Abstract
A fast, sensitive and selective liquid chromatography–tandem mass spectrometry (LCMS/MS) method for the determination of alfentanil and midazolam in human plasma has been developed and validated. Alfentanil and midazolam were extracted from plasma using a mixed-mode cation exchange solid phase extraction method, with recoveries of both compounds greater than 80% at 3 different concentrations (1, 10 and 100 ng/ml). Compounds were analyzed on a C18 column with a water and methanol mobile phase gradient with acetic acid as an additive, at a flow rate of 0.3 ml/min. The working assay range was linear from 0.25 to 100ng/ml for each compound. The signal to noise ratio was 80 and 40 for alfentanil and midazolam, respectively, at the lowest concentration calibration standard, with less than 10% matrix suppression by human plasma at this concentration. Alfentanil and midazolam were stable in human plasma during storage at -80°C, processing, and analysis. The procedure was validated and applied to the analysis of plasma samples from healthy human subjects administered oral and intravenous alfentanil and midazolam.
1. Introduction
The cytochrome P450 (CYP) superfamily is a large and diverse group of enzymes that is the most clinically important in the disposition of a large number of drugs. The function of most P450 enzymes is to catalyze the oxidation or detoxification of organic substances including endogenous substances such as lipids and hormones, as well as xenobiotic substances such as drugs and toxic chemicals.
Among the P450 families of enzymes, the CYP3A subfamily comprises approximately 30 to 60% of total P450 in human liver, the majority of P450 in human intestine, and a major fraction of P450 in kidneys.[1-3] The CYP3A enzymes are responsible for metabolizing half of all clinically used drugs, encompassing multiple drug classes. The expression of CYP3A enzymes, especially CYP 3A4 and 3A5, shows large interindividual variation, and that these variations can lead to variable clinical response to drugs that are CYP3A substrates.[2, 3] Adding to this variability is the exquisite sensitivity of CYP3A to drug interactions (i.e., induction and inhibition).[2, 4]
Because of the interindividual variability and the sensitivity of CYP3A to drug interactions, there is considerable interest in methods to measure the in vivo activities of CYP3A, to determine the mechanism and magnitude of such interactions and to predict such clinical interactions from in vitro studies. Several CYP3A substrates have been studies as in vivo probes.[5, 6] Midazolam is the most commonly used probe for CYP3A activity, but it has some general limitations, including intermediate hepatic extraction, variability in clearance with changes in hepatic blood flow, and pharmacologically active metabolites.[7-9] Conversely, previous studies suggested that alfentanil shows many characteristics of an ideal CYP3A probe.[10-13] Among the general advantages of alfentanil are elimination exclusively by metabolism, pharmacologically inactive metabolites, low hepatic extraction ratio, and low interday variability in systemic clearance that is not affected by liver blood flow.[13-17]
Assessment of intravenous and oral drug clearances typically require their administration by different routes on separate days. An alternative approach is the concurrent administration of two different drug probes, for example, alfentanil and midazolam, by two different routes, such as oral and intravenous. This reduces the number of study days, blood sampling and analytical determinations (typically by half), eliminates interday variability and thus improves results, shortens overall study duration, and reduces study costs. Concurrent administration of alfentanil and midazolam, by similar routes, has also been used to assess the ability of one CYP3A probe to report on the disposition of another CYP3A probe.[18]
Relatively few HPLC based methods for the quantification of alfentanil in blood or plasma have been reported. Early methods used gas chromatography with mass spectrometry detection to detect alfentanil in urine or plasma.[19, 20] An early HPLC method used ultraviolet detection, with limited sensitivity.[21] Subsequent methods used single quadrupole LCMS with liquid-liquid extraction,[12, 13] and then solid phase extraction.[22] Liquid chromatography-tandem mass spectrometry assays have to date used liquid-liquid extraction.[23-25] Conversely, there are innumerable HPLC assays for midazolam, using either single or tandem quadrupole mass spectrometry, and either liquid-liquid or solid phase extraction.
A sensitive and reliable analytical method for simultaneously quantifying alfentanil and midazolam concentrations in human plasma was needed for supporting clinical pharmacokinetic and CYP3A phenotyping studies. GC/MS and LC/MS methods for simultaneously analyzing alfentanil and midazolam from human plasma, both using liquid-liquid extraction, were developed and validated in our laboratory.[11, 13, 26] These methods are relatively susceptible to matrix effects, require intensive sample preparation steps, greater chromatographic optimization compared to LC-MS/MS methods.
The purpose of this investigation was to develop and validate a sensitive and robust high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for simultaneous determination of alfentanil and midazolam in human plasma, using solid phase extraction. The applicability of this method in assessing plasma alfentanil and midazolam following administration in healthy human volunteers is described.
