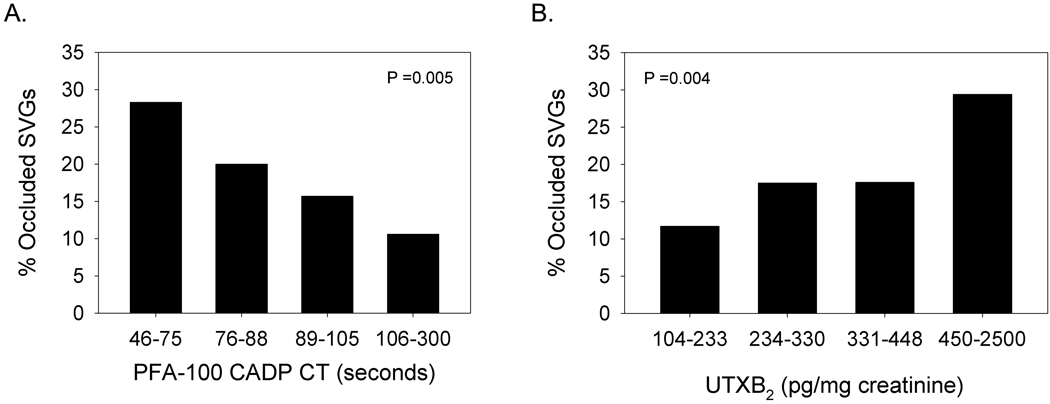
No laboratory parameter measured 3 days after CABG surgery could differentiate occluded from non-occluded SVGs or subjects with occluded versus patent SVGs (Table 2). When assessed 6 months after surgery, however, occluded SVGs were associated with a higher mean level of UTXB2 and lower median PFA-100 CADP CT (Table 3). Subjects with ≥1 occluded SVG also had a lower median PFA-100 CADP CT than subjects with patent SVGs. While SVG occlusion was inversely proportional to PFA-100 CADP CT, it was most prevalent in subjects with UTXB2 ≥450 pg/mg creatinine (Figure 3).
Table 2.
Relationship between platelet reactivity and ASA responsiveness, measured 3 days after CABG surgery, and 6-month SVG patency.
| Assay | Per SVG | Per Subject | ||||||
|---|---|---|---|---|---|---|---|---|
|
Occluded SVG |
Patent SVG | N |
P value |
≥1 Occluded SVG |
No Occluded SVG |
N |
P value |
|
| Platelet Aggregation (Ω) | ||||||||
| Arachidonic Acid, 0.5 mM (median, IQR) | 0 (0-0) | 0 (0-0) | 289 | NS | 0 (0-0) | 0 (0-0) | 154 | NS |
| ADP, 5 µM (mean ±SD) | 17.1 ± 5.6 | 18.8 ± 4.9 | 230 | NS | 17.9 ± 5.3 | 18.9 ± 4.9 | 124 | NS |
| ADP, 10 µM (mean ± SD) | 18.0 ± 4.8 | 18.6 ± 5.1 | 221 | NS | 18.0 ± 4.5 | 18.7 ± 5.2 | 118 | NS |
| ADP, 20 µM (mean ± SD) | 16.5 ± 4.6 | 16.6 ± 4.4 | 287 | NS | 16.8 ± 4.8 | 16.6 ± 4.7 | 153 | NS |
| Epinephrine, 50 µM (mean ± SD) | 10.7 ± 6.5 | 9.94 ± 6.56 | 289 | NS | 10.5 ±6.7 | 10.4 ±6.6 | 154 | NS |
| Collagen, 1 µg/mL (mean ± SD) | 14.1 ± 5.4 | 12.7 ± 6.3 | 289 | NS | 14.4 ± 5.2 | 12.9 ± 6.4 | 154 | NS |
| PFA-100 CT (seconds) | ||||||||
| CEPI (median, IQR) | 121 (94–203) | 136 (101–241) | 289 | NS | 135 (100–204) | 136 (101–263) | 156 | NS |
| CADP (median, IQR) | 96 (82–137) | 98 (83–145) | 305 | NS | 96 (81–139) | 102 (84–146 | 162 | NS |
| UTXB2 (ln pg/mg creatinine; mean ± SD) | 6.42 ± 0.71 | 6.43 ± 0.65 | 479 | NS | 6.33 ± 0.71 | 6.47 ± 0.60 | 219 | NS |
| VerifyNow Aspirin (ARU; mean ± SD) | 475 ± 59 | 484 ± 60 | 308 | NS | 478 ± 61 | 484 ± 61 | 164 | NS |
Abbreviations: ARU =aspirin reaction unit; CADP =collagen/ADP agonist cartridge; CEPI =collagen/epinephrine agonist cartridge; IQR =interquartile range; PFA-100 =Platelet Function Analyzer 100; UTXB2 =urine 11-dehydro-thromboxane B2.