2. Experimental
2.1 Chemicals and reagents
Alfentanil hydrochloride, midazolam, alfentanil d5 and midazolam d4 (all >98% chemical purity) was purchased from Cerilliant (Round Rock, TX). Oasis MCX 96-well solid phase extraction (SPE) plates were obtained from Waters Corp (Milford, MA). All other reagents were HPLC or better grade and purchased from Sigma Chemical Co. (St. Louis, MO).
2.2. Instrumentation
Analysis was performed on an API 3200 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) equipped with a Turbo Ion Spray ionization source operated at 550 °C. The HPLC system consisted of two LC-20AC pumps with a CTO-20A oven, SIL-20A autosampler, DGU-20A3 degasser, FCF-11AL valve and a CBM 20A controller (Shimadzu, Columbia, MD). The chromatographic separation was performed on a Sunfire C18 column (50 × 2.1 mm, 3.5 μm) (Waters Corp, Milford, MA) with a C18 guard cartridge (10 × 2 mm, 3.5 μm) (Varian, Palo Alto, CA). The injection volume was 20 μl and the oven temperature was 25 °C. Before each injection, the needle was washed with methanol:water (50:50, v:v). Mobile phase (0.3 ml/min) was (A) 0.01% acetic acid and (B) 0.01% acetic acid in methanol using the following program: 25% B for 0.01 min, linear gradient to 80% B between 0.01 and 1.0 min, held at 80% until 2 min, linear gradient to 100% until 3 min, held at 100% B until 4 min, then re-equilibrated to initial conditions between 4.01 and 5.5 min. Under these conditions, retention times for alfentanil and midazolam were 2.1 and 2.4 min, respectively (Table 1). Both Q1 and Q3 quadrupoles were optimized to unit mass resolution, and the mass spectrometer conditions were optimized for each analyte. The instrument was operated in positive-ion mode with an ion spray voltage of 5500 V. The curtain gas was set at 20, ion source gas 1 at 30, ion source gas 2 at 30 and the collision gas was set at 5. Multiple reaction monitoring (MRM) transitions monitored for each analyte, along with the analyte-specific parameters, are listed in Table 1.
Table 1.
Retention times, multiple-reaction monitoring transitions and compound specific settings for analytes and internal standard
| Compound | Retention time (min) | MRM transition (m/z) | Declustering potential (V) | Collision energy (V) |
|---|---|---|---|---|
| Alfentanil | 2.1 | 417.0 / 197.2 | 46 | 35 |
| Alfentanil-d5 | 2.1 | 422.0 / 197.2 | 46 | 39 |
| Midazolam | 2.5 | 326.0 / 291.0 | 71 | 47 |
| Midazolam-d4 | 2.5 | 330.0 / 295.0 | 66 | 45 |
2.3. Sample preparation
Plasma (330 μl of patient sample, calibrators, and QCs) was acidified with freshly prepared 4% phosphoric acid (660 μl, containing 1 ng/ml each of alfentanil d5 and midazolam d4; final concentration, 0.67ng/ml each in acidified plasma) and vortex mixed. Solid phase extraction was used to process samples. Oasis MCX 96 well SPE plates (30 mg per well, Waters Corp, Milford, MA) were conditioned with 1 ml methanol followed by 1 ml of water. An aliquot of acidified plasma (900 μl) was transferred to the SPE plate using a multi-channel pipette. The SPE plate was washed with 1ml 0.1N HCl followed by 1ml methanol. The SPE plate was dried at full vacuum for 2 min, then the samples were eluted with 0.5 ml of 95:5 acetonitrile:ammonium hydroxide (v:v). Samples were dried under nitrogen stream at 60°C. Dried samples were then reconstituted with 100 μl of 15% acetonitrile in 0.05% formic acid and kept at 4°C until analysis.
2.4. Standards and quality control samples
Methanolic solutions (1 mg/ml free base) were prepared of each analyte (alfentanil, alfentanil d5, midazolam and midazolam d4). Dilutions from these stock standards were prepared and used to make standard and quality control (QC) samples in plasma. Plasma calibration standards contained both alfentanil and midazolam at 0.25, 0.5, 1, 2.5, 5, 10, 25, 50 and 100 ng/ml, and plasma QC samples were contained both 1, 10 and 100 ng/ml alfentanil and 1.35, 12 and 100 ng/ml midazolam.
2.5. Assay validation
Analytical methods developed in this study were validated according to Food and Drug Administration (FDA) Guidance for Industry, Bioanalytical Method Validation [US Food and Drug Administration, Guidance for Industry, Bioanalytical Method Validation, Centre for Drug Evaluation and Research (CDER), Rockville, 2001]. Briefly, a minimum of 75% of the total number of standards in the calibration range must not deviate by greater than ±15% (±20% at the lower limit of quantitation, LLOQ) from their nominal values and standards that back-calculate to be greater than ±15% (±20% at the LLOQ) from their nominal value were excluded from the regression analysis. Intra- and interday accuracy and precision were determined by assay of six replicates of low, medium and high QC samples on 3 different days and a minimum of 2/3 of the total number of QCs must not deviate by more than ±15% from their nominal concentration, and at least half of the QC samples at each concentration must be within 100±15% of their nominal concentration. Selectivity was assessed by observing any potential interference peaks at the retention times of analytes and internal standards from six different lots from human plasma. Sample carryover was assessed by injecting an extracted blank sample immediately after the highest calibration standard (upper limit of quantitation, ULOQ) from the first set of calibration standards in each validation run. The peak area of any analyte observed in the carryover blank should be less than 20% of the mean of the corresponding analyte peak areas observed in the lowest accepted standard concentration. Matrix effect was evaluated by using 5 replicates of post extracted blank plasma samples spiked with low, medium and high QC level neat solutions with the QC level neat solutions. Sample recovery was determined by comparing the mean peak areas for 5 replicates analysis of low, medium and high QC samples with those of blank plasma extracts reconstituted with the corresponding neat QC solutions. Stability of analytes in plasma was assessed on storage at room temperature for 12 h (bench top stability), after three freeze-thaw cycles (freeze-thaw stability) and after long-term storage (-80°C for 12 mo). A comparison between the QC concentrations from the reinjected run of QC samples to their nominal concentrations was used to assess the post preparative sample stability. These post preparative samples were kept at 4°C for 72 hours prior to reinjection.
2.6. Method application
This method was applied to samples obtained from a clinical investigation of alfentanil and midazolam disposition. The study was approved by the Washington University Institutional Review Board and performed after obtaining written informed consent from the subject. The subject received 1 mg midazolam and 15 μg/kg alfentanil intravenously, or 3 mg midazolam and 75μg/kg alfentanil orally. Venous blood samples were obtained at 0, 0.02, 0.05, 0.1, 0.2, 0.3, 0.45, 1, 1.02, 1.05, 1.10, 1.15, 1.2, 1.3, 1.45, 2, 2.15, 2.30, 2.45, 3, 3.30, 4, 5, 6, 7, 8, and 9 h after dosing. Plasma samples were stored at -80°C prior to analysis.
3. Results and discussion
3.1. LC/MS/MS method development
The goal of LC-MS/MS method development process is to develop and validate a simple, fast and reliable analytical method to measure alfentanil and midazolam in human plasma. There were numerous individual reports based on HPLC assays for analyzing alfentanil or midazolam, but none of them analyzes these compounds simultaneously using tandem mass spectrometry. Furthermore, some previous methods used conventional analytical dimension HPLC columns (i.e., 150 or 250 mm in length). One assay goal was to use an HPLC column amendable to relatively high speed analysis (5-6 min total analysis time) with acceptable chromatographic performance (peak shape, peak tailing and retention time, etc). Several reversed phase type HPLC columns were initially evaluated, using common LC-MS/MS mobile phase systems (i.e., 0.1% formic acid or 0.1% acetic acid in methanol and water). Some HPLC columns under these mobile phase conditions showed little retention, long equilibration time, distorted peak shape, and/or excessive peak tailing. A Sunfire (Waters Corp) C18 column (50 × 2.1mm ID, 3.5 μm) was eventually chosen and optimal chromatographic performance achieved using methanol:water with 0.01% acetic acid as the mobile phase system. Under these conditions, alfentanil and midazolam were well resolved (retention times of 2.1 and 2.5 min, respectively, resolution=2).
Sample clean-up focused on solid phase extraction (SPE) since this technique generally provides the cleanest sample compared to protein precipitation or liquid-liquid extraction. Furthermore, assay specifications required a limit of quantitation (0.25 ng/ml) not easily achieved using protein precipitation or liquid-liquid extraction, due to excessive matrix effects. In a previous study, a mixed-mode cation exchange SPE (Waters Oasis MCX) plate was successfully used to extract alfentanil from human plasma with more than 80% recovery.[22] The use of MCX SPE for extracting both alfentanil and midazolam was successful without major modifications from the previously reported method. Reverse-phase type solid phase extraction media were also tested, but the matrix suppression was greater than with MCX type media. The use of stable labeled internal standards compensated for any possible matrix effect and further improved the robustness of the method.
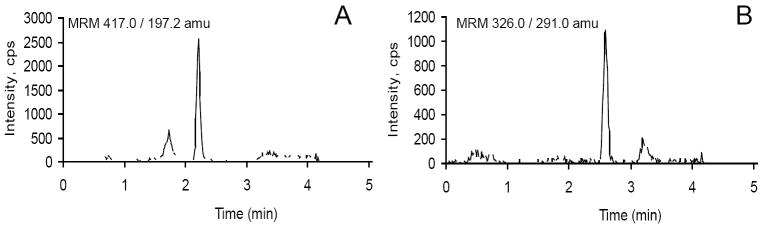
Figure 1 shows typical chromatograms of the lowest standard samples of alfentanil and midazolam spiked in human plasma. Signal intensity was more than adequate, with a signal to noise ratio at the lowest concentration calibration standard of about 80 for alfentanil and 40 for midazolam. There was no interference from other peaks at the retention times of alfentanil and midazolam. Further evaluation found no differences in results obtained from normal vs heavily hemolized or hyperlipdemic plasma samples (not shown).