Table 3.
Relationship between platelet reactivity and ASA responsiveness, measured 6 months after CABG surgery, and 6-month SVG patency.
| Assay | Per SVG | Per Subject | ||||||
|---|---|---|---|---|---|---|---|---|
|
Occluded SVG |
Patent SVG | N |
P value |
≥1 Occluded SVG |
No Occluded SVG |
N |
P value |
|
| Platelet Aggregation (Ω) | ||||||||
| Arachidonic Acid, 0.5 mM (median, IQR) | 0 (0-0) | 0 (0-0) | 495 | NS | 0 (0-0) | 0 (0-0) | 228 | NS |
| ADP, 5 µM (mean ±SD) | 17.5 ± 5.2 | 17.7± 4.7 | 463 | NS | 17.7 ± 5.5 | 17.8 ± 4.8 | 212 | NS |
| ADP, 10 µM (mean ± SD) | 17.5 ± 4.7 | 18.0 ± 4.9 | 457 | NS | 17.8 ± 4.8 | 17.7 ± 5.1 | 209 | NS |
| ADP, 20 µM (mean ± SD) | 16.1 ± 5.3 | 17.1 ± 4.7 | 483 | NS | 16.4 ± 5.3 | 17.1 ± 4.4 | 224 | NS |
| Epinephrine, 50 µM (mean ± SD) | 5.47 ± 5.59 | 5.23 ± 6.33 | 490 | NS | 5.20 ± 5.76 | 5.50 ± 6.45 | 227 | NS |
| Collagen, 1 µg/mL (mean ± SD) | 14.3 ± 5.6 | 14.9 ± 5.2 | 495 | NS | 15.1 ± 5.1 | 14.8 ± 5.2 | 228 | NS |
| PFA-100 CT (seconds) | ||||||||
| CEPI (median, IQR) | 288 (245–300) | 295 (260–300) | 491 | NS | 288 (245–300) | 300 (257–300) | 226 | NS |
| CADP (median, IQR) | 80 (72–98) | 88 (77–106) | 492 | <0.001 | 80 (71–95) | 92 (77–110) | 228 | 0.001 |
| UTXB2 (ln pg/mg creatinine; mean ± SD) | 5.98 ± 0.63 | 5.76 ± 0.55 | 495 | <0.001 | 5.90 ± 0.62 | 5.77 ± 0.52 | 228 | NS |
| VerifyNow Aspirin (ARU; mean ± SD) | 450 ± 51 | 458 ± 60 | 496 | NS | 452 ± 55 | 460 ± 60 | 228 | NS |
Abbreviations: ARU =aspirin reaction unit; CADP =collagen/ADP agonist cartridge; CEPI =collagen/epinephrine agonist cartridge; IQR =interquartile range; PFA-100 =Platelet Function Analyzer 100; UTXB2 =urine 11-dehydro-thromboxane B2
Figure 3. Prevalence of SVG occlusion by PFA-100 CADP CT and UTXB2.
Percentage of occluded SVGs in subjects stratified by quartiles of A) PFA-100 CADP CT and B) UTXB2 measured 6 months after CABG surgery.
The associations of PFA-100 CADP CT and UTXB2 to early SVG occlusion were then determined in relationship to a comprehensive array of known and potential risk factors. In addition to several patient- and graft-specific factors, PFA-100 CADP CT and UTXB2 measured 6 months after CABG surgery correlated with early SVG occlusion by univariate analysis (Data Supplement Tables 2–5) when expressed both as continuous variables and as binary variables based on respective median (88 seconds) and upper quartile values (450 pg/mg creatinine). When analyzed either as continuous (Model 1) and binary (Model 2) variables in multivariate analyses (Table 4), PFA-100 CADP CT and UTXB2 remained significantly associated with SVG outcome along with target vessel diameter ≤1.5 mm, historically recognized as one of the strongest risk factor for early SVG occlusion (16–18). SVG occlusion was also more prevalent in subjects with both CADP CT ≤88 seconds and UTXB2 ≥450 pg/mg creatinine than in subjects with CADP CT >88 seconds and UTXB2 <450 pg/mg creatinine (32% versus 10%, respectively, p <0.0001). By multivariate analysis, this equated to a 6.9-fold increased odds of SVG occlusion (confidence interval [CI] 2.16–21.7, p =0.001) between groups.