Figure 1.
Representative chromatograms of the lowest calibration standard (0.25 ng/ml) of (A) alfentanil and (B) midazolam in human plasma.
3.2. Method validation
Calibration standards at 9 concentrations (0.25, 0.5, 1, 2.5, 5, 10, 25, 50 and 100 ng/ml) were prepared (see Section 2.4). Linear regression analysis was performed with 1/x2 weighting using Analyst software v.1.4.2 (Applied Biosystems, Foster City, CA). Table 2 and 3 show the back-calculated calibration standard concentrations for alfentanil and midazolam in human plasma. The results show a linear fit from 0.25 to 100 ng/ml for alfentanil and midazolam (r2>0.99 for both).
Table 2.
Back-calculated concentrations of alfentanil calibration standards in human plasma
| Nominal Concentration (ng/ml) | Calculated Concentration (ng/ml)
|
Mean | SD | %CV | %Difference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Run 1 | Run 3 | Run 3 | ||||||||
| 0.25 | 0.24 | 0.26 | 0.25 | 0.25 | 0.23 | 0.26 | 0.25 | 0.01 | 4.8 | 0.6 |
| 0.5 | 0.54 | 0.48 | 0.50 | 0.52 | 0.44 | 0.49 | 0.50 | 0.03 | 6.8 | 1.1 |
| 1 | 1.17 | 1.00 | 1.07 | 0.93 | 0.90 | 1.02 | 1.02 | 0.10 | 9.5 | -1.6 |
| 2.5 | 2.43 | 2.56 | 2.58 | 2.87 | 2.27 | 2.45 | 2.53 | 0.20 | 8.0 | -1.1 |
| 5 | 4.90 | 4.74 | 5.25 | 5.32 | 5.36 | 5.14 | 5.12 | 0.25 | 4.8 | -2.4 |
| 10 | 9.48 | 9.82 | 10.4 | 10.4 | 9.39 | 10.3 | 10.0 | 0.5 | 4.7 | 0.4 |
| 25 | 24.5 | 22.7 | 23.8 | 26.1 | 22.1 | 23.2 | 23.7 | 1.4 | 6.0 | 5.0 |
| 50 | 50.8 | 55.1 | 50.0 | 54.5 | 51.0 | 51.5 | 52.2 | 2.1 | 4.1 | -4.3 |
| 100 | 100 | 96.7 | 97.8 | 106 | 91.9 | 97.2 | 98.3 | 4.6 | 4.7 | 1.7 |
Table 3.
Back-calculated concentrations of midazolam calibration standards in human plasma
| Nominal Concentration (ng/ml) | Calculated Concentration (ng/ml)
|
Mean | SD | %CV | %Difference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | ||||||||
| 0.25 | 0.25 | 0.26 | 0.24 | 0.27 | 0.26 | 0.27 | 0.26 | 0.01 | 4.4 | -3.6 |
| 0.5 | 0.51 | 0.50 | 0.42 | 0.50 | 0.48 | 0.49 | 0.48 | 0.03 | 6.6 | 3.6 |
| 0.98 | 0.90 | 0.99 | 0.94 | |||||||
| 1 | 1.06 | 1.03 | 0.99 | 0.06 | 5.7 | 1.2 | ||||
| 5 | 3 | 8 | 7 | |||||||
| 2.5 | 2.59 | 2.56 | 2.41 | 2.39 | 2.58 | 2.59 | 2.52 | 0.09 | 3.7 | -0.8 |
| 5 | 5.17 | 5.36 | 5.41 | 4.94 | 5.08 | 5.04 | 5.16 | 0.18 | 3.6 | -3.3 |
| 10 | 10.7 | 10.5 | 9.45 | 9.36 | 10.2 | 10.2 | 10.1 | 0.6 | 5.4 | -0.7 |
| 25 | 23.8 | 26.3 | 21.9 | 22.4 | 25.3 | 23.9 | 23.9 | 1.7 | 7.0 | 4.3 |
| 50 | 51.8 | 54.9 | 50.1 | 44.3 | 53.9 | 53.4 | 51.4 | 3.9 | 7.5 | -2.8 |
| 100 | 95.4 | 108 | 90.8 | 102 | 102 | 109 | 101 | 7 | 7.0 | -1.2 |
Although the method was only validated to 0.25 ng/ml, to match clinical study sensitivity requirements, greater sensitivity was actually possible. The lower limit of detection (based on a signal:noise ratio = 3) was less than 50 pg/ml for midazolam and 25 pg/ml for alfentanil. Preliminary evaluations showed that a lower limit of quantitation (LLOQ, S/N=10) of less than 100 pg/ml for midazolam and 50 pg/ml for alfentanil could easily be achieved. Nevertheless, 0.25 ng/ml was chosen as the LLOQ for routine assay implementation as a matter of practicality since greater sensitivity was not needed to analyze samples from clinical studies.