Table 4.
Multivariate models of factors associated with odds of SVG occlusion versus patency.
| Variable | Odds Ratio | P value | 95% confidence interval |
|---|---|---|---|
| Model 1 | |||
| PFA-100 CADP CT (seconds)* | 0.98 | 0.002 | 0.97–0.99 |
| Target vessel diameter ≤1.5 mm | 2.36 | 0.009 | 1.23–4.53 |
| lnUTXB2 (pg/mg creatinine)* | 1.73 | 0.047 | 1.10–2.99 |
| Female gender | 2.31 | 0.052 | 0.99–5.39 |
| Non-white race | 2.48 | 0.059 | 0.97–6.38 |
| Model 2 | |||
| PFA-100 CADP CT (≤88 vs. >88 seconds) | 2.85 | 0.006 | 1.36–6.00 |
| Target vessel diameter ≤1.5 mm | 2.38 | 0.011 | 1.22–4.61 |
| UTXB2 (≥450 vs. <450 pg/mg/creatinine) | 2.59 | 0.015 | 1.20–5.56 |
| Female gender | 2.13 | 0.089 | 0.89–5.12 |
Odds of occlusion for every variable unit increase.
Abbreviations: CADP =collagen/ADP agonist cartridge; CEPI =collagen/epinephrine agonist cartridge; PFA-100 =Platelet Function Analyzer 100; UTXB2 =urine 11-dehydro-thromboxane B2.
Discussion
Despite advances in surgical technique and post-operative management, including routine administration of ASA, the incidence of early SVG occlusion after CABG surgery remains substantial in a contemporary patient population. The rate of SVG occlusion 6 months after CABG surgery observed in the RIGOR study is consistent with results from several recent studies, including the PREVENT 4 (Project of Ex-vivo Vein Graft Engineering via Transfection 4) trial which reported an overall SVG occlusion rate of 26% in 2400 patients 12–18 months after CABG surgery (19). Among patient and graft-specific variables associated with SVG occlusion, bypass of small diameter (≤1.5 mm) target vessels has historically been considered one of the strongest (17,18). In addition to this traditional risk factor, we identified shear-dependent platelet activation, measured by PFA-100 CADP CT, and ASA-insensitive TXA2 generation, measured by UTXB2, as potent novel independent risk factors for early SVG thrombosis. The combination of PFA-100 CADP CT and UTXB2 was further able to stratify subjects into those at particularly high- and low-risk for SVG thrombosis.
In normal individuals, the overwhelming majority of TXA2 generated in the body is produced in platelets by metabolism of arachidonic acid by the COX-1 enzyme. Because ASA efficiently and irreversibly inhibits platelet COX-1 activity, it markedly suppresses TXA2 generation as measured by UTXB2 and blunts platelet reactivity to several agonists (15,20). Growing evidence suggests that some patients with cardiovascular disease have persistent TXA2 generation in spite of ASA therapy and are consequently at increased risk for adverse clinical events. This association was first noted in a subgroup analysis from the HOPE (Heart Outcomes Prevention Evaluation) study and later confirmed in the larger CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance) trial where subjects on ASA with the highest compared to the lowest quartile of UTXB2 at study entry had an approximate 1.7-fold increased risk (P ≤0.03 for both studies) of myocardial infarction, stroke or cardiovascular death over the subsequent 2–5 years (8,9). Our findings add to the body of evidence suggesting that ASA-insensitive TXA2 generation is a significant cardiovascular risk factor by demonstrating that it is independently associated with early SVG occlusion after CABG surgery. Follow up data on RIGOR subjects is currently being collected to determine if ASA-insensitive TXA2 generation after CABG surgery also adversely affects long-term clinical outcome.