Precision and accuracy of the method were determined by analyzing quality control samples at 3 concentrations (1, 10 and 100 ng/ml alfentanil and 1.35, 12 and 100 ng/ml midazolam) within the standard curve range to validate reproducibility. Intraday precision and accuracy represent how the precision and accuracy behave within an analytical run and were determined by analyzing six replicates for each QC sample in each of the three validation runs. The results for intraday precision and accuracy were acceptable under FDA guideline (Table 4). Interday precision and accuracy represent the precision and accuracy of the assay over different analytical runs and were determined by analyzing six replicates for each QC sample from three validation runs. The results for interday precision and accuracy were acceptable (Table 4), with inter- and intraday variability less than 10% at all analyte concentrations.
Table 4.
Intra- and inter day precision and relative errors (accuracy) for alfentanil and midazolam.
| Alfentanil | Midazolam | |||||
|---|---|---|---|---|---|---|
| Concentration (ng/ml) | ||||||
| 1 | 10 | 100 | 1.35 | 12 | 100 | |
| Intraday run 1-mean | 0.98 | 9.67 | 102.7 | 1.36 | 10.6 | 103 |
| SD | 0.04 | 0.77 | 5.6 | 0.11 | 0.5 | 7 |
| %CV | 4.1 | 8.0 | 5.4 | 8.1 | 5.1 | 7.2 |
| % RE | -2.0 | -3.3 | 2.7 | 0.7 | -11.3 | 2.9 |
|
| ||||||
| Intraday run 2-mean | 0.99 | 9.88 | 97.1 | 1.39 | 12.0 | 102 |
| SD | 0.05 | 0.42 | 3.2 | 0.09 | 0.6 | 6 |
| %CV | 5.1 | 4.3 | 3.3 | 6.5 | 5.3 | 5.4 |
| % RE | -1.0 | -1.2 | -2.9 | 3.0 | -0.2 | 2.3 |
|
| ||||||
| Intraday run 3-mean | 0.98 | 9.83 | 97.0 | 1.32 | 12.2 | 105 |
| SD | 0.05 | 0.61 | 2.8 | 0.05 | 0.7 | 4 |
| %CV | 5.1 | 6.2 | 2.9 | 3.8 | 6.0 | 3.7 |
| % RE | -2.0 | -1.7 | -3.0 | -2.2 | 1.8 | 4.6 |
|
| ||||||
| Interday run-mean | 0.98 | 9.79 | 98.9 | 1.36 | 11.6 | 103 |
| SD | 0.04 | 0.59 | 4.7 | 0.09 | 0.9 | 5 |
| %CV | 4.1 | 6.0 | 4.7 | 6.6 | 8.0 | 5.3 |
| % RE | -2.0 | -2.1 | -1.1 | 0.7 | -3.3 | 3.3 |
| N | 18 | 18 | 18 | 18 | 18 | 18 |
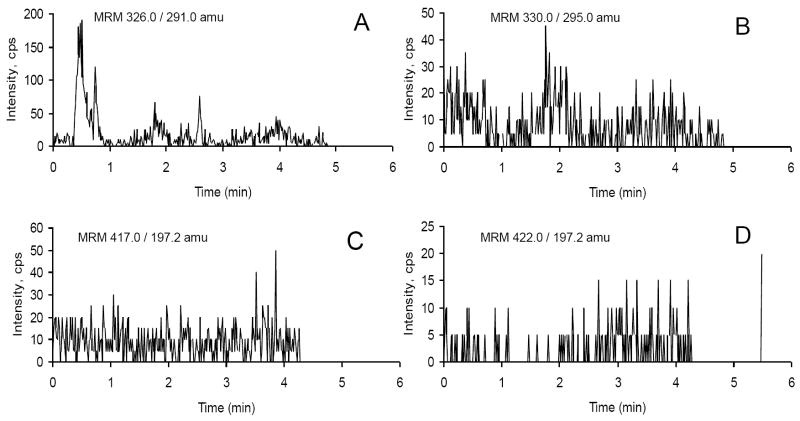
Selectivity is the ability of an analytical method to differentiate and quantify the analyte in the presence of other components in the sample. Six different lots of blank plasma without alfentanil/midazolam or internal standards were extracted and analyzed for evaluating the selectivity of this method. For selectivity to be considered acceptable, each blank sample was tested for any potential interference at the retention time of the peak for alfentanil/midazolam or their internal standards. Figure 2 shows chromatograms of pooled (6 different lots) blank human plasma used for testing selectivity. No interference peak was appeared at the retention times of alfentanil and midazolam.
Figure 2.
Representative chromatograms of blank human plasma to assess potential interfering peaks at the retention times and MRM transitions for (A) midazolam, (B) midazolam d4, (C) alfentanil, and (D) alfentanil d5.