A fundamental question arising from the above data is whether ASA-insensitive TXA2 generation in patients with cardiovascular disease represents a failure of ASA to inhibit platelet COX-1-mediated TXA2 biosynthesis, the strictest and most accurate definition of ASA resistance (5), or the presence of alternate TXA2 production pathways that are not effectively inhibited by ASA. Zimmerman found that platelets from patients taking 100 mg/day of ASA generate more TXA2 in the first week after CABG surgery than platelets from healthy ASA-treated controls (11). TXA2 generation was only partially suppressed by the further exposure of platelets in vitro to high concentrations of ASA, suggesting both incomplete COX-1 inhibition in vivo by low-dose ASA, as well as the presence of COX-1-independent TXA2 synthesis (10). Others have subsequently confirmed that platelets from ASA-treated subjects are capable of generating TXA2 via non-COX-1-mediated pathways, though the amounts appear relatively small in comparison to that produced by platelets from ASA-naïve patients (21). While we did not directly measure platelet TXA2 generation in our study, arachidonic acid-induced platelet aggregation is known to be inhibited when platelet TXA2 generation is suppressed by >90% (22). The suppression of arachidonic acid-induced platelet aggregation in ≥95% of subjects both early and late after CABG surgery argues for a high degree of platelet COX-1 inhibition by ASA and therefore a very low prevalence of true ASA resistance in our study population.
Despite evidence for effective platelet COX-1 suppression by ASA, UTXB2 was markedly elevated in a substantial number of subjects both early and late after CABG surgery. As UTXB2 reflects TXA2 generation in the entire body, not only from platelets, our data suggest that significant TXA2 production may originate from non-platelet sources that are not effectively inhibited by ASA. Potential sources include inflammatory and endothelial cells that are capable of producing TXA2 via both COX-1 and inducible COX-2-dependent pathways. ASA is a relatively weak inhibitor of COX-2 at standard doses (20) and, unlike platelets, both cell types have the capacity to regenerate COX-1 that is irreversibly inhibited by ASA. Another potential source is from non-enzymatic conversion of arachidonic acid to F2-isoprostanes under conditions of oxidative stress. F2-isoprostanes are capable of activating platelet and cellular TXA2 receptors and can also directly stimulate TXA2 production in endothelial cells (23,24). Additional studies are needed to identify the sources of this ASA-insensitive TXA2 generation and the mechanism by which its production adversely affects clinical outcome.
Various aspects of platelet function, including ASA responsiveness, can be characterized by a wide array of ex vivo assays. One of the most physiologic is the PFA-100 device which measures agonist-induced platelet aggregation under conditions of high shear by quantifying the closure time (CT) of a membrane aperture by the formation of a platelet plug (25). The PFA-100 was primarily designed to screen for defects in primary hemostasis with the CEPI agonist cartridge detecting the antiplatelet effects of ASA and the CADP agonist cartridge identifying patients with congenital and acquired platelet disorders (26). Among patients with cardiovascular disease, a CEPI CT in the normal range (94–193 seconds) despite ASA therapy is associated with an increased risk of recurrent cardiovascular events (27,28). However, the specificity and ultimate utility of the PFA-100 at detecting true aspirin resistance has been called into question by the observation that CEPI CTs are highly influenced by plasma levels of vWF (29). Our results not only confirm the poor correlation between ASA responsiveness defined by CEPI CT compared to arachidonic acid-induced platelet aggregometry but also reveal no association between CEPI CT and SVG outcome.
A major finding of our study was the strong association between low CADP CT and early SVG occlusion. Unlike CEPI CT, CADP CT is not influenced by ASA or thienopyridine use and is therefore considered an indicator of global platelet reactivity. Similar to CEPI CT, we found a correlation between low CADP CT and high plasma vWF levels (13) but did not find an independent association between vWF levels and early SVG occlusion (data not shown). A CADP CT below the normal range (71–118 seconds) suggests platelet hyper-reactivity and has been observed in patients with acute coronary syndromes where it correlates with the degree of myocardial necrosis and recurrent ischemic events (30–32). In a recent study of 700 patients undergoing percutaneous coronary intervention, a CADP CT <65 seconds measured before the procedure was associated with a 3.5-fold increase risk (CI 1.2–10.4, p<0.03) of major adverse cardiac events at 24 months (21). These studies along with our own suggest the potential of measuring shear-dependent platelet activation as a means to risk stratify patients with cardiovascular disease.
A limitation of the RIGOR study was that ASA responsiveness and platelet reactivity were not assessed preoperatively. This was by design as we anticipated not all eligible subjects would have been on chronic ASA therapy prior to CABG surgery and some would have been on concurrent non-ASA antiplatelet agents that would confound interpretation of many of the assays. It was reasoned that values obtained 6 months after CABG surgery, following resolution of the associated inflammatory response and hematologic changes, would be indicative of the subject’s baseline state. Now that ASA-insensitive thromboxane generation and shear-dependent platelet activation have been identified as important parameters, additional studies will be needed to determine if their preoperative measurement can identify patients at high risk for SVG thrombosis and what therapies might be useful to mitigate such risk.
In conclusion, we have identified ASA-insensitive TXA2 generation, measured by UTXB2, and shear-dependent platelet hyper-reactivity, measured by PFA-100 CADP CT, as novel independent risk factors for early SVG thrombosis. Both entities appear to represent pathways that are independent of platelet COX-1 activity and not effectively inhibited by ASA. We further found that the prevalence of ASA non-responsiveness varied widely by several commonly used assays and time relative to CABG surgery but was not generally associated with graft outcome. Using arachidonic acid-induced platelet aggregometry as a specific indicator of platelet TXA2 generation, however, the overwhelming majority of patients appear to have a high degree of platelet COX-1 inhibition by ASA and therefore a low incidence of ASA resistance both early and late after CABG surgery.
Supplementary Material
Acknowledgments
This study was supported by the Johns Hopkins Institute for Clinical and Translational Research (funded by UL1 RR025005 from the National Center for Research Resources, National Institutes of Health, Bethesda, MD, USA), grants from AstraZeneca Pharmaceuticals, Sanofi-BMS, the Flight Attendant Medical Research Foundation and material support from Siemens Healthcare Diagnostics, Inc. and GlaxoSmithKline.
Abbreviations and Acronyms
- ASA
aspirin
- COX-1
cyclooxygenase-1
- CABG
coronary artery bypass graft surgery
- SVG
saphenous vein graft
- UTXB2
urinary 11-dehydro-thromboxane B2
- PFA-100
Platelet Function Analyzer-100
- CADP
collagen/epinephrine agonist cartridge
- CEPI
collagen/ADP agonist cartridge
- CT
closure time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.FitzGibbons GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 3.Halabi AR, Alexander JH, Shaw LK, et al. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol. 2005;96:1254–1259. doi: 10.1016/j.amjcard.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 4.Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy-II:Maintenance of vascular graft or arterial patency by antiplatelet therapy. BMJ. 1994;308:168. [PMC free article] [PubMed] [Google Scholar]
- 5.Patrono C, Rocca B. Drug insight: aspirin resistance--fact or fashion? Nat Clin Pract Cardiovasc Med. 2007;4:42–50. doi: 10.1038/ncpcardio0728. [DOI] [PubMed] [Google Scholar]
- 6.Patrono C. Aspirin resistance: definition, mechanisms and clinical read-outs. J Thromb Haemost. 2003;1:1710–1713. doi: 10.1046/j.1538-7836.2003.00284.x. [DOI] [PubMed] [Google Scholar]
- 7.Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin "resistance" and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336:195–198. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eikelboom JW, Hankey GJ, Thom J, et al. Incomplete Inhibition of Thromboxane Biosynthesis by Acetylsalicylic Acid. Determinants and Effect on Cardiovascular Risk. Circulation. 2008;118:1690. doi: 10.1161/CIRCULATIONAHA.108.768283. [DOI] [PubMed] [Google Scholar]
- 9.Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann N, Wenk A, Kim U, et al. Functional and biochemical evaluation of platelet aspirin resistance after coronary artery bypass surgery. Circulation. 2003;108:542–547. doi: 10.1161/01.CIR.0000081770.51929.5A. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann N, Kienzle P, Weber AA, et al. Aspirin resistance after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2001;121:982–984. doi: 10.1067/mtc.2001.111416. [DOI] [PubMed] [Google Scholar]
- 12.Gluckman TJ, Segal JB, Schulman SP, et al. Effect of anti-platelet factor-4/heparin antibody induction on early saphenous vein graft occlusion after coronary artery bypass surgery. J Thromb Haemost. 2009;7:1457–1464. doi: 10.1111/j.1538-7836.2009.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazarian SM, Thompson JB, Gluckman TJ, et al. Clinical and laboratory factors associated with shear-dependent platelet hyper-reactivity in patients on chronic aspirin therapy. Thromb Res. 2009 doi: 10.1016/j.thromres.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelson AD, Frelinger AL, III, Furman MI. Current options in platelet function testing. Am J Cardiol. 2006;98:4N–10N. doi: 10.1016/j.amjcard.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Fritsma GA, Ens GE, Alvord MA, Carroll AA, Jensen R. Monitoring the antiplatelet action of aspirin. JAAPA. 2001;14:57–52. [PubMed] [Google Scholar]