The injection carryover test was to evaluate the extent of carryover of an analyte of interest from one sample to the next in every run. This was performed by placing an extracted blank after the highest calibration standard (ULOQ, 100 ng/ml alfentanil or midazolam). No potentially interfering peak (i.e., carryover) was found at the retention times of alfentanil and midazolam indicating no injection carryover was observed for this method.
Evaluation of matrix effects in LC-MS/MS assay is of particular interest since the analyte and internal standard response can be suppressed or enhanced by the matrix. The matrix effect was determined in human plasma at three concentrations (1, 10, and 100 ng/ml, n=5 each) for alfentanil and midazolam. Matrix effect was evaluated by comparing the peak areas of neat analytes spiked into extracted blank matrix with the peak areas of neat unextracted analytes at the same concentrations. The neat solutions served as reference samples. The matrix suppression for alfentanil and midazolam was all less than 10% at all tested concentrations (Table 5). In addition, over the range of analyte concentrations tested, the degree of matrix suppression varied less than 15% for alfentanil and less than 9% for midazolam, indicating that the matrix suppression met the acceptance criteria and had no significant impact on quantification. Hemolyzed plasma was also analyzed, but no difference from normal plasma samples was observed.
Table 5.
Matrix suppresion and recovery of alfentanil and midazolam in human plasma.
| Analyte | Concentration (ng/ml) | Matrix Suppression (%) | Recovery (%) |
|---|---|---|---|
| Alfentanil | 1 | 7.2 | 81 |
| 10 | 9.4 | 84 | |
| 100 | 7.8 | 85 | |
|
|
|||
| CV | 14.0 % | 2.5 % | |
| Midazolam | 1 | 8.4 | 86 |
| 10 | 9.8 | 84 | |
| 100 | 8.6 | 90 | |
|
|
|||
| CV | 8.5% | 3.5 % | |
In a previous assay, alfentanil recovery from human plasma using Oasis MCX SPE exceeded 80%.[22] The recovery of both alfentanil and midazolam extracted simultaneously in this assay was evaluated by comparing the mean peak areas from analyte added to and recovered from the biological matrix (extracted samples) to the peak areas from unextracted samples at three concentrations (1, 10, and 100 ng/ml, n=5 each), as described for the matrix effect test. The recoveries for alfentanil and midazolam were all more than 80% at all tested concentrations (Table 5). In addition, there were less than 5% variability in recovery across the range of concentrations tested. These indicated that the mixed-mode cation exchange SPE is an effective and reproducible sample preparation technique for simultaneously extracting alfentanil and midazolam from human plasma.
Analyte stability is a function of the storage conditions, the chemical properties of the analyte and the matrix. The purpose of stability tests is to evaluate the stability of analyte during the situations likely to be encountered during actual sample handling and analysis. Results of various stability tests were presented in Table 6 and no significant loss or deterioration was observed for alfentanil and midazolam.
Table 6.
Stability of alfentanil and midazolam in human plasma.
| Analyte | Concentration (ng/ml) | BenchTop (12 hours) | Freeze-thaw (3 times) | Autosampler (72hours) | Long Term (12 months) | ||||
|---|---|---|---|---|---|---|---|---|---|
| CV (%) | Accuracy (%) | CV (%) | Accuracy (%) | CV (%) | Accuracy (%) | CV (%) | Accuracy (%) | ||
| Alfentanil | 1 | 8.4 | 104 | 6.4 | 99.8 | 4.8 | 102 | 8.5 | 95.5 |
| 10 | 6.8 | 97.4 | 7.5 | 96.4 | 5.5 | 105 | 6.4 | 102 | |
| 100 | 4.5 | 102 | 5.6 | 95.6 | 4.9 | 102 | 6.2 | 97.4 | |
| Midazolam | 1 | 7.6 | 96.4 | 6.4 | 93.5 | 8.5 | 102 | 7.8 | 106 |
| 10 | 7.1 | 99.6 | 5.7 | 97.5 | 7.5 | 98.6 | 8.2 | 102 | |
| 100 | 5.4 | 93.6 | 4.9 | 94.6 | 7.1 | 95.8 | 5.1 | 102 | |
3.3. Method application
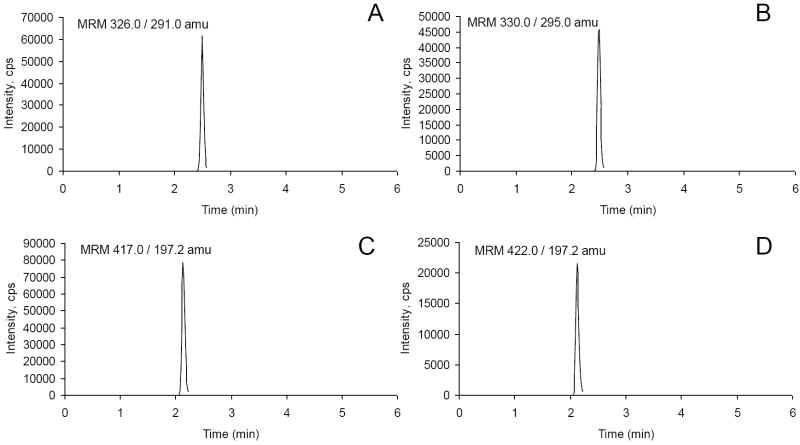
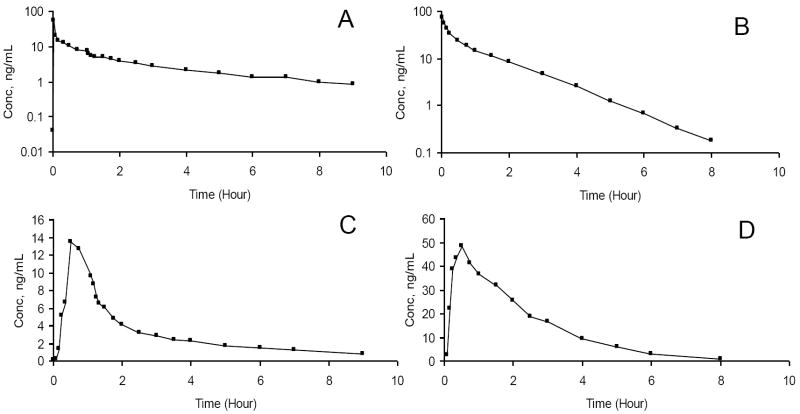
The validated LC-MS/MS method developed in this work was successfully applied to determine the plasma concentration of alfentanil and midazolam in healthy human subjects.Figure 3. shows typical chromatograms. The plasma concentration–time curves from a single human subject for intravenous (A,B) or oral (C,D) alfentanil and midazolam are presented in Figure 4.
Figure 3.
Representative LC-MS/MS chromatograms of human plasma from a subject administered alfentanil and midazolam. Shown are (A) midazolam, (B) midazolam d4, (C) alfentanil, and (D) alfentanil-d5.
Figure 4.
Plasma concentration vs time profiles of alfentanil and midazolam after administering (A) intravenous midazolam, (B) intravenous alfentanil, (C) oral midazolam, and (D) oral alfentanil.
4. Conclusion
An LC-MS/MS method has been validated for the quantitation of alfentanil and midazolam in human plasma. The assay was linear from 0.25 to 100 ng/ml for each compound. The lowest standards (i.e., 0.25ng/ml) generated typical signal to noise (S/N) ratios around 80 and 40 for alfentanil and midazolam, respectively. Recovery of alfentanil and midazolam from plasma was more than 80% while matrix suppression was less than 10%. Alfentanil and midazolam are stable during storage, processing, and analysis in human plasma samples. The results indicate the method to be sensitive, selective, accurate, and reproducible. The validated method was successfully applied to obtain concentration-time curves of oral and intravenous alfentanil and midazolam in human healthy subjects.
Acknowledgments
The authors thank Kristi Stubbert for her excellent technical assistance.
Supported by NIH grants R01-GM63674, R01-DA14211, and K24-DA00417
Footnotes
Conflict of interest: No author has any conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 2.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 3.Plant N. The human cytochrome P450 sub-family: transcriptional regulation, inter-individual variation and interaction networks. Biochim Biophys Acta. 2007;1770:478–488. doi: 10.1016/j.bbagen.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Harris R, Jang G, Tsunoda S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet. 2003;42:1071–1088. doi: 10.2165/00003088-200342130-00001. [DOI] [PubMed] [Google Scholar]
- 5.Streetman DS, Bertino JS, Jr, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Tucker GT, Houston JB, Huang S-M. Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential-toward a consensus. Clin Pharmacol Ther. 2001;70:103–114. doi: 10.1067/mcp.2001.116891. [DOI] [PubMed] [Google Scholar]
- 7.Rogers JF, Rocci ML, Jr, Haughey DB, Bertino JS., Jr An evaluation of the suitability of intravenous midazolam as an in vivo marker for hepatic cytochrome P4503A activity. Clin Pharmacol Ther. 2003;73:153–158. doi: 10.1067/mcp.2003.23. [DOI] [PubMed] [Google Scholar]
- 8.Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite a-hydroxymidazolam in healthly volunteers. Clin Pharmacol Ther. 1992;51:715–728. doi: 10.1038/clpt.1992.84. [DOI] [PubMed] [Google Scholar]
- 9.Kirwan C, Macphee I, Philips B. Using drug probes to monitor hepatic drug metabolism in critically ill patients: midazolam, a flawed but useful tool for clinical investigation of CYP3A activity? Expert Opin Drug Metab Toxicol. 2010;6:1–11. doi: 10.1517/17425255.2010.482929. [DOI] [PubMed] [Google Scholar]
- 10.Kharasch ED, Russell M, Mautz D, Thummel KE, Kunze KL, Bowdle TA, Cox K. The role of cytochrome P450 3A4 in alfentanil clearance. Implications for interindividual variability in disposition and perioperative drug interactions. Anesthesiology. 1997;87:36–50. doi: 10.1097/00000542-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kharasch ED, Jubert C, Senn T, Bowdle TA, Thummel KT. Intraindividual variability in male hepatic CYP3A4 activity assessed by alfentanil and midazolam clearance. J Clin Pharmacol. 1999;39:664–669. doi: 10.1177/00912709922008290. [DOI] [PubMed] [Google Scholar]