- 16.Bjork VO, Ekestrom S, Henze A, Ivert T, Landou C. Early and late patency of aortocoronary vein grafts. Scand J Thorac Cardiovasc Surg. 1981;15:11–21. doi: 10.3109/14017438109101020. [DOI] [PubMed] [Google Scholar]
- 17.Paz MA, Lupon J, Bosch X, Pomar JL, Sanz G. Predictors of early saphenous vein aortocoronary bypass graft occlusion. The GESIC Study Group. Ann Thorac Surg. 1993;56:1101–1106. doi: 10.1016/0003-4975(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 18.Shah PJ, Gordon I, Fuller J, et al. Factors affecting saphenous vein graft patency: clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg. 2003;126:1972–1977. doi: 10.1016/s0022-5223(03)01276-5. [DOI] [PubMed] [Google Scholar]
- 19.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 20.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 21.Frelinger AL, III, Li Y, Linden MD, et al. Association of cyclooxygenase-1-dependent and-independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation. 2009;120:2586–2596. doi: 10.1161/CIRCULATIONAHA.109.900589. [DOI] [PubMed] [Google Scholar]
- 22.Pulcinelli FM, Riondino S, Celestini A, et al. Persistent production of platelet thromboxane A2 in patients chronically treated with aspirin. J Thromb Haemost. 2005;3:2784–2789. doi: 10.1111/j.1538-7836.2005.01633.x. [DOI] [PubMed] [Google Scholar]
- 23.Davi G, Falco A, Patrono C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem Phys Lipids. 2004;128:149–163. doi: 10.1016/j.chemphyslip.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Hou X, Roberts LJ, Taber DF, et al. 2,3-Dinor-5,6-dihydro-15-F(2t)-isoprostane: a bioactive prostanoid metabolite. Am J Physiol Regul Integr Comp Physiol. 2001;281:R391–R400. doi: 10.1152/ajpregu.2001.281.2.R391. [DOI] [PubMed] [Google Scholar]
- 25.Hayward CP, Harrison P, Cattaneo M, Ortel TL, Rao AK. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J Thromb Haemost. 2006;4:312–319. doi: 10.1111/j.1538-7836.2006.01771.x. [DOI] [PubMed] [Google Scholar]
- 26.Favaloro EJ. Clinical application of the PFA-100. Curr Opin Hematol. 2002;9:407–415. doi: 10.1097/00062752-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Reny JL, de Moerloose P, Dauzat M, Fontana P. Use of the PFA-100 closure time to predict cardiovascular events in aspirin-treated cardiovascular patients: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:444–450. doi: 10.1111/j.1538-7836.2008.02897.x. [DOI] [PubMed] [Google Scholar]
- 28.Crescente M, Di Castelnuovo A, Iacoviello L, De Gaetano G, Cerletti C. PFA-100 closure time to predict cardiovascular events in aspirin-treated cardiovascular patients: a meta-analysis of 19 studies comprising 3,003 patients. Thromb Haemost. 2008;99:1129–1131. doi: 10.1160/TH08-03-0130. [DOI] [PubMed] [Google Scholar]
- 29.Chakroun T, Gerotziafas G, Robert F, et al. In vitro aspirin resistance detected by PFA-100 closure time: pivotal role of plasma von Willebrand factor. Br J Haematol. 2004;124:80–85. doi: 10.1046/j.1365-2141.2003.04727.x. [DOI] [PubMed] [Google Scholar]
- 30.Frossard M, Fuchs I, Leitner JM, et al. Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation. 2004;110:1392–1397. doi: 10.1161/01.CIR.0000141575.92958.9C. [DOI] [PubMed] [Google Scholar]
- 31.Harrison P, Mackie I, Mathur A, et al. Platelet hyper-function in acute coronary syndromes. Blood Coagul Fibrinolysis. 2005;16:557–562. doi: 10.1097/01.mbc.0000187252.09759.ba. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs I, Frossard M, Spiel A, Riedmuller E, Laggner AN, Jilma B. Platelet function in patients with acute coronary syndrome (ACS) predicts recurrent ACS. J Thromb Haemost. 2006;4:2547–2552. doi: 10.1111/j.1538-7836.2006.02239.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.