- 12.Kharasch ED, Hoffer C, Walker A, Sheffels P. Disposition and miotic effects of oral alfentanil: a potential noninvasive probe for first-pass cytochrome P4503A activity. Clin Pharmacol Ther. 2003;73:199–208. doi: 10.1067/mcp.2003.30. [DOI] [PubMed] [Google Scholar]
- 13.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass CYP3A activity. Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004;76:452–466. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Chauvin M, Lebrault C, Levron JC, Duvaldestin P. Pharmacokinetics of alfentanil in chronic renal failure Anesth. Analg. 1987;66:53–56. [PubMed] [Google Scholar]
- 15.Chauvin M, Bonnet F, Montembault C, Levron JC, Viars P. The influence of hepatic plasma flow on alfentanil plasma concentration plateaus achieved with an infusion model in humans: measurement of alfentanil hepatic extraction coefficient Anesth. Analg. 1986;65:999–1003. [PubMed] [Google Scholar]
- 16.Meuldermans W, Van Peer A, Hendrickx J, Woestenborghs R, Lauwers W, Heykants J, Vanden Bussche G, Van Craeyvelt H, Van der Aa P. Alfentanil pharmacokinetics and metabolism in humans. Anesthesiology. 1988;69:527–534. doi: 10.1097/00000542-198810000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, Whittington D, Ensign D. Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther. 2007;82:410–426. doi: 10.1038/sj.clpt.6100237. [DOI] [PubMed] [Google Scholar]
- 18.Kharasch ED, Thummel KE, Watkins PB. CYP3A probes can quantitatively predict the in vivo kinetics of other CYP3A substrates and can accurately assess CYP3A induction and inhibition. Mol Interv. 2005;5:151–153. doi: 10.1124/mi.5.3.3. [DOI] [PubMed] [Google Scholar]
- 19.Mautz DM, Labroo RB, Kharasch ED. Determination of alfentanil and noralfentanil in human plasma by gas chromatography-mass spectrometry. J Chromatogr B. 1994;658:149–153. doi: 10.1016/0378-4347(94)00201-0. [DOI] [PubMed] [Google Scholar]
- 20.Van Nimmen NF, Poels KL, Veulemans HA. Highly sensitive gas chromatographic-mass spectrometric screening method for the determination of picogram levels of fentanyl, sufentanil and alfentanil and their major metabolites in urine of opioid exposed workers. J Chromatogr B. 2004;804:375–387. doi: 10.1016/j.jchromb.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Kumar K, Morgan DJ, Crankshaw DP. Determination of fentanyl and alfentanil in plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1987;419:464–468. doi: 10.1016/0378-4347(87)80317-1. [DOI] [PubMed] [Google Scholar]
- 22.Kharasch ED, Walker A, Hoffer C, Sheffels P. Evaluation of first-pass cytochrome P4503A (CYP3A) and P-glycoprotein activities using alfentanil and fexofenadine in combination. J Clin Pharmacol. 2005;45:79–88. doi: 10.1177/0091270004269705. [DOI] [PubMed] [Google Scholar]
- 23.Baririan N, Horsmans Y, Desager JP, Verbeeck R, Vanbinst R, Wallemacq P, Van Obbergh L. Alfentanil-induced miosis clearance as a liver CYP3A4 and 3A5 activity measure in healthy volunteers: improvement of experimental conditions. J Clin Pharmacol. 2005;45:1434–1441. doi: 10.1177/0091270005282629. [DOI] [PubMed] [Google Scholar]
- 24.Gergov M, Nokua P, Vuori E, Ojanpera I. Simultaneous screening and quantification of 25 opioid drugs in post-mortem blood and urine by liquid chromatography-tandem mass spectrometry. Forensic Sci Int. 2009;186:36–43. doi: 10.1016/j.forsciint.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Cooreman S, Deprez C, Martens F, Van Bocxlaer J, Croes K. A comprehensive LCMS-based quantitative analysis of fentanyl-like drugs in plasma and urine. J Sep Sci. 2010;33:2654–2662. doi: 10.1002/jssc.201000330. [DOI] [PubMed] [Google Scholar]
- 26.Phimmasone S, Kharasch ED. A pilot evaluation of alfentanil-induced miosis as a noninvasive probe for hepatic cytochrome P450 3A4 (CYP3A4) activity in humans. Clin Pharmacol Ther. 2001;70:505–517. doi: 10.1067/mcp.2001.119994. [DOI] [PubMed] [Google Scholar]